Abstract
Background: Sac behavior after endovascular aneurysm repair (EVAR) for abdominal aortic aneurysms (AAAs) is considered as a surrogate for the risk of late rupture. The purpose of the study is to assess the sac behavior of AAAs after EVAR.
Methods and Results: Late sac enlargement (LSE) (≥5 mm) and late sac shrinkage (LSS) (≥5 mm) were analyzed in 589 consecutive patients who were registered at 14 national centers in Japan. The proportions of patients who had LSE at 1, 3 and 5 years were 2.6% ± 0.7%, 10.0% ± 1.6% and 19.0% ± 2.9%. The proportions of patients who had LSS at 1, 3 and 5 years were 50.1% ± 0.7%, 59.2% ± 2.3% and 61.7% ± 2.7%. Multiple logistic regression analysis identified two variables as a risk factor for LSE; persistent endoleak (Odds ratio 9.56 (4.84–19.49), P <0.001) and low platelet count (Odds ratio 0.92 (0.86–0.99), P = 0.0224). The leading cause of endoleak in patients with LSE was type II.
Conclusions: The incidence of LSE is not negligible over 5 year period. Patients with persistent endoleak and/or low platelet count should carefully be observed for LSE.
Clinical Trial Registration: UMIN-CTR (UMIN000008345).
Keywords: abdominal aortic aneurysm, endovascular aneurysm repair, sac behavior, endoleak, late rupture
Introduction
The first successful case of endovascular aneurysm repair (EVAR) for abdominal aortic aneurysm (AAA) was reported by Parodi et al. in 1991.1) EVAR is currently accepted as a less-invasive alternative of open surgical repair for AAA. Several prospective randomized trials that compared open surgical repair with EVAR reported that the short-term survival rate of EVAR was better than that of open surgical repair.2–4) However, such a survival advantage disappeared over time.4–6) Because the goal of EVAR is to prevent rupture of AAAs, long term follow-up is required for confirming this benefit.
As a surrogate endpoint, sac behavior of either enlargement or shrinkage is assumed to predict the risk of rupture. A previous study alarmingly reported that sac enlargement greater than or equal to 5 mm was 17% at 3 years and 41% at 5 years.7) The study includes the very early generation of EVAR devices that potentially cause late sac enlargement frequently. However, it is unknown how the late sac behavior is after EVAR that utilizes more recent generation of devices.
In Japan, EVAR was approved as a clinical procedure in July 2006 and its medical reimbursement for the device was granted in January 2007.8) Fourteen centers of the National Hospital Organization (NHO) joined to form registration of AAA cases retrospectively. The present study aimed to assess late sac behavior in patients who had endovascular repair of AAA in Japanese population.
Methods
This retrospective, observational study was conducted at 14 national medical centers participating in the NHO network study group. We reviewed and collected clinical data of AAA cases. Late events have been collected. All 14 databases were combined into one large database of registration, which was then analyzed. The study was approved by the central human rights ethical committee and by the institutional review board at each participating center. Progress of the study was assessed annually, and its extension was approved by the central human rights ethical committee of the NHO. All of the 14 participating institutions displayed the notice that they joined the NHO network study of AAA in their center according to the ethical guidelines for epidemiological research, which is published by the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour, and Welfare, Japan.9)
Patients and registration
Patients’ registration and management of the database have been previously described.10,11) In brief, indications for treating infra-renal AAA in the present study were as follows: a maximum diameter greater than or equal to 5 cm or 4–5 cm with rapid enlargement of 5 mm or more over 6 months or a saccular morphology that carried a high risk of rupture. Each patient had a preoperative examination, including a multi-detector computed tomography (CT) examination according to the requirements in each participating center. The choice of treatment modality between EVAR and open abdominal repair depended upon the decision made by individual surgeon and endovascular therapist in each institution. A total of 115 variables consisting of preoperative, intraoperative, and postoperative variables were collected. Parameters were selected based on 10 risk scores, which have been previously published, for predicting risks of open abdominal repair of AAAs.12) After anonymization in a linkable fashion, all databases at the 14 centers were connected to a large database.
From January 2007 to August 2012, a total of 2154 consecutive patients who had AAAs were treated and registered. Among them, 589 patients underwent EVAR.
