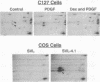
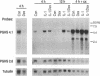
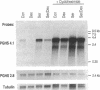
Abstract
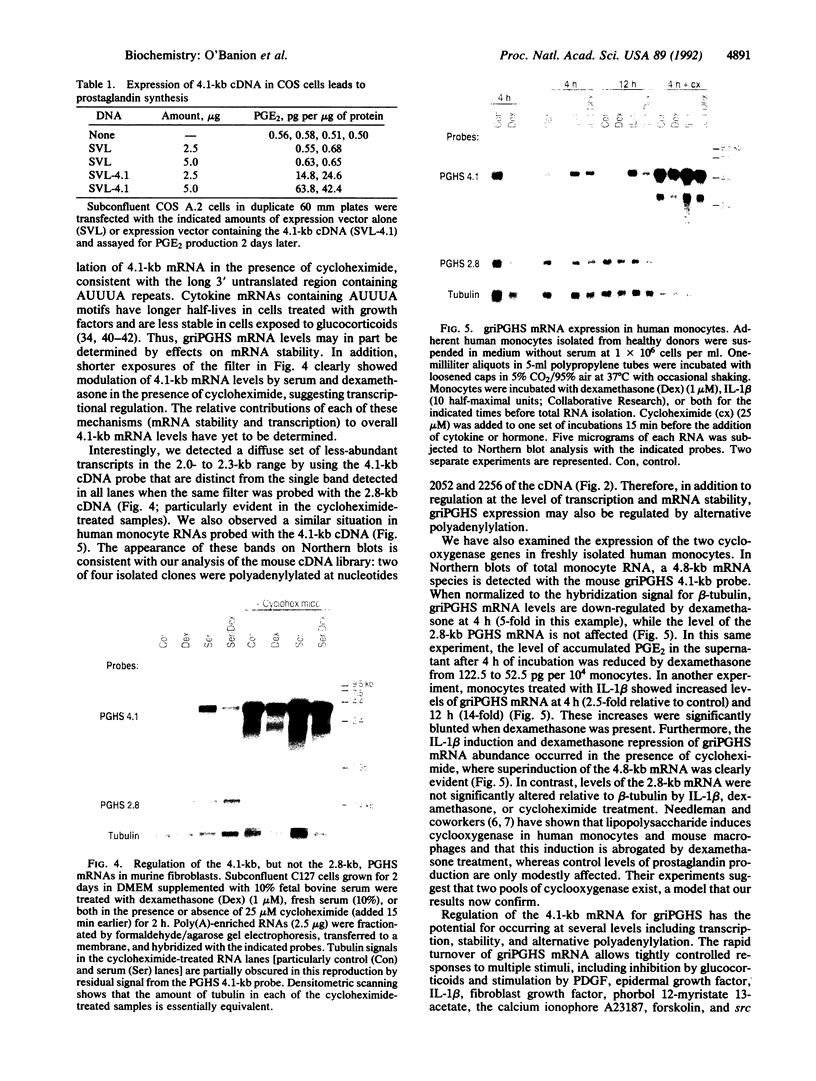
The antiinflammatory glucocorticoids are potent inhibitors of cyclooxygenase, a key regulator of prostaglandin synthesis; yet, the mechanism(s) by which this occurs is not fully understood. We have cloned a 4.1-kilobase (kb) cDNA, distinct from the previously cloned cyclooxygenase (2.8 kb), that confers cyclooxygenase activity to transfected cells. The mRNA for this newly discovered cyclooxygenase is unique for its long 3' untranslated region containing many AUUUA repeats. Levels of the 4.1-kb cyclooxygenase mRNA are rapidly increased by serum or interleukin 1 beta in mouse fibroblasts and human monocytes, respectively, and decreased by glucocorticoids, whereas levels of the 2.8-kb cyclooxygenase mRNA do not change. Similar effects are seen in the presence of cycloheximide where the 4.1-kb, but not the 2.8-kb, mRNA is greatly superinduced. Thus, there are both constitutive (2.8 kb) and regulated (4.1 kb) cyclooxygenase species, the latter most likely being a major mediator of inflammation.
