Abstract
The mechanism whereby phospholipids rapidly flip-flop in the endoplasmic reticulum (ER) membrane remains unknown. We previously demonstrated that the presence of a hydrophilic residue in the center of the model transmembrane peptide sequence effectively promoted phospholipid flip-flop and that hydrophilic residues composed 4.5% of the central regions of the membrane-spanning sequences of human ER membrane proteins predicted by SOSUI software. We hypothesized that ER proteins with hydrophilic residues might play a critical role in promoting flip-flop. Here, we evaluated the flip rate of fluorescently labeled lipids in vesicles containing each of the 11 synthetic peptides of membrane-spanning sequences, using a dithionite-quenching assay. Although the flippase activities of nine peptides were unexpectedly low, the peptides based on the EDEM1 and SPAST proteins showed enhanced flippase activity with three different fluorescently labeled lipids. The substitution of hydrophobic Ala with His or Arg in the central region of the EDEM1 or SPAST peptides, respectively, attenuated their ability to flip phospholipids. Interestingly, substituting Ala with Arg or His at a location outside of the central region of EDEM1 or SPAST, respectively, also affected the enhancement of flip-flop. These results indicated that both Arg and His are important for the ability of these two peptides to increase the flip rates. The EDEM1 peptide exhibited high activity at significantly low peptide concentrations, suggesting that the same side positioning of Arg and His in α-helix structure is critical for the flip-flop promotion and that the EDEM1 protein is a candidate flippase in the ER.
Introduction
Phospholipid transbilayer movement (flip-flop) plays an essential role in the proper functions of biological membranes in eukaryotes (1). Several kinds of membrane proteins control flip-flop in the plasma membrane (PM). An asymmetric composition of phospholipids in the PM is maintained through an energy-dependent process by the activities of amino phospholipid translocases and ATP-binding cassette transporters (2, 3). Disruption of the asymmetry by phospholipid scramblases is involved in apoptotic cell death or platelet-dependent coaggregation (4). A rapid rate of phospholipid flip-flop is observed in the endoplasmic reticulum (ER), with the half-time of the flip-flop ranging from seconds to minutes (5, 6, 7, 8). Phospholipids in eukaryotic cells are mainly synthesized at the cytoplasmic leaflet of the ER membrane (9, 10, 11). Therefore, newly synthesized phospholipids must be transferred to the luminal leaflet of the membrane to maintain membrane integrity in the ER. Flip-flop in the ER is energy-independent and shows low selectivity with respect to the headgroups of phospholipids (5, 6, 7). The observed sensitivities to proteases and protein-modification reagents have suggested that the rapid flip-flop process is protein-mediated (5, 6, 7). Although many studies have been conducted to investigate the mechanism of the rapid flip-flop in the ER, “flippases” responsible for flip-flop have yet to be identified (12, 13, 14, 15, 16).
Kol et al. reported that model transmembrane peptides increase the rate of flip-flop of phosphatidylethanolamine and phosphatidylglycerol in artificial membranes, and suggested that the mere presence of membrane proteins promote flip-flop (17). However, the hydrophobic peptides did not affect the flip-flop of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), which is a main component of eukaryotic membranes (18). We previously demonstrated that transmembrane model peptides with a hydrophilic residue located within the center of a hydrophobic Leu-Ala repeat sequence enhanced the flip-flop of POPC and that the hydrophilic residues accounted for 4.5% of the central region in the predicted membrane-spanning sequences of human ER membrane proteins (19). Based on these findings, we hypothesized that membrane proteins with hydrophilic residues in the transmembrane region might act as “flippases” in the ER.
To test our hypothesis, we extracted membrane-spanning sequences of human ER membrane proteins and searched for proteins with hydrophilic residues in the central regions of their transmembrane domains. In this study, we focused on single-pass membrane proteins because their transmembrane regions can be easily mimicked using synthetic peptides. We synthesized the transmembrane regions of 13 single-pass membrane proteins and investigated their ability to promote flip-flop of fluorescently labeled lipids in artificial membranes.
Here, we demonstrated that the membrane-spanning sequences of EDEM1 and SPAST increased the flip rate of all three kinds of fluorescent lipids. In particular, the EDEM1 peptide had the high activity in a significantly low peptide/lipid ratio. These results suggested that the EDEM1 protein might function as a “flippase” in the ER.
Materials and Methods
Materials
POPC, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine (POPS), 1-palmitoyl-2-(6-((7-nitro-2-1,3-benzoxadiazol-4-yl)amino)hexanoyl)-sn-glycero-3 (C6NBD)-phosphocholine (C6NBD-PC), -phosphoethanolamine (C6NBD-PE), and -phosphoserine (C6NBD-PS) were purchased from Avanti Polar Lipids (Alabaster, AL). Cholesterol was obtained from Sigma Chemical (St. Louis, MO). Methoxy-poly(ethylene glycol)4 N-hydroxysuccinimide ester (mPEG4-NHS) was obtained from Thermo Fisher Scientific (Waltham, MA). All other chemicals used were of the highest reagent grade.
Extraction of membrane-spanning sequences of human ER membrane proteins
SOSUI software was used to predict 588 proteins as membrane proteins out of human ER proteins deposited in the UniProt database. Of these 588 proteins, 356 contain hydrophilic residues (Arg, Lys, Glu, Asp, Gln, Asn, or His) in the central six or seven residues of their transmembrane domains, in cases where the transmembrane sequence was comprised of an even or odd number of residues, respectively. Although only charged residues (Lys and Glu) were assessed in our previous study (19), noncharged hydrophilic residues (Gln, Asn, and His) were also found to be effective in promoting flip-flop in artificial membranes (unpublished data). Among the 356 proteins, 27 were predicted to be single-pass membrane proteins. Among these 27 proteins, 13 are described as ER-specific and not multipass proteins in the UniProt database.