CT examination and follow-up schedule
Baseline schedule of follow-up visit and CT examination was conducted at 1 month, 6 months, 1 year and thereafter annually according to the recommendations of the Japanese Committee of Stentgraft Management (JCSM). Additional outpatient visit and examination were performed as needed. The enhancement of CT examination with contrast medium depended upon the patient condition such as renal function. The maximum short axis diameter of aneurysm sac by CT examination was measured by radiologist. The shrinkage or enlargement of aneurysmal sac was calculated by comparing with preoperative CT imaging as a reference.
Outcome measures and statistical analysis
The primary outcome measure was sac enlargement greater than or equal to 5 mm. Secondary outcome measures included sac shrinkage greater than or equal to 5 mm, surgical mortality, and aneurysm-related death. Surgical mortality was defined as either in-hospital death or death within 30 days.
Statistical analysis was conducted using a statistical software package (JMP version 11; SAS Institute, Tokyo, Japan). Two groups were compared using the chi-square test for categorical variables and the Student’s t-test for continuous variables. The results are expressed as a proportion for categorical variables and as mean ± standard deviation for continuous variables. Survival rates free of all-cause death, and of aneurysm-related death were calculated with the Kaplan–Meier survival estimates and comparisons were made by log-rank analysis. The cumulative incidence of sac enlargement and shrinkage in all patients was also calculated. Univariate analyses of preoperative and intraoperative variables for predicting late sac enlargement (LSE) were conducted. Variables of P value less than 0.2 were included for constructing multiple logistic regression model for LSE. P values of less than 0.05 were considered to indicate statistical significance.
Results
Patients’ characteristics and EVAR procedure
The patients’ characteristics and preoperative conditions are shown in Table 1. Mean age was 77.3 ± 7.4 year old. Maximum diameter of AAA was 50.9 ± 9.9 mm. Eighty-three percent of patients were male gender. Incidences of hypertension and diabetes mellitus were 81.8% and 17.2%. It is noted that among 589 patients who were treated with EVAR, 410 were classified as within instruction for use (IFU) and 179 were classified as outside IFU. The reason of outside IFU included angulated neck in 59 patients, short distal landing zone in 45, short neck in 30 and narrow access arteries in 17 and narrow terminal aorta in 10.
Table 1.
Patients’ characteristics and preoperative conditions
| N = 589 | |
|---|---|
| Age | 77.3 ± 7.4 |
| Maximum diameter of aneurysm (mm) | 50.9 ± 9.9 |
| Male gender | 487 (82.7%) |
| BMI ≥25 | 140 (23.8%) |
| BMI ≥30 | 18 (3.1%) |
| Smoking history | 373 (63.3%) |
| Current smoker | 89 (15.1%) |
| Hypertension | 482 (81.8%) |
| DM | 101 (17.2%) |
| Preoperative creatinine | 1.2 ± 2.7 |
| Coronary artery disease | 206 (35.0%) |
| Myocardial ischemia | 99 (16.8%) |
| History of stroke | 124 (21.1%) |
| NYHA III/IV | 17 (2.9%) |
| Steroid use | 25 (4.3%) |
| COPD on inhaled drug therapy | 37 (6.3%) |
| Preoperative VC | 933 ± 19.0 |
| FEV1.0 | 73.8 ± 15.8 |
| History of abdominal surgery | 149 (25.3%) |
BMI: body-mass index; DM: diabetes mellitus; NYHA: New York Heart Association functional class; COPD: chronic obstructive pulmonary disease; VC: vital capacity; FEV1.0: forced expiratory volume at 1.0 second
The four devices that were used for EVAR were the Gore Excluder AAA endoprosthesis (W. L. Gore & Associates, Flagstaff, AZ, USA) (52.5%), the COOK Zenith AAA endovascular system (Cook Medical, Bloomington, IN, USA) (30.1%), the Endologix PowerLink system (Endologix, Irvine, CA, USA) (14.0%), and the Endurant AAA Stent Graft system (Medtronic, Minneapolis, MN, USA) (3.4%).