Full text
PDF

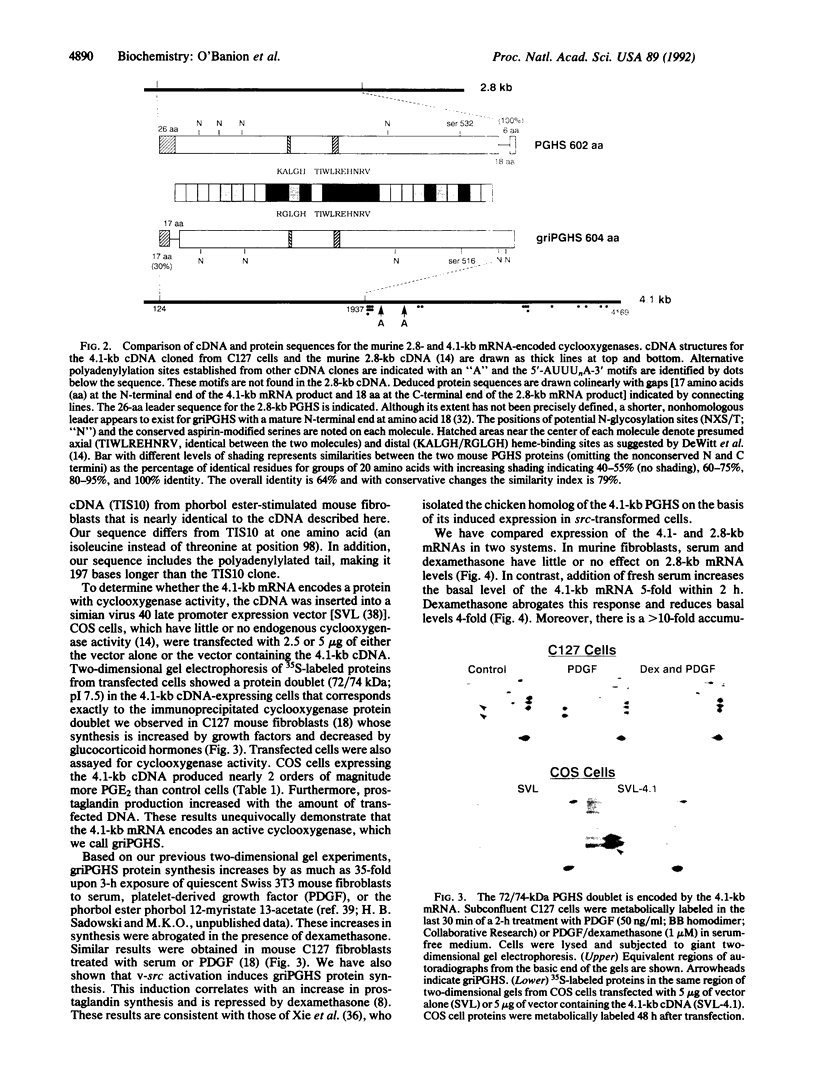

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Makheja A. N., Pash J., Verma M. Corticosteroids suppress cyclooxygenase messenger RNA levels and prostanoid synthesis in cultured vascular cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1159–1163. doi: 10.1016/s0006-291x(88)80995-1. [DOI] [PubMed] [Google Scholar]
- Boggaram V., Smith M. E., Mendelson C. R. Posttranscriptional regulation of surfactant protein-A messenger RNA in human fetal lung in vitro by glucocorticoids. Mol Endocrinol. 1991 Mar;5(3):414–423. doi: 10.1210/mend-5-3-414. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Harris B. A. Plasmids for the cloning and expression of full-length double-stranded cDNAs under control of the SV40 early or late gene promoter. Nucleic Acids Res. 1983 Oct 25;11(20):7119–7136. doi: 10.1093/nar/11.20.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DeWitt D. L., Smith W. L. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1412–1416. doi: 10.1073/pnas.85.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt D. L., el-Harith E. A., Kraemer S. A., Andrews M. J., Yao E. F., Armstrong R. L., Smith W. L. The aspirin and heme-binding sites of ovine and murine prostaglandin endoperoxide synthases. J Biol Chem. 1990 Mar 25;265(9):5192–5198. [PubMed] [Google Scholar]
- Del Sal G., Manfioletti G., Schneider C. The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages or plasmids suitable for sequencing. Biotechniques. 1989 May;7(5):514–520. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frasier-Scott K., Hatzakis H., Seong D., Jones C. M., Wu K. K. Influence of natural and recombinant interleukin 2 on endothelial cell arachidonate metabolism. Induction of de novo synthesis of prostaglandin H synthase. J Clin Invest. 1988 Dec;82(6):1877–1883. doi: 10.1172/JCI113805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [PubMed] [Google Scholar]
- Funk C. D., FitzGerald G. A. Eicosanoid forming enzyme mRNA in human tissues. Analysis by quantitative polymerase chain reaction. J Biol Chem. 1991 Jul 5;266(19):12508–12513. [PubMed] [Google Scholar]
- Han J. W., Sadowski H., Young D. A., Macara I. G. Persistent induction of cyclooxygenase in p60v-src-transformed 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1990 May;87(9):3373–3377. doi: 10.1073/pnas.87.9.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M., Lands W. E. Purification of the cyclooxygenase that forms prostaglandins. Demonstration of two forms of iron in the holoenzyme. J Biol Chem. 1976 Sep 25;251(18):5575–5579. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hla T., Farrell M., Kumar A., Bailey J. M. Isolation of the cDNA for human prostaglandin H synthase. Prostaglandins. 1986 Dec;32(6):829–845. doi: 10.1016/0090-6980(86)90093-6. [DOI] [PubMed] [Google Scholar]
- Iwai Y., Bickel M., Pluznik D. H., Cohen R. B. Identification of sequences within the murine granulocyte-macrophage colony-stimulating factor mRNA 3'-untranslated region that mediate mRNA stabilization induced by mitogen treatment of EL-4 thymoma cells. J Biol Chem. 1991 Sep 25;266(27):17959–17965. [PubMed] [Google Scholar]
