Highlights
-
•
DSC/ASL-MRI can be acquired in growing VS with sufficient image quality.
-
•
In most patients DSC and ASL techniques provide similar qualitative scores.
-
•
These techniques can be of importance in future decision-making.
Abbreviations: AAO-HNS, American Academy of Otolaryngology-Head and Neck Surgery; ASL, arterial spin labeling; CA, contrast agent; CBF, cerebral blood flow; CBV, cerebral blood volume; DSC, dynamic susceptibility contrast; EPI, echo planar imaging; FA, flip angle; FOV, field of view; Gd, gadolinium; MR, magnetic resonance; MRI, magnetic resonance imaging; PCASL, pseudo-continuous arterial spin labeling; RF, radiofrequency; rCBV, relative CBV; SNR, signal to noise ratio; TR, repetition time; TE, echo time; VS, vestibular schwannoma
Keywords: Vestibular schwannoma, Perfusion weighted MR, Decision making, Dynamic susceptibility contrast (DSC) and arterial spin labeling (ASL)
Abstract
Objective
The added value of perfusion MRI for decision-making in vestibular schwannoma (VS) patients is unknown. MRI offers two perfusion methods: the first employing contrast agent (dynamic susceptibility contrast (DSC)-MRI) that provides information on cerebral blood volume (CBV) and cerebral blood flow (CBF), the second by magnetic labeling of blood (arterial spin labeling (ASL)-MRI), providing CBF-images. The goal of the current study is to investigate whether DSC and ASL perfusion MRI provides complimentary information to current anatomical imaging in treatment selection process of VS.
Methods
Nine patients with growing VS with extrameatal diameter >9 mm were included (>2 mm/year and 20% volume expansion/year) and one patient with 23 mm extrameatal VS without growth. DSC and ASL perfusion MRI were obtained on 3 T MRI. Perfusion in VS was scored as hyperintense, hypointense or isointense compared to the contralateral region.
Results
Seven patients showed hyperintense signal on DSC and ASL sequences. Three patients showed iso- or hypointense signal on at least one perfusion map (1 patient hypointense on both DSC-MRI and ASL; 1 patient isointense on DSC-CBF; 1 patient isointense on ASL). All patients showed enhancement on post-contrast T1 anatomical scan.
Conclusion
Perfusion MR provides additional information compared to anatomical imaging for decision-making in VS.
1. Introduction
Vestibular schwannoma (VS) is a benign tumour that originates from the Schwann’s cell of the vestibular nerve, also known as the eighth cranial nerve. The vestibular nerve is located in the cerebellopontine angle, the space between brainstem, cerebellum and temporal bone. Clinical complaints of VS generally consist of progressive unilateral hearing loss, vertigo and tinnitus. Tumours that compress the brainstem give more general complaints, such as headache, vision disorders and hypoesthesia of the face. Data from Denmark shows an incidence of 19 VSs per 1 million people per year, these data are seen as most complete because of the referral of all VSs from one country to one single clinic [1]. Diagnosis is made by anatomical MRI examination with or without the use of contrast agent (CA).
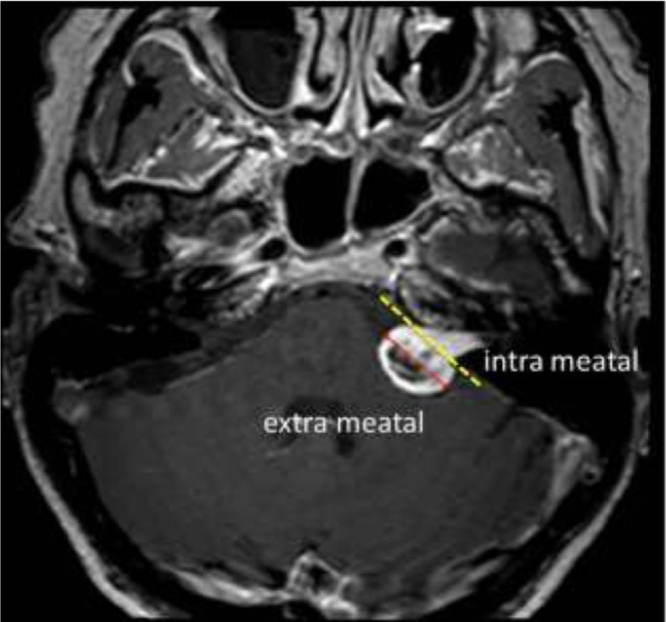
Treatment options for VS are radiotherapy, surgery or observation with regularly magnetic resonance (MR) preferable with the use of CA [2]. The choice for treatment is based both upon tumour characteristics and patient characteristics. Such as tumour size, growth rate, heterogeneity of the tumour and hearing loss. The average tumour growth was found to be 1–2 mm per year, but varies [3]. Tumour size is both measured intra- and extrameatal (Fig. 1) [2]. Besides the tumour characteristics the patients preferences are important to decide for a treatment option. If tumour size is stable the frequency of MR is decreased. The preferred treatment option for intrameatal tumours is observation, although occasionally patients with functional hearing are operated to preserve hearing [4]. Tumours localized extrameatal, which are larger than 20 mm are generally spoken, advised to undergo treatment, surgery or radiotherapy. Each treatment option has its specific benefits and side effects. The intention of radiotherapy is to freeze the growth rate; but hearing loss and other cranial nerve pathology are common side effects [5]. Surgery usually results in hearing loss at the operated side and can also affect the function of the facial nerve [6]. In addition infection and haemorrhage are common complications. Patients with small or medium sized tumours can experience a significant decrease of their quality-of-life in each treatment option with relatively small differences in quality-of-life between the treatment groups. It has been shown that not the treatment modality itself, but the actual diagnosis of VS is the main cause of decreasing quality-of-life in patients with VS [7], [8]. Observation is becoming the preferred initial treatment policy for VS [8], [9]. In order to give an objective advice for patient specific treatment during observation and to decrease side effects during radiotherapy or surgery, it would be of clinical relevance if the growth rate could be predicted.