Early and intermediate term results
Early results were detailed in Table 2. In-hospital mortality was observed in seven (1.2%) patients. Three patients died of multiple organ failure, two of acute myocardial infarction, one of renal failure and one of shower embolism. With respect to early morbidity, delayed wound healing or infection was the most frequent morbidity in 16 patients (2.72%); additional intervention for peripheral malperfusion in the index hospitalization was required in 14 patients (2.38%). New onset of dialysis was required in six patients (1.00%). Kaplan–Meier survival estimates were conducted. The mean duration of follow-up was 2.9 ± 1.6 years (median 3.0). Ninety-five patients died during the follow-up period. Twenty-six patients had aneurysm-related deaths, which included one late aneurysm rupture, four sudden death and 17 deaths due to unknown causes. Kaplan–Meier survival analysis showed that the rate of survival free from all-cause death in all patients was 78.1% ± 2.4% at 5 years. The survival rates free of aneurysm-related death in all patients was 94.4% ± 1.2% at 5 years.
Table 2.
Early mortality and morbidity
| N = 589 | |
|---|---|
| Operative mortality | 7 (1.20%) |
| Morbidity | |
| Postoperative liver dysfunction | 3 (0.51%) |
| Gastro-intestinal complication | 7 (1.20%) |
| Re-procedure for bleeding | 3 (0.51%) |
| Dialysis (postoperative new event) | 6 (1.00%) |
| Postoperative cardiac failure | 1 (0.17%) |
| Delayed wound healing/infection | 16 (2.72%) |
| Additional procedure for malperfusion | 14 (2.38%) |
| Stroke | 2 (0.34%) |
| Intermittent claudication | 2 (0.34%) |
| General infection (pneumonia/urinary tract infection) | 5 (0.85%) |
Late sac behavior
Twelve patients had type I endoleak and six had type III endoleak (2:4) as shown by enhanced CT examination immediate after EVAR. These patients had additional intervention during the index hospitalization or in the early stage of follow-up within 3 months, although sac enlargement was not observed during this period.
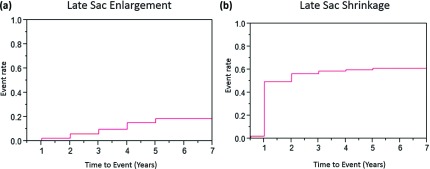
Fifty-two patients showed LSE and 273 patients did late sac shrinkage (LSS) after 4 months. The proportions of patients who showed LSE or LSS are shown in Figs. 1a and 1b. The proportions of patients who had LSE at 1, 2, 3, 4 and 5 years were 2.6% ± 0.7%, 6.2% ± 1.2%, 10.0% ± 1.6%, 15.5% ± 2.2% and 19.0% ± 2.9% respectively. While those of LSS at 1, 2, 3, 4, and 5 years were 50.1% ± 2.3%, 57.1% ± 2.3%, 59.2% ± 2.3%, 60.3% ± 2.4%, and 61.7% ± 2.7% respectively. Out of 52 patients who showed LSE, 33 patients had persistent endoleak at the time when LSE was diagnosed (type I: 6, type II: 26, type III: 1). Endoleak was not visualized in 8 patients and enhanced CT was not available in 11 patients. Type II endoleak is the leading cause of patients with LSE.
Fig. 1.
(a) Cumulative incidence of late sac enlargement. (b) Cumulative incidence of late sac shrinkage.
In order to identify risks of LSE, univariate analyses were conducted (Table 3). Then the variables of P value less than 0.2 were included for constructing the multiple logistic regression model. The results identified two variables as a risk factor for LSE; persistent endoleak (Odds ratio 9.56 (4.84–19.49), P <0.001) and preoperative low platelet count (Odds ratio 0.92 (0.86–0.99), P = 0.0224) (Table 4).
Table 3.
Univariate analysis for late sac enlargement (P <0.2)
| Odds ratio | Lower 95% CI | Upper 95% CI | P value | |
|---|---|---|---|---|
| Coronary artery disease | 1.7127 | 0.9098 | 3.4246 | 0.0972 |
| Cerebrovascular disease | 0.5592 | 0.3009 | 1.0788 | 0.0816 |
| Male gender | 1.7488 | 0.8590 | 3.3703 | 0.1193 |
| Atrial fibrillation/atrial flutter | 0.3522 | 0.1560 | 0.8743 | 0.026 |
| History of previous abdominal surgery | 1.9483 | 0.9369 | 4.5795 | 0.0758 |
| Procedure time | 1.0030 | 0.9986 | 1.0070 | 0.1782 |
| Smoking history | 1.7407 | 0.9715 | 3.1099 | 0.0623 |
| Platelet count | 0.9566 | 0.9032 | 1.0027 | 0.1174 |
| Pre-procedural maximum short axis diameter | 1.0202 | 0.9915 | 1.0484 | 0.1577 |
| Endoleak at the time of LSE | 11.2724 | 6.0693 | 21.5907 | <.0001 |
| OIFU | 1.0366 | 0.5631 | 1.9838 | 0.9102 |
CI: confidence interval; LSE: late sac enlargement; OIFU: outside instruction for use
Table 4.
