Abstract
The main structural component of the mucus in the gastrointestinal tract is the MUC2 mucin. It forms large networks that in colon build the loose outer mucous layer that provides the habitat for the commensal flora and the inner mucous layer that protects the epithelial cells by being impenetrable to bacteria. The epithelial cells in mice lacking MUC2 are not adequately protected from bacteria, resulting in inflammation and the development of colon cancer as found in human ulcerative colitis. Correct processing of the MUC2 mucin is the basis for the building of these protective networks. During the biosynthesis of the MUC2 mucin, post-translational modifications are formed resulting in reduction-insensitive bonds between MUC2 monomers. By the use of γ-glutamyltranspeptidase and isopeptidase activity in leech saliva, we could show that the molecular nature of these reduction-insensitive bonds is isopeptide bonds formed between side chains of lysine and glutamine. Transglutaminase 2 has an affinity to the MUC2 CysD2 domain in the nanomolar range and can catalyze its cross-linking. By using mass spectrometry, we identified MUC2 residues involved in this cross-linking. This shows for the first time that transamidation is not only stabilizing the skin and the fibrin clot, but is also important for the correct intracellular processing of MUC2 to generate protective mucus.
Keywords: intestine, mass spectrometry (MS), mucin, mucus, transglutaminase
Introduction
Protection of epithelial cells from the environment in the intestinal tract is maintained by the mucus, a gelatinous protein network. The MUC2 mucin is the major constituent of the two-layered mucous structure in the colon with a stratified inner layer that is attached to the epithelium and is impenetrable to bacteria, whereas the outer layer provides the habitat for the commensal flora (1, 2). The MUC2 monomer is made up of more than 5,000 amino acids with three complete domains and one partial von Willebrand domain (vWD)2 at the N terminus followed by the first CysD domain, two regions with a high amount of proline, threonine, and serine the so-called PTS domains that are separated by the second CysD domain, and the C-terminal part harbors the fourth vWD, a vWC, and a cysteine knot domain (Fig. 1a) (3). The primarily translation product forms C-terminal dimers via disulfide bonds in the endoplasmic reticulum (4). During their transport in the Golgi apparatus, the MUC2 dimers become heavily O-glycosylated thereby shifting their mass to ≈5 MDa. In the trans-Golgi-network MUC2 is sorted in the regulatory pathway and experiences disulfide bond-based trimerization of the dimers in their vWD3 domain (5). In the later stages of the secretory pathway, around or after this trimerization, reduction-insensitive bonds are formed (6). At that time MUC2 becomes insoluble in regular buffers, including SDS-containing ones when the biosynthesis was studied in LS174T cells (6). The more mature and secreted MUC2 mucin from the intestine is also insoluble in chaotropic salts (like guanidinium chloride) what was first discovered by Carlstedt and co-workers (7, 8). Ever since the first description of these non-reducible bonds, their nature and localization have remained a mystery.
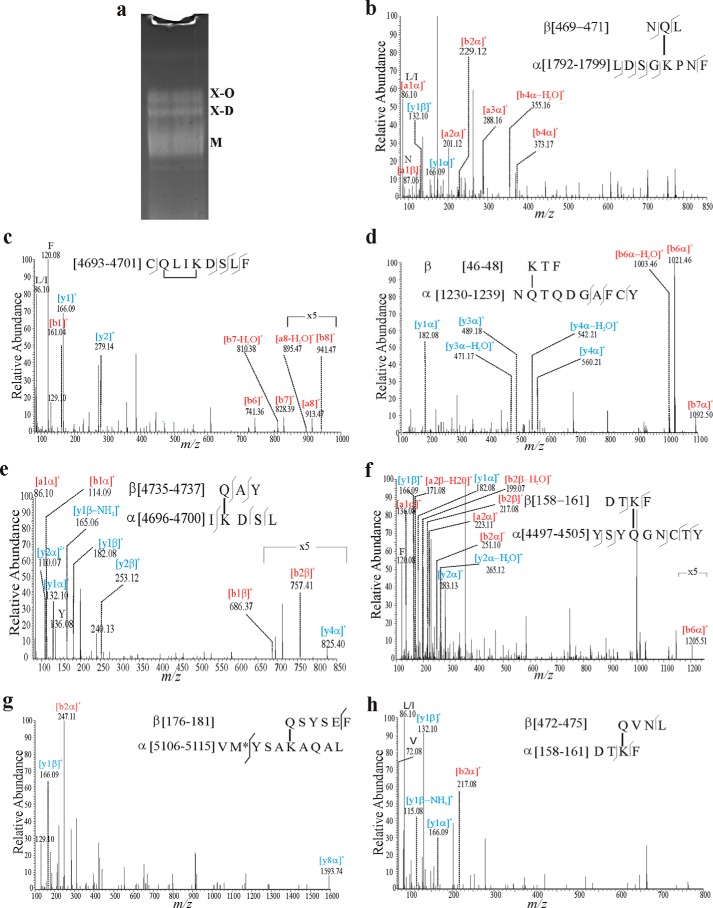
FIGURE 1.
Reduction-insensitive MUC2 oligomers are due to isopeptide bonds. The MUC2 mucin was isolated from LS174T cells. a, schematic figure of the MUC2 domain structure and sequence of the CysD2 domain. SS, signal sequence; D, von Willebrand D domain; CysD, CysD domain; PTS, proline/threonine/serine-rich domain; C, von Willebrand C domain; CK, cysteine-knot domain; GDPH, auto-catalytic cleavage site. MUC2N, CysD2, and MUC2C mark the localization of the recombinant proteins used. The numbers refer to the number in the MUC2 mucin (reference sequence Q02817 (MUC2_HUMAN) of the UniProt database). b, reduced and alkylated purified MUC2 from LS174T cells was treated with KOH for 5 h, separated by AgPAGE, and stained with Alcian blue. No cleavage of the reduction-insensitive oligomers was observed. c, reduced and alkylated purified MUC2 from LS174T cells was treated with γ-GT (1.6 units) or with for saliva from H. medicinalis saliva treated with protease inhibitors for 12 h followed by analysis with AgPAGE and staining with Alcian blue. The smaller size of the treated sample is due to the remaining protease or glycosidase activities. d, purified MUC2 from LS174T cells was incubated with γ-GT for 12 h at 37 °C, heated to 95 °C, centrifuged at 10,000 × g for 10 min, and the A600 of the supernatant was measured. The graph summarizes the results from three independent experiments. n.s., not significant. e, purified MUC2 from LS174T cells was incubated in the presence and absence of TGM2 at pH 5.5 and 8.0 as well as with TGM2 and the transglutaminase-inhibitor Z-DON for 12 h at 37 °C and analyzed as described in d. **, p < 0.01.
Transglutaminases (EC 2.3.2.13) are a family of structurally and functionally related proteins that catalyze Ca2+-dependent post-translational modification of proteins by introducing covalent bonds between the γ-carboxamide group of glutamine and the ϵ-amino group of protein-bound lysine (9). The human transglutaminase family consists of nine members. Although their primary sequence differs, with the exception of the inactive erythrocyte band 4.2, all share the same catalytic triad of Cys, His, and Asp enabling the enzymes to transamidate or deamidate proteins. Transglutaminases are pleiotropic enzymes. Besides their cross-linking activity, they can also act as GTPases and protein-disulfide isomerases (10, 11). Their different enzymatic activities allow these proteins to influence a number of cellular events like adhesion and autophagy (10, 12, 13). Among the members of the transglutaminase family, TGM2 represents the most widely distributed isoform. Its localization is predominantly in the cytosol, but it is also present at the plasma membrane. In addition, TGM2 is also found extracellularly where it can be activated by mechanical injury or inflammatory stimuli (14). Furthermore, highly sulfated glycosaminoglycans augment cross-linking activity of transglutaminases (15).
To shed light into the nature of the reduction-insensitive oligomers, we utilized different biochemical approaches to cleave and generate reduction-insensitive bonds and analyzed different domains/parts of the MUC2 mucin for the localization of these bonds. Furthermore, we were able to decipher some of the amino acid residues that are involved in the formation of these reduction-insensitive cross-links via mass spectrometric analyses.
Experimental Procedures
Expression and Purification of the MUC2 Mucin
Human LS174T cells were grown in Iscove's modified Dulbecco's medium (Gibco, Paisley, Renfrewshire, Scotland, UK) containing 10% (v/v) fetal calf serum and 1% (v/v) supplement (110 mg/liter sodium pyruvate, 36 mg/liter l-asparagine, 116 mg/liter l-arginine, 290 mg/liter l-glutamine, and 10 mg/liter folic acid) in roller bottles (1/min, 37 °C, 5% CO2) until confluent and harvested after additional growth for 10 days with daily medium change. Cells were resuspended in 50 ml of guanidinium chloride (6 m guanidinium hydrochloride, 10 mm sodium phosphate, 5 mm N-ethylmaleimide, 5 mm EDTA, 0.5 mm PMSF, pH 6.5) and homogenized by 5 strokes in a Dounce homogenizer. Lysis was completed by gentle stirring of the suspension for 12 h at 4 °C. Insoluble mucins were precipitated by centrifugation at 20,000 × g for 30 min followed by four washings of the pellet by centrifugation in the same guanidinium chloride buffer (7). The pellet containing the insoluble mucin was brought into solution by reduction in a buffer containing 6 m guanidinium chloride, 100 mm Tris, 5 mm EDTA, 10 mm freshly added dithiothreitol, pH 8.0, under gentle stirring for 5 h at 37 °C. The reduced cysteinyl groups were alkylated by the addition of 25 mm iodoacetamide under gentle stirring in the dark for 12 h at room temperature. Finally, the samples were dialyzed against water.