Fig. 1.
MR image of left sided vestibular schwannoma. Yellow dotted line is border between intra- and extrameatal portion of the tumour. Size quantified as the largest diameter measurable in the extrameatal portion (red line).
For brain tumours it is known that vascularization can be measured using perfusion MRI and that it can help in differentiation and staging of brain tumours [10], [11]. A tumour with a volume larger than 2 mm3 is dependent on angiogenesis for growth, since the tumour growth critically depends on influx of oxygen and nutrients [12], [13]. Perfusion MRI has been used for early detection and staging of many different tumour types, such as lung cancer and gliomas, although its added value for VS is yet unknown [14], [15].
MRI perfusion can be performed by two approaches, one with and the other without the use of CA, i.e. dynamic susceptibility contrast (DSC) MRI and arterial spin labeling (ASL). DSC relies on the intravenous injection of a CA and serial MRI measurement of signal loss during the passage of the bolus through the tissue, using T2 or T2* weighted sequences. Using this technique, cerebral blood volume (CBV) and cerebral blood flow (CBF) can be calculated. ASL is a non-invasive perfusion MRI method for quantitatively measuring cerebral perfusion, by employing blood itself as an endogenous tracer via inversion of longitudinal magnetization [16]. A difficulty in the depiction of perfusion of VS lies in the magnetic field inhomogeneities near the temporal bone, which could especially affect the measurements of the intrameatal portion of the VS. Such concerns on the imaging quality are probably the reason for the absence of perfusion MRI in many imaging protocols of VS patients. Only a few studies show examples of perfusion MRI in VS, and these studies are limited to single subject examples [11], [17].
The goal of the current study is to investigate the additional value of the different perfusion MRI methods to provide information on the vascularization in VS.
2. Material and methods
2.1. Patients
The Leiden University Medical Center is a tertiary referral centre for VS-patients in the Netherlands. Every other week all new patients with VS are being (multidisciplinary) discussed, and patient characteristics are documented in a database. From this database ten patients were selected to be included in this study, based upon the growth rate. Growth was assessed on two consecutive MRI’s, where the extrameatal component was measured using the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) criteria (Fig. 1) [2]. This means that in the axial plan on a T1 gadolinium-enhanced sequence the largest diameter was measured in anterior-posterior and medial-lateral dimension. Patients with a growth rate larger than 2 mm/year and a volume increase of more than 20% per year were included following literature procedures [18], [19]. A further inclusion criteria was that the extrameatal diameter of the VS needed to be larger than 9 mm; this to guarantee good depiction even when taking the relatively coarse resolution of perfusion MRI sequences into account. Patients with neurofibromatosis type 2 were excluded. The clinical state of the patients on follow-up was documented.
The internal ethical review board of the Leiden University Medical Center approved the study and all subjects provided written informed consent.
2.2. MR protocol
All experiments were performed on a clinical 3 T MRI scanner (Achieva 3 T, Philips Healthcare, Best, The Netherlands) equipped with software to enable ASL imaging. Pseudo-continuous labeling was performed with a labeling duration of 1650 ms (1650 RF pulses of 0.5 ms duration). ASL imaging was performed in combination with background suppression, which consisted of a saturation pulse immediately before labeling and inversion pulses at 1680 and 2830 ms after the saturation pulse [20]. This leads to optimal background suppression for the first acquired slice, whereas the background signal in the other slices will gradually increase due to T1-recovery. Imaging was performed with single-shot echo planar imaging (EPI) in combination with parallel imaging (SENSE factor 2.5). In total 17 slices of 5 mm slice thickness were acquired in ascending fashion with an in-plane resolution of 3 × 3 mm2 (TE of 14 ms, TR of 3.9 s). Imaging started 1525 ms after labeling stopped. The total scan was 4 min (Table 1).
