Abstract
Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) is a known activating kinase for AMP-activated protein kinase (AMPK). In vitro, CaMKKβ phosphorylates Thr172 in the AMPKα subunit more efficiently than CaMKKα, with a lower Km (∼2 μm) for AMPK, whereas the CaMKIα phosphorylation efficiencies by both CaMKKs are indistinguishable. Here we found that subdomain VIII of CaMKK is involved in the discrimination of AMPK as a native substrate by measuring the activities of various CaMKKα/CaMKKβ chimera mutants. Site-directed mutagenesis analysis revealed that Leu358 in CaMKKβ/Ile322 in CaMKKα confer, at least in part, a distinct recognition of AMPK but not of CaMKIα.
Keywords: AMP-activated kinase (AMPK), Ca2+/calmodulin-dependent protein kinase (CaMK), calmodulin (CaM), phosphorylation, substrate specificity, CaMKI, CaMKK, substrate recognition
Introduction
Ca2+/calmodulin-dependent protein kinase kinases (CaMKKs)2 were originally identified as members of the calmodulin (CaM) kinase (CaMK) family that phosphorylate and activate two multifunctional CaMKs, including CaMKI and CaMKIV, constituting Ca2+-dependent kinase cascades named CaMK cascades (1–3). CaMKK in mammals is derived from two genes (CaMKKα and CaMKKβ), with ∼70% of amino acid sequence homology in the catalytic domains (4–6). The CaMKK/CaMKIV cascade has been shown to be involved in the regulation of gene expression through the phosphorylation of transcription factors such as cAMP-response element-binding protein (7, 8) and serum response factor (9). In contrast, the CaMKK/CaMKI cascade plays an important role in neuronal development, including axon outgrowth (10), leptin-induced spine formation (11), and activity-dependent synaptogenesis (12). Except for the downstream CaMKs, AMP-activated protein kinase (AMPK) has been identified as a novel target kinase for CaMKKβ (13–15), and the CaMKKβ/AMPK pathway has been demonstrated to be functional in various Ca2+-dependent AMPK-mediated signal transduction processes, including regulation of appetite and glucose homeostasis (16), stimulation of mitochondrial fatty acid oxidation by thyroid hormone T3 (17), and regulation of autophagy by amino acid starvation (18). According to studies using either RNA interference or pharmacological inhibition of CaMKK in HeLa cells, which do not express LKB1, an alternative AMPK kinase, CaMKKβ has been shown to be responsible for Ca2+-dependent activation of AMPK in vivo, whereas both CaMKK isoforms are capable of phosphorylating the AMPKα subunit at Thr172 in vitro (13–15). This was confirmed by the fact that STO-609 (19), a CaMKK inhibitor, suppressed ionomycin-induced AMPK phosphorylation in A549 cells (a human lung adenocarcinoma epithelial cell line) expressing STO-609-resistant CaMKKα but not STO-609-resisitant CaMKKβ (20). These results indicate that the CaMKKβ isoform, rather than CaMKKα, preferably recognizes AMPK, suggesting that the recognition mechanism of AMPK by CaMKK isoforms as a substrate may differ from that of CaMKI. A previous report showed that CaMKKβ, but not CaMKKα, forms a stable complex with AMPK, which could explain why CaMKKβ is an AMPK kinase and CaMKKα is not (21). However, Fogarty et al. (22) reported that CaMKKβ activates AMPK without forming a stable complex with AMPK. Therefore, despite the well characterized regulatory mechanisms of CaMKK, including an autoinhibitory mechanism (23), and the role of Ca2+/CaM-binding in the expression of its kinase activity (24), little is known about the molecular mechanism of substrate recognition of CaMKKs. To clarify the differential substrate specificity of CaMKK isoforms, especially for AMPK, we investigated enzymatic characterization of CaMKKs using various chimeras and site-directed mutants of CaMKK isoforms and identified a single residue in subdomain VIII that may be essential for the discrimination of AMPK as a substrate.
Experimental Procedures
Materials
Recombinant rat CaMKKα and β, including WT and mutants, were expressed in Escherichia coli BL21 Star (DE3) and purified using CaM-Sepharose and Q-Sepharose chromatographies. The GST-rat CaMKKβ catalytic domain (162–470) and the GST-rat CaMKKα catalytic domain (126–434) were constructed, and recombinant GST-fused CaMKKs, including chimera mutants, were expressed in E. coli JM109 and purified as described previously (25). GST-rat CaMKIα 1–293, K49E (GST-CaMKIα 1–293, KE) was expressed in E. coli JM109 and purified as described previously (23). Recombinant kinase-dead AMPK (K45R) was expressed in E. coli strain BL21-CodonPlus (DE3) (Stratagene, La Jolla, CA) using the tricistronic pγ1β1His-α1 plasmid (provided by Dr. Dietbert Neumann, Swiss Federal Institute of Technology, Zurich, Switzerland) and purified as described previously (26). Recombinant rat CaM was expressed in the E. coli BL21 (DE3) strain using the plasmid pET-CaM (provided by Dr. Nobuhiro Hayashi, Tokyo Institute of Technology, Yokohama, Japan) and purified as described previously (27). Antibodies against the AMPK α subunit (2532) and AMPK α subunit phosphorylated at Thr172 (2535) were purchased from Cell Signaling Technology (Danvers, MA). An anti-FLAG antibody (clone M2) was obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals were obtained from standard commercial sources.
