Abstract
Background
In this study, we assessed whether red blood cell distribution width (RDW) was associated with all-cause mortality in patients on peritoneal dialysis (PD) and evaluated its prognostic value.
Methods
This study included 136 patients who had RDW levels at PD initiation from January 2007 to January 2014 at the Presbyterian Medical Center and Seoul St. Mary's Hospital. We divided these patients into 2 groups (survivors vs. nonsurvivors), compared their clinical characteristics, and analyzed the predictors of survival.
Results
The study included 79 men and 57 women, with a mean age of 54 years (range, 15–85 years). The mean follow-up duration was 32 months (range, 1–80 months). Of 136 patients, 14 died during the follow-up period. When clinical characteristics of survivors (n = 122) and nonsurvivors (n = 14) were compared, no differences were identified, with the exception of serum albumin, total iron-binding capacity (TIBC), left ventricular ejection fraction, total leukocyte count, and RDW value. Survivors had higher serum albumin (3.4 ± 0.5 vs. 3.0 ± 0.5 g/dL, P < 0.001) and left ventricular ejection fraction (56.8 ± 9.8 vs. 48.7 ± 12.8, P = 0.040) and lower TIBC (213.4 ± 40.9 vs. 252.8 ± 65.6, P = 0.010), total leukocyte counts (6.9 × 103/μL vs. 8.6 × 103/μL, P = 0.009), and serum RDW values (13.9 ± 1.7 vs. 16.0 ± 1.8, P < 0.001). Patients with high RDW levels (≥ 14.8) showed significantly higher all-cause mortality than patients with low RDW levels (< 14.8, P < 0.001). In multivariate-adjusted Cox analysis, RDW and TIBC at the start of PD were independent risk predictors for all-cause mortality.
Conclusion
RDW could be an additive predictor for all-cause mortality in patients on PD.
Keywords: Erythrocyte indices, Peritoneal dialysis, Red blood cell distribution width
Introduction
Red blood cell distribution width (RDW) is an index measurement of erythrocyte volume variability and is routinely reported as a part of a complete blood cell count [1], [2]. Elevated RDW reflects increased size variations of red blood cells, which indicates altered erythrocyte life span or dysfunctional erythrocytes. For a long time, RDW has been regarded as a useful index to differentiate the etiology of anemia, such as thalassemia and megaloblastic anemia, as well as iron deficiency–related anemia [3].
In recent years, it was observed that high RDW levels were associated with an increased risk of adverse outcomes in the general population [4], severe sepsis [5], heart failure [6], coronary artery disease [7], stroke [8], acute kidney injury that required renal replacement therapy [9], and kidney transplant recipients [10]. In addition, it was reported that high RDW is associated with early failure of arteriovenous fistula for hemodialysis access [11]. The exact mechanisms causing elevated RDW in these diverse conditions are unknown; however, it is assumed to be related to inflammatory processes that might interfere with the process of erythropoiesis [12]. A few authors reported that increased RDW is a predictor of mortality in end-stage renal disease (ESRD) patients [13], [14].
However, there are only a few studies that have investigated the association between RDW and adverse outcomes in patients on peritoneal dialysis. Therefore, we investigated the association between increased RDW and all-cause mortality in patients on peritoneal dialysis (PD).
Methods
Patient selection
Between 2007 and 2014, 458 patients received PD as a renal replacement therapy at the Presbyterian Medical Center and Seoul St. Mary's Hospital. Therefore, 136 patients were included in this study and were divided into 2 groups: survival (n = 122) and nonsurvival (n = 14). This study was approved by the institutional review boards of the Presbyterian Medical Center and Catholic University of Korea.
Clinical and laboratory information
The patients' baseline demographics as well as clinical and laboratory data were reviewed at the start of PD. The reference range for RDW levels in this study was 12.2–14.8%. The primary end point was all-cause mortality and the secondary end point was nonfatal cardiovascular (CV) events. CV events were defined as coronary artery disease (coronary artery bypass surgery, percutaneous intervention, or myocardial infarction), heart failure, ventricular arrhythmia, cerebrovascular accidents (cerebral infarction, transient ischemic attack, or cerebral hemorrhage), or peripheral arterial disease.
