Abstract
Background: Vitamin D exerts anti-inflammatory actions both in vitro and in murine models of colitis. In previous studies, we demonstrated that vitamin D protects against the development of colitis by maintaining the integrity of the intestinal mucosal barrier.
Objective: We sought to evaluate whether deficient serum 25 hydroxyvitamin D [25(OH)D] concentrations are associated with increased mucosal inflammation, a loss of epithelial junctional proteins, and an increase in mucosal inflammatory cytokines in patients with ulcerative colitis (UC).
Design: We prospectively enrolled 230 subjects with UC. Serum 25(OH)D concentrations were compared with the Mayo endoscopic score, the total Mayo score, and histologic activity. Colonic mucosal expression concentrations of vitamin D receptor (VDR), E-cadherin, zonula occluden 1 (ZO-1), occludin, claudin-2, tumor necrosis factor α (TNF-α), and interleukin 8 (IL-8) were compared between dichotomous groups with low or high serum 25(OH)D concentrations.
Results: The mean serum 25(OH)D concentration was 21.8 ng/mL. Subjects stratified by concentrations included 12.6% ≥30 ng/mL, 45.6% ≥20 to <30 ng/mL, 37.4% ≥10 to <20 ng/mL, and 4.4% <10 ng/mL. There was an inverse association between serum 25(OH)D concentrations and mucosal inflammation as assessed by the Mayo endoscopy score (P = 0.01), disease activity as indicated by the total Mayo score (P = 0.001), and histologic activity (P = 0.02). A serum 25(OH)D concentration <20 ng/mL was associated with decreased mucosal transcript and protein expression concentrations of VDR, E-cadherin, and occludin as well as decreased protein expression of ZO-1, whereas TNF-α and IL-8 mucosal transcript expression concentrations were increased.
Conclusions: In UC patients, serum 25(OH)D concentration is inversely correlated with mucosal inflammation and disease activity. These results, coupled with the findings that serum 25(OH)D concentrations correlate with the mucosal expression of VDR as well as epithelial junction proteins and inversely with proinflammatory cytokines, suggest that vitamin D deficiency may contribute to UC inflammation by disrupting epithelial barrier function.
Keywords: inflammatory bowel disease, mucosal inflammation, tight junction, ulcerative colitis, vitamin D
INTRODUCTION
Ulcerative colitis (UC)9 is a chronic inflammatory disorder that affects the colon. Although its pathogenesis remains unknown, UC is thought to develop from a complex interaction between genetics, environmental triggers, and aberrant immune responses (1). Epidemiologic evidence supports the hypothesis that a low serum 25-hydroxyvitamin D [25(OH)D] concentration is one such environmental factor involved in inflammatory bowel disease (IBD) risk. In the Northern Hemisphere, the symptomatic onset of UC peaks in the winter months, the season with the least availability of UVB radiation (2, 3). In addition, Northern Hemisphere populations that reside at higher latitudes where daily sunlight exposure is lower have a higher prevalence of IBD, particularly Crohn disease (4, 5). Previous analyses have also reported a high prevalence of vitamin D deficiency in patients with both Crohn disease and UC (6–11). Furthermore, several studies have demonstrated an inverse correlation between clinical symptoms and serum vitamin concentrations in patients with Crohn disease (12–14). In addition, a randomized placebo-controlled trial that examined the impact of 1200 IU vitamin D3/d on maintaining remission in 94 patients with Crohn disease demonstrated a trend toward lower relapse rates in the vitamin D–treated group (15). Less is known, however, regarding the relation between serum 25(OH)D and mucosal inflammation in patients with UC.
The vitamin D endocrine system is a pleiotropic multifunctional system. 1,25-Dihydroxyvitamin D3 [1,25(OH)2D3], the hormonally active form of vitamin D, has several effects on the immune system, including the suppression of T cell activation and modulation of antigen-presenting cell migration, differentiation, and apoptosis (16). The biological activity of 1,25(OH)2D3 is mediated by the vitamin D receptor (VDR) (17). In vitro studies and experiments in animal models have also concluded that vitamin D supplementation suppresses inflammation in colitis by reducing aberrant T cell–mediated immune responses in the colon (18–21).
In addition to its anti-inflammatory effects, our group has reported that vitamin D-VDR signaling protects against colitis by maintaining intestinal epithelial barrier integrity by suppressing epithelial apoptosis and abrogating myosin light-chain kinase-dependent tight junction dysfunction (22–24). Although there are 2 studies that have demonstrated an inverse correlation between serum 25(OH)D and fecal calprotectin in patients with IBD, there are no data to our knowledge comparing serum 25(OH)D concentrations with endoscopic mucosal disease activity or epithelial barrier function in patients with IBD (25, 26). As such, we sought to investigate 25(OH)D concentrations in a prospectively collected cohort of patients with UC with well-characterized endoscopic, clinical, and histologic disease activity. We also investigated colonic mucosal expression levels of epithelial junction proteins and proinflammatory cytokines to evaluate whether sufficient concentrations of serum 25(OH)D are associated with the preservation of epithelial junctional proteins and a decrease in mucosal inflammatory cytokines in patients with UC.
METHODS
Subjects
The study was approved by the institutional review board at the University of Chicago. Subjects consented for the study at the time of endoscopic examination. Inclusion criteria included subjects aged ≥18 y with a history of UC confirmed by histologic evaluation and a disease extent >20 cm proximal to the anal verge. Subjects were excluded if they had indeterminate colitis or if the clinical diagnosis after the procedure was changed to Crohn disease based on endoscopic or pathologic data.
Study procedures
Clinical data were collected through a questionnaire that included data on the subject’s age, disease duration, family history of IBD, smoking status, and history, as well as current medication and dose, including vitamin D supplementation. The Mayo disease activity index was scored at the time of the procedure (27). For subjects who had varying disease activity throughout the colon, mucosal inflammation in the most severe segment was recorded for the Mayo endoscopy score. Histologic severity was determined based on a review of the pathology from standard-of-care biopsies. Histologic scores were graded as histologically normal, quiescent, mild, moderate, or severe based on an evaluation by a gastrointestinal pathologist. If there were varying degrees of histologic inflammation in the colon, the most severe score was recorded.