Construction of CaMKKβ/α Chimera Mutants and CaMKKα Point Mutants
GST-CaMKKβ-(162–470), GST-CaMKKα-(126–434), and CaMKKβ/α-1; GST-CaMKKβ-(162–364)/CaMKKα-(329–434) and CaMKKβ/α-2; GST-CaMKKβ-(162–303)/CaMKKα-(268–434) were constructed using a pGEX-PreS vector as described previously (25). CaMKKβ/α/β-2, GST-CaMKKβ-(162–303)/CaMKKα-(268–326)/CaMKKβ-(363–470); CaMKKβ/α/β-3, GST-CaMKKβ-(162–303)/CaMKKα-(268–322)/CaMKKβ-(359–470); and CaMKKβ/α/β-4, GST-CaMKKβ-(162–303)/CaMKKα-(268–311)/CaMKKβ-(348–470) were constructed as follows. N-terminal fragments amplified by PCR using a sense primer (5′-GGTCTAGAGAATCAGTACACGCTG-3′) and various phosphorylated antisense primers (CaMKKβ/α/β-2, 5′-pGCCGGTGTCAGAGATGGCCTC-3′; CaMKKβ/α/β-3, 5′-pGATGGCCTCCGGGGCCATGAA-3′; and CaMKKβ/α/β-4, 5′-pTGCCGTACTGGACAGCTGAGC-3′) and CaMKKβ/α-2 as a template were digested by XbaI and C-terminal fragments amplified by PCR using an antisense primer (5′-CCGTCGACTAGACCTCCTCTTCGGT-3′) and the appropriate phosphorylated primers (CaMKKβ/α/β-2, 5′-pAAGATCTTCTCCGGAAAGGCC-3′; CaMKKβ/α/β-3, 5′-pTCAGAGACCCGGAAGATCTTC-3′; and CaMKKβ/α/β-4, 5′-pGGCACGCCTGCCTTCATGGCG-3′) were digested by SalI, and then both fragments were ligated into an XbaI/Sal-digested pGEX-PreS vector. CaMKKβ/α/β-1:GST-CaMKKβ-(162–303)/CaMKKα-(268–328)/CaMKKβ-(365–470) was constructed by overlapping PCR using CaMKKβ/α/β-2 as a template and PCR primers (5′-CCGGCCAGAGCTTCTCCGGAAAGGCCTT-3′ and 5′-AGAAGCTCTGGCCGGTGTCAGAGATGGC-3′). Point mutants of CaMKKα were generated by inverse PCR using pET-CaMKKα as a template and PCR primers as follows: CaMKKα A321S, 5′-ATGGCCCCGGAGTCCATTTCTGACACC-3′ and 5′-GAATGCTGGGGTCCCTGCCGTACTGGA-3′; CaMKKα I322L, 5′-ATGGCCCCGGAGGCCCTTTCTGACACC-3′ and 5′-GAATGCTGGGGTCCCTGCCGTACTGGA-3′.
The retroviral transfer vectors (pMSCV-MCS-IRES-EGFP) harboring the FLAG-CaMKKα triple mutant (A292T/L233F/I322L) was constructed by inverse PCR using primers as described above and the retroviral transfer vector harboring FLAG-CaMKKα double mutant (A292T/L233F) (20) as a template. The nucleotide sequences of all constructs were confirmed by sequencing using an ABI Prism 310 genetic analyzer (Applied Biosystems, Foster City, CA).
In Vitro CaMKK Activity Assay
Purified recombinant CaMKK isoforms, including site-directed mutants and GST-CaMKK chimera mutants (∼1 μg/ml unless otherwise indicated), were incubated individually with GST-CaMKIα 1–293, KE (0.5 mg/ml) or AMPK K45R (0.5 mg/ml) at 30 °C for the indicated time periods in a solution containing 50 mm HEPES (pH 7.5), 10 mm Mg(CH3COO)2, 1 mm DTT, and 200 μm [γ-32P]ATP (200–700 cpm/pmol) in the presence of either 4 mm CaCl2/10.0–16.5 μm CaM or 2 mm EGTA. Each reaction was initiated by addition of [γ-32P]ATP and terminated by addition of 2× SDS-PAGE sample buffer. Samples were then subjected to 10% SDS-PAGE followed by autoradiography. 32P incorporation into each substrate was estimated by Cerenkov counting of the excised gels.
AMPK Phosphorylation in A549 Cells
Human lung adenocarcinoma epithelial cell line A549 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS, 100 units/ml penicillin, and 100 units/ml streptomycin at 37 °C in 5% CO2. A549 cells in 6-well dishes were infected with FLAG-CaMKKα mutants expressing retroviruses that were generated by transfection of the pVSV-G vector and retroviral transfer vectors (pMSCV-MCS-IRES-EGFP) harboring either the FLAG-CaMKKα double mutant (A292T/L233F) as described previously (20) or the FLAG-CaMKKα triple mutant (A292T/L233F/I322L) into the GP2–293 packaging cell line. After 18 h of culture, the cells were cultured in the absence of FBS for 6 h and then treated with 1 μm ionomycin for 5 min. The cells were extracted with 1× SDS-PAGE sample buffer (100 μl), followed by immunoblot analysis using indicated antibodies.
Statistics
Student's t tests were used to evaluate statistical significance when two groups were compared. p < 0.05 was considered to be statistically significant.
Other Methods
The CaM overlay method was performed using 0.5 μg/ml biotinylated CaM in the presence of 1 mm CaCl2, followed by detection of the CaM-binding signal using a chemiluminescence reagent (PerkinElmer Life Sciences) as described previously (28). Protein concentration was estimated by staining the samples with Coomassie Brilliant Blue (Bio-Rad) using bovine serum albumin as a standard.
Results and Discussion
CaMKKβ, but Not CaMKKα, Preferentially Phosphorylates AMPK in Vitro
It has been reported that the purified CaMKKβ from rat brain activated AMPK 7-fold more rapidly than purified CaMKKα in vitro (14), whereas both CaMKK isoforms equally phosphorylated CaMKI. To confirm a distinct substrate preference of CaMKK isoforms, we attempted to measure the direct phosphorylation of the activation-loop Thr (Thr172) of the α subunit of the AMPK heterotrimeric complex by purified recombinant CaMKK isoforms and compared this with the phosphorylation of the catalytic domain of rat CaMKIα (GST-CaMKIα 1–293, K49E) at Thr177. Recombinant CaMKK isoforms were expressed in the E. coli BL21 Star (DE3) strain and purified by CaM-coupled Sepharose column chromatography. The amounts of the enzymes for measuring substrate phosphorylation were comparable, as judged by the CaM overlay method (Fig. 1A), because both CaMKK isoforms contain similar CaM-binding sequences (24). Both CaMKK substrates were mutated at a residue in the ATP-binding site (K45R in AMPKα and K49E in CaMKIα) to generate kinase-dead enzymes to avoid the feedback phosphorylation of CaMKKs by activated CaMKK target kinases (29). Time course experiments of phosphorylation of the catalytic domain of GST-CaMKIα 1–293, K49E (Fig. 1B), and AMPK (Fig. 1C) were performed with the same concentrations (1 μg/ml) of CaMKK isoforms in the presence of Ca2+/CaM. Although the phosphorylation profiles of CaMKI by both CaMKK isoforms were indistinguishable (Fig. 1B), CaMKKβ was shown to phosphorylate the Thr172 of AMPK 14-fold more rapidly than CaMKKα (Fig. 1C) under this experimental condition. When we compared the kinetic constant (Km) of CaMKK isoforms for AMPK based on the double reciprocal plots of phosphorylation data using various concentrations of AMPK (Fig. 2A), it was clear that the main reason for the distinct activities of CaMKK isoforms for AMPK phosphorylation was the significantly higher Km (13.1 μm) of CaMKKα for AMPK compared with that of CaMKKβ (1.5 μm). These results are in good agreement with a previous report using purified CaMKKs from rat brain (14), suggesting that CaMKKβ is the dominant isoform with respect to the regulation of AMPK.