Table 1.
Summary of acquisition parameters for ASL perfusion imaging.
| Acquisition parameter | ASL |
|---|---|
| Labeling | PCASL |
| Labeling duration | 1650 ms |
| Background suppression | 1680 & 2830 ms |
| Pulse sequence | Single shot EPI |
| TR | 3.9 s |
| Voxel-size | 3 × 3 × 5 mm |
| Slices | 17 |
| Slice thickness | 5 mm |
| Total scan time | 4 min |
For DSC-MRI a single-shot spin-echo echo planar imaging (SE-EPI) sequence with echo time (TE) 30 ms, flip angle (FA) 90°, was used to cover an imaging volume of 13 slices for 96 s at a temporal resolution of 1.64 s 15 mL of gadolinium-based CA (Dotarem, Guerbet, France) was injected at a rate of 5 mL/sec followed by a chaser of 25 mL saline, also injected at 5 mL/sec [21], [22]. A pre-bolus of 8 mL CA was given 5 min before dynamic imaging (see Table 2).
Table 2.
Summary of acquisition parameters for DSC perfusion imaging.
| Acquisition parameter | DSC |
|---|---|
| Pulse sequence | SE-EPI |
| TR | 1.6 s |
| TE | 30 ms |
| Flip angle | 90° |
| Preload Gd- based contrast agent dose | 8 mL dose |
| Slices | 13 |
| Slice thickness | 5 mm |
| Slice gap | 0.5 mm |
| Voxel-size | 2.5 × 2.5 × 4.5 mm |
| FOV | 240 mm |
| IV catheter gauge | 18 gauge |
| Injection rate | 5 mL/s |
| Total acquisition time | 96 s |
Note: TR = repetition time, TE = echo time, FOV = field of view.
2.3. Post processing and statistical analysis
The label images of the ASL-sequence were subtracted from the control images and averaging over the repeated measurements was performed. No further quantification was performed, since this study employed a qualitative comparison of VS perfusion compared to a contralateral region in the same slice. With vendor supplied software (Philips Healthcare, Best, The Netherlands) the DSC-data were analysed. This was done by converting the MRI-signal changes in time to ΔR2(t) curves which were considered to reflect concentration time curves by assuming linearity:
| ΔR2(t) = {−In(S(t)/S(0))}/TE |
TE is the echo time, S(0) baseline signal intensity and S(t) MRI signal intensity as a function of time. From these concentration-time curves relative CBV and CBF-maps were reconstructed using standard tracer kinetic theory [23].
2.4. MR perfusion characteristics
Tumours where rated using a three point scale; hyperintense, isointense and hypointense as compared to the contralateral side. Using OsiriX© v5.5.2 32 bit, images of DSC-CBF, DSC-CBV and ASL-CBF sequences where registered with the T1 post contrast sequence for accurate co-localization of hemodynamic and anatomical information. This resulted in 4 MR images per patient: T1 post contrast, DSC-CBF, DSC-CBV and ASL-CBF. These images where reviewed by a radiologist to define whether the tumour was hyperintense, isointense or hypointense compared to contralateral side.
3. Results
3.1. Patients
Ten patients were included in this study (Table 3). Nine patients with an unilateral fast growing (≥2 mm/year) extrameatal vestibular schwannoma, ≥9 mm in diameter (axial measured) were included. The VS of one patients showed minimal growth after the perfusion MRI, although the patient did full fill the inclusion criteria of a growth of more than >2 mm per year based upon 2 consecutives MRI’s. However, looking back at the consecutives MR’s it was deemed debatable by a second observer whether this VS grew actually more than 2 mm and having a volume increase of 20% per year. As of poor image quality the caudal part of the tumour was overestimated for the second MRI. Therefore this patient was excluded for further analyses for fast growing tumours. Since the perfusion MRI of this patient was made, the results were analysed and discussed as an (single-subject) example of a slow growing tumour. This patient was categorized as having a stable unilateral vestibular schwannoma of 24 mm.
Table 3.
Patient characteristics.
| Number | 9 | |
|---|---|---|
| Mean age (years) | 62 (45–74) | |
| Gender | male | 6 |
| female | 3 | |
| Mean max. diameter 1 st MR | mm | 13.4 |
| range | mm | 9.0–19.5 |
| Mean max. diameter 2nd MR | mm | 15.9 |
| range | mm | 12.2–22.4 |
| Mean time between MR’s | months | 7.6 |
| Mean volume 1st MR | cm3 | 1.47 |
| range | cm3 | 0.72–3.42 |
| Mean volume 2nd MR | cm3 | 2.2 |
| range | cm3 | 0.97–4.84 |
| Mean growth | mm/yr | 4.1 |
| range | mm/yr | 2.3-7.0 |
| volume/yr (%) | 53.3 | |
| range | volume/yr (%) | 24.0–120.0 |
In Table 3 characteristics of all patients are shown. The mean maximum diameter was 15.9 mm and the mean growth rate was 4.1 mm per year, the stable patient was excluded from this mean.