Multiple logistic regression analysis for LSE
| Odds ratio | Lower 95% CI | Upper 95% CI | P value | |
|---|---|---|---|---|
| Endoleak at the time of LSE | 9.5550 | 4.8401 | 19.4939 | <.0001 |
| Platelet count | 0.9242 | 0.8597 | 0.9892 | 0.0224 |
| History of previous abdominal surgery | 1.7151 | 0.7592 | 4.3007 | 0.2016 |
| Procedure time | 1.0023 | 0.9970 | 1.0074 | 0.389 |
| OIFU | 1.4233 | 0.6635 | 3.2048 | 0.3706 |
| Atrial fibrillation/atrial flutter | 0.6504 | 0.2310 | 2.0098 | 0.4401 |
| Male gender | 1.4082 | 0.5273 | 3.6885 | 0.4891 |
| Pre-procedural maximum short axis diameter | 0.9888 | 0.9521 | 1.0302 | 0.5796 |
| Coronary artery disease | 1.2216 | 0.5774 | 2.6964 | 0.6056 |
| Cerebrovascular disease | 0.8168 | 0.3856 | 1.8141 | 0.6092 |
| Smoking history | 1.0582 | 0.4669 | 2.3171 | 0.8895 |
CI: confidence interval; LSE: late sac enlargement; OIFU: outside instruction for use
For 52 patients who had LSE, the following treatments were conducted; surgical graft replacement in 3 patients, additional endovascular graft placement in 3, sacotomy in 2 (surgical ligation of type II endoleak) and coil embolization in 8. The rest of 36 patients were under observation.
Discussion
Endovascular stent-graft repair has become an established therapeutic modality for AAA repair. As EVAR is recognized as a less invasive procedure than open abdominal repair, it has become the preferred procedure, accounting for 70%–80% of AAA repair in European countries and North America.13) In Japan where EVAR was introduced as a commercially available device in 2007, approximately 50% of patients with AAA were treated with EVAR in recent years.14) However, the EVAR procedure leaves the aneurysmal sac. Therefore, blood supply to this sac is sometimes maintained, resulting in sac enlargement. Enlargement and shrinkage of the sac are considered as surrogate endpoints for assessing the risk of late rapture. Schanzer et al.7) previously reported that the 5-year post-EVAR rate of AAA sac enlargement was alarmingly high at 41%. In the present study, the incidence of sac enlargement at 3 and 5 years was 10.0% and 19.0%. This may be interpreted by the fact that devices used in the present study were more recent generation with better device performance. Modification of devices must have reduced late sac enlargement. Although 10% of LSE at 3 years in the present study is similar with 8%–10% in other studies,13,15,16) such proportion is steadily increased from 1 year up to 5 years after EVAR. On the other hand, incidence of sac shrinkage at 3 and 5 years were 59.2% and 61.7% respectively. It appeared to be plateaued around 60% after 2 years. Accordingly, it must be still a concern for endovascular therapist if proportion of patients who have sac enlargement is increased further more after 5 years.
In order to identify the risk factors for LSE, multiple logistic regression analysis was conducted. The result identified persistent endoleak at the time of LSE and pre-procedural low platelet count as a risk factor for LSE. In the present study, endoleak that was observed at the time of enhanced CT examination in patients with LSE was mostly type II. Sixty nine percent of patients with LSE were conservatively observed. A conservative approach to manage type II endoleak has been accepted because most of them are relatively benign.17,18) Persistent type II endoleaks lead to significant aneurysmal sac enlargement, but there is no increase in mortality or rupture rates.17,18) Persistent type II endoleak is often treated by coil embolization once the aneurysmal sac is expanded.15,19,20,21) However, eradicating all channels of blood supply to the sac is sometimes difficult because this procedure requires access to the inflow vessels, and to the communicating vessels to inflow ones, as well as the outflow vessels.22) In such cases, surgical conversion is required if the aneurysmal sac is further enlarged, despite repeated coil embolization. Close imaging follow-ups are mandatory in patients with type II endoleak.18,19)
There is no established method for reducing type II endoleak at the time of EVAR. A patent inferior mesenteric artery (IMA) has been suggested to be one of the sources of blood supply to the aneurysmal sac.22,23) The additional procedure to occlude IMA by coil embolization or to cover it with a larger size of aortic extension device at the time of EVAR was started.24) The results of such a strategy are awaited.