Statistical analysis
All the data are presented as the mean ± standard deviation unless otherwise specified. Comparisons of continuous variables between groups were carried out using the Wilcoxon–Mann–Whitney test. Comparisons between groups for categorical variables were carried out using the chi-square test. Kaplan–Meier curves and log-rank tests were used to describe and compare the event-free survival rates for all-cause mortality. Prognostic variables for mortality were analyzed by using the univariate Cox proportional hazards model, and variables with P < 0.1 in univariate analysis were used in the multivariate Cox proportional hazards model. The univariate and multivariate Cox regression analysis results are presented as hazard ratios and 95% confidence intervals. A P value of < 0.05 was considered statistically significant. Statistical analysis was carried out using SPSS software, version 21 (IBM corporation, New York, NY, USA).
Results
Baseline characteristics
The study included 79 (58%) men and 57 (42%) women, with a mean age of 54 years (range, 15–85 years). Sixty-nine patients had diabetes. The initial hemoglobin and mean RDW levels were 8.9 g/dL and 14.2%, respectively. The serum albumin was 3.3 g/dL. One hundred twenty-two patients (90%) received erythropoiesis-stimulating agents. During the mean follow-up of 32 months (range, 1–80 months), 14 deaths (9%) and 18 nonfatal CV events (14%) occurred.
Comparison of clinical characteristics between survivors and nonsurvivors
Survivors had higher serum albumin (3.4 ± 0.5 vs. 3.0 ± 0.5 g/dL, P < 0.001) and left ventricular ejection fraction (LVEF; 56.8 ± 9.8 vs. 48.7 ± 12.8, P = 0.040) and lower total iron-binding capacity (TIBC; 213.4 ± 40.9 vs. 252.8 ± 65.6, P = 0.010), total leukocyte counts (6.9 × 103/μL vs. 8.6 × 103/μL, P = 0.009), and serum RDW values (13.9 ± 1.7 vs. 16.0 ± 1.8, P < 0.001) than nonsurvivors (Table 1).
Table 1.
Comparison of baseline characteristics between survivors and nonsurvivors
| Survivors (N = 122) | Nonsurvivors (N = 14) | P | |
|---|---|---|---|
| Age (y) | 54.0 ± 12.0 | 58.0 ± 12.0 | 0.342 |
| Male | 74 (61) | 5 (39) | 0.301 |
| Duration of dialysis (mo) | 34.0 ± 23.0 | 15.0 ± 21.0 | 0.007 |
| Diabetes | 62 (51) | 7 (50) | 0.592 |
| Previous CV disease | 6 (5) | 1 (7) | 0.540 |
| RDW (%) | 13.9 ± 1.7 | 16.0 ± 1.8 | < 0.001 |
| Hemoglobin (g/dL) | 9.0 ± 1.8 | 9.5 ± 1.4 | 0.421 |
| Ferritin (ng/mL) | 360.4 ± 333.8 | 571.2 ± 808.9 | 0.138 |
| Iron (μg/dL) | 67.5 ± 31.3 | 58.5 ± 25.9 | 0.380 |
| TIBC (μg/dL) | 213.4 ± 40.9 | 252.8 ± 65.6 | 0.010 |
| Erythropoietin-stimulating agent | 109 (89) | 13 (93) | 0.260 |
| Total leukocyte count (×103/μL) | 6.9 ± 2.2 | 8.6 ± 3.6 | 0.009 |
| Platelet (103/μL) | 191.0 ± 74.0 | 227.0 ± 92.0 | 0.140 |
| C-reactive protein (mg/dL) | 1.3 ± 2.3 | 2.4 ± 4.1 | 0.142 |
| Intact PTH (pg/mL) | 320.3 ± 420.3 | 296.4 ± 337.6 | 0.349 |
| Albumin (mg/dL) | 3.4 ± 0.5 | 3.0 ± 0.5 | < 0.001 |
| Total cholesterol (mg/dL) | 160.0 ± 52.0 | 166.0 ± 60.0 | 0.680 |
| Triglyceride (g/dL) | 170.0 ± 200.0 | 136.0 ± 63.0 | 0.521 |
| Na (mEq/L) | 138.3 ± 3.6 | 136.5 ± 3.3 | 0.156 |
| CO2 (mEq/L) | 21.4 ± 5.4 | 23.3 ± 3.4 | 0.192 |
| Echocardiographic data∗ | |||
| LA diameter (mm2) | 40.6 ± 11.0 | 44.6 ± 2.7 | 0.561 |
| LV ejection fraction | 56.8 ± 9.8 | 48.7 ± 12.8 | 0.040 |
| E/E′ ratio | 13.7 ± 5.6 | 16.6 ± 9.7 | 0.213 |
Data are presented as means ± SD or number (%).