Synthesis of peptides corresponding to the transmembrane domains of single-pass ER proteins
The amino acid sequences of the 13 peptides used in this study are shown in Fig. 1. Two Lys residues were placed at each terminus of the peptides to improve their solubility. A Trp residue was appended to the N-terminus of the peptides unless a Trp residue was already included in the native sequence, and the absorbance of the Trp residues was used for peptide quantification. These peptides were synthesized using Fmoc-based chemistry and purified by reverse-phase high-performance liquid chromatography (Shimadzu, Kyoto, Japan) with an Inertsil WP300 C18 column (GL sciences, Tokyo, Japan). The purity of the peptides was more than 90%, which was confirmed by the use of high-performance liquid chromatography and matrix-assisted laser desorption/ionization time-of-flight-mass spectrometry (Bruker Daltonics, Kanagawa, Japan). Concentrations of the peptides in trifluoroethanol were determined from the absorbance at 280 nm, using the molar extinction coefficients of Trp and Tyr (20).
Figure 1.
Amino acid sequences of the synthesized peptides. The residues surrounded by the rectangular box represent the central regions of the sequences. Hydrophilic residues in the central region are shown in bold text. ATF6, activating transcription factor 6; EDEM1, ER degradation-enhancing α-mannosidase-like protein 1; FACL4, long-chain fatty acid-CoA ligase 4; FMO2, flavin-containing monooxygenase 2; JP3, junctophilin-3; JP4, junctophilin-4; MMP23, matrix metalloproteinase-23; ORP5, oxysterol-binding protein-related protein 5; P4HTM, transmembrane prolyl 4-hydroxylase; RNF180, ring finger protein 180; SPAST, spastin; TMED7, transmembrane emp24 domain-containing protein 7; UBE2J2, ubiquitin-conjugating enzyme E2 J2.
Vesicle preparation
The required amounts of chloroform-methanol solutions containing POPC, POPE, POPS, and cholesterol, as well as trifluoroethanol solutions containing the peptides were mixed in a round-bottomed glass flask to prepare large unilamellar vesicles (LUVs). We used a lipid mixture of PC, PE, and PS at a 6:3:1 molar ratio without cholesterol, unless otherwise indicated. After the evaporation of organic solvents, the sample was dried overnight under vacuum. The film was hydrated with a Tris-HCl buffer (10 mM Tris, 150 mM NaCl, 1 mM EDTA, and 0.01 g/mL NaN3, pH 7.4), a HEPES-NaOH buffer (10 mM HEPES, pH 7.4), or a Tricine-NaOH buffer (10 mM Tricine, pH 7.5). The suspension was freeze-thawed several times and extruded through a 100-nm pore polycarbonate filter using a LiposoFast extruder (Avestin, Ottawa, Canada). The vesicle size of the suspension was confirmed to be ∼110–150 nm with dynamic light scattering analysis, using an FPAR-1000 particle analyzer (Otsuka Electronics, Osaka, Japan). The concentration of phospholipids was determined using a phosphorus assay (21). We confirmed that the extrusion of the suspension did not change the peptide/lipid ratio by measuring the Trp fluorescence of the peptides (Fig. S1 in the Supporting Material).
Circular dichroism spectroscopy
For circular dichroism (CD) measurements, LUVs were prepared using a HEPES-NaOH buffer, with a peptide/lipid ratio of 0.2 mol %. Each LUV suspension (3 mM) was placed in a quartz cell with a 0.1-cm path length. The CD spectra of the peptides in the LUVs were recorded at 25°C from 205 to 250 nm with a J-805 spectropolarimeter (JASCO, Tokyo, Japan). The spectra obtained represented the signal-averaged accumulation of 10 scans. The peptide spectra were converted to mean-residue ellipticity values after subtracting the spectra of LUVs without peptides. The α-helix content was estimated from the equation (22)
| (1) |
where [θ222] is the mean molar residue ellipticity at 222 nm.
Flip assay
An ethanol solution of C6NBD-phospholipid (PC, PE, or PS) at a molar concentration (fluorescent lipid/nonfluorescent lipid) of 0.2 mol % was added to LUVs with various peptide/lipid ratios in a Tris-HCl buffer to enable asymmetric incorporation of the fluorescent lipids into the outer LUV leaflets. We found that >98% of C6NBD-PC (at 37°C) and C6NBD-PS (at 31°C and 37°C) were incorporated immediately (<30 s) into LUVs, whereas >98% incorporation of C6NBD-PC at 25°C and 31°C; C6NBD-PE at 25°C, 31°C, and 37°C; and C6NBD-PS at 25°C took ∼5, 2, 37, 30, 10, and 4 min, respectively (Fig. S2). Therefore, LUVs were preincubated under the latter conditions for the indicated durations to promote complete incorporation. After incubation at 25°C, 31°C, or 37°C for several different time periods (10–360 min) to allow the C6NBD-phospholipids to translocate (flip) to the inner leaflet, 320 mM sodium dithionite in 2 M Tris was added to 50 μM LUV suspension to obtain a final dithionite concentration of 32 mM, which led to the reduction (and fluorescence quenching) of C6NBD-phospholipids in the outer leaflet of the LUVs. After the addition of dithionite, the fluorescence of NBD was monitored on an F-2500 spectrofluorometer (Hitachi, Tokyo, Japan) for 500 s at excitation and emission wavelengths of 460 and 534 nm, respectively.