Expression and Purification of Recombinant MUC2N, CysD2, and MUC2C
Expression of the different recombinant MUC2 domains in permanently expressing CHO-Lec3 cells was performed by the Mammalian Protein Expression Core Facility (University of Gothenburg), and purification was performed as described (4, 5, 16).
Saponification
MUC2 (15 μg) was mixed with KOH leading to end concentrations of 25, 50, 75, and 100 mm and incubated for 5 h at room temperature. Reaction products were analyzed by composite Ag-PAGE (17).
Cleavage of the Non-reducible Bond by γ-Glutamyltranspeptidase and Saliva of Hirudo medicinalis
γ-Glutamyltranspeptidase (1.6–4 units, Sigma) was added to 15 μg of MUC2 from the insoluble fraction in a buffer containing 50 mm Tris-HCl, pH 8.0, 150 mm NaCl, and 2 mm Gly-Gly (Sigma). The reaction was incubated for 12 h at 37 °C. Reaction products were analyzed by composite agarose-PAGE and visualized via Alcian blue staining. Saliva from H. medicinalis was collected according to the method of Friedrich et al. (18). Briefly, 2 days before the collection of saliva, the leeches were stimulated with a solution containing 20 mm sodium phosphate, pH 7.4, 150 mm NaCl, and 10 mm l-Arg. After 48 h, leeches were incubated in the same solution with 1% (w/v) pilocarbine for 1 h at room temperature. The solution with the secreted saliva was dialyzed against water and lyophilized. The crude extract showed an activity of 2.6 nmol/min/mg as determined by the cleavage of γ-glutamyl-p-nitroanilide. One unit of isopeptidase activity was defined as the enzymatic activity producing 1 μmol of p-nitroaniline from γ-glutamyl-p-nitroanilide per min at room temperature. MUC2 (15 μg) from the MUC2 insoluble fraction was incubated in 50 mm Tris-HCl, pH 8.0, 150 mm NaCl containing 5 milliunits of isopeptidase and cOmpleteTM protease inhibitor mixture (Roche Applied Science) for 12–16 h at 37 °C. Reaction products were analyzed by composite agarose-PAGE and visualized with Alcian blue.
Composite Agarose-PAGE, SDS-PAGE, and Western Blot Analyses
Composite agarose-polyacrylamide (AgPAGE) gels were prepared according to the method of Schulz et al. (19). Purified MUC2 from LS174T cells was mixed with 2-fold concentrated loading buffer (100 mm Tris-HCl, pH 6.8, 4% (w/v) SDS, 200 mm DTT, 20% (v/v) glycerol) and heated for 5 min at 95 °C before separation via AgPAGE for 3.5 h at 30 mA and 6 °C and stained with Alcian blue or by zinc imidazole (20).
Separated proteins from SDS-PAGE were transferred onto PVDF membranes (Immobilin-PSQ, 0.2 μm; Millipore) with 0.9 mA/cm2 for 1.5 h using a transfer buffer containing 48 mm Tris, 38 mm glycine, 1.3 mm SDS, and 20% (v/v) methanol. The membrane was blocked in 5% (w/v) nonfat milk in TBS containing 0.1% (v/v) Tween 20 (TBS-T) and 0.05% NaN3 or 3% (w/v) BSA in TBS-T for the biotin-containing molecules for 1 h at room temperature. Membranes were incubated with an antibody against a Myc tag (Ab 9106; Abcam, Cambridge, UK). After five washing steps in TBS-T, the respective secondary antibody (α-rabbit-IgG, 1:1,000, Southern Biotech, Solna, Sweden) coupled to alkaline phosphatase was incubated for 1 h at room temperature, rinsed five times in TBS-T, and visualized with the nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate substrate (Promega). Biotin incorporation in MUC2 domains after cross-linking was visualized by streptavidin-coupled alkaline phosphatase (1:1,000, Southern Biotech) after blocking of the membrane in 3% (w/v) BSA in TBS-T.
Analysis of MUC2 Depolymerization by Measuring Turbidity
Insoluble MUC2 (∼90 μg) from LS174 T cells was incubated in the presence and absence of 2 units of TGM2 (Zedira, Darmstadt, Germany) or together with the transglutaminase inhibitor Z-DON (Zedira) in 50 mm Tris, 10 mm CaCl2, pH 5.5 or pH 8.0, respectively (total volume 150 μl), for 12 h at 37 °C. The same experimental setup was used for incubation with 1.6 units of γ-glutamyltranspeptidase in a buffer containing 50 mm Tris-Cl, pH 8.0, 150 mm NaCl, and 2 mm Gly-Gly. The reactions were stopped by heating for 5 min at 95 °C and then centrifuged for 10 min at 10,000 × g, and the supernatants (100 μl) were transferred to a microcuvette, and the A600 was determined.
Cross-linking of MUC2 Domains/Parts and Western Blot Analyses
Aliquots (500 ng) of recombinant MUC2 N and C termini and the CysD2 domain were incubated either in the presence of 0.15 μmol N-(biotinyl)-cadaverine (Zedira) or 0.15 μmol of biotinyl-Thr-Val-Gln-Gln-Glu-OH (Zedira) in 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 10 mm CaCl2 with or without 2 units of TGM2 (Zedira) for 30 min at 37 °C. Negative controls were performed by adding the TGM2 inhibitor Z-DON (Zedira) to a final concentration of 20 μm and without adding the enzyme. The reactions were stopped by 2-fold concentrated loading buffer and heating at 95 °C for 5 min. The samples were electrophoresed on 4–12% SDS-polyacrylamide gels. For time course analyses, the CysD2 domain was incubated with TGM2 at 37 °C, and sample aliquots were taken after different time periods. The reactions were terminated by adding SDS-PAGE loading buffer and heating for 5 min to 95 °C. For mass spectrometry (MS) analyses of CysD2 homo-oligomers, reaction products were reduced for 30 min at 60 °C in the presence of 10 mm DTT and alkylated at room temperature by the addition of 25 mm iodoacetamide.
Microscale Thermophoresis
Interaction experiments were carried out on a Monolith NT115.1 Microscale Thermophoresis instrument (Nanotemper, Munich, Germany) equipped with a blue-green fluorescent reader using 50% LED and 60% IR power. Interaction partners were prepared in 50 mm Tris, pH 7.5, 150 mm NaCl, 5 mm EDTA, 0.05% Tween 20 with decreasing concentrations of the non-fluorescent-labeled interaction partner. A dilution series ranging from 3.2 μm to 1.6 nm of TGM2 was prepared, and fluorescently labeled CysD2 was added at a final concentration of 110 nm. Microscale Thermophoresis instrument traces were followed for 30 s after heat induction, and the ratio difference was plotted against the concentration of the titrated interaction partner.
In-gel/In-solution Digestion and Mass Spectrometric Analyses of Cross-linked Peptides
Protein bands of interest were cut into ∼1-mm3 gel pieces. Zinc imidazole-stained gel pieces were first washed in 50 mm Tris, pH 8.1, 200 mm glycine, 30% (v/v) acetonitrile followed by three washing steps in 50 mm Tris, pH 8.1. Gel pieces were dried in a vacuum centrifuge (Techtum, Umeå, Sweden) and digested by chymotrypsin (1:60) (Promega) in 25 mm Tris, pH 8.0, 10 mm CaCl2 for 12 h at 25 °C. Peptides were extracted by 50% (v/v) acetonitrile, 2% trifluoroacetic acid (TFA) for 30 min under constant shaking (800 rpm) followed by a second extraction step in 50% (v/v) acetonitrile, 0.1% TFA. The extracts were combined, dried to completeness, and resolubilized in 0.2% TFA. Salts and buffer were removed by in-house stage tips with C18 resin (C18 Empore, 3 m, Minneapolis, MN) and the samples resolved in 0.2% formic acid (21). For in-solution digest of cross-linked CysD, chymotrypsin (1:60) was added to the buffer as described above, and after 12 h of incubation at 25 °C, 1 mm PMSF was added followed by the addition of AspN (1:50, Promega) and incubation for 12 h at 37 °C. Peptides were separated on nanoRP-HPLC coupled to an ESI source on a Q-Exactive mass spectrometer (Thermo) with a gradient of 5–60% B (solvent A, 0.2% formic acid; solvent B, 0.2% formic acid, 80% (v/v) acetonitrile) (22). Cross-linked products were identified using the StavroX software tool (version 3.5.1) (23). MS data files were searched for cross-linked peptides using the following settings: mass tolerance of the precursor ion of 2 ppm; tolerance for fragment ions 20 ppm; cleavage C terminus of Trp; Tyr, Phe, and Leu for chymotrypsin as cleaving enzyme; cleavage N terminus of Asp and cleavage C terminus of Trp; Tyr, Phe, and Leu for combined chymotrypsin/AspN proteolysis (for the analysis of CysD oligomers) up to two missed cleavages were allowed; carbamidomethylation of Cys as fixed modification; oxidized methionine as variable modification; ammonia loss for cross-linked products. Fragmentation spectra of putative candidates were further analyzed manually.