3.2. Tumour characteristics
In Table 4 data regarding size, volume and growth are given for every patient.
Table 4.
Tumour characteristics.
| Patient | max diameter (mm) | Volume (cm3) |
growth (%/yr) | growth (mm/yr) |
|---|---|---|---|---|
| 1* | 23.5 | 8.00 | 12.0 | 1.0 |
| 2 | 12.5 | 1.04 | 120.0 | 7.0 |
| 3 | 12.0 | 0.93 | 48.0 | 4.0 |
| 4 | 12.5 | 1.33 | 36.0 | 4.0 |
| 5 | 10.9 | 0.87 | 24.0 | 2.6 |
| 6 | 9.0 | 0.72 | 36.0 | 2.8 |
| 7 | 10.9 | 0.80 | 60.0 | 3.6 |
| 8 | 19.5 | 2.79 | 84.0 | 5.8 |
| 9 | 15.1 | 1.33 | 24.0 | 2.3 |
| 10 | 18.6 | 3.42 | 48.0 | 4.8 |
Note: *stable patient.
3.3. MRI characteristics
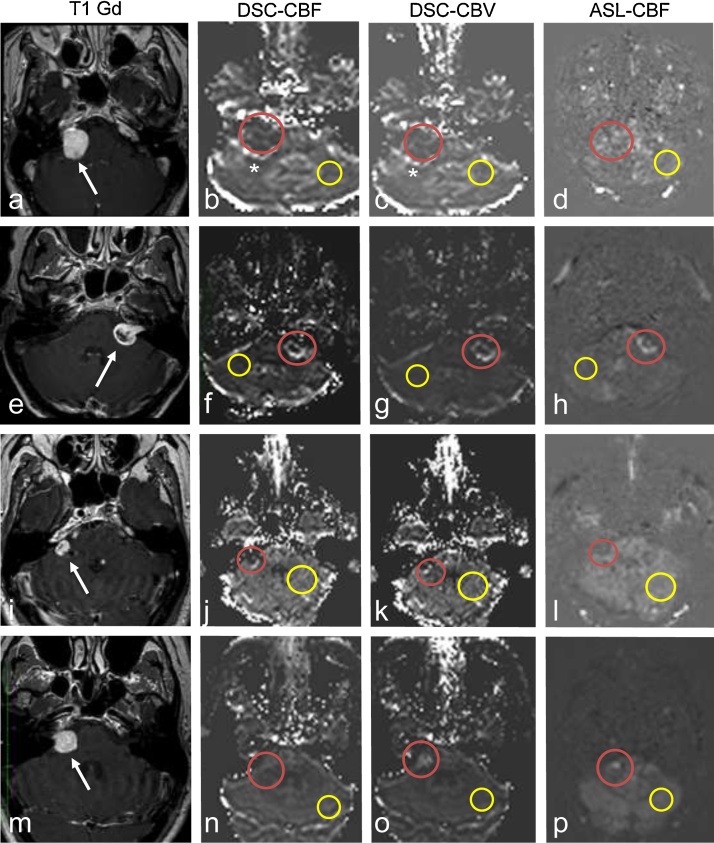
In Table 5 the results are shown for perfusion MRI. Perfusion was rated on a three-point intensity scale, hyperintense, isointense and hypointense as compared to a mirror location contralaterally. In Fig. 2 example images are shown for 4 patients.
Table 5.
MRI characteristics.
| Patient | DSC-CBF | DSC-CBV | ASL-CBF | T1 post contrast |
|---|---|---|---|---|
| 1* | hypointense | hypointense | hypointense | hyperintense |
| 2 | hyperintense | hyperintense | hyperintense | hyperintense |
| 3 | hyperintense | hyperintense | hyperintense | hyperintense |
| 4 | hyperintense | hyperintense | hyperintense | hyperintense |
| 5 | hyperintense | hyperintense | isointense | hyperintense |
| 6 | hyperintense | hyperintense | hyperintense | hyperintense |
| 7 | hyperintense | hyperintense | hyperintense | hyperintense |
| 8 | hyperintense | hyperintense | hyperintense | hyperintense |
| 9 | isointense | hyperintense | hyperintense | hyperintense |
| 10 | hyperintense | hyperintense | hyperintense | hyperintense |
Note: DSC-CBF = Dynamic susceptibility contrast—cerebral blood flow, DSC-CBV = dynamic susceptibility contrast- cerebral blood volume, AS-CBF = arterial spin labeling—cerebral blood flow, * stable patient.
