Abstract
Background
Uremic pruritus is a common, but unpleasant, complication of end-stage renal disease. The uremic burden may differ between hemodialysis (HD) and peritoneal dialysis (PD) patients. This difference may also change the clinical characteristics of uremic pruritus between the 2 modalities. In this study, we investigated the uremic pruritus between patients on HD and PD.
Methods
A total of 425 HD and 223 PD patients from the Clinical Research Center registry in Korea were included. Patients were assessed for pruritus intensity, scratching activity, pruritus distribution, and frequency of pruritus-related sleep disturbance using the visual analog scale and questionnaire.
Results
The prevalence of uremic pruritus was higher in PD patients than that in HD patients (62.6% vs. 48.3%, P = 0.001). In the multivariable logistic analysis, PD treatment was significantly associated with the prevalence of uremic pruritus (odds ratio, 1.76; 95% confidence interval, 1.20–2.57, P = 0.004) after adjustment for clinical variables. The visual analog scale score, representing a subjective intensity of itchiness, was significantly higher in PD patients (PD 2.11 ± 2.32 vs. HD 1.65 ± 2.28, P = 0.013) compared with HD patients. The intensity of uremic pruritus was independently related with serum albumin levels (β = –0.143, P = 0.006) in HD patients and total weekly Kt/V (β = –0.176, P = 0.028) in PD patients.
Conclusion
Our data demonstrate the difference in prevalence, intensity, and risk factors of uremic pruritus between HD and PD patients. These findings suggest that careful consideration for uremic pruritus might be needed in end-stage renal disease patients according to the dialysis modality.
Keywords: Hemodialysis, Peritoneal dialysis, Uremic pruritus
Introduction
Uremic pruritus is a common and disabling complication that affects the quality of life in end-stage renal disease (ESRD) patients undergoing hemodialysis (HD) and peritoneal dialysis (PD) [1], [2], [3], [4], [5]. The prevalence of uremic pruritus has been reported from 22% to 84% in HD patients [2], [3], [4], [5], [6], [7], [8], [9].
The degrees of intensity and the spatial distribution of uremic pruritus are influenced by multiple factors and vary over time [3], [6]. Although its pathogenesis is not well understood, factors such as uremic burden (i.e., increased inflammation), secondary hyperparathyroidism, iron-deficiency anemia, neuropathy, and neurophysiological factors or allergic sensitization may contribute to the development of uremic pruritus [2], [3], [4], [5], [6], [7], [8], [9]. Severe uremic pruritus negatively affects the quality of life and is associated with a poor outcome in HD patients [3].
The uremic burden may differ between HD and PD patients [10], which may make a difference in clinical characteristics of uremic pruritus between patients on HD and PD. Therefore, it may be postulated that clinical characteristics of uremic pruritus may be different between patients on HD and PD. A previous study reported a higher prevalence of uremic pruritus in PD patients than in HD patients [11]. However, the study is limited by a relatively small sample size.
In this study, we determined the differences in the prevalence and the clinical characteristics of uremic pruritus in patients with uremic pruritus undergoing PD and HD from the Clinical Research Center registry for ESRD, a multicenter cohort study in Korea.
Methods
Study population
Patients were selected from the Clinical Research Center registry for ESRD, which is a multicenter, observational, prospective cohort study on patients with ESRD in Korea. Adult patients (aged > 18 years) with ESRD undergoing PD or HD were included from 31 medical centers in Korea. The study was performed between April 2009 and April 2015. Only patients who had completed a questionnaire about uremic pruritus were included. A total of 648 patients from 9 medical centers were included in the final analysis. Of these, 425 patients were undergoing HD and 223 were undergoing PD. Demographic and clinical data were collected at enrollment. The study protocol was approved by the medical ethics committees of all participating hospitals. Written informed consent was obtained from all patients before inclusion.
Pruritus assessment
A survey was used to measure uremic pruritus by 2 scoring systems. A detailed scoring system modified by Pauli-Magnus [12] was used to assess the characteristics of pruritus including intensity, scratching activity, pruritus distribution, and the frequency of pruritus-related sleep disturbances. The visual analog scale (VAS) was used to assess the subjective intensity of itchiness. A survey was done by trained investigators. These parameters were graded as follows:
-
1.