Statistical Analysis
Statistical analyses were performed using Student's t test for pairwise comparison analysis. Significance was accepted when p values were below 0.05. Data are expressed as means ± S.D.
Results
Determination of the Molecular Nature of the Reduction-insensitive Bonds in MUC2
One of the possibilities for the reduction-insensitive bonds that could cross-link MUC2 monomers are ester bonds. MUC2 can undergo an autocatalytic cleavage at the GD↓PH sequence in its fourth vWD domain thereby forming a new C terminus with an aspartate internal anhydride that easily could react with the numerous glycan hydroxyl groups (arrow in Fig. 1a) (24). To test this hypothesis, we treated the MUC2-insoluble fraction with different concentrations of KOH that cleave ester bonds. However, even after 5 h of incubation with 100 mm KOH, we could not detect any loss of the MUC2 dimer (X-D) or oligomer (X-O, Fig. 1b). This indicates that ester bonds are not involved in the formation of MUC2 oligomers.
Other possibilities for the linkage of the MUC2 monomers are regular amide bonds between the N and the C termini of MUC2 monomers or isopeptide linkages between the side chains of Lys and with Asn, Gln, Asp, or Glu where the pair Lys-Gln is the most common. To test this, we took advantage of γ-glutamyltranspeptidase (γ-GT; EC 2.3.2.2) that can cleave the isopeptide bond between the γ-glutamyl group of glutamate and the amine group of cysteine in the tripeptide glutathione (25). We treated reduced insoluble MUC2 with γ-GT, and indeed the oligomeric forms (X-D and X-O) of the MUC2 mucin disappeared, and only the monomeric form (M) remained (Fig. 1c). The saliva of the medicinal leech H. medicinalis contains isopeptidase activity as shown by its ability to cleave the isopeptide bonds between the A and B polypeptide of cross-linked fibrin (18, 26). We incubated reduced insoluble MUC2 mucin with the saliva from the leech in the presence of protease inhibitors. Also, this treatment led to loss of the MUC2 oligomers (Fig. 1c). The results from the γ-GT and H. medicinalis saliva experiments suggest that MUC2 monomers are linked together via isopeptide bonds.
As non-reduced MUC2 polymers are insoluble and easily precipitable by centrifugation, we analyzed the influence γ-GT on this feature. After incubation with γ-GT at pH 8.0, we observed a trend toward increased turbidity of the supernatant after centrifugation at 10,000 × g, which would indicate that more MUC2 became soluble due to the cleavage of isopeptide bonds (Fig. 1d) Another way to analyze this was to utilize the isopeptidase activity described for transglutaminases at acidic pH (27, 28). Therefore, purified MUC2 was incubated with TGM2 at pH 5.5 and 8.0, and the turbidity of the supernatant was determined as for the treatment with γ-GT. A significant increase of the turbidity was observed when MUC2 was incubated with TGM2 at pH 5.5 compared with the control conditions (Fig. 1e). This result further suggests a disruption of the MUC2 gel based on the isopeptidase activity of TGM2.
MUC2 CysD Domain Can Be Part of Isopeptide Bonds
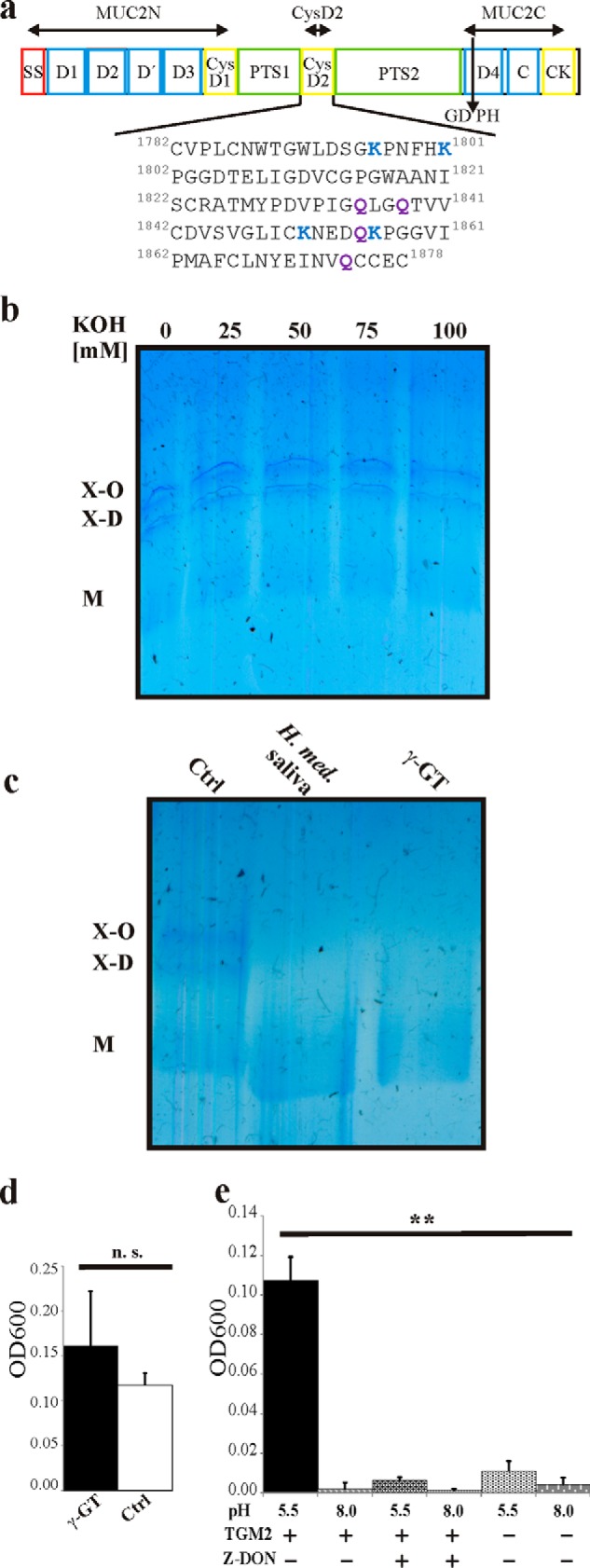
As the cleavage experiments suggested isopeptide bonds, we wondered whether such cross-links could be formed between recombinantly expressed MUC2 parts via transglutaminase-catalyzed reactions. Therefore, recombinant proteins containing the N terminus (MUC2N), the C terminus (MUC2C), or the CysD2 domain of MUC2 (Fig. 1a) were incubated with biotinylated acyl or amine donors and transglutaminase 2 (TGM2). When the MUC2 parts were used as acyl donors in the presence of the biotinylated peptide TVQQEL (B-TVQQEL) and analyzed by PAGE followed by Western blotting, several biotin bands were detected (Fig. 2a). When the reaction was performed in presence of the transglutaminase inhibitor Z-DON and in the absence of TGM2, no bands were detected. Most of the bands observed with either MUC2N or MUC2C were also present in the control reaction where TGM2 was only incubated with B-TVQQEL suggesting that TGM2 was conjugated with the biotinylated peptide. Therefore, specific signals reflecting TGM2-catalyzed biotin incorporation into MUC2N or MUC2C in the mass range of 250 kDa (MUC2N) and 220 kDa (MUC2C) could not be detected. However, in the CysD-containing reaction, a signal in the mass range of 20 kDa corresponding to the size of CysD was observed (Fig. 2a). This band corresponds to the size of the CysD2 domain and suggests that the CysD2 domain of MUC2 can act as an acyl donor in TGM2-catalyzed reactions. To test whether the MUC2 mucin parts could also act as acyl acceptors in transglutaminase-catalyzed cross-linking reactions, the different MUC2 parts were incubated with biotinylated cadaverine (5-BP). Also in this case, the CysD became biotinylated as a band corresponding to CysD was detected (Fig. 2b). A few bands, in addition to the control without the MUC2 parts, were observed in the reactions containing MUC2N and MUC2C and were weaker than the CysD band. The results so far suggest that the CysD2 domain can serve both as acyl acceptor and acyl donor in TGM2-based cross-linking reactions suggesting that this domain can form cross-linked homo-oligomers.