At the time of endoscopy, colonic mucosal biopsies were obtained 20 cm proximal to the anus. Four biopsies were placed in RNAlater (Life Technologies), and one biopsy was placed in 10% buffered formalin. Biopsies for transcript and protein expression concentrations were analyzed only in subjects who had concordance between the recorded Mayo endoscopy score and the histologic score at the site of biopsies (20 cm proximal to the anus). Peripheral blood was also drawn before endoscopy, and serum was separated into aliquots after centrifugation.
Serum 25(OH)D analysis
Serum 25(OH)D concentration was measured with the use of the Food and Drug Administration–approved DiaSorin radioimmunoassay kit in a Clinical Laboratory Improvement Amendments-certified laboratory by Heartland Assays that participates in the vitamin D external-quality assessment scheme (28).
Real-time polymerase chain reaction
Tissue was homogenized with the use of a bullet blender (Next Advance) and extracted with an AllPrep DNA/RNA/miRNA Universal Kit (Qiagen). cDNA was synthesized from RNA extracted from 60 subjects with the use of a high-capacity cDNA reverse-transcriptase kit (Life Technologies). Real-time quantitative polymerase chain reaction (qPCR) was performed with Fast SYBR Green Master Mix (Thermo Fisher Scientific) with the use of primers for β-actin, claudin-2, E-cadherin, occludin, TNF-α, VDR, and zonula occluden 1 (ZO-1) in a Roche 480 LightCycler (Supplemental Table 1). The thermal profile included activation at 95°C for 5 min followed by 45 amplification cycles (denaturation at 95°C for 10 s, annealing either from 59°C to 53°C or from 57°C to 51°C with a 0.25°C decrease/cycle, touch down for 15 s, and extension at 72°C for 20 s). This was followed by a melt curve (95°C for 5 s, 65°C for 1 min, and 97°C continuous acquisition) and then cooling (40°C for 30 s). As shown in Supplemental Table 2, subjects included in the analysis were evenly distributed by Mayo endoscopy score and categorical vitamin D concentration [high (>25 ng/mL) compared with low (<20 ng/mL)].
Immunohistochemistry
We cut 5-μm-thick sections from formalin-fixed, paraffin-embedded tissue and mounted them on Vectabond-coated Superfrost Plus slides (Fisher Scientific) from 74 subjects evenly distributed by Mayo endoscopy score and categorical vitamin D concentration (high compared with low). The distribution of subjects by endoscopic activity and serum 25(OH)D concentrations is included in Supplemental Table 3. After deparaffinization and blocking of endogenous peroxidase, nonspecific binding was prevented with a protein block (Dako). Antigen was retrieved with a 0.01M citrate buffer (pH 6) in a steamer for 20 min for claudin-2, E-cadherin, and VDR. ZO-1 antigen retrieval was performed with 0.04% protease treatment at 37°C for 15 min. Occludin antigen was retrieved with 0.04% protease treatment at 37°C for 15 min followed by a 0.01M citrate buffer in a steamer for 15 min. Slides were incubated overnight with primary antibody at 4°C. The primary antibody dilution was 1:100 for claudin-2 (Life Technologies), 1:100 for E-cadherin (BD Biosciences), 1:100 for occludin (Life Technologies), 1:100 for VDR (Santa Cruz Biotechnology), and 1:500 for ZO-1 (Life Technologies). The slides were incubated with a 1:200 dilution of secondary antibody (Dako) for 35 min, stained with 3,3′-diaminobenzidine solution, and counterstained with hematoxylin and bluing reagent (Supplemental Figure 1).
Semiquantitative scoring was performed by 3 investigators who were blinded to clinical, endoscopic, and histologic scores as well as to serum 25(OH)D concentrations. Staining concentrations for E-cadherin, VDR, and ZO-1 were scored as 0–3 based on the intensity. Occludin and claudin-2 staining were scored as weak or strong. When there was a disagreement among investigators in scores, the slides were reviewed again, and a consensus was reached.
Statistical analysis
Analyses were performed with Stata version 14 software (StataCorp LP). Mean 25(OH)D concentration was compared between baseline demographic and medication-use groups with the use of the Wilcoxon rank-sum or Kruskal-Wallis tests. Post hoc comparisons were done with the use of Dunn’s test, and Bonferroni’s correction was used to adjust for multiple comparisons. Categorical 25(OH)D concentrations (<10, ≥10 to <20, ≥20 to <30, and ≥30 ng/mL) were compared across groups with the use of Fisher’s exact test. The nonparametric trend test was used to test for the trend in vitamin D concentrations across ordinal categories in the Mayo endoscopy score (0–3), the total Mayo score (0–12), and histologic inflammation (normal, quiescent, mild, moderate, and severe) (29). Univariate and multivariate logistic regression models were used to evaluate the effect of 25(OH)D concentration as a continuous variable or supplemental vitamin D dose on binary outcomes (disease activity).
PCR analysis was performed with the use of the 2−ΔΔCT method after normalizing each sample to β-actin (30). Normalization between PCR plates was performed by comparing each sample with 5 normal control samples that were analyzed for each gene on every PCR plate. A 2-group comparison was made between low (<25 ng/mL) and high (>25 ng/mL) 25(OH)D concentrations with the use of Student’s t test. For immunohistochemistry, ordinal intensity scores were compared between subjects with low and high serum 25(OH)D concentrations with the use of the Wilcoxon rank-sum test for VDR, E-cadherin, and ZO-1. The chi-square test was used to compare weak- and strong-intensity staining for occludin and claudin-2. For both qPCR and immunohistochemistry, low serum 25(OH)D was defined as <20 ng/mL and high serum 25(OH)D as >25 ng/mL. The cutoff of 25 ng/mL was selected because of the limited number of patients available for analysis with active inflammation and 25(OH)D concentrations >30 ng/mL.