Fig. 2.
Imaging examples of 4 patients, from left to right: T1 post contrast, DSC-CBF, DSC-CBV and ASL-CBF. From top to bottom: patient 1, 2, 5, and 9. Red circle indicates the region-of-interest in the vestibular schwannoma (VS); yellow circle is the contralateral reference area. Arrows indicates the VS. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
a–d shows the result of patient 1, on the posterior border there is hyperintensity visible (asterisk b-c), which is indicative for a vessel. All perfusion images are hypointense. e–h shows the results of patient 2. All perfusion images where hyperintense. i–l patient 5. Both the DSC perfusion images are hyperintense. ASL-CBF is isointense. m–p patient 9. DSC-CBF is isointense, DSC-CBV and ASL-CBF where hyperintense.
Seven patients showed on all the MR perfusion scans hyperintense signal. The stable patient showed hypointense signal for all modalities. One patient showed hyperintense signal in de DSC-CBF and DSC-CBV sequences, but isointense signal in the ASL-CBF sequence. Finally, one patient showed isointense signal in the DSC-CBF sequences and hyperintense signal in the DSC-CBV and ASL-CBF sequences. All patients had an enhancement on post-contrast T1 anatomical scan (Table 5).
Fig. 2a–d shows the results of the patient with slowly growing VS. All perfusion images were hypointense. The T1 post contrast was used for navigation purposes and showed hyperintense signal intensity as compared to the contralateral mirror-location. Images 2b and 2c show hyperintense signal on the posterior border, which is indicative for a vessel as confirmed on the post contrast T1 sequence. Fig. 2e–h shows the results of patient 2. All perfusion images showed hyperintense signal. On the T1 post contrast the tumour is cystic, in the perfusion images this cystic area is hypointense. Fig. 2i–l gives an overview of the result of patient 5. Both the DSC perfusion images are hyperintense at the posterior border with cerebellum. ASL-CBF is isointense. It should be stated that the full extend of the complete tumour was not included in the image data. Fig. 2m–p resembles the result of patient 9. DSC-CBF is isointense, DSC-CBV and ASL-CBF where hyperintense.
3.4. Follow up data
For all patients included in this study the current clinical status on follow-up was reviewed. An overview of the information is provided in Table 6. Since inclusion in the study the tumour of patient 1 remained stable and no invasive treatment has been performed and is clinically still in the observation group. Patient 2 showed progressive growth and surgical removal was performed within one month after perfusion MRI, histology confirmed the radiological diagnosis of VS. The residual VS after surgery was stable for 24 months after surgery. Patient 3 showed no growth between the perfusion MRI and the most recent MRI (interval 3 months), but because of patient preferences, radiotherapy was performed. During follow up the tumour grew after radiotherapy; this patient is still under observation. Patient 4 showed progressive growth between the perfusion MRI and the most recent MRI (interval 3 months) and surgery was performed within one month after perfusion MRI. The histological examination showed VS. Patient 5 stayed under observation and is still stable, volumetric growth could not be assessed because of incomplete imaging of the tumour. Patient 6 had minimal growth, but because of invalidating vertigo and patients’ preferences surgery was performed. Again histopathology revealed VS. Patient 7 showed progressive growth and radiotherapy was performed because of patients’ preferences. In the 6 months of radiological follow up the tumour stayed stable. Patient 8 had also progressive growth and radiotherapy was initiated, currently there is no radiological follow up data. Patient 9 had progressive growth, patient’s preferences results in continuing observation. After 14 months, growth was still progressive and surgery is currently planned. Patient 10 had progressive growth and radiotherapy was performed. After radiotherapy, growth stayed progressive and because of hydrocephalus an intracranial drain was placed. Currently, the patient is under observation whether the post-radiotherapy oedema will diminish.
Table 6.
Follow up data.
| Patient | max diameter pre perfusion MR (mm) | max diameter Perfusion MR (mm) | Post-MR examination growth (mm/yr) | Treatment | histology | Radiological follow up after perfusion MR (Months) | Current state of the tumour |
|---|---|---|---|---|---|---|---|
| 1* | 24.0 | 24.0 | 0.0 | Observation | – | 13 | Stable |
| 2 | 16.0 | 16.8 | 2.1 | Surgery | VS | 24 | Stable residual |
| 3 | 14.0 | 14.0 | 0.0 | Radiotherapy | 16 | Growth | |
| 4 | 14.2 | 15.6 | 4.4 | Surgery | VS | 12 | Stable residual |
| 5 | 12.2 | 12.2 | 0,0 | Observation | – | 9 | Stable |
| 6 | 12.5 | 13.0 | 1.2 | Surgery | VS | 16 | Stable residual |
| 7 | 13.6 | 19.2 | 5.6 | Radiotherapy | – | 6 | Stable |
| 8 | 22.4 | 24.4 | 4.8 | Radiotherapy | – | – | – |
| 9 | 16.6 | 18.6 | 2.1 | Observation | – | 14 | Growth, surgery |
| 10 | 21.0 | 22 | 2.0 | Radiotherapy | – | 17 | Growth, hydrocephalus |
4. Discussion
This study investigated whether it is possible to obtain perfusion-weighted images of sufficient quality in patients with growing VS. The main findings are that growing VS (≥9 mm extrameatal) are visible on perfusion weighted MR, both ASL and DSC-MRI are suitable for assessing vascularization in VS, and that these techniques should be evaluated in future research for their added-value in the decision-making process of the treatment-strategy in the individual VS patient.