CV, cardiovascular; LA, left atrium; LV, left ventricular; PTH, parathyroid hormone; RDW, red blood cell distribution width; TIBC, total iron-binding capacity.
Echocardiographic data were available in 99 patients.
Comparison of clinical characteristics between high- and low-RDW groups
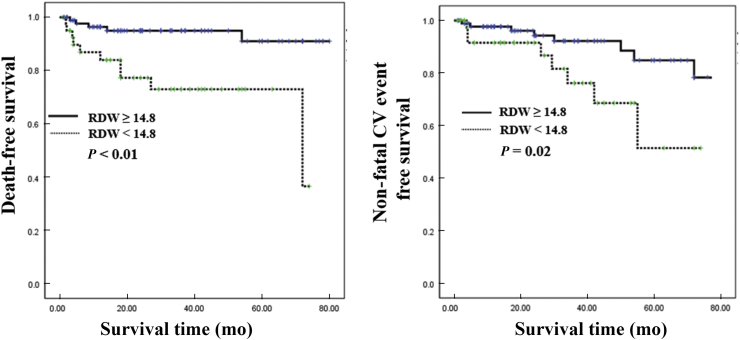
According to RDW normal reference in our hospital, patients with measured RDW values were divided into 2 groups: low-RDW group (< 14.8) and high-RDW group (≥ 14.8). When we compared the clinical characteristics of the low- (n = 94) and high-RDW (n = 42) groups, no differences were observed, with the exception of C-reactive protein, TIBC, LVEF, all-cause mortality, and nonfatal CV events (Table 2). While CV mortality was not different between the 2 groups, the high-RDW group showed significantly lower event-free survival rates for all-cause mortality and nonfatal CV events than the low-RDW group (Fig. 1).
Table 2.
Comparison of baseline characteristics between high and low RDW value
| High RDW (N = 42) | Low RDW (N = 94) | P | |
|---|---|---|---|
| Age (y) | 56.0 ± 12.0 | 53.0 ± 13.0 | 0.200 |
| Male | 26 (63) | 53 (56) | 0.261 |
| Duration of dialysis (mo) | 25.0 ± 20.0 | 34.0 ± 24.0 | 0.064 |
| Diabetes | 25 (61) | 44 (46) | 0.153 |
| Previous CV disease | 2 (4.8) | 5 (5.3) | 0.531 |
| Hemoglobin (g/dL) | 8.9 ± 1.6 | 9.1 ± 1.9 | 0.731 |
| Ferritin (ng/mL) | 418.0 ± 546.0 | 368.7 ± 330.5 | 0.632 |
| Iron (μg/dL) | 63.3 ± 27.3 | 68.4 ± 32.2 | 0.491 |
| TIBC (μg/dL) | 229.3 ± 54.3 | 211.3 ± 39.4 | 0.091 |
| Erythropoietin-stimulating agent | 39 (93) | 84 (88) | 0.230 |
| Total leukocyte count (×103/μL) | 7.5 ± 2.4 | 7.0 ± 2.4 | 0.231 |
| Platelet (103/μL) | 207.0 ± 85.0 | 188.0 ± 73.0 | 0.225 |
| C-reactive protein | 2.2 ± 3.6 | 1.1 ± 0.9 | 0.027 |
| Intact PTH (pg/mL) | 281.2 ± 255.1 | 337.2 ± 320.2 | 0.051 |
| Albumin (g/dL) | 3.1 ± 0.5 | 3.3 ± 0.5 | 0.050 |
| Na (mEq/L) | 138.7 ± 3.9 | 137.9 ± 3.5 | 0.271 |
| CO2 (mEq/L) | 21.8 ± 4.2 | 21.5 ± 5.7 | 0.832 |
| Echocardiographic data∗ | |||
| LA diameter (mm2) | 38.9 ± 13.2 | 42.6 ± 11.0 | 0.431 |
| LV ejection fraction | 54.5 ± 11.8 | 56.8 ± 10.4 | 0.041 |
| E/E′ ratio | 16.1 ± 7.2 | 13.0 ± 7.1 | 0.102 |
| Nonfatal cardiovascular event | 10 (25) | 8 (8) | 0.021 |
| Fatal cardiovascular event | 1 (2.43) | 2 (2.22) | 0.532 |
| Death | 10 (24.4) | 5 (5.6) | < 0.001 |
Data are presented as means ± SD or number (%).