FIGURE 1.
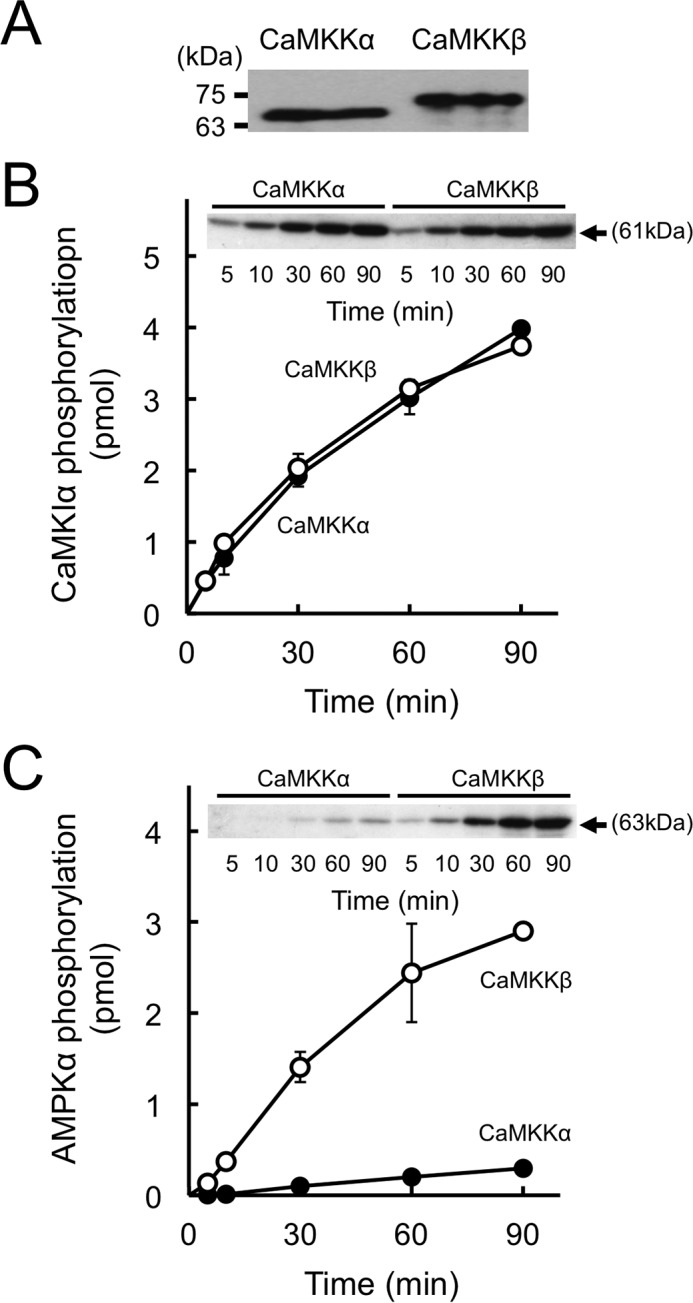
In vitro phosphorylation of CaMKI and AMPK by recombinant CaMKK isoforms. A, CaM overlay analysis of recombinant rat CaMKKs (20 ng), including wild-type CaMKKα (CaMKKα) and wild-type CaMKKβ (CaMKKβ) was performed as described under “Experimental Procedures.” The molecular masses in kilodaltons are indicated on the left. B and C, phosphorylation of either GST-CaMKIα 1–293, K49E (B), or AMPKα K45R (C) by recombinant rat CaMKKα (closed circles) and CaMKKβ (open circles) was measured at 30 °C for various time points with 4 mm CaCl2/10 μm CaM and 200 μm [γ-32P]ATP. After termination of the reaction, the samples were subjected to 10% SDS-PAGE followed by Coomassie Brilliant Blue staining and autoradiography (insets). 32P incorporation into each substrate (arrows) was measured by Cerenkov counting of the excised gels. Results are expressed as the mean ± S.D. of three experiments.
FIGURE 2.
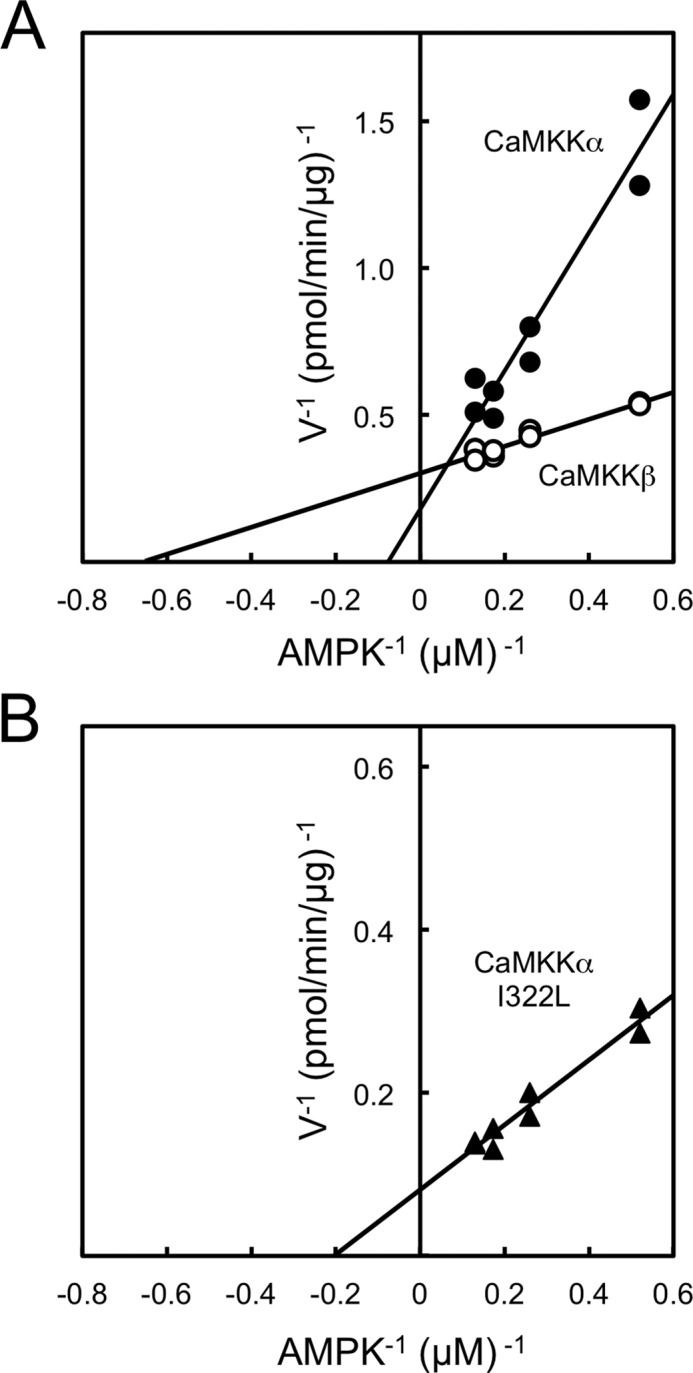
Double reciprocal plots of CaMKKα and β activities. Protein kinase activities of recombinant rat CaMKKα (closed circles, 8 μg/ml), rat CaMKKβ (open circles, 1 μg/ml) (A) and rat CaMKKα I322L mutant (closed triangles, 1 μg/ml, B) were measured as described in Fig. 1B with various concentrations of AMPK (1.9–7.7 μm) at 30 °C for 30 min in the presence of 200 μm [γ-32P]ATP. The results represent duplicate experiments and are presented as double reciprocal plots (Lineweaver-Burk).