RESULTS
Baseline demographics
In total, 248 patients consented for the study, and 230 with UC were included in the analysis after removing 18 patients who had a change in disease diagnosis after the procedure or insufficient serum available for the 25(OH)D analysis (Supplemental Figure 2). The mean age of the subjects was 45.8 y, and the mean disease duration was 16.6 y. Women accounted for 47% of the total enrollment; men accounted for 53%. Ninety percent of the subjects were Caucasian, 5% were African American, and 4% were Asian or of Mideast/Indian descent. Three percent of the patients identified their ethnicity as Hispanic or Latino. Fifty-four percent of the subjects were never smokers, 37% were former smokers, and 8% were current smokers. Eighteen percent of the subjects had a family history of IBD in a first-degree relative, and 6% had a co-diagnosis of primary sclerosing cholangitis. Sixty-seven percent of patients had pancolitis and 33% had left-sided disease. Seventy-one percent of patients were taking oral 5-aminosalcylates (5-ASAs), 20% were taking rectal 5-ASAs, 25% were taking a thiopurine analogue, 17% were receiving anti-TNF-α treatment, and 15% were taking corticosteroids at the time of enrollment (Table 1).
TABLE 1.
Mean 25(OH)D concentrations by baseline subject characteristics and medication usage1
| Patients, n (%) | 25(OH)D concentration, ng/mL | P value | |
| All | 230 (100) | 21.8 ± 7.5 | |
| Sex | NS | ||
| Women | 107 (46.5) | 22.3 ± 7.4 | |
| Men | 123 (53.5) | 21.4 ± 7.6 | |
| Race | 0.003 | ||
| African American | 12 (5.2) | 15.9 ± 6.3 | |
| Caucasian | 208 (90.4) | 22.4 ± 7.4 | |
| Asian | 10 (4.3) | 17.2 ± 6.7 | |
| Ethnicity | 0.04 | ||
| Hispanic | 6 (2.6) | 15.8 ± 6.9 | |
| Not Hispanic | 224 (97.4) | 22.0 ± 7.5 | |
| Smoking history | NS | ||
| Never | 125 (54.3) | 22.1 ± 7.1 | |
| Former | 86 (37.4) | 21.8 ± 7.8 | |
| Current | 19 (8.3) | 19.6 ± 8.4 | |
| Family history of IBD | NS | ||
| No | 189 (82.2) | 21.7 ± 7.6 | |
| Yes | 41 (17.8) | 22.6 ± 6.8 | |
| Primary sclerosing cholangitis | NS | ||
| No | 216 (93.9) | 21.7 ± 7.5 | |
| Yes | 14 (6.1) | 23.4 ± 7.3 | |
| Disease extent | NS | ||
| Pancolitis | 152 (66.1) | 21.7 ± 6.7 | |
| Left-sided colitis | 60 (26.1) | 21.6 ± 8.5 | |
| Proctosigmoiditis | 18 (7.8) | 23.9 ± 9.6 | |
| BMI, kg/m2 | NS | ||
| <18.5 | 4 (1.8) | 21.3 ± 6.7 | |
| 18.5–25 | 92 (40.9) | 22.0 ± 8.1 | |
| >25–30 | 80 (35.6) | 22.0 ± 7.3 | |
| >30 | 49 (21.8) | 20.9 ± 7.2 | |
| Season of enrollment | NS | ||
| December–February | 62 (27.0) | 20.4 ± 6.8 | |
| March–May | 63 (27.4) | 21.2 ± 8.7 | |
| June–August | 47 (20.4) | 23.1 ± 7.3 | |
| September–November | 58 (25.2) | 22.9 ± 6.7 | |
| Any IBD medication | 0.09 | ||
| No | 16 (7.0) | 18.4 ± 7.3 | |
| Yes | 214 (93.0) | 22.1 ± 7.5 | |
| Oral 5-ASA | 0.03 | ||
| No | 67 (29.1) | 20.2 ± 7.6 | |
| Yes | 163 (70.9) | 22.5 ± 7.4 | |
| Rectal 5-ASA | 0.04 | ||
| No | 184 (80.0) | 21.2 ± 7.0 | |
| Yes | 46 (20.0) | 24.1 ± 8.9 | |
| Corticosteroids | NS | ||
| No | 195 (84.8) | 21.9 ± 7.6 | |
| Yes | 35 (15.2) | 21.5 ± 6.8 | |
| Thiopurine | 0.1 | ||
| No | 171 (74.3) | 21.4 ± 7.6 | |
| Yes | 59 (25.7) | 23.1 ± 7.0 | |
| Anti-TNF-α | NS | ||
| No | 191 (83) | 22.1 ± 7.6 | |
| Yes | 39 (17.0) | 20.3 ± 6.7 | |
| Supplemental vitamin D (any dose) | <0.001 | ||
| No | 98 (42.6) | 18.7 ± 6.6 | |
| Yes | 132 (57.4) | 24.8 ± 7.3 | |
| Supplemental vitamin D dose | <0.001 | ||
| None | 98 (49.0) | 18.7 ± 6.6 | |
| <2000 IU/d | 82 (41.0) | 23.6 ± 6.7 | |
| ≥2000 IU/d | 20 (10.0) | 27.3 ± 9.8 |
Mean ± SD 25(OH)D concentrations were compared, and P values were calculated with the use of the Kruskal-Wallis test. Five subjects did not have a height recorded at the time of enrollment to calculate BMI. Thirty subjects reported taking vitamin D supplementation daily, although their dose could not be confirmed. These subjects are reported as taking vitamin D in the analysis of patients taking any dose of supplemental vitamin D dose but are not included in the comparison of 25(OH)D concentration by vitamin D dose. IBD, inflammatory bowel disease; 25(OH)D, 25-hydroxyvitamin D; 5-ASA, 5-aminosalcylate.