The mole fraction of C6NBD-phospholipids that translocated to the inner leaflet (Φinner) was calculated from the dithionite-quenching profile, F(t)/F(0), where F(0) and F(t) are the fluorescence intensities before and after the addition of dithionite, respectively. The fluorescence quenching caused by dithionite displayed a biphasic decay profile (Fig. S3); the faster decay indicated the quenching of C6NBD-phospholipids located in the outer leaflet, and the slower decay represented the quenching of C6NBD-phospholipids that had been located in the inner leaflet and flopped after the addition of dithionite. Therefore, we fitted the fluorescence decay data with a double-exponential function to determine the Φinner value (Fig. S3).
Theoretically, if the number of phospholipids included in the outer and inner leaflets is the same, Φinner is given by:
| (2) |
where kflip and kflop are the rate constants of flip and flop, respectively. If flip and flop occur at the same rate (kflip = kflop):
| (3) |
Experimentally, we found that the Φinner values at t = 0 and ∞ did not equal 0 and 1/2, respectively. Therefore, we used the following equation instead:
| (4) |
Results
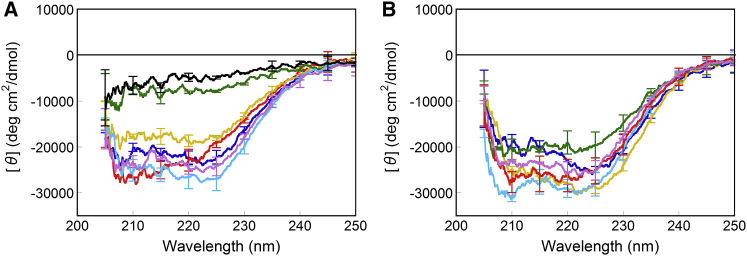
In this study, we synthesized 13 peptides predicted to represent membrane-spanning domains in single-pass ER membrane proteins. However, whether the synthesized peptides would adopt a transmembrane conformation in an artificial membrane in practice was unclear because of the hydrophilic residues in the central regions of the sequences. Therefore, we first investigated the secondary structure of the peptides in LUVs using CD spectroscopy (Fig. 2). The CD spectra of the MMP23 and FMO2 peptides indicated that the structure of the peptides was almost entirely that of a random coil, suggesting that neither peptide interacted with the membrane. The spectra of the other 11 peptides were typical of an α-helix-rich structure. The α-helix content of the peptides was calculated using Eq. 1, as shown in Table 1. The hydrophobic thickness of the POPC membrane, whose acyl chain composition matched that of the membranes used here, is 25.8 Å (23). At least 17 residues were necessary for an α-helix to span the bilayer, which correspond to 64% or 61% helix content for 27- or 28-residue peptides, respectively. Sufficient α-helix content was observed with 10 out of the 11 peptides. However, the α-helix content of FACL4 peptide was slightly lower, presumably due to the presence two Pro residues, which can disrupt α-helices. Moreover, fluorescence from Trp residues in the EDEM1 and RNF180 peptides in the LUVs exhibited blue-shifted spectra, compared with that from free tryptophan in aqueous solution (Fig. S4). These results suggested that the 11 peptides with high helix content most probably had transmembrane conformations in LUVs. Therefore, we evaluated the flip-promotion abilities of the 11 peptides.
Figure 2.
CD spectra of 13 synthetic peptides in LUVs. The peptide/lipid ratio was 0.2 mol %. Spectra are provided for (A) ATF6 (blue), EDEM1 (red), FACL4 (yellow), FMO2 (green), JP3 (light blue), JP4 (purple), and MMP23 (black), and (B) ORP5 (blue), P4HTM (red), RNF180 (yellow), SPAST (green), TMED7 (light blue), UBE2J2 (purple). Error bars represent the mean ± SD from n = 2 independent experiments. To see this figure in color, go online.
Table 1.
α-Helix Content of the Peptides in LUVs
| Peptide | α-Helix Content (%) |
|---|---|
| ATF6 | 70 ± 7 |
| EDEM1 | 67 ± 7 |
| FACL4 | 55 ± 4 |
| FMO2 | 18 ± 0 |
| JP3 | 82 ± 8 |
| JP4 | 75 ± 8 |
| MMP23 | 11 ± 1 |
| ORP5 | 74 ± 5 |
| P4HTM | 81 ± 12 |
| RNF180 | 89 ± 3 |
| SPAST | 61 ± 9 |
| TMED7 | 91 ± 5 |
| UBE2J2 | 82 ± 6 |
The SD values shown are representative of two independent experiments.
Flip promotion by membrane-spanning sequences
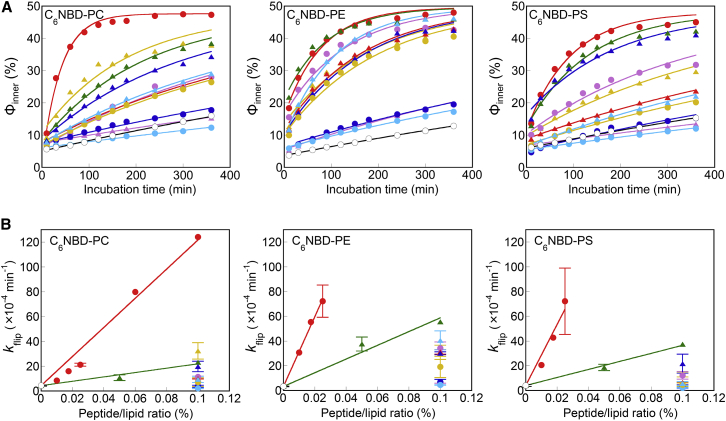
We measured flip of three fluorescent-labeled phospholipids (C6NBD-PC, C6NBD-PE, and C6NBD-PS) in LUVs in the presence of each synthetic peptide (Fig. 3; Table S1). Overall, the profiles shown in Fig. 3 revealed that the mole fraction of the fluorescent lipids in the inner leaflet (Φinner) increased and approached ∼50%, suggesting that flip and flop occurred at a similar rate. Importantly, the introduction of Lys residues at both termini of peptides little altered the flip-flop-promotion ability of peptides with a native sequence (Fig. S5).