FIGURE 2.
Analyses of recombinant MUC2 domains as acyl and amine donors in a transglutaminase 2-catalyzed cross-linking reaction. a, recombinant MUC2N, CysD2, and MUC2C were incubated with biotinylated TVQQEL peptide and recombinant TGM2 (2 units) alone and together with the transglutaminase inhibitor Z-DON for 30 min at 37 °C. The reaction products were analyzed by SDS-PAGE and transferred to PVDF membranes, and biotin incorporation was visualized by alkaline phosphatase coupled to streptavidin. b, recombinant MUC2N, CysD2, and MUC2C were incubated with biotinylated cadaverine (5-BP) and recombinant TGM2 (2 units) alone and together with Z-DON for 30 min at 37 °C. Analysis as in b. c, recombinant CysD2 was incubated with TGM2 (0.5 units) for different times, and the reaction products were analyzed by SDS-PAGE, Western blotted, and detected with an anti-Myc tag antibody. The TGM2 lane shows its migration illustrates that TGM2 disappeared relatively fast due to polymerization.
To test this, we incubated purified Myc-tagged CysD2 with TGM2 for different time periods and analyzed the reaction products by SDS-PAGE and Western blot using an anti-Myc antibody. Signals corresponding to CysD2 dimers (CysD2 × 2, ∼40 kDa) and tetramers (CysD2 × 4, ∼80 kDa) were observed after 5–10 min, which increased over time (Fig. 2c). Furthermore, heterodimers of CysD and TGM2 were observed after 120 min as well as higher multimers in the wells of the respective gel lanes. Thus, the CysD2 domain can form TGM2-catalyzed oligomeric complexes with itself.
Affinity of TGM2 to MUC2 CysD2
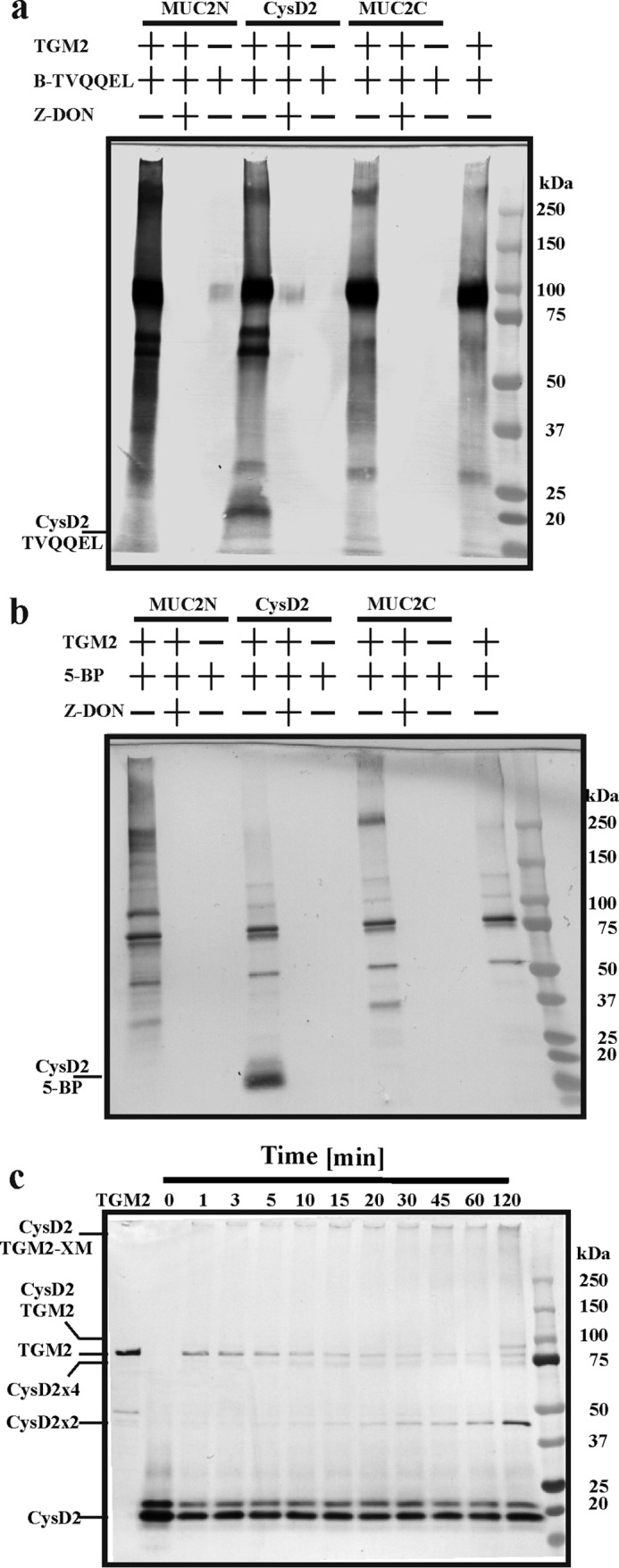
The fast reaction of TGM2 with CysD2 could suggest direct binding. The binding affinity of TGM2 to different parts of the MUC2 mucin was studied by thermophoresis. For this, three independent serial dilutions of unlabeled TGM2 (1.6 nm to 3.2 μm) were incubated with fluorescently labeled CysD and analyzed in triplicate. TGM2 bound to CysD2 with a KD of 113 ± 24 nm (Fig. 3a). No interaction between TGM2 and either the MUC2N or the MUC2C parts could be detected as shown for MUC2C (Fig. 3b). These experiments indicate a direct interaction between CysD2 and TGM2 and are in line with the formation of covalent CysD2-homo-oligomers and CysD2-TGM2 dimers (Fig. 2c).
FIGURE 3.
Thermophoretic analyses of the affinity between recombinant CysD2 and TGM2. a, fluorescently labeled CysD2 in constant amounts was added to titrated TGM2 from 1.6 nm to 3.2 μm and analyzed by thermophoresis. Normalized fluorescence intensities plotted against the TGM2 concentrations revealed a KD of 113 ± 24 nm. b, fluorescently labeled MUC2C in constant amounts was titrated against 0.15 nm to 5 μm TGM2 and analyzed by thermophoresis. No binding was observed.
Mass Spectrometric Identification of Isopeptide Bonds in CysD2
As the CysD2 domain formed oligomers by itself, at least one of the four Gln and one of the four Lys in the CysD2 sequence must be involved in the formation of isopeptide bonds (Fig. 1a). To determine the localization of the residues involved, recombinant CysD2 was incubated with TGM2 and subsequently digested with the proteases chymotrypsin and Asp-N followed by LC-MS analysis. When the obtained results were analyzed with the StavroX software, two cross-links could be detected. One was found between Gln-1838 and Lys-1851 (Fig. 4a). The MS2 mass spectra showed a characteristic b-ion series, including parts of the α-peptide and the full β-peptide at m/z 831, 932, and 1031. The second cross-link was observed between Gln-1835 and Lys-1851 (Fig. 4b). The mass spectra show a characteristic [a3β]2+ peak at m/z 871. The Lys at Lys-1851 can thus be used as an acyl donor in both CysD cross-links. As two different Glns in CysD2 can be utilized as acyl acceptors, it is possible to form higher CysD oligomers than dimers. This is in line with the observed tetramers in Fig. 2c, and a possible CysD2 tetramer is explained in Fig. 4c. Gln-1835 and Gln-1838 are localized close to each other, and it is possible that the use of one could interfere with the other.
FIGURE 4.