Subdomain VIII of CaMKK is involved in discrimination of AMPK as a substrate
To clarify the distinct substrate preference of CaMKK isoforms, we analyzed the activities of catalytic domain mutants of CaMKKβ (residues 162–470) and CaMKKα (residues 126–434) including the wild-type enzyme, which each lacks both the N-terminal extension domain and C-terminal domain containing an autoinhibitory segment and a CaM-binding segment (23). These catalytic domain mutants were expressed in E. coli JM109 and purified as GST fusion proteins (Fig. 3A). Thus, all of recombinant enzyme phosphorylated the protein substrates in a complete Ca2+/CaM-independent manner. When we compared the AMPK phosphorylation activity of GST-CaMKK catalytic domain mutants, including wild-type and CaMKKβ/α chimera mutants, we used the same concentrations (∼1 μg/ml) of the enzymes as those used for measuring GST-CaMKIα 1–293 K49E phosphorylation activity, which were equalized (Fig. 3B, top panel). Despite the ∼70% amino acid sequence homology of the catalytic domain between rat CaMKKα and β (5, 6), CaMKKβ phosphorylated AMPK ∼6-fold more rapidly than CaMKKα (Fig. 3B, bottom panel), consistent with the result with full-length CaMKK isoforms, as shown in Fig. 1C. Although the activity of the CaMKKβ-(162–364)/CaMKKα-(329–434) mutant (CaMKKβ/α-1) was comparable with that of CaMKKβ-(162–470), the CaMKKβ-(162–303)/CaMKKα-(268–434) mutant (CaMKKβ/α-2) showed a significantly reduced activity compared with that of CaMKKβ-(162–470) (Fig. 3B, bottom panel). These data suggest that residues 304–364 in CaMKKβ are involved in the efficient phosphorylation of AMPKα. Indeed, we could confirm that the CaMKKβ mutant, in which residues 304–364 were replaced by an equivalent region (residues 268–328) in CaMKKα (CaMKKβ/α/β-1), exhibited a significantly lower activity toward the substrate AMPK than CaMKKβ-(162–470) (Fig. 3B, bottom panel) did, similar to CaMKKα-(126–434).
FIGURE 3.
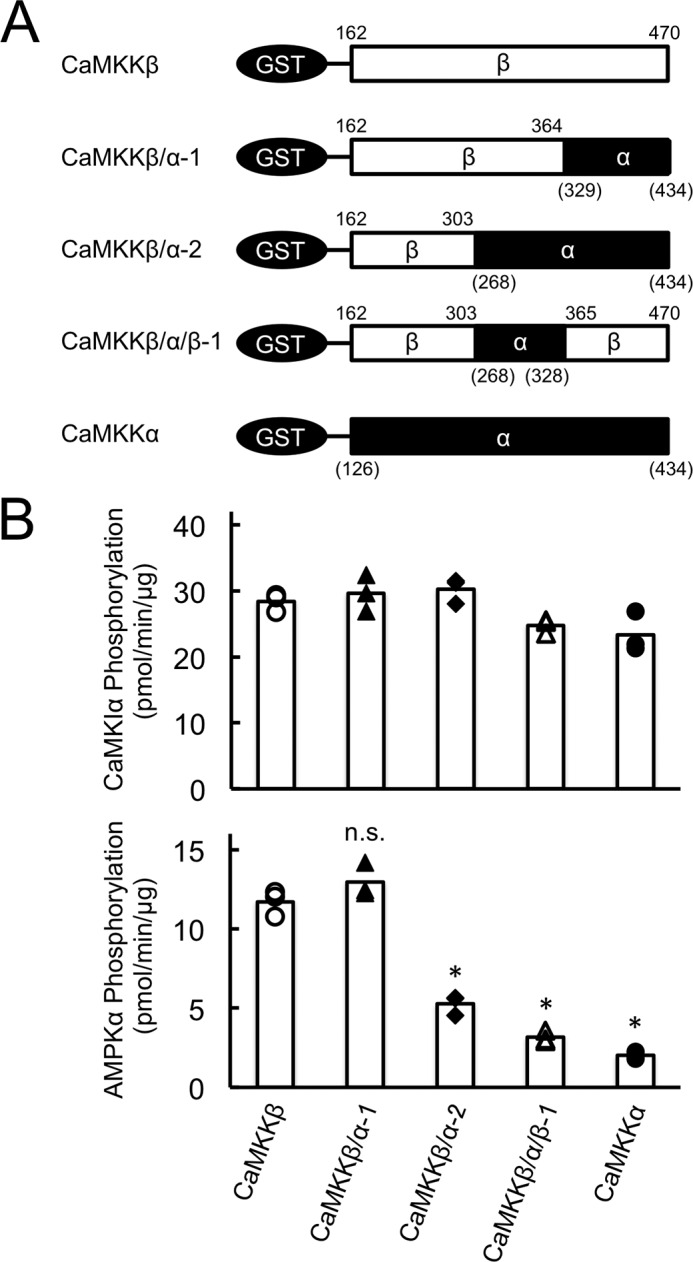
Substrate phosphorylation by CaMKK catalytic domain chimera mutants. A, schematic of GST-CaMKK catalytic domain mutants, including wild-type and chimera mutants (CaMKKβ/α-1, CaMKKβ/α-2, and CaMKKβ/α/β-1). GST-fused CaMKK catalytic domain mutants, including wild-type, were constructed and expressed in E. coli JM109 and purified as described under “Experimental Procedures.” B, phosphorylation of either GST-CaMKIα 1–293, K49E (top panel), or AMPKα K45R (bottom panel) by CaMKK catalytic domain chimera mutants (CaMKKβ/α-1, closed triangles; CaMKKβ/α-2, closed diamonds; CaMKKβ/α/β-1, open triangles), including wild-type enzyme (CaMKKβ, open circles; CaMKKα, closed circles), was measured at 30 °C in the presence of 2 mm EGTA and 200 μm [γ-32P]ATP for 20 min. CaMKK activity was measured as shown in Fig. 1. CaMKK activities are displayed as scatterplots. The averages of three experiments are plotted as columns. *, p < 0.01 versus CaMKKβ WT. n.s., not significant.