Figure 3.
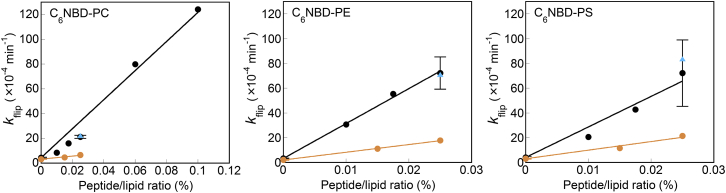
(A) Flip assays for C6NBD-PC, C6NBD-PE, and C6NBD-PS in LUVs without peptides (open circles), containing 0.1 mol % (for C6NBD-PC), or 0.025 mol % (for C6NBD-PE and -PS) EDEM1 (red circles), or containing 0.1 mol % ATF6 (blue circles), FACL4 (yellow circles), JP3 (light blue circles), JP4 (purple circles), ORP5 (blue triangles), P4HTM (red triangles), RNF180 (yellow triangles), SPAST (green triangles), TMED7 (light blue triangles), or UBE2J2 (purple triangles). The lines are fitting curves generated using Eq. 2. (B) Flip rate constants of C6NBD-PC, C6NBD-PE, and C6NBD-PS in LUVs containing 0–0.1 mol % peptides. The symbols are the same as in (A). The error bars represent the mean ± SD from n = 2 experiments. To see this figure in color, go online.
The flip rate of C6NBD-PE increased markedly with 8 out of the 11 peptides, whereas 4 and 3 peptides were associated with high flippase activities with C6NBD-PC and C6NBD-PS, respectively. The higher degree of C6NBD-PE flip promoted by the peptides might be due to lower hydrophilicity of the headgroup, as suggested previously (24). Although hydrophilic residues were located in the central region of all peptides, the flip-promotion ability of most peptides was unexpectedly low. The JP4 peptide (with a charged Asp residue in the central region of the sequence) did not efficiently facilitate the flip of C6NBD-PC and C6NBD-PS. The UBE2J2, ATF6, and JP3 peptides had almost no effect on the flip of the three C6NBD-phospholipids. These results suggest that not all single-pass transmembrane proteins with a hydrophilic residue in the middle of the transmembrane domain exhibited flippase activity. However, compared with the other peptides, the EDEM1 and SPAST peptides considerably enhanced the flip of all three C6NBD-phospholipids. In particular, EDEM1 promoted flip even at a significantly low peptide/lipid ratio (4.9-, 22-, and 18-fold for PC, PE, and PS, respectively, at a peptide/lipid ratio of 0.025 mol %). The flip rates were proportional to the concentrations of these peptides (Fig. 3 B), indicating that the monomeric forms of the EDEM1 and SPAST peptides facilitated phospholipid flip-flop.
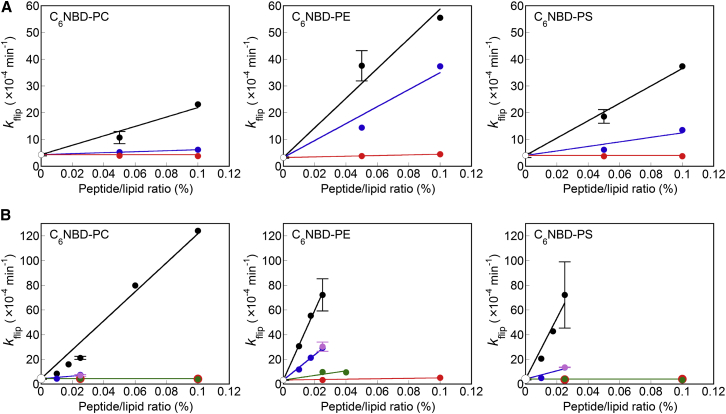
Flip promotion by mutant variants of the EDEM1 and SPAST peptides
To elucidate the mechanism by which the EDEM1 and SPAST peptides promoted phospholipid flip-flop, we substituted a hydrophobic Ala residue for the hydrophilic residues in the central region of both sequences. We confirmed that all mutant peptides used in this study exhibited CD spectra similar to the wild-type (WT) peptides (Fig. S6). Substituting Arg with Ala in the SPAST peptide abolished its ability to promote flip of fluorescent lipids (Fig. 4 A; Table S2). However, substituting His with Ala in the EDEM1 peptide only partially inhibited this activity (Fig. 4 B; Table S2). It should be noted that EDEM1 has an Arg residue located outside of the central region (Fig. 1). Interestingly, a mutant peptide having this Arg replaced with Ala completely lost its flip-promoting function (Fig. 4 B; Table S2). Similarly, a limited loss of function was observed for a SPAST mutant, in which a His residue located outside of the central region was substituted with Ala (Fig. 4 A; Table S2). These results suggested that the high activities of the EDEM1 and SPAST peptides depend on the two hydrophilic residues within the sequences. Incorporation of the same quantities of the Arg-to-Ala and His-to-Ala mutants of EDEM1 into LUVs resulted in no synergistic enhancement of phospholipid flip (Fig. 4 B; Table S2), suggesting that intrapeptide, not interpeptide, interactions play a critical role in flip-flop promotion by the EDEM1 peptide, which was consistent with our results showing the linear concentration dependence of the activity. In addition, both the EDEM1 and SPAST peptides promoted C6NBD-PS flip more efficiently than C6NBD-PC flip. Considering that the positively charged Arg residue is located in both sequences, the selectivity for PS might depend on electrostatic interaction(s) between Arg and the negative charge(s) of the headgroup of PS. Indeed, replacement of Arg in EDEM1 with a negatively charged Glu residue almost completely abolished the flip-promotion ability (Fig. 4 B; Table S2).