- Koehler L., Hass R., DeWitt D. L., Resch K., Goppelt-Struebe M. Glucocorticoid-induced reduction of prostanoid synthesis in TPA-differentiated U937 cells is mainly due to a reduced cyclooxygenase activity. Biochem Pharmacol. 1990 Sep 15;40(6):1307–1316. doi: 10.1016/0006-2952(90)90397-4. [DOI] [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson R., Iwata K., Klagsbrun M., Young D. A. Growth factor- and dexamethasone-induced proteins in Swiss 3T3 cells. Relationship to DNA synthesis. J Biol Chem. 1985 Jul 5;260(13):8056–8063. [PubMed] [Google Scholar]
- Maier J. A., Hla T., Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem. 1990 Jul 5;265(19):10805–10808. [PubMed] [Google Scholar]
- Masferrer J. L., Zweifel B. S., Seibert K., Needleman P. Selective regulation of cellular cyclooxygenase by dexamethasone and endotoxin in mice. J Clin Invest. 1990 Oct;86(4):1375–1379. doi: 10.1172/JCI114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Fagan D., Mudd J., Needleman P. Isolation and characterization of the complementary DNA for sheep seminal vesicle prostaglandin endoperoxide synthase (cyclooxygenase). J Biol Chem. 1988 Mar 15;263(8):3550–3553. [PubMed] [Google Scholar]
- O'Banion M. K., Sadowski H. B., Winn V., Young D. A. A serum- and glucocorticoid-regulated 4-kilobase mRNA encodes a cyclooxygenase-related protein. J Biol Chem. 1991 Dec 5;266(34):23261–23267. [PubMed] [Google Scholar]
- O'Banion M. K., Young D. A. Bovine papillomavirus type 1 alters the processing of host glucose- and calcium-modulated endoplasmic reticulum proteins. J Virol. 1991 Jul;65(7):3481–3488. doi: 10.1128/jvi.65.7.3481-3488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppel K., Vinci J. M., Baglioni C. The AU-rich sequences in the 3' untranslated region mediate the increased turnover of interferon mRNA induced by glucocorticoids. J Exp Med. 1991 Feb 1;173(2):349–355. doi: 10.1084/jem.173.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Wyche A., Fu J., Seibert K., Needleman P. Regulation of prostanoids synthesis in human fibroblasts and human blood monocytes by interleukin-1, endotoxin, and glucocorticoids. Adv Prostaglandin Thromboxane Leukot Res. 1990;20:22–27. [PubMed] [Google Scholar]
- Raz A., Wyche A., Needleman P. Temporal and pharmacological division of fibroblast cyclooxygenase expression into transcriptional and translational phases. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1657–1661. doi: 10.1073/pnas.86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Wyche A., Siegel N., Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988 Feb 25;263(6):3022–3028. [PubMed] [Google Scholar]
- Roberts N. J., Jr, Steigbigel R. T. Effect of in vitro virus infection on response of human monocytes and lymphocytes to mitogen stimulation. J Immunol. 1978 Sep;121(3):1052–1058. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Smith W. L., DeWitt D. L., Kraemer S. A., Andrews M. J., Hla T., Maciag T., Shimokawa T. Structure-function relationships in sheep, mouse, and human prostaglandin endoperoxide G/H synthases. Adv Prostaglandin Thromboxane Leukot Res. 1990;20:14–21. [PubMed] [Google Scholar]
- Wu K. K., Sanduja R., Tsai A. L., Ferhanoglu B., Loose-Mitchell D. S. Aspirin inhibits interleukin 1-induced prostaglandin H synthase expression in cultured endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2384–2387. doi: 10.1073/pnas.88.6.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. A., Voris B. P., Maytin E. V., Colbert R. A. Very-high-resolution two-dimensional electrophoretic separation of proteins on giant gels. Methods Enzymol. 1983;91:190–214. doi: 10.1016/s0076-6879(83)91017-0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]