CV, cardiovascular; LA, left atrium; LV, left ventricular; PTH, parathyroid hormone; RDW, red blood cell distribution width; TIBC, total iron-binding capacity.
Echocardiographic data were available in 99 patients.
Figure 1.
Kaplan–Meier plots for all-cause mortality and nonfatal CV events. Patients with high RDW levels (≥ 14.8) showed significantly higher all-cause mortality and nonfatal CV events than patients with low RDW levels.
CV, cardiovascular; RDW, red blood cell distribution width.
Predicting survival in patients on PD
Of the 14 patients who died during the study period, 10 patient deaths were caused by sepsis and 3 were related to CV events. The causes of sepsis were as follows: 5 were pneumonia, 1 was bowel perforation, 1 was encapsulating peritoneal sclerosis, and 3 were of unknown origin. Univariate analysis indicated that serum albumin, RDW values, TIBC, C-reactive protein, total leukocyte count, and LVEF were significant predictors of mortality in patients on PD. After adjusting for these factors in a multivariate-adjusted Cox analysis, total leukocyte counts, serum albumin, and RDW values were the most important prognostic factors in ESRD patients who received PD treatment (Table 3). In case of nonfatal CV events, age and presence of diabetes were the significant predictors (Table 4).
Table 3.
Cox proportional hazards analysis for all-cause mortality
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| RDW (per 1% increase) | 1.687 (1.342–2.100) | < 0.001 | 2.908 (1.346–6.284) | 0.007 |
| TIBC (μg/dL) | 1.016 (1.003–1.028) | 0.014 | 1.019 (1.002–1.037) | 0.032 |
| Albumin (g/dL) | 0.343 (0.147–0.803) | 0.014 | 0.396 (0.013–12.473) | 0.609 |
| CRP (mg/dL) | 1.148 (1.000–1.317) | 0.050 | 1.153 (0.864–1.539) | 0.333 |
| TLC (×103/mL) | 1.244 (1.044–1.483) | 0.015 | 0.958 (0.676–1.358) | 0.809 |
| LVEF (%) | 0.946 (0.896–0.998) | 0.041 | 0.958 (0.817–1.013) | 0.809 |
CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; LVEF, left ventricular ejection fraction; RDW, red blood cell distribution width; TIBC, total iron-binding capacity; TLC, total leukocyte count.
Table 4.
Cox proportional hazards analysis for nonfatal CV events
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| RDW (per 1% increase) | 1.513 (1.161–1.971) | 0.002 | 1.464 (0.870–2.464) | 0.152 |
| Age | 1.122 (1.061–1.187) | < 0.001 | 1.099 (1.005–1.201) | 0.038 |
| Presence of diabetes | 9.256 (1.176–72.86) | 0.035 | 10.428 (1.243–87.482) | 0.031 |
| LVEF (%) | 0.983 (0.931–1.038) | 0.530 | 0.111 (0.880–1.013) | 0.111 |
CI, confidence interval; CV, cardiovascular; HR, hazard ratio; LVEF, left ventricular ejection fraction; RDW, red blood cell distribution width.