Previous studies compared DSC perfusion images between various intracranial space occupying lesions [11], [17]. VS was one of the studied lesions, although it was limited to a single subject example. Hakyemez et al. showed that rCBV ratios of VS were lower compared to meningiomas and metastases. Therefore they concluded that perfusion MRI could be helpful for discriminating schwannomas from meningiomas or other masses. Zimny et al. confirmed that low rCBV values could differentiate schwannoma from meningioma. Although these studies are important by proving the value of perfusion MRI in differentiating between different types of intracranial lesions, they lack the comparison of different perfusion MRI techniques in the context of imaging VS, nor did they focus on predicting future growth of VS. Table 4 shows that the three patients with the lowest volume increase per year (i.e. patient 1, 5 and 9 with a volume expansion of 0%, 24%, 24% per year, respectively), showed at least for one of the perfusion MR approach iso- or hypointense signal intensity compared to the contralateral region. This in contradiction with the post-contrast T1-weighted images that showed hyperintense signal in the VS for all patients. Although our sample-size was relatively small, these observations seem to indicate that perfusion MRI could show additional information as compared to traditional MRI.
Several studies of intracranial masses compared findings obtained by ASL and DSC perfusion MRI. Rau et al. investigated the use of ASL and DSC imaging in the use of patients diagnosed with high-grade gliomas. They found that relative CBF measurements seemed to provide the best sensitivity and specificity to predict tumour recurrence and survival times in these patients, there was no difference between DSC and ASL [24]. Another study compared ASL and DSC perfusion imaging for diagnosis in brain metastasis and meningiomas showing comparable results for both perfusion methods [25]. Furthermore, ASL and DSC might have similar predictive value for treatment outcome in brain metastases [26]. In this study, Weber et al. found that after stereotactic radiosurgery alteration of CBF was highly predictive for treatment outcome [26]. Another comparative analysis of ASL and DSC found a close correlation between ASL and DSC perfusion imaging in patients with proven brain tumours [27]. These authors concluded that ASL possesses the potential to be a non-invasive alternative for DSC perfusion imaging particular in patients with renal failure; these patients have a contraindication for CA.
The current study adds to the literature that also in the setting of VS both perfusion MRI approaches provide sufficient image quality for assessing perfusion in VS and that in most patients both approaches provided similar readings. Future clinical trials with a higher number of included patients as well as with a longer follow-up time would be necessary to proof superior behaviour of one of the two perfusion techniques. Furthermore, such a clinical trial would be essential to proof whether inclusion of perfusion information into the treatment selection process would improve clinical outcome as compared to current standard clinical care. When comparing ASL with DSC, there are some important differences. ASL is a completely non-invasive approach, but suffers from difficulty in providing reliable measurements in low-CBF regions like the cerebral white matter, and it only provides information on CBF whereas blood volume measurements have been found to be more informative for diagnosis and staging of brain tumours [28], [29]. On the other hand, DSC is invasive because of the use of CA and its accuracy can be affected due to leakage of CA, but it provides a more holistic view on tumour hemodynamics by providing information on both CBV and CBF. Leakage of CA violates the applied tracer kinetic model since it is intrinsically a model for intravascular tracers. Furthermore, leakage of contrast agent leads to a significant decrease in T1 of the extravascular compartment, resulting in a signal increase, which can lead to quantification errors in the measurement of the concentration of CA. By giving a pre-load of CA the T1-effect can be minimized and this approach was adopted in the current study. This study included one patient, which in retrospect did not meet the inclusion criteria.
However, since the perfusion MRI was already been made, the data was analysed and included in order to serve as a reference to the data of patients with growing tumours. For future studies it is worthwhile to investigate in a larger group of patients whether growing and stable tumours differ significantly in their perfusion characteristics.