The mean serum 25(OH)D concentration in the cohort was 21.8 ng/mL (median: 21.5; range: 5.1–49.5). As shown in Table 1, Caucasians (P = 0.003), non-Hispanics (P = 0.04), and those who were taking oral (P = 0.03) and rectal 5-ASAs (P = 0.04) had significantly higher mean serum 25(OH)D concentrations. In a post hoc analysis, Caucasians had significantly higher 25(OH)D concentrations than subjects who identified as African American (P = 0.006) and demonstrated a trend toward significantly higher 25(OH)D concentrations than those who identified as Asian (P = 0.07). There were no differences in 25(OH)D concentrations between Asian and African American subjects. Subjects taking any dose of supplemental vitamin D had higher serum 25(OH)D concentrations than those not taking vitamin D supplementation (P < 0.001). Furthermore, those taking doses ≥2000 IU/d were noted to have higher serum 25(OH)D concentrations than those taking doses <2000 IU/d or receiving no vitamin D supplementation (P < 0.001). When examining the association of categorical 25(OH)D concentrations (<10, >10–20, >20–30, and >30 ng/mL) with subject baseline variables, only Caucasians (P = 0.02), those taking any dose of vitamin D supplementation compared with no supplementation (P < 0.001), and differences in supplemental vitamin D dose (P < 0.001) demonstrated a significant association with higher 25(OH)D concentrations (Supplemental Table 4).
Serum 25(OH)D concentrations are associated with disease activity in UC
To evaluate the association between disease activity and serum vitamin D, mean serum 25(OH)D concentrations were compared to disease activity as defined by the Mayo endoscopy score, the total Mayo score, and histologic disease activity grade. There was a significant inverse association between 25(OH)D concentrations and mucosal inflammation as characterized by the Mayo endoscopy score (P = 0.01), disease activity as indicated by the total Mayo score (P = 0.001), and histologic activity (P = 0.02) (Table 2).
TABLE 2.
Comparison of mean serum 25(OH)D concentrations by endoscopic, clinical, and histologic disease activity1
| n | Vitamin D concentration, ng/mL | P value | |
| Mayo endoscopy score | 0.01 | ||
| 0 | 107 | 22.3 ± 7.5 | |
| 1 | 59 | 23.0 ± 7.5 | |
| 2 | 38 | 21.1 ± 7.2 | |
| 3 | 26 | 17.9 ± 6.9 | |
| Total Mayo score | 0.001 | ||
| 0–2 | 149 | 22.6 ± 7.3 | |
| 3–5 | 33 | 22.4 ± 8.4 | |
| 6–9 | 29 | 21.0 ± 6.8 | |
| 10–12 | 19 | 15.8 ± 5.7 | |
| Histologic score | 0.02 | ||
| Normal | 15 | 20.3 ± 6.2 | |
| Quiescent | 96 | 23.3 ± 7.4 | |
| Mild | 55 | 22.0 ± 7.7 | |
| Moderate | 40 | 20.6 ± 7.8 | |
| Severe | 24 | 18.1 ± 6.3 |
Mean ± SD serum 25(OH)D concentrations were compared with ordinal values in the Mayo endoscopy score, total Mayo score, and histologic score. P values were calculated with the use of the nonparametric trend test and represent the trend in serum 25(OH)D concentrations across ordinal values in the represented scoring index. 25(OH)D, 25-hydroxyvitamin D.
In patients with no histologic inflammation (histologic normalization and quiescent disease), mean serum 25(OH)D concentrations were significantly higher than in subjects with histologic inflammation (mild, moderate, or severe activity) (22.9 compared with 20.7 ng/mL; P = 0.04). Likewise, subjects with no or mild histologic activity had significantly higher 25(OH)D concentrations than those with moderate or severe histologic activity (22.6 compared with 19.7 ng/mL; P = 0.01). After controlling for sex, race, smoking status, 5-ASA use, and season of enrollment, higher 25(OH)D concentrations were associated with a decrease in disease activity in a logistic regression analysis. For each 1-ng/mL increase in serum 25(OH)D concentration, the OR of having a Mayo endoscopy score >1 was 0.95 (95% CI: 0.90, 0.99; P = 0.03), a total Mayo score >5 was 0.93 (95% CI: 0.88, 0.98; P = 0.009), and moderate or severe histologic activity was 0.95 (95% CI: 0.91, 1.00; P = 0.05) (Table 3). No association was observed, however, in a logistic regression analysis comparing subjects taking supplemental vitamin D doses <2000 and ≥2000 IU/d to those not taking supplemental vitamin D on these outcomes of disease activity (Supplemental Table 5).
TABLE 3.
Logistic regression analysis comparing serum 25(OH)D to endoscopic, clinical, and histologic disease activity1
| OR (95% CI) | P value | |
| Mean endoscopy score (>1) | 0.95 (0.90, 0.99) | 0.03 |
| Total Mayo score (>5) | 0.93 (0.88, 0.98) | 0.009 |
| Histology (moderate/severe) | 0.95 (0.91, 1.00) | 0.05 |
Serum 25(OH)D concentration was evaluated as a continuous variable in a logistic regression analysis compared with a Mayo endoscopy score >1, a total Mayo score >5, and moderate or severe histologic inflammation. ORs and significance controlled for sex, race, smoking status, 5-aminosalcylate use, and season. 25(OH)D, 25-hydroxyvitamin D.