Discussion
RDW, routinely reported as part of a complete blood cell count, is a simple laboratory test that is used to evaluate variability in the size and form of red blood cells [1], [2]. Several studies have recently shown that elevated RDW is a predictor of morbidity and mortality in CV diseases, such as acute and chronic congestive heart failure, acute myocardial infarction, pulmonary hypertension, peripheral artery disease, and stroke [6], [7], [8], [15]. In addition, high RDW has emerged as a risk factor in clinical nephrology, including hemodialysis patients and patients with acute kidney injuries that require continuous renal replacement therapy. Yoon et al [13] reported that a progressive rise in RDW predicts mortality and CV events in ESRD. In our study, the RDW value was also higher in nonsurvivors than in survivors, and it was an independent risk predictor for all-cause mortality.
The US Renal Data System (USRDS) and ANZDATA Registry reported that the most common cause of death is cardiovascular disease, in which infectious disease was the second leading cause [16], [17], [18]. However, Choi et al [19] reported that infection was the most common cause of death in Korean patients on PD. Of the 14 patients that died during this study period, 10 of them were attributable to infectious diseases, such as sepsis. It has also been reported that RDW is associated with inflammatory and infectious conditions. Ku et al [20] and Braun et al [21] reported that RDW is an independent predictor of mortality among patients with gram-negative bacteremia and community-acquired pneumonia. The 2 primary causes of death in PD patients in this study were infection and CV disease, and we believe that RDW could be an additive predictor for all-cause mortality in patients on PD.
Previous literature has reported that RDW is a predictor of CV mortality in various populations [22]. However, only a few reports have discussed the relevance of RDW and CV mortality and morbidity in ESRD patients [7], [8]. Recently, Yoon et al [13] reported that a progressive rise in RDW predicts mortality and CV events in ESRD patients. Peng et al [23] also reported that a high baseline RDW value was associated with CV mortality in incidental PD patients. However, high RDW was not associated with CV mortality in our study. We believe that this finding might be due to different causes of death such as infection in our study. In addition, although patients with high RDW levels showed significantly more frequent nonfatal CV events than patients with low RDW levels, the RDW was not a significant predictor in the present study. Therefore, to clarify the association between RDW and CV mortality, large prospective controlled studies are needed.
Although the mechanism underlying the association between higher RDW and adverse outcomes in PD patients is not completely understood, several mechanisms can be postulated. First, increasing RDW may indicate impaired iron metabolism and is assumed to be related to inflammation which might interfere with the process of erythropoiesis [12], [24]. Impaired iron metabolism is reported to be associated with heart failure [25]. In the present study, TIBC was higher in the high-RDW group than in the low-RDW group and was the independent risk predictor for all-cause mortality. In addition, inflammatory response plays a critical role in the development and progression of heart failure [26], [27] and inhibits erythrocyte maturation and accelerates the migration of reticulocytes into the peripheral circulation, thereby increasing RDW [28]. Therefore, RDW may bridge the relationship between inflammation and poorer heart failure prognosis. In this study, C-reactive protein, which is regarded as an inflammatory marker, was also higher in the high-RDW group than in the low-RDW group. We think that such findings might contribute to the lower event-free survival rate for nonfatal CV events and all-cause mortality in the high-RDW group.
Second, a mechanism that could be involved in such a process is the release of cytokines in response to inflammatory stress. These cytokines might block the activity of erythropoietin, inhibit erythrocyte maturation, and cause production of ineffective red blood cells and elevated RDW [12]. The expansion of proinflammatory cytokines may increase susceptibility to infections [29]. In addition, Duranay et al [30] reported that oxidative stress seems to increase peritonitis in continuous ambulatory peritoneal dialysis patients. In our study, no patients expired because of PD-associated peritonitis; however, infection was an important cause of death. Four patients of 10 who expired because of infection experienced sepsis because of pneumonia within 6 months after starting PD.
Our study has some limitations. First, this is a retrospective study and the study population comprised only Asian people. Second, despite a single measurement of RDW levels that could have reflected acute changes in RDW induced by blood loss or hemolysis, we did not evaluate fluctuations in RDW levels and thus could not account for possible variation over time.
In our study, RDW, albumin, and total leukocyte counts when patients started PD were associated with the incidence of mortality. RDW is a widely available and inexpensive test performed as part of the complete blood cell count. Therefore, measuring the RDW at initiation of PD is helpful for predicting all-cause mortality in patients on PD.