Preoperative low platelet count is another risk factor identified in the present study. Anti-platelet treatment after EVAR is reported as risks for type II endoleak.25) The function of platelet must be important factor for type II endoleak. Low platelet count may prevent occlusion of collateral channel with clot formation. Further investigation is required.
Study limitation
The present study had several limitations. It is obvious that the study was conducted based on the data collected retrospectively. In addition, the follow up term was still short and insufficient. With respect to the potential association between sac enlargement and medications after EVAR, regimen of anti-platelet or anti-coagulant drugs, β-blocker and statins which may affect the late sac enlargement are not available in the current design of registry. Further investigation of the role of those drugs is required.
Conclusion
The incidence of sac enlargement after EVAR had increased over 5 year period, which is not negligible. The cause of LSE is mostly type II endoleak. The management of type II endoleak needs to be further investigated.
Funding Sources
This study was supported by a Grant-in-aid from the National Hospital Organization in Japan.
Participants of the National Hospital Organization Network Study Group for Abdominal Aortic Aneurysm in Japan
Ishibashi Y, Masakazu Kawasaki M (Hokkaido Medical Center);
Handa N, Nishina T, Mizuno A, Ueno Y (Nagara Medical Center);
Endo M, Kasashima F (Kanazawa Medical Center);
Takahashi T, Suhara H, Sakaki M (Osaka Medical Center);
Okada M, Nakai M (Okayama Medical Center);
Shimoe Y (Shikoku Medical Center for Children and Adults);
Yamamoto T (Iwakuni Medical Center);
Onohara T, Furuyama T (Kyusyu Medical Center);
Kei J, Okamoto M (Kumamoto Medical Center);
Yamashita M (Kagoshima Medical Center);
Ishigura S, Urata Y (Hamada Medical Center);
Sato K (Higashi-hiroshima Medical Center);
Ryugo M (Kure Medical Center);
Kishimoto J (Kyusyu University: Biostatistician)
Disclosure Statement
The authors have no conflict of interest to disclose.
Authors Contribution
Study conception: NH
Data collection: TO, MO, TY, YS, YI, MY, TT, AM, JK, MN, MS, HS, FK, ME, TN, TF, MK,KI, YU, SI, KS, MR
Analysis: JK
Investigation: MO
Writing: MO
References
- 1).Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 1991; 5: 491-9. [DOI] [PubMed] [Google Scholar]
- 2).Greenhalgh RM, Brown LC, Kwong GP, et al. The EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 2004; 364: 843-8. [DOI] [PubMed] [Google Scholar]
- 3).Blankensteijn JD, de Jong SE, Prinssen M, et al. Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med 2005; 352: 2398-405. [DOI] [PubMed] [Google Scholar]
- 4).Lederle FA, Freischlag JA, Kyriakides TC, et al. Open Versus Endovascular Repair (OVER) Veterans Affairs Cooperative Study Group. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA 2009; 302: 1535-42. [DOI] [PubMed] [Google Scholar]
- 5).Greenhalgh RM, Brown LC, Powell JT, et al. United Kingdom EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010; 362: 1863-71. [DOI] [PubMed] [Google Scholar]
- 6).De Bruin JL, Baas AF, Buth J, et al. DREAM Study Group. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med 2010; 362: 1881-9. [DOI] [PubMed] [Google Scholar]
- 7).Schanzer A, Greenberg RK, Hevelone N, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011; 123: 2848-55. [DOI] [PubMed] [Google Scholar]
- 8).Obitsu Y, Ishimaru S, Shigematsu H. The education system to master endovascular aortic repair in Japan-the Japanese Committee for Stentgraft Management. Eur J Vasc Endovasc Surg 2010; 39 Suppl 1: S5–9. [DOI] [PubMed] [Google Scholar]
- 9).The Ministry of Education, Culture, Sports, Science and Technology, and the Ministry Health, Labor and Welfare. Ethical Guidelines for Epidemiological Research. 17 June 2002.