Limitations of this study are that the studied patient group is very small, that perfusion information was not used in treatment decisions and that therefore no conclusion can be drawn on the added value of perfusion MRI on the decision making. Furthermore, we employed a simplistic three-point scale to score perfusion images. Such a simple scoring system reflects current approach in the radiological clinic in which quantitative analysis is only rarely performed due to large intra- and intersubject variations in quantification. This study was meant to provide initial evidence whether perfusion MRI might help in the clinical treatment strategy of VS patients and based on the result of the current study, further research seems to be justified since perfusion MRI provided sufficient image quality, provided insight into the heterogeneity of our patients, which was informative over standard post-contrast MRI that in all patients showed signal enhancement independent of past and future growth rates.
In conclusion, this study showed that growing VS (≥9 mm extrameatal) are visible on perfusion-weighted MR, that the two techniques DSC and ASL provide similar imaging quality and that future research is needed to prove whether these techniques are of importance in the decision-making in VS-patients.
Conflict of interest
None.
Acknowledgements
The scientific guarantor of this publication is M.C. Kleijwegt. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. No study subjects or cohorts have been previously reported. Methodology: prospective, diagnostic, performed at one institution.
References
- 1.Stangerup S.E., Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryngol. Clin. N. Am. 2012;45 doi: 10.1016/j.otc.2011.12.008. 257–268 vii. [DOI] [PubMed] [Google Scholar]
- 2.Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 113 (1995) 179–180. [DOI] [PubMed]
- 3.Stangerup S.E., Caye-Thomasen P., Tos M., Thomsen J. The natural history of vestibular schwannoma otology & neurotology: official publication of the American Otological Society. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2006;27:547–552. doi: 10.1097/01.mao.0000217356.73463.e7. [DOI] [PubMed] [Google Scholar]
- 4.Chamoun R., MacDonald J., Shelton C., Couldwell W.T. Surgical approaches for resection of vestibular schwannomas: translabyrinthine, retrosigmoid, and middle fossa approaches. Neurosurg. Focus. 2012;33:E9. doi: 10.3171/2012.6.FOCUS12190. [DOI] [PubMed] [Google Scholar]
- 5.Jian B.J., Kaur G., Sayegh E.T., Bloch O., Parsa A.T., Barani I.J. Fractionated radiation therapy for vestibular schwannoma. J. Clin. Neurosci. 2014;21:1083–1088. doi: 10.1016/j.jocn.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Godefroy W.P., van der Mey A.G., de Bruine F.T., Hoekstra E.R., Malessy M.J. Surgery for large vestibular schwannoma: residual tumor and outcome otology & neurotology: official publication of the American Otological Society. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2009;30:629–634. doi: 10.1097/MAO.0b013e3181a8651f. [DOI] [PubMed] [Google Scholar]
- 7.Godefroy W.P., Kaptein A.A., Vogel J.J., van der Mey A.G. Conservative treatment of vestibular schwannoma: a follow-up study on clinical and quality-of-life outcome otology & neurotology: official publication of the American Otological Society. Am. Neurot. Soc. Eur. Acad. Otol. Neurotol. 2009;30:968–974. doi: 10.1097/MAO.0b013e3181b4e3c9. [DOI] [PubMed] [Google Scholar]
- 8.Carlson M.L., Tveiten O.V., Driscoll C.L., Goplen F.K., Neff B.A., Pollock B.E. Long-term quality of life in patients with vestibular schwannoma: an international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. J. Neurosurg. 2015;122:833–842. doi: 10.3171/2014.11.JNS14594. [DOI] [PubMed] [Google Scholar]
- 9.Carlson M.L., Habermann E.B., Wagie A.E., Driscoll C.L., Van Gompel J.J., Jacob J.T. The changing landscape of vestibular schwannoma management in the United States-A shift toward conservatism. Otolaryngol. Head Neck Surg. 2015;153(3):440–446. doi: 10.1177/0194599815590105. [DOI] [PubMed] [Google Scholar]
- 10.Cha S., Knopp E.A., Johnson G., Wetzel S.G., Litt A.W., Zagzag D. Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology. 2002;223:11–29. doi: 10.1148/radiol.2231010594. [DOI] [PubMed] [Google Scholar]
- 11.Hakyemez B., Erdogan C., Bolca N., Yildirim N., Gokalp G., Parlak M. Evaluation of different cerebral mass lesions by perfusion-weighted MR imaging. J. Magn. Reson. Imaging: JMRI. 2006;24:817–824. doi: 10.1002/jmri.20707. [DOI] [PubMed] [Google Scholar]
- 12.Moller M.N., Werther K., Nalla A., Stangerup S.E., Thomsen J., Bog-Hansen T.C. Angiogenesis in vestibular schwannomas: expression of extracellular matrix factors MMP-2, MMP-9, and TIMP-1. Laryngoscope. 2010;120:657–662. doi: 10.1002/lary.20834. [DOI] [PubMed] [Google Scholar]