Involvement of Ser357-Leu358 of CaMKKβ in Efficient AMPK Phosphorylation
Among residues 304–364 in CaMKKβ and 268–328 in CaMKKα, 17 amino acid residues are different from their corresponding counterparts in these two kinases (Fig. 4A). To narrow down the primary sequence of CaMKKβ, which is involved in the efficient phosphorylation of AMPK, we produced serial chimera mutants based on CaMKKβ/α/β-1 and measured their activities (Fig. 4A). We performed a phosphorylation assay against AMPKα K45R using the same concentration of CaMKKβ/α/β chimera mutants as that used for GST-CaMKIα 1–293 K49E phosphorylation (Fig. 4B) as described in Fig. 3B. CaMKKβ/α/β-2 and CaMKKβ/α/β-3 were shown to phosphorylate AMPK with a lower efficiency similar to that of CaMKKβ/α/β-1, but CaMKKβ/α/β-4 exhibited significantly increased activity to phosphorylate AMPKα (Fig. 4B, bottom panel). These results indicate that Ser357-Leu358 in CaMKKβ apparently plays an important role for the efficient phosphorylation of AMPKα but not for CaMKIα as a substrate.
FIGURE 4.
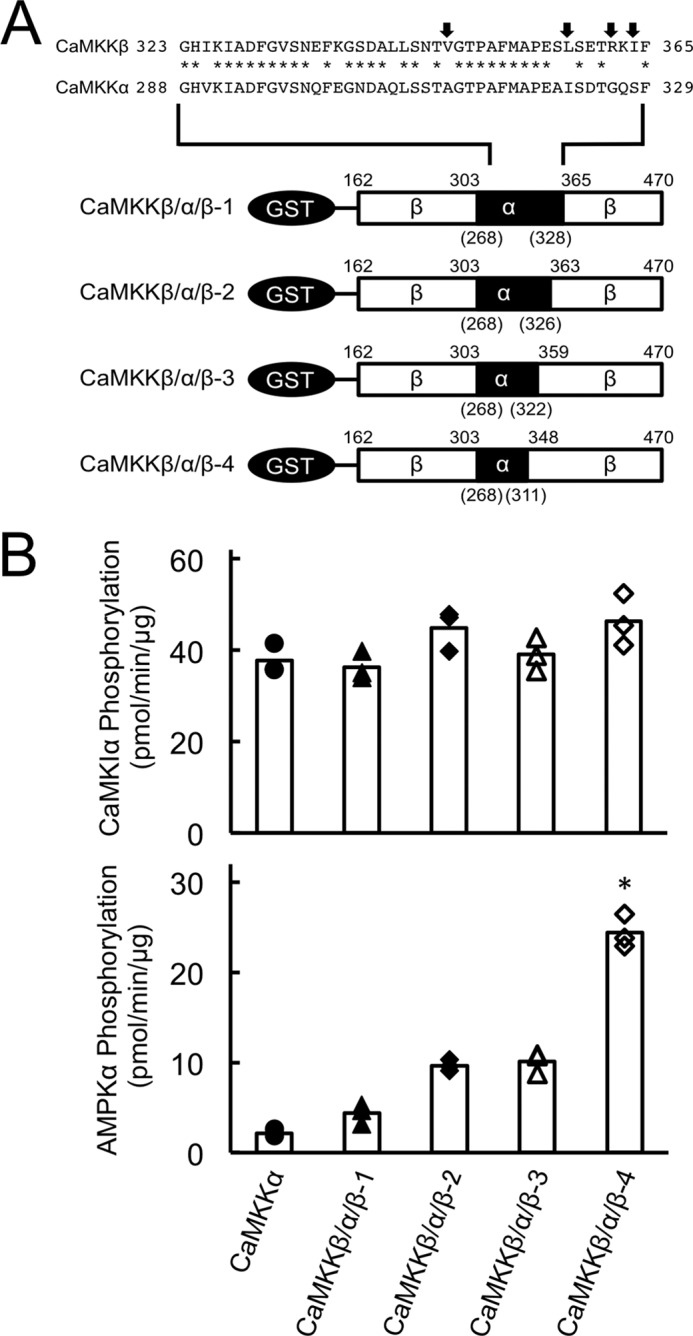
Involvement of Ser357-Leu358 of CaMKKβ in efficient AMPK phosphorylation. A, schematic of GST-CaMKK catalytic domain chimera mutants (CaMKKβ/α/β-1, CaMKKβ/α/β-2, CaMKKβ/α/β-3, and CaMKKβ/α/β-4). GST-fused CaMKK catalytic domain mutants were constructed and expressed in E. coli JM109 and purified as described under “Experimental Procedures.” B, phosphorylation of either GST-CaMKIα 1–293, K49E (top panel) or AMPKα K45R (bottom panel) by CaMKK catalytic domain chimera mutants (CaMKKβ/α/β-1, closed triangles; CaMKKβ/α/β-2, closed diamonds; CaMKKβ/α/β-3, open triangles; and CaMKKβ/α/β-4, open diamonds), and the CaMKKα catalytic domain mutant (closed circles) was measured as described in Fig. 3B. CaMKK activities are displayed as scatterplots. The averages of three experiments are plotted as columns. *, p < 0.01 versus CaMKKβ/α/β-3.