Figure 4.
(A) Flip-rate constants of C6NBD-PC, C6NBD-PE, and C6NBD-PS in LUVs containing 0–0.1 mol% SPAST WT (black), R13A (red), or H18A (blue). (B) Flip-rate constants of C6NBD-PC, C6NBD-PE, and C6NBD-PS in LUVs containing 0–0.1 mol% EDEM1 WT (black), R10A (red), H14A (blue), R10E (green), or 0.025 mol% of both EDEM1 R10A and H14A (purple). Error bars represent the mean ± SD from n = 2 experiments. To see this figure in color, go online.
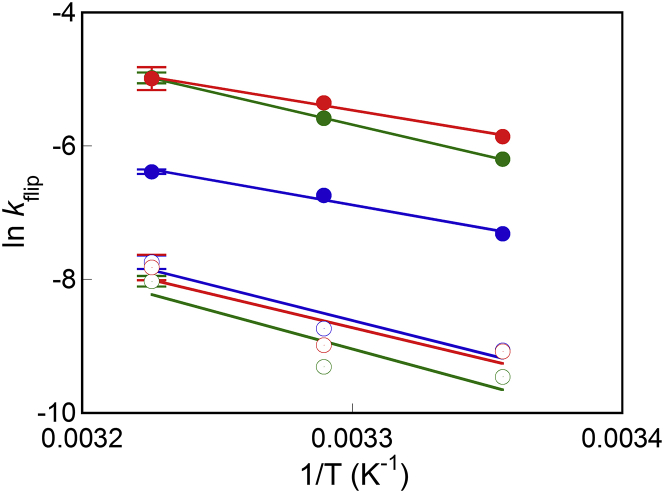
Kinetics of EDEM1-mediated flip
We evaluated the temperature dependence of phospholipid flip-flop with or without EDEM1 to gain insight into the flip-promotion mechanism of EDEM1. Lower flip-rate constants were obtained at lower temperatures, both in the absence and presence of the peptide (Table S3). Arrhenius plots of the rate constants exhibited a linear relationship (Fig. 5), from which the thermodynamic activation parameters at 37°C were calculated, using the method described by Homan and Pownall (25) (Table 2). Intrinsic flip-flop of C6NBD-phospholipids in peptide-free LUVs was unfavorable both enthalpically and entropically, which agreed with previous results obtained for 1,2-dimyristoyl-sn-glycero-3-phosphocholine (26). EDEM1 reduced the activation free energy (ΔΔG‡ = –3.88, –8.36, and –7.82 kJ/mol for C6NBD-PC, C6NBD-PE, and C6NBD-PS, respectively). The decrease in the activation free energy was accompanied by negative enthalpic and entropic changes (ΔΔH‡ = –25.0, –13.3, and –24.5 kJ/mol and TΔΔS‡ = –21.2, –4.96, and –16.7 kJ/mol, for C6NBD-PC, C6NBD-PE, and C6NBD-PS, respectively). This was most probably due to electrostatic stabilization of the transition state, where phospholipid headgroups interact with hydrophilic residues in EDEM1.
Figure 5.
Arrhenius plots of the rate constants of intrinsic flip (open circles) or EDEM1 (0.025 mol %)-mediated flip (solid circles) of C6NBD-PC (blue), C6NBD-PE (green), and C6NBD-PS (red). Error bars represent the mean ± SD from n = 2 experiments. The rate constants of EDEM1-mediated flip were calculated as the difference of the kflip values in the presence and absence of 0.025 mol % EDEM1. To see this figure in color, go online.
Table 2.
Thermodynamic Activation Parameters of C6NBD-PC, C6NBD-PE, and C6NBD-PS Flip-Flop at 37°C
| Fluorescent Lipid | Peptide (mol %) | Ea (kJ/mol) | ΔG‡ (kJ/mol)a | ΔH‡ (kJ/mol)a | TΔS‡ (kJ/mol)a |
|---|---|---|---|---|---|
| C6NBD-PC | no peptide | 84.4 | 96.3 | 81.8 | –14.5 |
| EDEM1 (0.025) | 59.4 | 92.4 | 56.8 | –35.6 | |
| C6NBD-PE | no peptide | 91.3 | 97.2 | 88.7 | –8.52 |
| EDEM1 (0.025) | 78.0 | 88.9 | 75.4 | –13.5 | |
| C6NBD-PS | no peptide | 80.2 | 96.7 | 77.6 | –19.0 |
| EDEM1 (0.025) | 55.7 | 88.8 | 53.1 | –35.7 |
Thermodynamic parameters of the activated states were calculated using the method described by Homan and Pownall (25).
Effect of cholesterol on flip promotion by the EDEM1 peptide
Cholesterol has been reported to decrease the ability of hydrophobic transmembrane peptides to promote phospholipid flip-flop (24). Here, we assessed the influence of cholesterol to inhibit the activity of EDEM1 peptide. Initially, the secondary structure and topology of the peptide were investigated in the presence of 20 mol % cholesterol. Similar CD spectra were obtained for the EDEM1 peptide in the presence and absence of cholesterol, suggesting that cholesterol has little effect on the secondary structure (Fig. S6 A). The transmembrane orientation of the peptide in the cholesterol-containing membrane was established using a membrane-impermeable, amino group-labeling reagent (Fig. S7). Because the peptide has two Lys residues at each end, labeling of two of the four Lys residues in the peptides occurs when they adopt a membrane-spanning orientation. Indeed, an increase in the mass (m)/charge (z) value by double the molecular mass of the labeling reagent was observed for the EDEM1 peptide in the membrane, whereas all Lys residues were labeled in the case of the MMP23 peptide, which was found not to interact with the membrane (Fig. S7).