- 10).Handa N, Onohara T, Akaiwa K, et al. Early outcomes of endovascular aneurysm repair for abdominal aortic aneurysm: first preliminary report of national hospital organization network study in Japan. Ann Vasc Dis 2011; 4: 218-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Handa N, Onohara T, Okamoto M, et al. Early outcomes of open abdominal repair versus endovascular repair for abdominal aortic aneurysm: report from national hospital organization network study in Japan. Ann Vasc Dis 2012; 5: 172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Patterson BO, Holt PJ, Hinchliffe R, et al. Predicting risk in elective abdominal aortic aneurysm repair: a systematic review of current evidence. Eur J Vasc Endovasc Surg 2008; 36: 637-45. [DOI] [PubMed] [Google Scholar]
- 13).Brewster DC, Jones JE, Chung TK, et al. Long-term outcomes after endovascular abdominal aortic aneurysm repair: the first decade. Ann Surg 2006; 244: 426-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Handa N, Yamashita M, Takahashi T, et al. Impact of introducing endovascular aneurysm repair on treatment strategy for repair of abdominal aortic aneurysm-National Hospital Organization network study in Japan. Circ J 2014; 78: 1104-11. [DOI] [PubMed] [Google Scholar]
- 15).Peppelenbosch N, Buth J, Harris PL, et al. Diameter of abdominal aortic aneurysm and outcome of endovascular aneurysm repair: does size matter? A report from EUROSTAR. J Vasc Surg 2004; 39: 288-97. [DOI] [PubMed] [Google Scholar]
- 16).Hobo R, Buth J: EUROSTAR collaborators. Secondary interventions following endovascular abdominal aortic aneurysm repair using current endografts. A EUROSTAR report. J Vasc Surg 2006; 43: 896-902. [DOI] [PubMed] [Google Scholar]
- 17).Nolz R, Teufelsbauer H, Asenbaum U, et al. Type II endoleaks after endovascular repair of abdominal aortic aneurysms: fate of the aneurysm sac and neck changes during long-term follow-up. J Endovasc Ther 2012; 19: 193-9. [DOI] [PubMed] [Google Scholar]
- 18).Steinmetz E, Rubin BG, Sanchez LA, et al. Type II endoleak after endovascular abdominal aortic aneurysm repair: a conservative approach with selective intervention is safe and cost-effective. J Vasc Surg 2004; 39: 306-13. [DOI] [PubMed] [Google Scholar]
- 19).Golzarian J, Maes EB, Sun S. Endoleak: treatment options. Tech Vasc Interv Radiol 2005; 8: 41-9. [DOI] [PubMed] [Google Scholar]
- 20).Gallagher KA, Ravin RA, Meltzer AJ, et al. Midterm outcomes after treatment of type II endoleaks associated with aneurysm sac expansion. J Endovasc Ther 2012; 19: 182-92. [DOI] [PubMed] [Google Scholar]
- 21).Solis MM, Ayerdi J, Babcock GA, et al. Mechanism of failure in the treatment of type II endoleak with percutaneous coil embolization. J Vasc Surg 2002; 36: 485-91. [DOI] [PubMed] [Google Scholar]
- 22).Velazquez OC, Baum RA, Carpenter JP, et al. Relationship between preoperative patency of the inferior mesenteric artery and subsequent occurrence of type II endoleak in patients undergoing endovascular repair of abdominal aortic aneurysms. J Vasc Surg 2000; 32: 777-88. [DOI] [PubMed] [Google Scholar]
- 23).Görich J, Rilinger N, Sokiranski R, et al. Embolization of type II endoleaks fed by the inferior mesenteric artery: using the superior mesenteric artery approach. J Endovasc Ther 2000; 7: 297-301. [DOI] [PubMed] [Google Scholar]
- 24).Chikazawa G, Yoshitaka H, Hiraoka A, et al. Preoperative Coil Embolization to Aortic Branched Vessels for Prevention of Aneurysmal Sac Enlargement Following EVAR: Early Clinical Result. Ann Vasc Dis 2013; 6: 175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Aoki A, Suezawa T, Sangawa K, et al. Effect of type II endoleaks and antiplatelet therapy on abdominal aortic aneurysm shrinkage after endovascular repair. J Vasc Surg 2011; 54: 947-51. [DOI] [PubMed] [Google Scholar]