CaMKKβ Leu358 Plays Important Roles in Efficiently Phosphorylating AMPK
To identify the crucial amino acid(s) in CaMKKβ required for efficiently phosphorylating AMPKα, we produced a full-length CaMKKα mutant in which Ala321-Ile322 was replaced by the corresponding amino acid residues in CaMKKβ (Ser357-Leu358) (Fig. 5A) and measured the CaMKK activity toward CaMKIα and AMPKα as substrates. The amounts of the enzymes for measuring substrate phosphorylation were comparable, as judged by the CaM overlay method (Fig. 5B). The CaMKKα A321S mutant was shown to phosphorylate AMPKα with a lower efficiency, in a similar manner as the CaMKKα wild type. In contrast, the CaMKKα I322L mutant exhibited significantly enhanced kinase activity toward AMPKα in a similar manner as the CaMKKβ wild type. We observed the AMPK phosphorylating activity of the CaMKKα I322L mutant in a complete Ca2+/CaM-dependent manner (supplemental Fig. 1), indicating that the enhanced kinase activity toward AMPKα as a substrate (Fig. 5C) was not likely due to disruption of the autoinhibitory mechanism of CaMKKα (23) by this mutation. Therefore, we measured the kinetic constant (Km) of the CaMKKα I322L mutant for AMPK based on the double reciprocal plots of phosphorylation data using various concentrations of AMPK (Fig. 2B) and obtained a Km value (4.9 μm) that was significantly lower than that of CaMKKα (13.1 μm, Fig. 2A). Finally, to confirm the data obtained with in vitro experiments as described above by using living cells, we attempted to test the ionomycin-induced AMPK phosphorylation in A549 cells (a human lung adenocarcinoma epithelial cell line) in which FLAG-tagged CaMKKα mutants were exogenously expressed with a retrovirus expression system (Fig. 6). When we expressed a FLAG-tagged CaMKKα double mutant (A292T/L233F, AT/LF) in A549 cells, which was a CaMKK inhibitor (STO-609)-resistant mutant (20), 10 μg/ml STO-609 (19) treatment completely inhibited ionomycin-induced AMPK phosphorylation because CaMKKβ was thought to be responsible for AMPK phosphorylation in A549 cells but not CaMKKα. In contrast, we could observe significant ionomycin-induced AMPK phosphorylation in cells expressing a CaMKKα triple mutant (A292T/L233F/I322L, AT/LF/IL) even in the presence of STO-609, indicating that the CaMKKα triple mutant (AT/LF/IL) acquired an ability for phosphorylating AMPK in living cells. These results suggest that a single amino acid difference (Leu358 in CaMKKβ/Ile322 in CaMKKα) in the catalytic domain of CaMKK isoforms dictates the efficiency of the kinase to phosphorylate AMPKα in vivo as well as in vitro.
FIGURE 5.
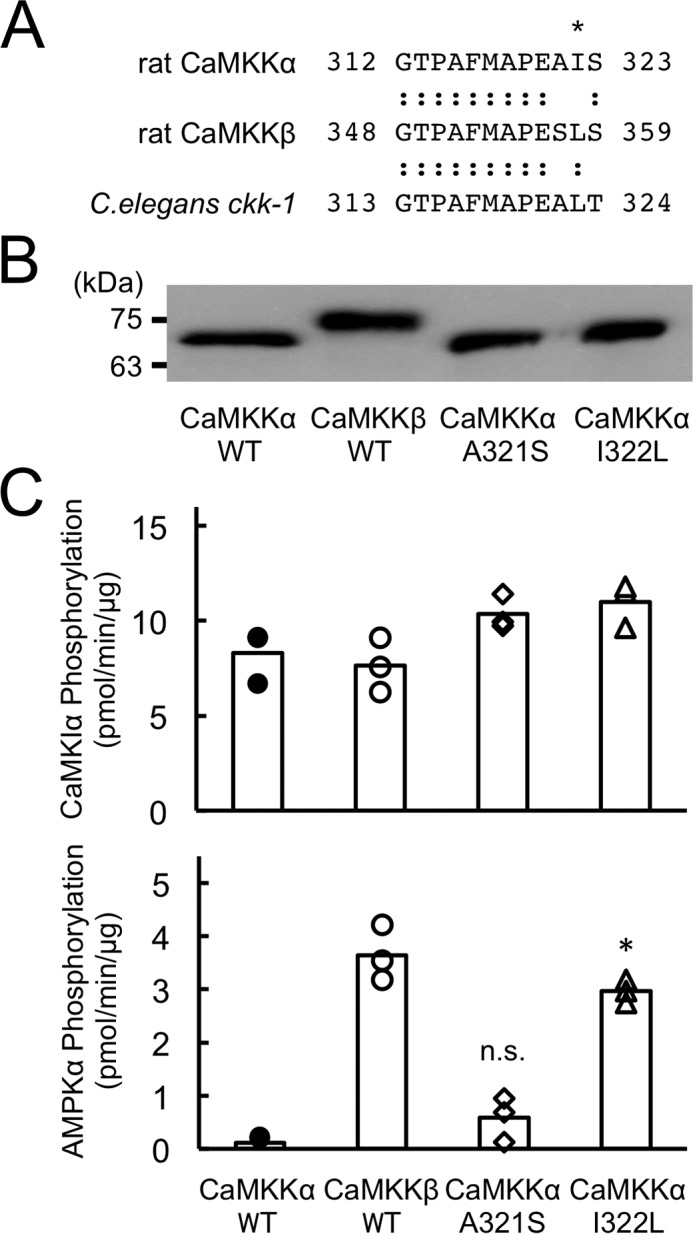
Identification of Leu358 as a critical residue in the discrimination of AMPK as a substrate for CaMKKβ. A, the amino acid sequences of subdomain VIII of rat CaMKKα (residues 312–323) (4–6), β (residues 348–359) (4–6), and C. elegans ckk-1 (residues 313–324) (4–6) are aligned. An asterisk indicates Ile322 in CaMKKα/Leu358 in CaMKKβ. B, CaM overlay analysis of recombinant rat CaMKKs (20 ng), including wild-type CaMKKα (CaMKKα WT), wild-type CaMKKβ (CaMKKβ WT), CaMKKα A321S (CaMKKα A321S), and CaMKKα I322L (CaMKKα I322L). The molecular masses in kilodaltons are indicated on the left. C, kinase activities of recombinant rat CaMKKs (1 μg/ml), including wild-type CaMKKα (CaMKKα WT, closed circles), wild-type CaMKKβ (CaMKKβ WT, open circles), CaMKKα A321S (CaMKKα A321S, open diamonds), and CaMKKα I322L (CaMKKα I322L, open triangles) using either GST-CaMKIα 1–293, K49E (top panel) or AMPKα K45R (bottom panel) as a substrate at 30 °C for 30 min with 4 mm CaCl2/12 μm CaM and 200 μm [γ-32P]ATP as described under “Experimental Procedures.” CaMKK activities are displayed as scatterplots. The averages of three experiments are plotted as columns. *, p < 0.001 versus CaMKKα WT. n.s., not significant.