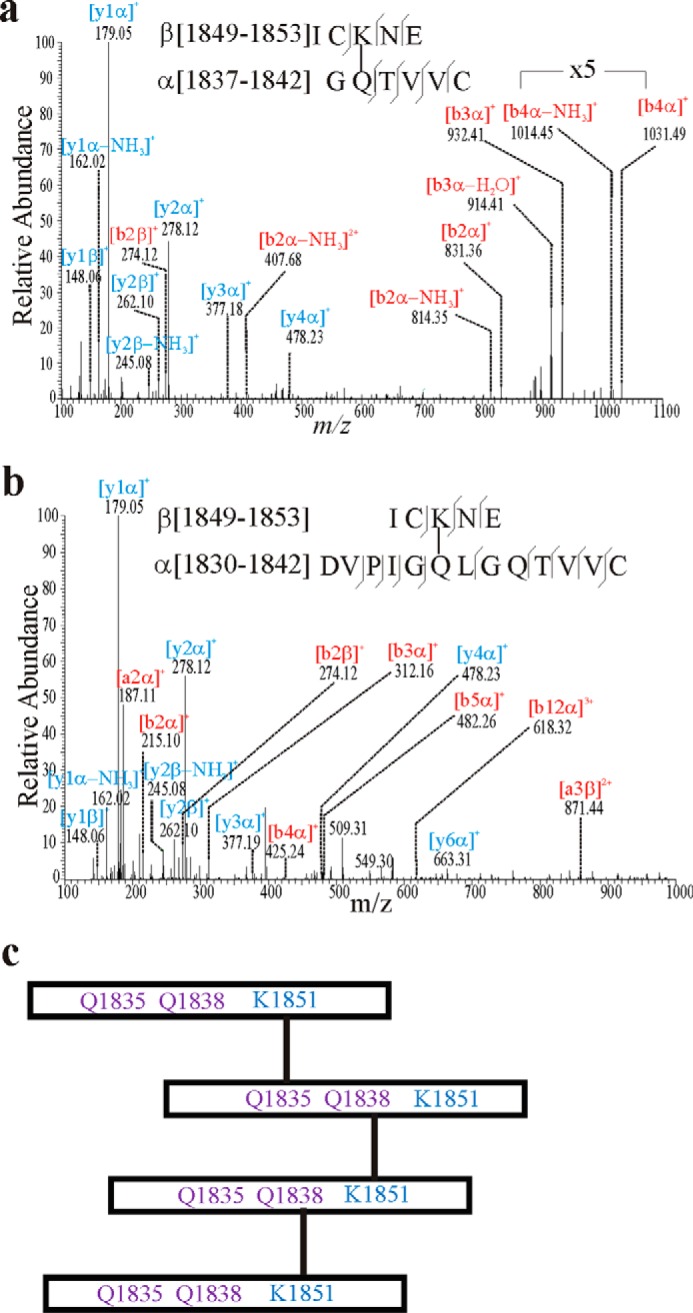
Mass spectrometric analysis of TGM2 introduced isopeptide cross-links in CysD2 homodimers. Recombinant CysD2 (2 μg) was incubated with TGM2 (0.5 units) in 50 mm Tris buffer, pH 7.5, for 30 min at 37 °C. The reaction products were reduced and alkylated followed by chymotrypsin and AspN digestion followed by LC-MS analysis. a, MS2 fragment spectrum of the molecular ion [M + 2H]2+ 654.80 showing the isopeptide bond between Lys-1851 and Gln-1838 (numbers as in Fig. 1a). b-ions are labeled in red, and y-ions are labeled in blue. b, MS2 fragment spectrum of parent ion [M + 3H]3+ 677.67 showing the isopeptide bond between Lys-1851 and Gln-1835. c, potential model of how the cross-links within CysD2 could generate higher oligomers.
Mass Spectrometric Determination of Isopeptide Cross-links in the Full-length MUC2 Mucin
To analyze localization of the isopeptide-based cross-links in the full-length MUC2 mucin, the purified insoluble intracellular MUC2 from LS174T cells was reduced, alkylated, and separated by AgPAGE (Fig. 5a). The three bands were cut out and analyzed by mass spectrometry for the presence of isopeptide bonds after in-gel digestion with chymotrypsin. Table 1 summarizes the observed cross-linked peptides in the MUC2 mucin, and the corresponding peptide spectra are collected in Fig. 5, b--h. In total seven different cross-links were observed. However, only one of the isopeptide-based cross-links was exclusively found in the dimeric band (X-D) representing the MUC2 dimer formed between Gln-470 and Lys-1796 (Fig. 5b). All other cross-linked peptides were also found in at least two of the replicates of the monomer. This might be due to the limited resolving power of AgPAGE and smearing of the multimeric bands. However, these linkages are most likely intramolecular of the monomer, but we cannot exclude that also the so-called monomeric form represents an already cross-linked dimer because it is not possible to calculate the correct molecular mass of the monomer due to lack of protein markers and the poor separation capacity of these gels. The same acyl donor Lys-160 was found linked to two different acceptors at Gln-4500 and Gln-472 (Fig. 5, f and h). The pair Gln-4694 and Lys-4697 formed an intramolecular bond within a monomer as they localized to the same peptide (Fig. 5c). This suggests that also other isopeptide bonds are found within one and the same molecule, for example the monomer. Together the mass spectrometric study showed that several Gln and Lys residues could be involved in the intracellular formation of isopeptide bonds, some of which are forming oligomers.
FIGURE 5.
Isopeptide cross-links observed in the MUC2 mucin isolated from intracellular granulae of LS174T cells. a, insoluble MUC2 complex was isolated from confluent LS174T cells, reduced-alkylated, separated by AgPAGE, and stained with zinc imidazole. X-O represents the oligomeric MUC2; X-D represents the dimeric, and M represents the monomeric isoform of MUC2. A representative separation of three replicates is shown. The separated bands were cut out, treated with chymotrypsin, and followed by analysis by LC-MS. b–h, annotated MS2 fragment spectra of the parent ions of the observed cross-linked peptides. y-ions are labeled in blue; b and a ions are labeled in red. Immonium ions for leucine or isoleucine, asparagine, phenylalanine, tyrosine, and valine are labeled with their one-letter amino acid code, respectively. b, MS2 fragment spectrum of parent ion [M + 2H]2+ 617.31. c, MS2 fragment spectrum of parent ion [M + 2H]2+ 553.78. d, MS2 fragment spectrum of parent ion [M + 2H]2+ 790.84. e, MS2 fragment spectrum of parent ion [M + 2H]2+ 469.75. f, MS2 fragment spectrum of parent ion [M + 2H]2+ 824.33. g, MS2 fragment spectrum of parent ion [M + H]2+ 920.43. h, MS2 fragment spectrum of parent ion [M + 2H]2+ 483.25.
TABLE 1.
Identified isopeptide-based cross-links in the different isoforms of intracellular MUC2
| m/z | z | [M + H+] obs. | [M + H+] calc. | Peptide α | Peptide β | Donor sitea | Acceptor site | X-M | X-D | X-O |
|---|---|---|---|---|---|---|---|---|---|---|
| 617.31 | 2 | 1233.61 | 1233.61 | LDSGKPNF | NQL | Lys-1796 | Gln-470 | 1,2,3 | ||
| 553.78 | 2 | 1106.55 | 1106.56 | CQLIKDSLF | Lys-4697 | Gln-4694 | 1,2,3 | 3 | ||
| 790.84 | 2 | 1580.67 | 1580.67 | NQTQDGAFCY | KTF | Lys-46 | Gln-1231/Gln-1233 | 1,2,3 | 2,3 | 2 |
| 469.75 | 2 | 938.48 | 938.48 | IKDSL | QAY | Lys-4697 | Gln-4735 | 1,2,3 | 1,2,3 | 1,3 |
| 824.33 | 2 | 1647.67 | 1647.66 | YSYQGNCTY | DTKF | Lys-160 | Gln-4500 | 1,2,3 | ||
| 920.43 | 2 | 1839.85 | 1839.85 | VMYSAKAQAL | QSYSEF | Lys-5111 | Gln-176 | 1,2 | 1,2,3 | 2 |
| 483.25 | 2 | 965.49 | 965.49 | DTKF | QVNL | Lys-160 | Gln-472 | 1,2,3 | 1,2,3 | 1,2,3 |
a Acyl donor and acceptor sites are given as well as their appearance in the different replicates of the MUC2 monomer (M), dimer (X-D), and oligomer (X-O).
Intracellular MUC2 Can Be Further Processed by the Transamidating Activity of TGM2
The LS174T cells only secrete minor amounts of mucus, and the purified MUC2 from the cells thus represents the intracellular stored protein in their mucin granulae. We were interested whether the MUC2 mucin can be further processed by transglutaminases after secretion to form a more cross-linked and stabilized network. We treated reduced and purified intracellular MUC2 from LS174T cells with TGM2 with or without Z-DON inhibitor for 12 h. The reaction products were reduced and analyzed by composite AgPAGE (Fig. 6). The TGM2-treated MUC2 revealed an additional band (X-OX) migrating slower than the X-D and X-O MUC2 oligomers. When the reaction was blocked by the addition of Z-DON or no enzyme was added, this reaction product was not observed. Furthermore, the monomeric and oligomeric isoforms of MUC2 also migrated slower. Analysis of the isolated X-OX band by mass spectrometry after trypsinization confirmed that this diffuse band represented MUC2 (Table 2). As the X-OX multimer as well as the smaller oligomers migrated slower than non-treated MUC2 indicates that additional isopeptide-based cross-links can be introduced to the already cross-linked intracellular MUC2. Therefore, MUC2 is a putative substrate for further covalent cross-linking by TGM2 after the MUC2 mucin has been expanded after secretion from the goblet cell.
FIGURE 6.