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.Simel D.L., DeLong E.R., Feussner J.R., Weinberg J.B., Crawford J. Erythrocyte anisocytosis. Visual inspection of blood films vs automated analysis of red blood cell distribution width. Arch Intern Med. 1988;148:822–824. doi: 10.1001/archinte.148.4.822. [DOI] [PubMed] [Google Scholar]
- 2.Karnad A., Poskitt T.R. The automated complete blood cell count. Use of the red blood cell volume distribution width and mean platelet volume in evaluating anemia and thrombocytopenia. Arch Intern Med. 1985;145:1270–1272. doi: 10.1001/archinte.145.7.1270. [DOI] [PubMed] [Google Scholar]
- 3.Lin C.K., Lin J.S., Chen S.Y., Jiang M.L., Chiu C.F. Comparison of hemoglobin and red blood cell distribution width in the differential diagnosis of microcytic anemia. Arch Pathol Lab Med. 1992;116:1030–1032. [PubMed] [Google Scholar]
- 4.Perlstein T.S., Weuve J., Pfeffer M.A., Beckman J.A. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;23:588–594. doi: 10.1001/archinternmed.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim C.H., Park J.T., Kim E.J., Han J.H., Han J.S., Choi J.Y., Han S.H., Yoo T.H., Kim Y.S., Kang S.W., Oh H.J. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit Care. 2013;17:R282. doi: 10.1186/cc13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen L.A., Felker G.M., Mehra M.R., Chiong J.R., Dunlap S.H., Ghali J.K., Lenihan D.J., Oren R.M., Wagoner L.E., Schwartz T.A., Adams K.F., Jr. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010;16:230–238. doi: 10.1016/j.cardfail.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonelli M., Sacks F., Arnold M., Moye L., Davis B., Pfeffer M., for the Cholesterol and Recurrent Events (CARE) Trial Investigators Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 8.Ani C., Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009;277:103–108. doi: 10.1016/j.jns.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Oh H.J., Park J.T., Kim J.K., Yoo D.E., Kim S.J., Han S.H., Kang S.W., Choi K.H., Yoo T.H. Red blood cell distribution width is an independent predictor of mortality in acute kidney injury patients treated with continuous renal replacement therapy. Nephrol Dial Transplant. 2012;27:589–594. doi: 10.1093/ndt/gfr307. [DOI] [PubMed] [Google Scholar]
- 10.Mucsi I., Ujszaszi A., Czira M.E., Novak M., Molnar M.Z. Red cell distribution width is associated with mortality in kidney transplant recipients. Int Urol Nephrol. 2014;46:641–651. doi: 10.1007/s11255-013-0530-z. [DOI] [PubMed] [Google Scholar]
- 11.Bojakowski K., Dzabic M., Kurzejamska E., Styczynski G., Andziak P., Gaciong Z., Söderberg-Nauclér C., Religa P. A high red blood cell distribution width predicts failure of arteriovenous fistula. PLoS One. 2012;7:e36482. doi: 10.1371/journal.pone.0036482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce C.N., Larson D.F. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion. 2005;20:83–90. doi: 10.1191/0267659105pf793oa. [DOI] [PubMed] [Google Scholar]
- 13.Yoon H.E., Kim S.J., Hwang H.S., Yang C.W., Shin S.J. Progressive rise in red blood cell distribution width predicts mortality and cardiovascular events in end-stage renal disease patients. PLoS One. 2015;10:e0126272. doi: 10.1371/journal.pone.0126272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sičaja M., Pehar M., Đerek L., Starčević B., Vuletić V., Romić Z., Božikov V. Red blood cell distribution width as a prognostic marker of mortality in patients on chronic dialysis: a single center, prospective longitudinal study. Croat Med J. 2013;54:25–32. doi: 10.3325/cmj.2013.54.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Z., Smith C., Kullo I.J. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol. 2011;107:1241–1245. doi: 10.1016/j.amjcard.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Renal Data System . The National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease; 2011. USRDS 2011 Annual Data Report; pp. 217–224. [Google Scholar]