Higher serum 25(OH)D concentrations are associated with increased colonic VDR
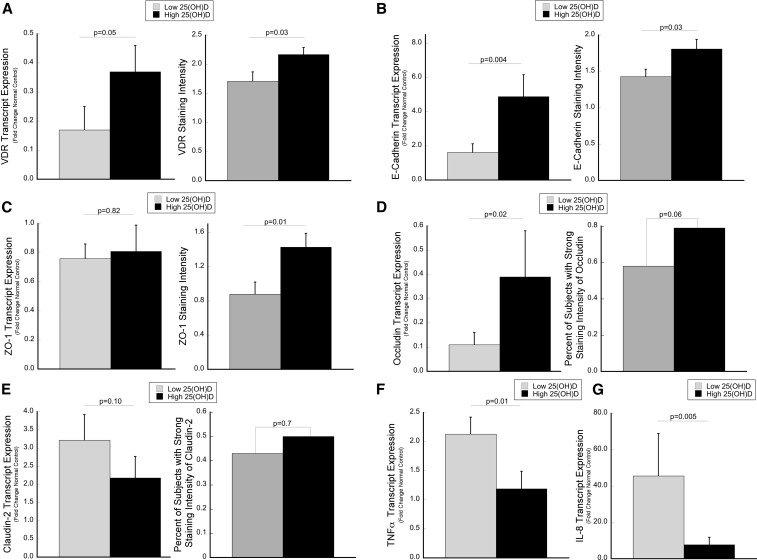
To explore the relation between serum 25(OH)D and colonic mucosal VDR, receptor expression was assessed by qPCR and immunohistochemistry. Among all UC patients, subjects with high serum 25(OH)D (>25 ng/mL) had a 2.1-fold increase in colonic mucosal VDR transcript expression compared with UC patients with a low serum 25(OH)D concentration (<20 ng/mL) (P = 0.05). Similarly, patients with high serum 25(OH)D concentrations had an increase in VDR staining intensity in the surface epithelium compared with subjects with low serum 25(OH)D concentrations (P = 0.03) (Figure 1A, Supplemental Figure 1A).
FIGURE 1.
Tissue expression levels of VDR and epithelial junction proteins are increased in association with serum 25(OH)D concentrations, whereas proinflammatory cytokine levels are decreased. Transcript expression by qPCR and protein expression by immunohistochemistry were compared between subjects with low (<20 ng/mL) and high (>25 ng/mL) 25(OH)D concentrations for (A) VDR, (B) E-cadherin, (C) ZO-1, (D) occludin, and (E) claudin-2. (F and G) Transcript abundance of proinflammatory cytokines is shown. The median 25(OH)D concentration within each group was 13.2 (IQR: 10.3–15.8) and 31.9 ng/mL (IQR: 28.7–35.8), respectively, for the transcript analysis and 12.4 (IQR: 10.2–14.6) and 32.8 ng/mL (IQR: 28.3–35.7), respectively, for the protein analysis. For qPCR, a 2-group comparison was made between low and high 25(OH)D concentrations with the use of Student’s t test after each sample was normalized to β-actin with the use of the 2−ΔΔCT method (30). Gene expression was analyzed in 60 subjects, 45 of whom had active inflammation. Protein expression of VDR, E-cadherin, and ZO-1 are presented as the mean staining intensity score, with P values calculated by the Wilcoxon rank-sum test (error bars represent SEMs). Occludin and claudin-2 were scored as strong compared with weak, and P values were calculated with the use of the chi-square test. Protein expression was analyzed in 74 subjects, 54 of whom had active inflammation. qPCR, quantitative polymerase chain reaction; VDR, vitamin D receptor; ZO-1, zonula occluden 1; 25(OH)D, 25-hydroxyvitamin D.
Elevated serum 25(OH)D is associated with increased colonic mucosal E-cadherin, occludin, and ZO-1
Subjects with high serum 25(OH)D concentrations had on average a 3.0-fold increased expression of E-cadherin compared with subjects with a serum 25(OH)D concentration <20 ng/mL (P = 0.004). In concordance with transcript changes, high serum 25(OH)D concentrations were associated with increased staining intensity of E-cadherin in the colonic epithelium (P = 0.03) (Figure 1B, Supplemental Figure 1B).
No association was observed between colonic mucosal transcript expression of ZO-1 and serum 25(OH)D concentrations. In contrast, the staining intensity of ZO-1 was significantly increased in association with elevated 25(OH)D concentrations (P = 0.01) (Figure 1C, Supplemental Figure 1C).
There was a direct relation between occludin transcript expression and serum 25(OH)D concentrations (3.4-fold; P = 0.02). Supporting the qPCR findings, 79% of subjects with serum 25(OH)D concentrations >25 ng/mL had strong occludin staining compared with 57% of subjects with 25(OH)D concentrations <20 ng/mL (P = 0.057) (Figure 1D, Supplemental Figure 1D).
Subjects with high serum 25(OH)D concentrations demonstrated a trend toward a statistically significant decrease in tissue claudin-2 transcript expression compared with subjects with low serum 25(OH)D concentrations (1.5-fold; P = 0.1). However, no differences in the staining intensity of claudin-2 by serum 25(OH)D concentrations were observed (Figure 1E, Supplemental Figure 1E).
Elevated serum vitamin D is associated with a decrease in proinflammatory cytokines, TNF-α, and IL-8
There was a significant inverse association between serum 25(OH)D concentrations and TNF-α, with a 1.8-fold decrease in expression in subjects with high serum 25(OH)D concentrations compared with those with concentrations <20 ng/mL (P = 0.01) (Figure 1F). Similarly, subjects with high serum 25(OH)D had a 6.0-fold decrease in IL-8 expression (P = 0.005) (Figure 1G).
DISCUSSION
Previous studies have reported a high incidence of vitamin D deficiency in patients with IBD (9–11, 31). In Crohn disease, several groups have demonstrated an association between vitamin D and clinical disease activity (12–14). Although to our knowledge there are no data comparing vitamin D to endoscopic or histologic inflammation in patients with Crohn disease, 2 recent analyses reported an inverse correlation between fecal calprotectin and serum 25(OH)D concentrations (25, 26). Similarly, retrospective data indicate that low serum 25(OH)D concentrations are associated with an increase in clinical disease activity, as well as an increase in the risk of operative procedures and hospitalizations in patients with UC (12, 32). Although there is a lack of previous data comparing 25(OH)D concentrations to mucosal disease activity in patients with UC, an inverse correlation between 25(OHD) and fecal calprotectin concentrations was noted in a recent study that included 31 patients (26).
In this prospectively collected cohort of 230 patients, we demonstrated that serum 25(OH)D concentrations have an inverse relation with disease activity as measured by endoscopic and histologic inflammation in patients with ulcerative colitis. To our knowledge, this is the largest prospective study to evaluate serum 25(OH)D concentrations in patients with UC and the first to compare 25(OH)D with endoscopic and histologic disease activity. For this analysis, we used the DiaSorin radioimmunoassay kit. Although HPLC followed by ultraviolet detection is considered the gold standard in determining circulating 25(OH)D concentration, the assay is time-consuming and expensive. Previous work has demonstrated a strong correlation between these 2 methods, and the DiaSorin radioimmunoassay kit is commonly used clinically in many large laboratories around the world (33, 34).