The intrinsic flip rates of C6NBD-phospholipids in the peptide-free LUVs were little affected by cholesterol, which was consistent with previous results (27) (Fig. 6; Table S4). The EDEM1-mediated flip of C6NBD-phospholipids decreased in the presence of 20 mol % cholesterol compared with that observed in the absence of cholesterol (0.10-, 0.22-, and 0.28-fold for PC, PE, and PS, respectively), suggesting an inhibitory effect of cholesterol on the flippase activity of the peptide (Fig. 6; Table S4). In contrast, 5 mol % cholesterol did not have any effect on the EDEM1 activity.
Figure 6.
Flip-rate constants of C6NBD-PC, C6NBD-PE, and C6NBD-PS in LUVs containing 0–0.1 mol% EDEM1 WT in the absence (black circles) or presence of 5 (light blue triangles) or 20 (orange circles) mol % cholesterol. Error bars represent the mean ± SD from n = 2 experiments. To see this figure in color, go online.
Discussion
The main barrier against phospholipid flip-flop in membranes is that hydrophilic headgroups of phospholipids must traverse the hydrophobic hydrocarbon region of lipid bilayers. Our previous results obtained using model transmembrane peptides with hydrophilic residues supported the hypothesis that the presence of hydrophilic residues in the hydrocarbon region promotes flip-flop by reducing the activation energy. Menon et al. reported that each chromatographic fraction of ER proteins enhanced the flip-flop of phospholipids and that the activity of the different fractions displayed differing sensitivity to proteolysis (12). Chang et al. showed additive inhibition of ER flippase activity by N-ethylmaleimide and diethylpyrocarbonate (14). These data suggested that various kinds of proteins are involved in rapid flip-flop in the ER. Therefore, we hypothesized that ER membrane proteins with hydrophilic residues in their transmembrane regions, which have other specific functions, might exhibit flippase activity. In this study, we evaluated the flippase activities of membrane-spanning sequences derived from ER-resident proteins.
The 11 membrane-spanning sequences showed quite different activities despite the presence of hydrophilic residues in the central regions of all peptides. Four (FACL4, JP4, P4HTM, and TMED7) and three (ATF6, JP3, and UBE2J2) peptides exhibited small and marginal flippase activities against C6NBD-PC, respectively. Transmembrane model peptides with a hydrophilic residue in the center of the 17 residues of a hydrophobic sequence exhibited flippase activity, whereas similar peptides with 21 residues in the hydrophobic sequence did not (unpublished data), presumably due to the hydrophobic matching between the membrane and the peptides. We have previously suggested that membrane deformation by hydrophobic mismatch increases the flip-flop rate (27). SOSUI software predicted that 23 residues formed a transmembrane region with the peptides used in this study, which can sufficiently span the bilayer with no mismatch created. Taken together, we conclude that a single hydrophilic residue in the transmembrane region is not sufficient to promote flip-flop in the absence of hydrophobic mismatch.
The RNF180 peptide showed moderate flippase activity, which was more effective against C6NBD-PC than against other C6NBD-phospholipids. Langer et al. recently reported that replacement of a helix-stabilizing Leu residue with a bulky helix-destabilizing Val residue increased the ability of the peptides to flip lipids and that this effect was most pronounced for PC (28). The RNF180 peptide sequence includes nine bulky β-branched residues (four Val and five Ile) and only one Leu. Thus, the backbone dynamics of RNF180 could be related to the moderate flippase activity.
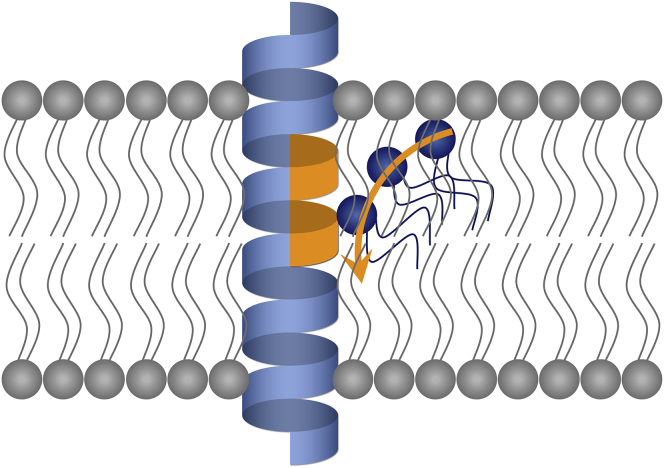
The EDEM1 peptide increased the flip rates of all three fluorescent lipids at a significantly low peptide/lipid ratio (0.025 mol %). The flip-flop of the fluorescent lipids was also enhanced in LUVs containing 0.1 mol % of the SPAST peptide. These peptides contain an additional hydrophilic residue (Arg or His for EDEM1 or SPAST, respectively) beyond that in the central region (His and Arg, respectively; Fig. 1). Experiments with mutant peptides revealed that both residues were important for flip-flop promotion. The promotion of flip-flop by both peptides was more effective against PE and PS than against PC. The selectivity for PE was likely due to the lower hydrophilicity of its headgroup, as mentioned above. We previously demonstrated that a peptide with a positively charged Lys residue in the center of the transmembrane region promoted flip of a negatively charged fluorescent lipid, C6NBD-phosphoglycerol, more effectively than it promoted flip of C6NBD-PC (19). Thus, the negatively charged headgroup of PS could be also effectively drawn into the bilayer via the electrostatic interaction with Arg and/or His. Interestingly, the EDEM1 peptide exhibited much higher activity than the SPAST peptide. The distance between Arg and His in the EDEM1 sequence is four residues, whereas this distance in the SPAST sequence is five residues. Considering that α-helices have 3.6 residues per turn, both residues in EDEM1 will face to the same side of the helix (Fig. 7), which composes a hydrophilic milieu within the membrane to allow the headgroups of phospholipids to easily enter the hydrocarbon region and flip across the membrane (Fig. 7). A similar mechanism was suggested in a recent report, which revealed the crystal structure of an energy-independent phospholipid scramblase (29). The thermodynamic activation parameters obtained for EDEM1-mediated flip-flop strongly support this model: The interaction of phospholipid headgroups with hydrophilic Arg/His residues in EDEM1 at the transition state renders the flip process enthalpically less unfavorable.