- 13.Kiessling F., Morgenstern B., Zhang C. Contrast agents and applications to assess tumor angiogenesis in vivo by magnetic resonance imaging. Curr. Med. Chem. 2007;14:77–91. doi: 10.2174/092986707779313516. [DOI] [PubMed] [Google Scholar]
- 14.Hakyemez B., Erdogan C., Ercan I., Ergin N., Uysal S., Atahan S. High-grade and low-grade gliomas: differentiation by using perfusion MR imaging. Clin. Radiol. 2005;60:493–502. doi: 10.1016/j.crad.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Huang H., Shen L., Ford J., Gao L., Pearlman J. Early lung cancer detection based on registered perfusion MRI. Oncol. Rep. 2006 doi: 10.3892/or.15.4.1081. 15 Spec no.:1081-4. [DOI] [PubMed] [Google Scholar]
- 16.Welker K., Boxerman J., Kalnin A., Kaufmann T., Shiroishi M., Wintermark M. ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. AJNR Am. J. Neuroradiol. 2015;36:E41–E51. doi: 10.3174/ajnr.A4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimny A., Sasiadek M. Contribution of perfusion-weighted magnetic resonance imaging in the differentiation of meningiomas and other extra-axial tumors: case reports and literature review. J. Neurooncol. 2011;103:777–783. doi: 10.1007/s11060-010-0445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolopoulos T.P., Fortnum H., O'Donoghue G., Baguley D. Acoustic neuroma growth: a systematic review of the evidence otology & neurotology: official publication of the American Otological Society. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2010;31:478–485. doi: 10.1097/MAO.0b013e3181d279a3. [DOI] [PubMed] [Google Scholar]
- 19.van de Langenberg R., de Bondt B.J., Nelemans P.J., Baumert B.G., Stokroos R.J. Follow-up assessment of vestibular schwannomas: volume quantification versus two-dimensional measurements. Neuroradiology. 2009;51:517–524. doi: 10.1007/s00234-009-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye F.Q., Frank J.A., Weinberger D.R., McLaughlin A.C. Noise reduction in 3D perfusion imaging by attenuating the static signal in arterial spin tagging (ASSIST) Magn. Reson. Med. 2000;44:92–100. doi: 10.1002/1522-2594(200007)44:1<92::aid-mrm14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Ostergaard L., Sorensen A.G., Kwong K.K., Weisskoff R.M., Gyldensted C., Rosen B.R. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: experimental comparison and preliminary results. Magn. Reson. Med. 1996;36:726–736. doi: 10.1002/mrm.1910360511. [DOI] [PubMed] [Google Scholar]
- 22.Ostergaard L., Weisskoff R.M., Chesler D.A., Gyldensted C., Rosen B.R. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: mathematical approach and statistical analysis. Magn. Reson. Med. 1996;36:715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 23.Peter B., Barker X.G., Greg Zaharchuk . Cambridge University Press; 2013. Clinical Perfusion MRI: Techniques and Applications. [Google Scholar]
- 24.Rau M.K., Braun C., Skardelly M., Schittenhelm J., Paulsen F., Bender B. Prognostic value of blood flow estimated by arterial spin labeling and dynamic susceptibility contrast-enhanced MR imaging in high-grade gliomas. J. Neurooncol. 2014;120:557–566. doi: 10.1007/s11060-014-1586-z. [DOI] [PubMed] [Google Scholar]
- 25.Lehmann P., Monet P., de Marco G., Saliou G., Perrin M., Stoquart-Elsankari S. A comparative study of perfusion measurement in brain tumours at 3 Tesla MR: arterial spin labeling versus dynamic susceptibility contrast-enhanced MRI. Eur. Neurol. 2010;64:21–26. doi: 10.1159/000311520. [DOI] [PubMed] [Google Scholar]
- 26.Weber M.A., Thilmann C., Lichy M.P., Gunther M., Delorme S., Zuna I. Assessment of irradiated brain metastases by means of arterial spin-labeling and dynamic susceptibility-weighted contrast-enhanced perfusion MRI: initial results. Invest. Radiol. 2004;39:277–287. doi: 10.1097/01.rli.0000119195.50515.04. [DOI] [PubMed] [Google Scholar]
- 27.Jiang J., Zhao L., Zhang Y., Zhang S., Yao Y., Qin Y. Comparative analysis of arterial spin labeling and dynamic susceptibility contrast perfusion imaging for quantitative perfusion measurements of brain tumors. Int. J. Clin. Exp. Pathol. 2014;7:2790–2799. [PMC free article] [PubMed] [Google Scholar]
- 28.Mabray M.C., Barajas R.F., Jr., Cha S. Modern brain tumor imaging. Brain Tumor Res Treat. 2015;3:8–23. doi: 10.14791/btrt.2015.3.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alsop D.C., Detre J.A., Golay X., Gunther M., Hendrikse J., Hernandez-Garcia L. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2014;73(1):102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]