It should be noted that there is considerable debate over what concentration constitutes vitamin D deficiency and sufficiency. Although rickets and osteomalacia are more commonly observed in patients with 25(OH)D concentrations <10 ng/mL, most investigators advocate for maintaining 25(OH)D concentrations ≥20 ng/mL (35, 36). An Institute of Medicine panel concluded in 2010 that a serum concentration of 20 ng/mL is sufficient for most of the population to maintain bone health (37). The Endocrine Society also recommends defining vitamin D deficiency as <20 ng/mL and suggests that concentrations between 21 and 29 ng/mL constitute vitamin D insufficiency (38). In concordance, both the National Geriatric Society and International Osteoporosis Foundation recommend maintaining serum 25(OH)D >30 ng/mL, a concentration at which parathyroid hormone concentrations begin to plateau in relation to 25(OH)D (39–41). Although these categorical recommendations for defining vitamin D deficiency and sufficiency are mainly based on investigations focused on bone metabolism, most studies to our knowledge that have examined patients with IBD have used a similar definition of a 25(OH)D concentration <20 ng/mL to represent vitamin D deficiency (12, 14, 31, 42–44).
In our cohort of patients, the mean vitamin D concentration was 21.8 ng/mL, with only 13% of the population having concentrations >30 ng/mL. Because normal controls were not included in the analysis, we could not draw conclusions regarding the prevalence of vitamin D deficiency in UC compared with controls from the same center. However, the prevalence of subjects with a 25(OH)D concentration <20 ng/mL reported in our study is greater than that described in many previous studies that have examined patients with UC (10, 26, 43). Although not reported in these previous investigations, the percentage of patients with a serum 25(OH)D concentration <10 ng/mL was relatively small in our cohort compared with those who had concentrations between 10 and 20 ng/mL. Because of the limited number of patients with 25(OH)D concentrations <10 ng/mL, however, the study was not powered to assess differences in disease activity between these 2 groups of patients.
In addition to demonstrating an inverse association between 25(OH)D concentrations and disease activity, a second key conclusion of this study is the finding that serum 25(OH)D concentrations had a direct association with expression of colonic mucosal VDR and several tight junction proteins, as well as an inverse association with proinflammatory cytokines. Taken together with our results demonstrating the association between serum 25(OH)D concentrations and disease activity, these conclusions support data from in vitro cell culture studies and in vivo animal models that the vitamin D-VDR axis exerts dual roles in protecting against colitis by maintaining epithelial barrier function and decreasing mucosal inflammation (22, 23). 1,25(OH)2D3 upregulates VDR by promoting gene transcription and stabilizing the VDR protein (45–47). Previous work has demonstrated that the VDR-retinoid X receptor heterodimer binds at the VDR gene itself, and VDR transcripts have been reported to increase with vitamin D3 treatment in lymphoblastoid cells (48). Consistent with the critical role of VDR in maintaining mucosal homeostasis, we also showed that mucosal inflammation suppresses concentrations of epithelial VDR via a microRNA-mediated downregulation (49). Although 1,25(OH)2D3 has been shown to increase E-cadherin, occludin, and ZO-1, specific VDR-regulated cis-acting elements in the promoters of these genes have not been identified, suggesting these vitamin D effects may be indirect.
In addition to inducing these genes by indirect mechanisms, vitamin D suppression of apoptosis may also play a role. Specifically, our group has demonstrated that, as a protective mechanism against mucosal inflammation, vitamin D signaling inhibits gut epithelial cell apoptosis by downregulating nuclear transcription factor κB (NF-κB), which in turn downregulates the p53 modulator of apoptosis, a key positive regulator of gut epithelial cell apoptosis (23). As such, we hypothesize that this inhibition of apoptosis contributes to preserving epithelial tight junction proteins. In support of this hypothesis, higher 25(OH)D concentrations were associated with increased tissue E-cadherin, occludin, and ZO-1. Although we have shown previously that 1,25(OH)2D3 blocks claudin-2 upregulation in colonic epithelial cells, we surprisingly did not detect downregulation of claudin-2 in association with elevated serum 25(OH)D concentrations in this study (50). Because NF-κβ also upregulates the expression of TNF-α and IL-8, the 1,25(OH)2D3 blockade of NF-κβ activation through the interaction between liganded VDR and Iκβ kinase may also contribute to a decrease in expression of these proinflammatory cytokines (51–53).
We have identified an association between 25(OH)D concentrations and mucosal inflammation, and our data suggest that low vitamin D contributes to the pathogenesis of IBD. We should note, however, that this study has several limitations. With our observational study design, we could not establish the causative role of low serum 25(OH)D in mucosal inflammation in UC. As such, low serum 25(OH)D concentrations may be secondary to more severe disease activity. Moreover, patients in this study receive care in a tertiary referral center in the northern United States and are more likely to have treatment refractory disease with additional comorbidities than the general population with UC, which may limit the applicability of these findings to other populations. In addition, we were limited in comparing tissue expression concentrations of VDR and epithelial junction proteins between subjects with serum 25(OH)D concentrations >25 and <20 ng/mL, which has debatable clinical significance. A concentration of >25 ng/mL was chosen to represent “high” serum 25(OH)D for these comparisons because of the limited number of patients with active inflammation who would traditionally be considered vitamin D sufficient (>30 ng/mL). It is possible that greater differences in the expression of VDR as well as epithelial junction proteins by serum 25(OH)D concentration would be seen if a more standardized cutoff of >30 ng/mL was used. It is worth noting, however, that the median serum 25(OH)D concentrations for patients classified as <20 and >25 ng/mL and used to compare transcript expression was 13.2 (IQR: 10.3–15.8) and 31.9 ng/mL (IQR: 28.7–35.8), respectively, and those used for immunohistochemistry values were 12.4 (IQR: 10.2–14.6) and 32.8 ng/mL (IQR: 28.3–35.7), respectively. Although we demonstrated a relation between serum 25(OH)D concentrations and transcript as well as protein expression for epithelial junction proteins, a final limitation of this study is that we did not directly assess epithelial barrier function. As such, future prospective vitamin D intervention studies should examine the effects of this secosteroid on colonic mucosal permeability in conjunction with measurements of tight junction proteins and proinflammatory cytokines.