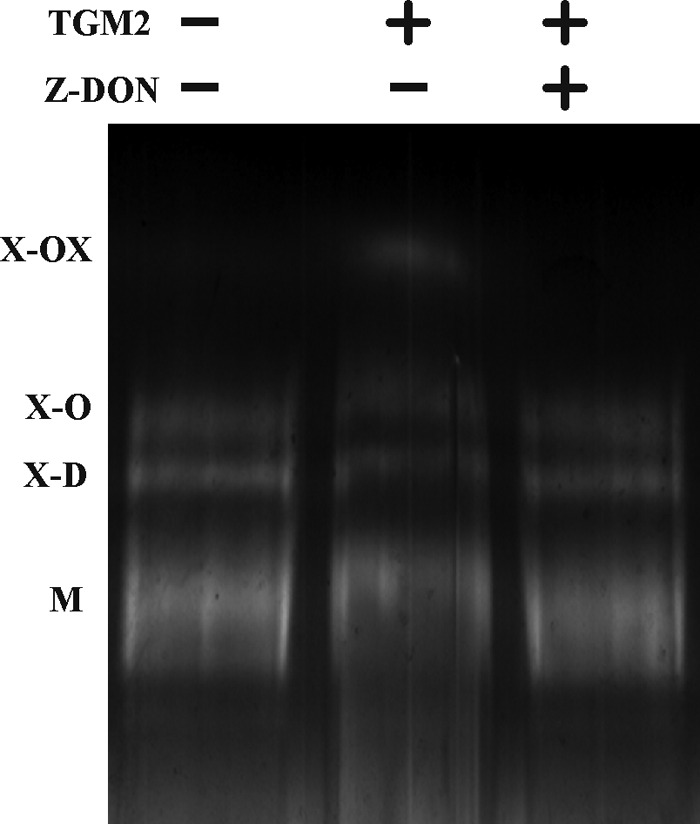
TGM-catalyzed in vitro cross-linking of purified intracellular MUC2. Purified reduced and alkylated intracellular MUC2 from LS174T cells was treated with TGM2 in the presence and absence of the TGM inhibitor Z-DON for 12 h at 37 °C. The reaction was terminated by loading buffer and heating followed by separation via AgPAGE and zinc imidazole staining.
TABLE 2.
MUC2 peptides identified by mass spectrometry in the TGM2-induced X-OX MUC2 multimer
| Peptide | Residues | Mass observed | Mass calculated | Ion score |
|---|---|---|---|---|
| TFDGDVFR | 47–54 | 478.7274 (2+) | 955.439 | 40 |
| KTETPFGR | 593–600 | 468.2508 (2+) | 934.487 | 23 |
| LIGQSCTAPK | 754–763 | 537.7841 (2+) | 1073.554 | 41 |
| ECPCVHNNDLYSSGAK | 819–834 | 617.5981 (3+) | 1849.772 | 19 |
| TELKLEDK | 927–934 | 488.2713 (2+) | 974.528 | 35 |
| HETQEVLIK | 4543–4551 | 548.8033 (2+) | 1095.592 | 38 |
| EYQACGPAEEPTCK | 4767–4780 | 820.3396 (2+) | 1638.665 | 62 |
| TCGCVGPDNVPR | 4814–4825 | 666.295 (2+) | 1330.576 | 42 |
Discussion
Since the first description of the reduction-insensitive bond that cross-linked the MUC2 mucin polypeptide chains, the nature of this has remained a mystery. We can now show that the inter-polypeptide cross-links of MUC2 are caused by transglutaminase-formed isopeptide bonds. These linkages occur between the side chains of Gln and Lys catalyzed by transglutaminases. The primary alternative was cross-linking via the reactive internal Asp anhydride formed by cleavage at the sequence GD↓PH in the vWD4 domain of MUC2 (24). The possibility that this anhydride forms ester bonds with hydroxyl groups was ruled out by the negative saponification experiments, but the possibility that the anhydride reacts with a primary amine remains still open. However, further experiments also ruled out this possibility. Cleavage with γ-GT depolymerized the MUC2 oligomers as did treatment with leech saliva. Saliva from H. medicinalis contains isopeptidase activity enabling hydrolysis of γ-glutamyl-p-anilide (data not shown).
It was not only the oligomeric nature of MUC2 that was affected, but depolymerization also affected its insolubility. The MUC2 mucin polymer becomes insoluble in ordinary buffers during its passage through the later secretory pathway (6). Further storage in the goblet cell granulae renders MUC2 also insoluble in urea and guanidinium chloride (7, 8). Treatment of the non-reduced insoluble MUC2 with γ-GT partly increased the supernatant's turbidity after centrifugation reflecting less precipitated MUC2 and a partial disruption of the MUC2 insoluble gel. Because transglutaminases also have isopeptidase activity, which is favored at acidic pH (27, 28), we also tested whether incubation at alkaline or acidic pH influences the breakdown of the MUC2 gel network. Indeed, we observed a significant disintegration of the MUC2-insoluble gel by treatment with TGM2 under acidic conditions as evaluated by turbidity measurement of the supernatant. Incubation with TGM2 under alkaline conditions or incubation in the presence of the TGase inhibitor Z-DON did not disrupt the gel network (Fig. 1e). This result further indicates that isopeptide bonds are not only forming the cross-links, but also the insolubility of MUC2. Because the intracellular MUC2 is first insoluble in normal buffers and after prolonged cell culture also in chaotropic salts suggests that the longer the mucin is stored in the goblet cell granulae, the more cross-links are formed and the mucus becomes less soluble. This could have biological implications for mucus quality and properties.
The most well studied example of isopeptide cross-linking is the blood coagulation cascade where thrombin-activated factor XIII catalyzes the formation of γ-γ dimers between two fibrin peptides to stabilize the fibrin clot (10). The presence of isopeptide bonds in the MUC2 mucin is in striking analogy to human skin where transamidation is important for stabilizing the multilayered cornified outer skin surface built by dead cells (29). This is very important for protecting us from outer environmental damage. TGM1, -3, and -5 are found in the skin and are responsible for the interprotein cross-linking of different proteins to form the cornified envelope, whereas the TGM2 isoform is only found in keratinocytes under certain conditions like vitamin A treatment (30, 31). Loss-of-function mutations in TGM1 give the common disease ichthyosis, where the cornified skin is easily lost (32). Because transamidation is that important for stabilizing the outer skin surface and increases the protective capacity of skin, it is logical that a similar mechanism has been used to stabilize the intestinal mucus.
The intestine secretes both the TGM2 and TGM3 transglutaminases as shown by studies of the mucous proteome (33). The TGM1 can also be membrane-associated by lipid anchors (34). The TGM2 enzyme is predominant in the small intestine, whereas the TGM3 dominates in the large intestine. Furthermore, patients with active ulcerative colitis show a reduced expression of TGM1 and a significantly lower transglutaminase activity in their plasma (35).
The isopeptide bonds of MUC2 can be cleaved by γ-GT, which is of special interest because this enzyme is found and is active at the brush-border membrane of the small intestine with its activity directed toward the extracellular lumen (36, 37). γ-GT shows a weak expression in colon, something that might be another potential reason for the difference between small and large intestinal mucous properties (38).
Transglutaminases lack signal sequences and are cytoplasmic proteins. However, they are also found extracellularly and as indirectly suggested here also in the secretory pathway compartments. The mechanism for their translocation from the cytoplasm is not known, but a recent observation suggested that the P2X7 receptor can translocate TGM2 (39). Although this receptor is not present in the intestine, a family of related P2 receptors is expressed there.
The fastest migrating mucin band of the reduced insoluble MUC2 has been suggested to be the monomeric form (6, 8, 40). Treatment with H. medicinalis saliva in the presence of protease inhibitors caused this band to move even faster. Because the isopeptidase from leech saliva contains also glycosidase activity (41), the small MUC2 monomer could be due to the remaining protease or glycosidase activities. This raises the question whether the fastest moving band is really the monomeric form or a higher oligomer. The studies of cross-links present in MUC2 by mass spectrometry revealed one confident intramolecular isopeptide bond as it was localized within one peptide. Therefore, it is not unlikely that some of the other isopeptide bonds could be intramolecular. Such intramolecular isopeptide bonds can affect the migration, and it is not possible to definitely determine whether the monomeric band is actually monomeric or of oligomeric nature.
TGM2 was able to bind the CysD2 domain with a relatively high affinity (KD = 113 nm) and utilizes CysD2 as both an acyl donor and acyl acceptor. Mass spectrometric analyses specifically identified Lys-1851 as acyl donor and Gln-1835 or Gln-1838 as acyl acceptors. These reactions were TGM2-dependent and preventable with the enzyme inhibitor Z-DON. The recombinant MUC2 N- and C-terminal parts did not show any TGM2 binding or specific reaction products with the used cross-linking partners. In addition, also self-multimerization of TGM2 and incorporation of acyl acceptor molecules could be observed as recently observed (42). These reactions led to biotinylated TGM2 multimers of around 250 kDa and were therefore not distinguishable from cross-linked MUC2N or MUC2C products. These results suggest that the CysD2 domains have the possibility to form isopeptide cross-links between its Gln and Lys residues and by this also larger oligomers. Indeed, when this MUC2 domain was incubated with TGM2, isopeptide-based homo- and heterodimers were formed already after 5 min. These were further transformed into higher oligomeric structures after prolonged incubation periods. Mass spectrometric analyses confirmed that the putative oligomers indeed consisted of reduction-insensitive cross-linked CysD2 (data not shown). The results suggest that CysD2 domains could be especially important for the localization of the inter-polypeptide cross-links. The MUC2 CysD1 domain also contains the ICKNE sequence and could also act as an acyl donor. However, when intracellular MUC2 from LS174T cells was analyzed for isopeptide bonds, none of the inter-CysD cross-links could be detected. Only one of the cross-linked peptides contained a CysD fragment, i.e. the Lys-1796 linked to Gln-470 of the N-terminal vWD2 assembly. This was also the only linkage that was exclusively found in the MUC2 dimer (X-D). The other six were also found in the MUC2 monomeric band suggesting that these are intramolecular monomeric cross-links as for the Lys-4697–Gln-4694 bond. This isopeptide bond seems to be specific for the monomer because it has been found in all monomeric replicates and only in one replicate of the dimeric forms. If the five cross-links detected in the MUC2 monomer are really intramolecular or due to the limited resolution of composite AgPAGE cannot be finally answered. The reduction-insensitive bonds appear during the later stages of the secretory pathway and probably in the fully glycosylated protein (6).