Figure 7.
Schematic representation of flip-flop promotion by the EDEM1 peptide. Arg and His residues (orange) in the α-helix of EDEM1 form a hydrophilic milieu in the hydrocarbon region, through which headgroups of phospholipids can enter the membrane with a reduced activation energy and flip-flop. To see this figure in color, go online.
The EDEM1 peptide exhibited the highest activity among the peptides studied. However, the slopes of the rate constants in Fig. 6 give an estimation that a single EDEM1 peptide translocates 0.2–0.5 lipids/s. These rates are much lower than those reported for lipid scramblases such as opsin and afTMEM16 from Aspergillus fumigatus (30, 31), although they cannot be directly compared because of differences in the lipid composition used. At least ∼0.12 mol % EDEM1 protein is required in the ER to achieve flip-flop with a half-time ranging from seconds to minutes. This concentration seems rather high considering that the protein/lipid ratio of the ER membrane is ∼1.2 mol % (32). Thus, the EDEM1 protein may function as one of the ER flippases, with other membrane proteins having similar physicochemical properties.
Cholesterol is widely distributed in membrane organelles in eukaryotes (33). The amount of cholesterol in the ER is only 5 mol %, whereas other organelles exhibit higher concentrations of cholesterol (i.e., 16 or 30–35 mol % in the Golgi apparatus or PM, respectively) (33, 34). We showed that 20 mol % of cholesterol suppressed the EDEM1-promoted flip-flop of fluorescent lipids. The presence of cholesterol in the membrane increases the order of acyl chains of lipids, as well as the membrane thickness (23, 35), which could inhibit phospholipid flip-flop (18, 36). Increased lipid packing by cholesterol would reduce vacancies for lipid headgroups to penetrate. A cholesterol concentration of 5 mol %, which corresponds to the concentration present in the ER, had no inhibitory effect. The results suggested that cholesterol controls the flippase activity and that a low cholesterol content is a prerequisite for rapid flip-flop in the ER.
In this study, we demonstrated that the membrane-spanning sequence of EDEM1, which is an ER-resident membrane protein, robustly promoted flip-flop in artificial membranes. Considering that marked activity of the EDEM1 peptide was observed at significantly low peptide concentrations, the EDEM1 protein might act as a flippase.
Author Contributions
Conceptualization, design, acquisition, analysis, and interpretation of data, and writing of the manuscript, H.N.; design, analysis, and interpretation of data, K.I.; Supervision, analysis, and interpretation of data, Y.I.; conceptualization, design, supervision, analysis, and interpretation of data, and writing of the manuscript, M.N.
Acknowledgments
This study was supported by the Japan Society for the Promotion of Sciences KAKENHI grants 26287098 and 26860020.
Editor: Paulo Almeida.
Footnotes
Seven figures and four tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30332-0.
Supporting Material
References
- 1.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zachowski A. Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem. J. 1993;294:1–14. doi: 10.1042/bj2940001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman J.A., Quazi F., Molday R.S. Mammalian P4-ATPases and ABC transporters and their role in phospholipid transport. Biochim. Biophys. Acta. 2013;1831:555–574. doi: 10.1016/j.bbalip.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasubramanian K., Schroit A.J. Aminophospholipid asymmetry: a matter of life and death. Annu. Rev. Physiol. 2003;65:701–734. doi: 10.1146/annurev.physiol.65.092101.142459. [DOI] [PubMed] [Google Scholar]
- 5.Bishop W.R., Bell R.M. Assembly of the endoplasmic reticulum phospholipid bilayer: the phosphatidylcholine transporter. Cell. 1985;42:51–60. doi: 10.1016/s0092-8674(85)80100-8. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann A., Zachowski A., Devaux P.F. Protein-mediated phospholipid translocation in the endoplasmic reticulum with a low lipid specificity. Biochemistry. 1990;29:2023–2027. doi: 10.1021/bi00460a010. [DOI] [PubMed] [Google Scholar]
- 7.Buton X., Morrot G., Seigneuret M. Ultrafast glycerophospholipid-selective transbilayer motion mediated by a protein in the endoplasmic reticulum membrane. J. Biol. Chem. 1996;271:6651–6657. doi: 10.1074/jbc.271.12.6651. [DOI] [PubMed] [Google Scholar]
- 8.Marx U., Lassmann G., Herrmann A. Rapid flip-flop of phospholipids in endoplasmic reticulum membranes studied by a stopped-flow approach. Biophys. J. 2000;78:2628–2640. doi: 10.1016/S0006-3495(00)76807-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vance D.E., Choy P.C., Schneider W.J. Asymmetry of phospholipid biosynthesis. Nature. 1977;270:268–269. doi: 10.1038/270268a0. [DOI] [PubMed] [Google Scholar]
- 10.Bell R.M., Ballas L.M., Coleman R.A. Lipid topogenesis. J. Lipid Res. 1981;22:391–403. [PubMed] [Google Scholar]
- 11.Fagone P., Jackowski S. Membrane phospholipid synthesis and endoplasmic reticulum function. J. Lipid Res. 2009;50(Suppl):S311–S316. doi: 10.1194/jlr.R800049-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menon A.K., Watkins W.E., 3rd, Hrafnsdottir S. Specific proteins are required to translocate phosphatidylcholine bidirectionally across the endoplasmic reticulum. Curr. Biol. 2000;10:241–252. doi: 10.1016/s0960-9822(00)00356-0. [DOI] [PubMed] [Google Scholar]