FIGURE 6.
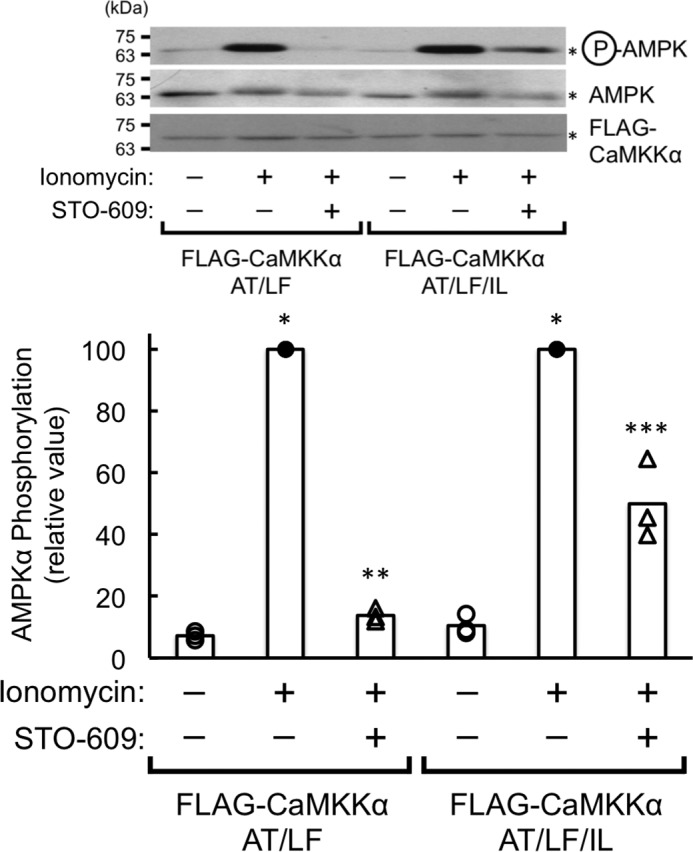
Phosphorylation of AMPK by the CaMKKα I322L mutant in A549 cells. Either the FLAG-CaMKKα A292T, L233F mutant (FLAG-CaMKKα AT/LF) or the FLAG-CaMKKα A292T/L233F/I322L mutant (FLAG-CaMKKα AT/LF/IL) was expressed with a retrovirus expression system as described under “Experimental Procedures” and then stimulated without (−, open circles) or with 1 μm ionomycin for 5 min (+) in the absence (−, closed circles) or presence (+, open triangles) of 10 μg/ml STO-609. Stimulation was terminated, and then AMPK phosphorylation at Thr172 was analyzed by immunoblotting with anti-phospho-AMPK antibody (top blot), anti-AMPK antibody (center blot), and anti-FLAG antibody (bottom blot), followed by quantification of the phosphorylation signal. The results are expressed as a percentage of the value in the absence of STO-609 (−) with ionomycin treatment (+) and displayed as scatterplots. The averages of three experiments are plotted as columns. *, p < 0.01 versus control cells without stimulation in the absence of STO-609; **, p < 0.01 versus ionomycin stimulated cells in the absence of STO-609; ***, p < 0.05 versus control cells without stimulation in the absence of STO-609.
In conclusion, CaMKKβ, but not CaMKKα, was shown to be an upstream activating kinase for AMPK (13–15) because of its efficient phosphorylating activity of CaMKKβ with an ∼9-fold higher affinity for AMPK than CaMKKα had. It is noteworthy that the kinase activities of both CaMKK isoforms toward CaMKIα were indistinguishable. Our mutagenesis studies clearly indicated that a single amino acid (Leu358 in CaMKKβ/Ile322 in CaMKKα) in subdomain VIII plays a role, at least in part, in the discrimination of AMPK as a native substrate but not in the discrimination of CaMKIα. This finding is in good agreement with a previous report demonstrating that subdomain VIII in MAPK/ERK kinase kinase 1 (MEKK1) was a contact site for its substrate, MKK4, thereby discriminating the substrate (30). In addition, Leu358 in CaMKKβ is also conserved in mammalian CaMKKβs and in its counterpart (ckk-1) in the roundworm Caenorhabditis elegans (31) (Fig. 5A) but not in mammalian CaMKKα isoforms, suggesting that the CKK-1/AMPK pathway may be functional in nematodes.
Author Contributions
Y. F., Y. K., and T. F. performed the experiments. N. K. and M. M. supervised the experiments and helped to edit the manuscript. H. T. designed the study and wrote the manuscript.
Supplementary Material
This work was supported by Grant-in-aid for Scientific Research 26440056 (to H. T.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Fig. 1.
- CaMKK
- Ca2+/calmodulin-dependent protein kinase kinase
- CaM
- calmodulin
- CaMK
- Ca2+/calmodulin-dependent protein kinase
- AMPK
- AMP-activated protein kinase.