We have previously presented a model for the MUC2 mucin packing in the goblet cell granulae that is mediated by Ca2+ and low pH (43). Upon release, the MUC2 polymers were predicted to flip open and form flat and extended net-like structures. This model does not take into account the type of cross-links discovered here. As these were found in material from the stored granulae of the LS174T cells, this suggests a more complex expansion upon secretion. Such cross-links, except the intramolecular one (Gln-4694–Lys-4697) and between amino acids close together (Gln-4735–Lys-4697 and Gln-472–Lys-160), should interfere with the unfolding. Of course, cross-links at longer distances should alter the MUC2 polymeric structure.
Here, we have only studied cross-linking that happens intracellularly. To address whether further extracellular cross-linking can take place, we also analyzed whether the intracellular MUC2 from LS174T cells can be further cross-linked by TGM2. As this resulted in even larger multimers, it is likely that MUC2 can be further processed extracellularly, but also that additional isopeptide bonds can be introduced in the different MUC2 isoforms as these migrated slower after treatment with TGM2. However, one has to take into account that the purified MUC2 was not in its native state because it has to be reduced during the purification procedure to become soluble. Together, this suggests that further isopeptide-based cross-links could be possible in vivo to generate a mucous network with pores that are small enough to keep bacteria away from the epithelial surface. The importance of further stabilization of an expanded MUC2 network is easily understood as this should enforce the intermolecular interactions of the laminated and size discriminating inner colon mucous layer (2, 43). The CysD domains are of special interest for such interactions as we have previously shown that the CysD2 domain can form dimers (16). If such CysD dimers were further enforced extracellularly by transamidation once the MUC2 polymeric network has expanded, the network should become more stable. Such interactions are suggested in Fig. 7 by sketching the isopeptide bonds, which define the reduction-insensitive MUC2 dimer. Such TGM-catalyzed cross-links should help to decrease the pore size. According to the model by Ambort (43), pore sizes of ∼2.6 μm2 are theoretically predicted after the secretion, a size too large to exclude bacteria as observed for the inner mucous layer. Shifting the CysD from different sheets and with different acyl donor/acceptor pairs would form dislocated MUC2 hexagon rings and decrease the pore sizes (Fig. 7). This model can explain why the inner mucous layer is devoid of bacteria (2). Further studies of MUC2 have to be performed in secreted mucus to shed more light on how transamidation will enforce the mucus of the intestine.
FIGURE 7.
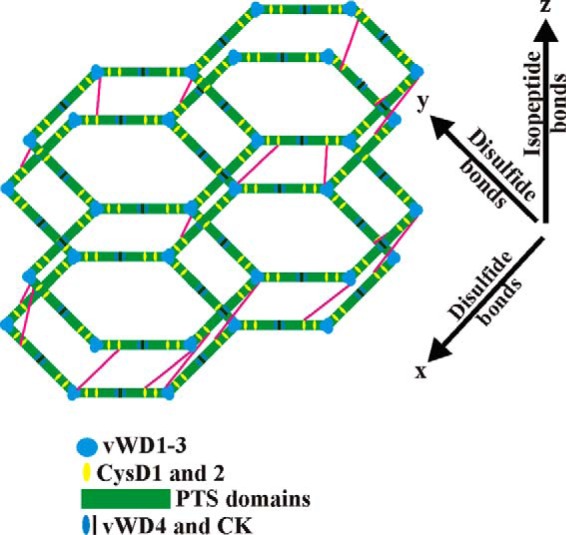
Schematic sketch showing how isopeptide cross-links could stabilize MUC2 polymer rings and alter their pore sizes. The symbols for the MUC2 domains are listed below the model. Isopeptide cross-links between the CysD2 and the vWD2 domains are shown as magenta lines.
It is now shown that isopeptide bonds are the molecular basis behind the formation of the reduction-insensitive MUC2 oligomers and most likely its insolubility in chaotropic salts. The intestinal milieu is harsh and needs further enforcement of the mucin and mucous stability. Therefore, it should not come as a surprise and reflects that the mucus is sometimes named the inner skin. These findings will open new potential therapeutic strategies for enforcing the mucous system of the colon and for novel treatments of inflammatory bowel diseases such as ulcerative colitis.
Author Contributions
C. R. designed, performed, and analyzed the experiments; G. C. H. conceived and coordinated the study; C. R. and G. C. H. wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
This work was supported by Swedish Research Council Grant 746, the Swedish Cancer Foundation, the Knut and Alice Wallenberg Foundation, the IngaBritt and Arne Lundberg Foundation, Sahlgren's University Hospital (ALF), the Wilhelm and Martina Lundgren's Foundation, Torsten Söderbergs Stiftelse, The Sahlgrenska Academy, National Institutes of Health Grant U01AI095473 from NIAID, the Swedish Foundation for Strategic Research-The Mucus-Bacteria-Colitis Center of the Innate Immunity Program, the Swedish Cystic Fibrosis Foundation, the Erica Lederhausen's Foundation, and the Lederhausen's Center for Cystic Fibrosis Research. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- vWD
- von Willebrand D domain
- vWC
- von Willebrand C domain
- AgPAGE
- composite agarose-PAGE
- γ-GT
- γ-glutamyltranspeptidase.
References
- 1. Atuma C., Strugala V., Allen A., and Holm L. (2001) The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G922–G929 [DOI] [PubMed] [Google Scholar]
- 2. Johansson M. E., Phillipson M., Petersson J., Velcich A., Holm L., and Hansson G. C. (2008) The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. U.S.A. 105, 15064–15069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gum J. R. Jr., Hicks J. W., Toribara N. W., Siddiki B., and Kim Y. S. (1994) Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J. Biol. Chem. 269, 2440–2446 [PubMed] [Google Scholar]
- 4. Lidell M. E., Johansson M. E., Mörgelin M., Asker N., Gum J. R. Jr., Kim Y. S., and Hansson G. C. (2003) The recombinant C-terminus of the human MUC2 mucin forms dimers in CHO cells and heterodimers with full-length MUC2 in LS 174T cells. Biochem. J. 372, 335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Godl K., Johansson M. E., Lidell M. E., Mörgelin M., Karlsson H., Olson F. J., Gum J. R. Jr., Kim Y. S., and Hansson G. C. (2002) The N termini of the MUC2 mucin form trimers that are held together within a trypsin-resistant core fragment. J. Biol. Chem. 277, 47248–47256 [DOI] [PubMed] [Google Scholar]
- 6. Axelsson M. A., Asker N., and Hansson G. C. (1998) O-Glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J. Biol. Chem. 273, 18864–18870 [DOI] [PubMed] [Google Scholar]
- 7. Carlstedt I., Herrmann A., Karlsson H., Sheehan J., Fransson L. A., and Hansson G. C. (1993) Characterization of two different glycosylated domains from the insoluble mucin complex of rat small intestine. J. Biol. Chem. 268, 18771–18781 [PubMed] [Google Scholar]
- 8. Herrmann A., Davies J. R., Lindell G., Mårtensson S., Packer N. H., Swallow D. M., and Carlstedt I. (1999) Studies on the “insoluble” glycoprotein complex from human colon. J. Biol. Chem. 274, 15828–15836 [DOI] [PubMed] [Google Scholar]
- 9. Eckert R. L., Kaartinen M. T., Nurminskaya M., Belkin A. M., Colak G., Johnson G. V., and Mehta K. (2014) Transglutaminase regulation of cell function. Physiol. Rev. 94, 383–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lorand L., and Graham R. M. (2003) Transglutaminases: cross-linking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 4, 140–156 [DOI] [PubMed] [Google Scholar]
- 11. Feng J. F., Rhee S. G., and Im M. J. (1996) Evidence that phospholipase δ1 is the effector in the Gh (transglutaminase II)-mediated signaling. J. Biol. Chem. 271, 16451–16454 [DOI] [PubMed] [Google Scholar]
- 12. Belkin A. M. (2011) Extracellular TG2: emerging functions and regulation. FEBS J. 278, 4704–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ozpolat B., Akar U., Mehta K., and Lopez-Berestein G. (2007) PKCδ and tissue transglutaminase are novel inhibitors of autophagy in pancreatic cancer cells. Autophagy 3, 480–483 [DOI] [PubMed] [Google Scholar]