- 17.Johnson D.W., McDonald S., Excell L., Livingston B., Shtangey V. Australia and New Zealand Dialysis and Transplant Registry; Adelaide (South Australia): 2010. ANZDATA Registry Report 2010. [Google Scholar]
- 18.Johnson D.W., Dent H., Hawley C.M., McDonald S.P., Rosman J.B., Brown F.G., Bannister K.M., Wiggins K.J. Associations of dialysis modality and infectious mortality in incident dialysis patients in Austria and New Zealand. Am J Kidney Dis. 2009;53:290–297. doi: 10.1053/j.ajkd.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Choi J.Y., Jang H.M., Park J., Kim Y.S., Kang S.W., Yang C.W., Kim N.H., Cho J.H., Park S.H., Kim C.D., Kim Y.L., Clinical Research Center for End Stage Renal Disease (CRC for ESRD) Investigators Survival advantage of peritoneal dialysis relative to hemodialysis in the early period of incident dialysis patients: a nationwide prospective propensity-matched study in Korea. PLoS One. 2013;8:e84257. doi: 10.1371/journal.pone.0084257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ku N.S., Kim H.W., Oh H.J., Kim Y.C., Kim M.H., Song J.E., Oh D.H., Ahn J.Y., Kim S.B., Jeong S.J., Han S.H., Kim C.O., Song Y.G., Kim J.M., Choi J.Y. Red blood cell distribution width is an independent predictor of mortality in patients with gram-negative bacteremia. Shock. 2012;38:123–127. doi: 10.1097/SHK.0b013e31825e2a85. [DOI] [PubMed] [Google Scholar]
- 21.Braun E., Kheir J., Mashiach T., Naffaa M., Azzam Z.S. Is elevated red cell distribution width a prognostic predictor in adult patients with community acquired pneumonia? BMC Infect Dis. 2014;14:129. doi: 10.1186/1471-2334-14-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zalawadiya S.K., Veeranna V., Panaich S.S., Afonso L., Ghali J.K. Gender and ethnic differences in red cell distribution width and its association with mortality among low risk healthy United State adults. Am J Cardiol. 2012;109:1664–1670. doi: 10.1016/j.amjcard.2012.01.396. [DOI] [PubMed] [Google Scholar]
- 23.Peng F., Li Z., Zhong Z., Luo Q., Guo Q., Huang F., Yu X., Yang X. An increasing of red blood cell distribution width was associated with cardiovascular mortality in patients on peritoneal dialysis. Int J Cardiol. 2014;176:1379–1381. doi: 10.1016/j.ijcard.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Chiari M.M., Bagnoli R., De Luca P.D., Monti M., Rampoldi E., Cunietti E. Influence of acute inflammation on iron and nutritional status indexes in older inpatients. J Am Geriatr Soc. 1995;43:767–771. doi: 10.1111/j.1532-5415.1995.tb07047.x. [DOI] [PubMed] [Google Scholar]
- 25.Klip I.T., Comin-Colet J., Voors A.A., Ponikowski P., Enjuanes C., Banasiak W., Lok D.J., Rosentryt P., Torrens A., Polonski L., van Veldhuisen D.J., van der Meer P., Jankowska E.A. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165:575–582. doi: 10.1016/j.ahj.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Yndestad A., Damås J.K., Øie E., Ueland T., Gullestad L., Aukrust P. Role of inflammation in the progression of heart failure. Curr Cardiol Rep. 2007;9:236–241. doi: 10.1007/BF02938356. [DOI] [PubMed] [Google Scholar]
- 27.Chen W., Frangogiannis N.G. The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail Rev. 2010;15:415–422. doi: 10.1007/s10741-010-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okonko D.O., Marley S.B., Anker S.D., Poole-Wilson P.A., Gordon M.Y. Suppression of erythropoiesis in patients with chronic heart failure and anaemia of unknown origin: evidence of an immune basis. Int J Cardiol. 2013;166:664–671. doi: 10.1016/j.ijcard.2011.11.081. [DOI] [PubMed] [Google Scholar]
- 29.Sarnak M.J., Jaber B.L. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58:1758–1764. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 30.Duranay M., Yilmaz F.M., Yilmaz G., Akay H., Parpucu H., Yücel D. Association between nitric oxide and oxidative stress in continuous ambulatory peritoneal dialysis patients with peritonitis. Scand J Clin Lab Invest. 2007;67:654–660. doi: 10.1080/00365510701253350. [DOI] [PubMed] [Google Scholar]