- 13.Gummadi S.N., Menon A.K. Transbilayer movement of dipalmitoylphosphatidylcholine in proteoliposomes reconstituted from detergent extracts of endoplasmic reticulum. Kinetics of transbilayer transport mediated by a single flippase and identification of protein fractions enriched in flippase activity. J. Biol. Chem. 2002;277:25337–25343. doi: 10.1074/jbc.M203809200. [DOI] [PubMed] [Google Scholar]
- 14.Chang Q.L., Gummadi S.N., Menon A.K. Chemical modification identifies two populations of glycerophospholipid flippase in rat liver ER. Biochemistry. 2004;43:10710–10718. doi: 10.1021/bi049063a. [DOI] [PubMed] [Google Scholar]
- 15.Sanyal S., Frank C.G., Menon A.K. Distinct flippases translocate glycerophospholipids and oligosaccharide diphosphate dolichols across the endoplasmic reticulum. Biochemistry. 2008;47:7937–7946. doi: 10.1021/bi800723n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanyal S., Menon A.K. Flipping lipids: why an’ what’s the reason for? ACS Chem. Biol. 2009;4:895–909. doi: 10.1021/cb900163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kol M.A., de Kroon A.I., de Kruijff B. Membrane-spanning peptides induce phospholipid flop: a model for phospholipid translocation across the inner membrane of E. coli. Biochemistry. 2001;40:10500–10506. doi: 10.1021/bi010627+. [DOI] [PubMed] [Google Scholar]
- 18.Nakano M., Fukuda M., Handa T. Flip-flop of phospholipids in vesicles: kinetic analysis with time-resolved small-angle neutron scattering. J. Phys. Chem. B. 2009;113:6745–6748. doi: 10.1021/jp900913w. [DOI] [PubMed] [Google Scholar]
- 19.Kaihara M., Nakao H., Nakano M. Control of phospholipid flip-flop by transmembrane peptides. Chem. Phys. 2013;419:78–83. [Google Scholar]
- 20.Pace C.N., Vajdos F., Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartlett G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- 22.Chen Y.H., Yang J.T. A new approach to the calculation of secondary structures of globular proteins by optical rotatory dispersion and circular dichroism. Biochem. Biophys. Res. Commun. 1971;44:1285–1291. doi: 10.1016/s0006-291x(71)80225-5. [DOI] [PubMed] [Google Scholar]
- 23.Nezil F.A., Bloom M. Combined influence of cholesterol and synthetic amphiphillic peptides upon bilayer thickness in model membranes. Biophys. J. 1992;61:1176–1183. doi: 10.1016/S0006-3495(92)81926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kol M.A., van Laak A.N., de Kruijff B. Phospholipid flop induced by transmembrane peptides in model membranes is modulated by lipid composition. Biochemistry. 2003;42:231–237. doi: 10.1021/bi0268403. [DOI] [PubMed] [Google Scholar]
- 25.Homan R., Pownall H.J. Transbilayer diffusion of phospholipids: dependence on headgroup structure and acyl chain length. Biochim. Biophys. Acta. 1988;938:155–166. doi: 10.1016/0005-2736(88)90155-1. [DOI] [PubMed] [Google Scholar]
- 26.Nakano M., Fukuda M., Handa T. Determination of interbilayer and transbilayer lipid transfers by time-resolved small-angle neutron scattering. Phys. Rev. Lett. 2007;98:238101. doi: 10.1103/PhysRevLett.98.238101. [DOI] [PubMed] [Google Scholar]
- 27.Nakao H., Ikeda K., Nakano M. pH-dependent promotion of phospholipid flip-flop by the KcsA potassium channel. Biochim. Biophys. Acta. 2015;1848(1 Pt A):145–150. doi: 10.1016/j.bbamem.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Langer M., Sah R., Langosch D. Structural properties of model phosphatidylcholine flippases. Chem. Biol. 2013;20:63–72. doi: 10.1016/j.chembiol.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Brunner J.D., Lim N.K., Dutzler R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature. 2014;516:207–212. doi: 10.1038/nature13984. [DOI] [PubMed] [Google Scholar]
- 30.Malvezzi M., Chalat M., Accardi A. Ca2+-dependent phospholipid scrambling by a reconstituted TMEM16 ion channel. Nat. Commun. 2013;4:2367. doi: 10.1038/ncomms3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goren M.A., Morizumi T., Menon A.K. Constitutive phospholipid scramblase activity of a G protein-coupled receptor. Nat. Commun. 2014;5:5115. doi: 10.1038/ncomms6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinn P., Griffiths G., Warren G. Density of newly synthesized plasma membrane proteins in intracellular membranes II. Biochemical studies. J. Cell Biol. 1984;98:2142–2147. doi: 10.1083/jcb.98.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mesmin B., Maxfield F.R. Intracellular sterol dynamics. Biochim. Biophys. Acta. 2009;1791:636–645. doi: 10.1016/j.bbalip.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Meer G. Lipids of the Golgi membrane. Trends Cell Biol. 1998;8:29–33. doi: 10.1016/s0962-8924(97)01196-3. [DOI] [PubMed] [Google Scholar]
- 35.Bittman R., Blau L. The phospholipid-cholesterol interaction. Kinetics of water permeability in liposomes. Biochemistry. 1972;11:4831–4839. doi: 10.1021/bi00775a029. [DOI] [PubMed] [Google Scholar]
- 36.Redelmeier T.E., Hope M.J., Cullis P.R. On the mechanism of transbilayer transport of phosphatidylglycerol in response to transmembrane pH gradients. Biochemistry. 1990;29:3046–3053. doi: 10.1021/bi00464a022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.