In conclusion, patients with UC have a high prevalence of 25(OH)D concentrations <20 ng/mL, and a decrease in serum 25(OH)D concentration is associated with increased disease activity. Given the known association between vitamin D deficiency with impaired calcium homeostasis, bone metabolism, and immune function, these findings support the routine monitoring of 25(OH)D concentrations and treatment of vitamin D deficiency in patients with UC, including those with inactive disease. We also demonstrated that serum 25(OH)D concentrations correlate with the tissue expression of epithelial junction proteins and inversely with proinflammatory cytokines. These results extend conclusions from previous in vitro cell culture experiments and in vivo animal model studies that indicated a protective role for vitamin D on inflammation and epithelial barrier function in patients with UC and lend support to further examining the efficacy of vitamin D supplementation on clinical outcomes in an intervention trial in this population.
Acknowledgments
The authors’ responsibilities were as follows—KM, YCL, JK, EY, MB, and JP: designed the research; KM, JL, CW, AA, XC, AK, FS, SBH, RDC, JK, DTR, IH, AS, and JP: conducted the research; KM, YCL, MK, MB, and JP: analyzed the data; JP: wrote the manuscript and had primary responsibility for the final content; KM, YCL, SBH, RDC, JK, DTR, IH, AS, EY, and MB: provided critical review of the manuscript; and all authors: read and approved the final version of the manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: IBD, inflammatory bowel disease; NF-κB, nuclear transcription factor κB; PCR, polymerase chain reaction; qPCR, quantitative polymerase chain reaction; UC, ulcerative colitis; VDR, vitamin D receptor; ZO-1, zonula occluden 1; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 25(OH)D, 25-hydroxyvitamin D; 5-ASA, 5-aminosalcylate.
REFERENCES
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002;347:417–29. [DOI] [PubMed] [Google Scholar]
- 2.Sellu DP. Seasonal variation in onset of exacerbations of ulcerative proctocolitis. J R Coll Surg Edinb 1986;31:158–60. [PubMed] [Google Scholar]
- 3.Moum B, Aadland E, Ekbom A, Vatn MH. Seasonal variations in the onset of ulcerative colitis. Gut 1996;38:376–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nerich V, Monnet E, Etienne A, Louafi S, Ramee C, Rican S, Weill A, Vallier N, Vanbockstael V, Auleley GR, et al. . Geographical variations of inflammatory bowel disease in France: a study based on national health insurance data. Inflamm Bowel Dis 2006;12:218–26. [DOI] [PubMed] [Google Scholar]
- 5.Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, van Blankenstein M. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut 1996;39:690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogelsang H, Ferenci P, Woloszczuk W, Resch H, Herold C, Frotz S, Gangl A. Bone disease in vitamin D-deficient patients with Crohn’s disease. Dig Dis Sci 1989;34:1094–9. [DOI] [PubMed] [Google Scholar]
- 7.Driscoll RH Jr, Meredith SC, Sitrin M, Rosenberg IH. Vitamin D deficiency and bone disease in patients with Crohn’s disease. Gastroenterology 1982;83:1252–8. [PubMed] [Google Scholar]
- 8.Harries AD, Brown R, Heatley RV, Williams LA, Woodhead S, Rhodes J. Vitamin D status in Crohn’s disease: association with nutrition and disease activity. Gut 1985;26:1197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahnsen J, Falch JA, Mowinckel P, Aadland E. Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scand J Gastroenterol 2002;37:192–9. [DOI] [PubMed] [Google Scholar]
- 10.Pappa HM, Langereis EJ, Grand RJ, Gordon CM. Prevalence and risk factors for hypovitaminosis D in young patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2011;53:361–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu YT, Chatur N, Cheong-Lee C, Salh B. Hypovitaminosis D in adults with inflammatory bowel disease: potential role of ethnicity. Dig Dis Sci 2012;57:2144–8. [DOI] [PubMed] [Google Scholar]
- 12.Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, Issa M. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr 2011;35:308–16. [DOI] [PubMed] [Google Scholar]
- 13.Ham M, Longhi MS, Lahiff C, Cheifetz A, Robson S, Moss AC. Vitamin D levels in adults with Crohn’s disease are responsive to disease activity and treatment. Inflamm Bowel Dis 2014;20:856–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raffner Basson A, Swart R, Jordaan E, Mazinu M, Watermeyer G, Vitamin D. Deficiency increases the risk for moderate to severe disease activity in Crohn’s disease patients in South Africa, measured by the Harvey Bradshaw Index. J Am Coll Nutr 2016;35:163–74. [DOI] [PubMed] [Google Scholar]
- 15.Jørgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn’s disease—a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther 2010;32:377–83. [DOI] [PubMed] [Google Scholar]
- 16.May E, Asadullah K, Zugel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy 2004;3:377–93. [DOI] [PubMed] [Google Scholar]
- 17.Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, Jurutka PW. Molecular mechanisms of vitamin D action. Calcif Tissue Int 2013;92:77–98. [DOI] [PubMed] [Google Scholar]
- 18.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr 2000;130:2648–52. [DOI] [PubMed] [Google Scholar]
- 19.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol 2003;17:2386–92. [DOI] [PubMed] [Google Scholar]
- 20.Martinesi M, Treves C, d’Albasio G, Bagnoli S, Bonanomi AG, Stio M. Vitamin D derivatives induce apoptosis and downregulate ICAM-1 levels in peripheral blood mononuclear cells of inflammatory bowel disease patients. Inflamm Bowel Dis 2008;14:597–604. [DOI] [PubMed] [Google Scholar]
- 21.Ardizzone S, Cassinotti A, Trabattoni D, Manzionna G, Rainone V, Bevilacqua M, Massari A, Manes G, Maconi G, Clerici M, et al. . Immunomodulatory effects of 1,25-dihydroxyvitamin D3 on TH1/TH2 cytokines in inflammatory bowel disease: an in vitro study. Int J Immunopathol Pharmacol 2009;22:63–71. [DOI] [PubMed] [Google Scholar]