References
- 1. Soderling T. R., and Stull J. T. (2001) Structure and regulation of calcium/calmodulin-dependent protein kinases. Chem. Rev. 101, 2341–2352 [DOI] [PubMed] [Google Scholar]
- 2. Means A. R. (2008) The year in basic science: calmodulin kinase cascades. Mol. Endocrinol. 22, 2759–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wayman G. A., Lee Y. S., Tokumitsu H., Silva A. J., Silva A., and Soderling T. R. (2008) Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron 59, 914–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tokumitsu H., Enslen H., and Soderling T. R. (1995) Characterization of a Ca2+/calmodulin-dependent protein kinase cascade: molecular cloning and expression of calcium/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 270, 19320–19324 [DOI] [PubMed] [Google Scholar]
- 5. Kitani T., Okuno S., and Fujisawa H. (1997) Molecular cloning of Ca2+/calmodulin-dependent protein kinase kinase β. J. Biochem. 122, 243–250 [DOI] [PubMed] [Google Scholar]
- 6. Anderson K. A., Means R. L., Huang Q. H., Kemp B. E., Goldstein E. G., Selbert M. A., Edelman A. M., Fremeau R. T., and Means A. R. (1998) Components of a calmodulin-dependent protein kinase cascade: molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase β. J. Biol. Chem. 273, 31880–31889 [DOI] [PubMed] [Google Scholar]
- 7. Enslen H., Sun P., Brickey D., Soderling S. H., Klamo E., and Soderling T. R. (1994) Characterization of Ca2+/calmodulin-dependent protein kinase IV: role in transcriptional regulation. J. Biol. Chem. 269, 15520–15527 [PubMed] [Google Scholar]
- 8. Bito H., Deisseroth K., and Tsien R. W. (1996) CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87, 1203–1214 [DOI] [PubMed] [Google Scholar]
- 9. Miranti C. K., Ginty D. D., Huang G., Chatila T., and Greenberg M. E. (1995) Calcium activates serum response factor-dependent transcription by a Ras- and Elk-1-independent mechanism that involves a Ca/calmodulin-dependent kinase. Mol. Cell. Biol. 15, 3672–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horigane S., Ageta-Ishihara N., Kamijo S., Fujii H., Okamura M., Kinoshita M., Takemoto-Kimura S., and Bito H. (2016) Facilitation of axon outgrowth via a Wnt5a-CaMKK-CaMKIα pathway during neuronal polarization. Mol. Brain 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dhar M., Wayman G. A., Zhu M., Lambert T. J., Davare M. A., and Appleyard S. M. (2014) Leptin-induced spine formation requires TrpC channels and the CaM kinase cascade in the hippocampus. J. Neurosci. 34, 10022–10033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saneyoshi T., Wayman G., Fortin D., Davare M., Hoshi N., Nozaki N., Natsume T., and Soderling T. R. (2008) Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/βPIX signaling complex. Neuron 57, 94–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., and Carling D. (2005) Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2, 21–33 [DOI] [PubMed] [Google Scholar]
- 14. Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., and Hardie D. G. (2005) Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2, 9–19 [DOI] [PubMed] [Google Scholar]
- 15. Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., and Witters L. A. (2005) The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280, 29060–29066 [DOI] [PubMed] [Google Scholar]
- 16. Anderson K. A., Ribar T. J., Lin F., Noeldner P. K., Green M. F., Muehlbauer M. J., Witters L. A., Kemp B. E., and Means A. R. (2008) Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 7, 377–388 [DOI] [PubMed] [Google Scholar]
- 17. Yamauchi M., Kambe F., Cao X., Lu X., Kozaki Y., Oiso Y., and Seo H. (2008) Thyroid hormone activates adenosine 5′-monophosphate-activated protein kinase via intracellular calcium mobilization and activation of calcium/calmodulin-dependent protein kinase kinase-β. Mol. Endocrinol. 22, 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghislat G., Patron M., Rizzuto R., and Knecht E. (2012) Withdrawal of essential amino acids increases autophagy by a pathway involving Ca2+/calmodulin-dependent kinase kinase-β (CaMKK-β). J. Biol. Chem. 287, 38625–38636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tokumitsu H., Inuzuka H., Ishikawa Y., Ikeda M., Saji I., and Kobayashi R. (2002) STO-609, a specific inhibitor of the Ca2+/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 277, 15813–15818 [DOI] [PubMed] [Google Scholar]
- 20. Fujiwara Y., Hiraoka Y., Fujimoto T., Kanayama N., Magari M., and Tokumitsu H. (2015) Analysis of distinct roles of CaMKK isoforms using STO-609-resistant mutants in living Cells. Biochemistry 54, 3969–3977 [DOI] [PubMed] [Google Scholar]
- 21. Green M. F., Anderson K. A., and Means A. R. (2011) Characterization of the CaMKKβ-AMPK signaling complex. Cell. Signal. 23, 2005–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fogarty S., Hawley S. A., Green K. A., Saner N., Mustard K. J., and Hardie D. G. (2010) Calmodulin-dependent protein kinase kinase-β activates AMPK without forming a stable complex: synergistic effects of Ca2+ and AMP. Biochem. J. 426, 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tokumitsu H., Muramatsu M., Ikura M., and Kobayashi R. (2000) Regulatory mechanism of Ca2+/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 275, 20090–20095 [DOI] [PubMed] [Google Scholar]
- 24. Osawa M., Tokumitsu H., Swindells M. B., Kurihara H., Orita M., Shibanuma T., Furuya T., and Ikura M. (1999) A novel target recognition revealed by calmodulin in complex with Ca2+-calmodulin-dependent kinase kinase. Nat. Struct. Biol. 6, 819–824 [DOI] [PubMed] [Google Scholar]
- 25. Tokumitsu H., Inuzuka H., Ishikawa Y., and Kobayashi R. (2003) A single amino acid difference between α and β Ca2+/calmodulin-dependent protein kinase kinase dictates sensitivity to the specific inhibitor, STO-609. J. Biol. Chem. 278, 10908–10913 [DOI] [PubMed] [Google Scholar]
- 26. Neumann D., Woods A., Carling D., Wallimann T., and Schlattner U. (2003) Mammalian AMP-activated protein kinase: functional, heterotrimeric complexes by co-expression of subunits in Escherichia coli. Protein Expr. Purif. 30, 230–237 [DOI] [PubMed] [Google Scholar]
- 27. Hayashi N., Matsubara M., Takasaki A., Titani K., and Taniguchi H. (1998) An expression system of rat calmodulin using T7 phage promoter in Escherichia coli. Protein Expr. Purif. 12, 25–28 [DOI] [PubMed] [Google Scholar]
- 28. Tokumitsu H., Hatano N., Tsuchiya M., Yurimoto S., Fujimoto T., Ohara N., Kobayashi R., and Sakagami H. (2010) Identification and characterization of PRG-1 as a neuronal calmodulin-binding protein. Biochem. J. 431, 81–91 [DOI] [PubMed] [Google Scholar]
- 29. Matsushita M., and Nairn A. C. (1999) Inhibition of the Ca2+/calmodulin-dependent protein kinase I cascade by cAMP-dependent protein kinase. J. Biol. Chem. 274, 10086–10093 [DOI] [PubMed] [Google Scholar]
- 30. Tu Z., and Lee F. S. (2003) Subdomain VIII is a specificity-determining region in MEKK1. J. Biol. Chem. 278, 48498–48505 [DOI] [PubMed] [Google Scholar]
- 31. Kimura Y., Corcoran E. E., Eto K., Gengyo-Ando K., Muramatsu M. A., Kobayashi R., Freedman J. H., Mitani S., Hagiwara M., Means A. R., and Tokumitsu H. (2002) A CaMK cascade activates CRE-mediated transcription in neurons of Caenorhabditis elegans. EMBO Rep. 3, 962–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