- 14. Siegel M., Strnad P., Watts R. E., Choi K., Jabri B., Omary M. B., and Khosla C. (2008) Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS ONE 3, e1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sane D. C., Moser T. L., Parker C. J., Seiffert D., Loskutoff D. J., and Greenberg C. S. (1990) Highly sulfated glycosaminoglycans augment the cross-linking of vitronectin by guinea pig liver transglutaminase. Functional studies of the cross-linked vitronectin multimers. J. Biol. Chem. 265, 3543–3548 [PubMed] [Google Scholar]
- 16. Ambort D., van der Post S., Johansson M. E., Mackenzie J., Thomsson E., Krengel U., and Hansson G. C. (2011) Function of the CysD domain of the gel-forming MUC2 mucin. Biochem. J. 436, 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomsson K. A., and Hansson G. C. (2011) Identification and quantification of mucin expression. Methods Mol. Biol. 742, 127–141 [DOI] [PubMed] [Google Scholar]
- 18. Friedrich T., Kröger B., Koerwer W., Strube K. H., Meyer T., and Bialojan S. (1998) An isopeptide bond splitting enzyme from Hirudo medicinalis similar to γ-glutamyl transpeptidase. Eur. J. Biochem. 256, 297–302 [DOI] [PubMed] [Google Scholar]
- 19. Schulz B. L., Packer N. H., and Karlsson N. G. (2002) Small-scale analysis of O-linked oligosaccharides from glycoproteins and mucins separated by gel electrophoresis. Anal. Chem. 74, 6088–6097 [DOI] [PubMed] [Google Scholar]
- 20. Hardy E., and Castellanos-Serra L. R. (2004) “Reverse-staining” of biomolecules in electrophoresis gels: analytical and micropreparative applications. Anal. Biochem. 328, 1–13 [DOI] [PubMed] [Google Scholar]
- 21. Rappsilber J., Ishihama Y., and Mann M. (2003) Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 75, 663–670 [DOI] [PubMed] [Google Scholar]
- 22. Johansson M. E. V., Jakobsson H. E., Holmén-Larsson J., Schütte A., Ermund A., Rodríguez-Piñeiro A. M., Arike L., Wising C., Svensson F., Bäckhed F., and Hansson G. C. (2015) Normalization of the host intestinal mucus systems requires long-term colonization. Cell Host Microbe 18, 582–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Götze M., Pettelkau J., Fritzsche R., Ihling C. H., Schäfer M., and Sinz A. (2015) Automated assignment of MS/MS cleavable cross-links in protein 3D-structure analysis. J. Am. Soc. Mass Spectrom. 26, 83–97 [DOI] [PubMed] [Google Scholar]
- 24. Lidell M. E., Johansson M. E., and Hansson G. C. (2003) An autocatalytic cleavage in the C terminus of the human MUC2 mucin occurs at the low pH of the late secretory pathway. J. Biol. Chem. 278, 13944–13951 [DOI] [PubMed] [Google Scholar]
- 25. Allison R. D., and Meister A. (1981) Evidence that transpeptidation is a significant function of γ-glutamyl transpeptidase. J. Biol. Chem. 256, 2988–2992 [PubMed] [Google Scholar]
- 26. Baskova I. P., and Nikonov G. I. (1985) Destabilize: an enzyme of medicinal leech salivary gland secretion hydrolyzes the isopeptide bonds in stabilized fibrin. Biokhimiia 50, 424–431 [PubMed] [Google Scholar]
- 27. Király R., Thangaraju K., Nagy Z., Collighan R., Nemes Z., Griffin M., and Fésüs L. (2016) Isopeptidase activity of human transglutaminase 2: disconnection from transamidation and characterization by kinetic parameters. Amino Acids 48, 31–40 [DOI] [PubMed] [Google Scholar]
- 28. Fleckenstein B., Molberg Ø., Qiao S. W., Schmid D. G., von der Mülbe F., Elgstøen K., Jung G., and Sollid L. M. (2002) Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. Role of enzyme specificity and pH influence on the transamidation versus deamidation process. J. Biol. Chem. 277, 34109–34116 [DOI] [PubMed] [Google Scholar]
- 29. Eckhart L., Lippens S., Tschachler E., and Declercq W. (2013) Cell death by cornification. Biochim. Biophys. Acta 1833, 3471–3480 [DOI] [PubMed] [Google Scholar]
- 30. Lichti U., Ben T., and Yuspa S. H. (1985) Retinoic acid-induced transglutaminase in mouse epidermal cells is distinct from epidermal transglutaminase. J. Biol. Chem. 260, 1422–1426 [PubMed] [Google Scholar]
- 31. Eckert R. L., Sturniolo M. T., Broome A. M., Ruse M., and Rorke E. A. (2005) Transglutaminase function in epidermis. J. Invest. Dermatol. 124, 481–492 [DOI] [PubMed] [Google Scholar]
- 32. Huber M., Rettler I., Bernasconi K., Frenk E., Lavrijsen S. P., Ponec M., Bon A., Lautenschlager S., Schorderet D. F., and Hohl D. (1995) Mutations of keratinocyte transglutaminase in lamellar ichthyosis. Science 267, 525–528 [DOI] [PubMed] [Google Scholar]
- 33. Rodríguez-Piñeiro A. M., Bergström J. H., Ermund A., Gustafsson J. K., Schütte A., Johansson M. E., and Hansson G. C. (2013) Studies of mucus in mouse stomach, small intestine, and colon. II. Gastrointestinal mucus proteome reveals Muc2 and Muc5ac accompanied by a set of core proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G348–G356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nemes Z., Marekov L. N., Fésüs L., and Steinert P. M. (1999) A novel function for transglutaminase 1: attachment of long-chain ω-hydroxyceramides to involucrin by ester bond formation. Proc. Natl. Acad. Sci. U.S.A. 96, 8402–8407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. D'Argenio G., Calvani M., Della Valle N., Cosenza V., Di Matteo G., Giorgio P., Margarucci S., Petillo O., Jori F. P., Galderisi U., and Peluso G. (2005) Differential expression of multiple transglutaminases in human colon: impaired keratinocyte transglutaminase expression in ulcerative colitis. Gut 54, 496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hagen T. M., Wierzbicka G. T., Bowman B. B., Aw T. Y., and Jones D. P. (1990) Fate of dietary glutathione: disposition in the gastrointestinal tract. Am. J. Physiol. 259, G530–G535 [DOI] [PubMed] [Google Scholar]
- 37. Darbouy M., Chobert M. N., Lahuna O., Okamoto T., Bonvalet J. P., Farman N., and Laperche Y. (1991) Tissue-specific expression of multiple γ-glutamyl transpeptidase mRNAs in rat epithelia. Am. J. Physiol. 261, C1130–C1137 [DOI] [PubMed] [Google Scholar]
- 38. Schütte A., Ermund A., Becker-Pauly C., Johansson M. E., Rodriguez-Pineiro A. M., Bäckhed F., Müller S., Lottaz D., Bond J. S., and Hansson G. C. (2014) Microbial induced meprin β cleavage in MUC2 mucin and functional CFTR channel are required to release anchored small intestinal mucus. Proc. Natl. Acad. Sci. U.S.A. 111, 12396–12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adamczyk M., Griffiths R., Dewitt S., Knäuper V., and Aeschlimann D. (2015) P2X7 receptor activation regulates rapid unconventional export of transglutaminase-2. J. Cell Sci. 128, 4615–4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Herrmann A., Carlstedt I., Shirazi T., Longman R., and Corfield A. (2003) A high-density putative monomeric mucin is the major 35S-labelled macromolecular product of human colorectal mucins in organ culture. Biochimie 85, 381–390 [DOI] [PubMed] [Google Scholar]
- 41. Zaidi S. M., Jameel S. S., Zaman F., Jil ani S., Sultana A., and Khan S. A. (2011) A systematic overview of the medicinal importance of sanguivorous leeches. Altern. Med. Rev. 16, 59–65 [PubMed] [Google Scholar]
- 42. Stamnaes J., Iversen R., du Pré M. F., Chen X., and Sollid L. M. (2015) Enhanced B-cell receptor recognition of the autoantigen transglutaminase 2 by efficient catalytic self-multimerization. PLoS ONE 10, e0134922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ambort D., Johansson M. E., Gustafsson J. K., Nilsson H. E., Ermund A., Johansson B. R., Koeck P. J., Hebert H., and Hansson G. C. (2012) Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl. Acad. Sci. U.S.A. 109, 5645–5650 [DOI] [PMC free article] [PubMed] [Google Scholar]