- 22.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 2008;294:G208–16. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Chen Y, Golan MA, Annunziata ML, Du J, Dougherty U, Kong J, Musch M, Huang Y, Pekow J, et al. . Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest 2013;123:3983–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du J, Chen Y, Shi Y, Liu T, Cao Y, Tang Y, Ge X, Nie H, Zheng C, Li YC. 1,25-Dihydroxyvitamin D protects intestinal epithelial barrier by regulating the myosin light chain kinase signaling pathway. Inflamm Bowel Dis 2015;21:2495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raftery T, Merrick M, Healy M, Mahmud N, O’Morain C, Smith S, McNamara D, O’Sullivan M, Vitamin D status is associated with intestinal inflammation as measured by fecal calprotectin in Crohn’s disease in clinical remission. Dig Dis Sci 2015;60:2427–35. [DOI] [PubMed] [Google Scholar]
- 26.Garg M, Rosella O, Lubel JS, Gibson PR. Association of circulating vitamin D concentrations with intestinal but not systemic inflammation in inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2634–43. [DOI] [PubMed] [Google Scholar]
- 27.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- 28.Hollis BW. Comparison of commercially available (125)I-based RIA methods for the determination of circulating 25-hydroxyvitamin D. Clin Chem 2000;46:1657–61. [PubMed] [Google Scholar]
- 29.Cuzick J. A Wilcoxon-type test for trend. Stat Med 1985;4:87–90. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 31.Leslie WD, Miller N, Rogala L, Bernstein CN. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Am J Gastroenterol 2008;103:1451–9. [DOI] [PubMed] [Google Scholar]
- 32.Ananthakrishnan AN, Cagan A, Gainer VS, Cai T, Cheng SC, Savova G, Chen P, Szolovits P, Xia Z, De Jager PL, et al. . Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn’s disease. Inflamm Bowel Dis 2013;19:1921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollis BW, Napoli JL. Improved radioimmunoassay for vitamin D and its use in assessing vitamin D status. Clin Chem 1985;31:1815–9. [PubMed] [Google Scholar]
- 34.Hollis BW. Measuring 25-hydroxyvitamin D in a clinical environment: challenges and needs. Am J Clin Nutr 2008;88:507S–10S. [DOI] [PubMed] [Google Scholar]
- 35.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999;69:842–56. [DOI] [PubMed] [Google Scholar]
- 36.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 37.Institue of Medicine. Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academic Press; 2010. [Google Scholar]
- 38.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 39.American Geriatrics Society Workgroup on Vitamin DSfOA. Recommendations abstracted from the American Geriatrics Society consensus statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc 2014;62:147–52. [DOI] [PubMed] [Google Scholar]
- 40.Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, Josse RG, Lips P, Morales-Torres J, Yoshimura N. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int 2010;21:1151–4. [DOI] [PubMed] [Google Scholar]
- 41.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 1997;7:439–43. [DOI] [PubMed] [Google Scholar]
- 42.Torki M, Gholamrezaei A, Mirbagher L, Danesh M, Kheiri S, Emami MH, Vitamin D. Deficiency associated with disease activity in patients with inflammatory bowel diseases. Dig Dis Sci 2015;60:3085–91. [DOI] [PubMed] [Google Scholar]
- 43.Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, Richter JM, Fuchs CS, Chan AT. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology 2012;142:482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ananthakrishnan AN, Cheng SC, Cai T, Cagan A, Gainer VS, Szolovits P, Shaw SY, Churchill S, Karlson EW, Murphy SN, et al. . Association between reduced plasma 25-hydroxy vitamin D and increased risk of cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014;12:821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiese RJ, Uhland-Smith A, Ross TK, Prahl JM, DeLuca HF. Up-regulation of the vitamin D receptor in response to 1,25-dihydroxyvitamin D3 results from ligand-induced stabilization. J Biol Chem 1992;267:20082–6. [PubMed] [Google Scholar]
- 46.Miyamoto K, Kesterson RA, Yamamoto H, Taketani Y, Nishiwaki E, Tatsumi S, Inoue Y, Morita K, Takeda E, Pike JW. Structural organization of the human vitamin D receptor chromosomal gene and its promoter. Mol Endocrinol 1997;11:1165–79. [DOI] [PubMed] [Google Scholar]
- 47.Kim S, Yamazaki M, Zella LA, Shevde NK, Pike JW. Activation of receptor activator of NF-kappaB ligand gene expression by 1,25-dihydroxyvitamin D3 is mediated through multiple long-range enhancers. Mol Cell Biol 2006;26:6469–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, Handunnetthi L, Handel AE, Disanto G, Orton SM, et al. . A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res 2010;20:1352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Du J, Zhang Z, Liu T, Shi Y, Ge X, Li YC. MicroRNA-346 mediates tumor necrosis factor alpha-induced downregulation of gut epithelial vitamin D receptor in inflammatory bowel diseases. Inflamm Bowel Dis 2014;20:1910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du J, Chen Y, Shi Y, Liu T, Cao Y, Tang Y, Ge X, Nie H, Zheng C, Li YC. 1,25-Dihydroxyvitamin D protects intestinal epithelial barrier by regulating the myosin light chain kinase signaling pathway. Inflamm Bowel Dis 2015;21:2495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raskatov JA, Meier JL, Puckett JW, Yang F, Ramakrishnan P, Dervan PB. Modulation of NF-kappaB-dependent gene transcription using programmable DNA minor groove binders. Proc Natl Acad Sci USA 2012;109:1023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol 1990;10:1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC. Vitamin D receptor inhibits nuclear factor kappaB activation by interacting with IkappaB kinase beta protein. J Biol Chem 2013;288:19450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]