Pruritus scoring system modified by Pauli-Magnus
Severity: A slight itchy sensation without the need to scratch received 1 point. The necessity to scratch, but in the absence of excoriations received 2 points. Scratching accompanied by excoriation received 4 points. Finally, pruritus causing total restlessness received 5 points.
Distribution: Itching at fewer than 2 locations received 1 point, at 2 locations 2 points, and generalized itching 3 points. The scores for pruritus severity and distribution were recorded and multiplied separately based on those from the morning and afternoon. A maximum of 30 points could be achieved.
Sleep disturbance: Each arousal from sleep due to itching received 2 points (maximum 10 points). Every nighttime scratching episode that led to excoriations received 1 point (maximum 5 points). The final score was obtained by adding the sleep disturbance score and the severity–distribution product. There was a maximum of 45 points.
-
2.
Visual Analog Scale
In addition to the pruritus scoring system modified by Pauli-Magnus, we assessed uremic pruritus using the VAS. The VAS has been previously used to assess itching intensity in clinical trials [13], [14]. Patients were asked to grade their itching intensity on a 10-cm VAS (0 = no pruritus to 10 = unbearable pruritus). Patients without uremic pruritus were defined by a score of 0.
Data collection
The following baseline demographic and clinical data were recorded: age, sex, height, weight, body mass index (BMI), causes of ESRD, comorbidities, systolic blood pressure (BP), diastolic BP, laboratory investigations, and therapeutic characteristics. Blood samples were drawn to measure serum hemoglobin, albumin, creatinine, blood urea nitrogen, potassium, total cholesterol, calcium, phosphorous, high-sensitivity C-reactive protein, intact parathyroid hormone, and β2-microglobulin.
Statistical analysis
Continuous variables with normal distributions are expressed as means ± standard deviations. Those without normal distributions are presented as medians and interquartile ranges. Student t tests were used to compare continuous variables. Categorical variables are presented as numbers with percentages. The Pearson chi-square test was used to compare the categorical variables. Univariate and multivariate logistic regression analyses were used to assess the clinical factors associated with uremic pruritus in HD patients. Multivariate logistic regression analysis was adjusted for significant or nearly significant (P < 0.05) predictors of uremic pruritus in univariate logistic regression analysis including BMI and serum albumin levels. To achieve adequate confounder control, important covariates known to be influential based on prior studies and clinical insight were retained in the multivariate logistic regression model, regardless of their statistical significance. These covariates included age, sex, and diabetes mellitus (DM).
Survival curves were estimated by the Kaplan–Meier method and compared by the log-rank test according to the presence of uremic pruritus. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Baseline characteristics of the study population according to dialysis modality are shown in Table 1. PD patients were older than HD patients. PD patients had higher BMI, diastolic BP, serum levels of creatinine, phosphorus, calcium–phosphorus product, intact parathyroid hormone, β2-microglobulin, and ferritin than HD patients. There was a lower prevalence of DM in the PD patients than in the HD patients. In addition, the systolic BP, serum albumin levels, and serum high-sensitivity C-reactive protein levels were lower in the PD patients than those in the HD patients. There were no significant differences in sex, duration of dialysis therapy, fasting blood glucose, hemoglobin, blood urea nitrogen, serum calcium, total cholesterol levels, or use of medications such as calcium-containing or non–calcium-containing phosphate binders, active vitamin D compounds, and calcimimetics between the HD and PD patients.
Table 1.
Baseline characteristics of patients
| Characteristics | Hemodialysis (n = 425) | Peritoneal dialysis (n = 223) | P |
|---|---|---|---|
| Age (y) | 58.0 ± 12.0 | 53.0 ± 11.0 | < 0.001 |
| Male | 237 (55.8) | 127 (59.2) | 0.402 |
| Body mass index (kg/m2) | 22.6 ± 3.5 | 23.9 ± 3.4 | < 0.001 |
| Diabetes mellitus | 215 (55.0) | 91 (41.2) | 0.001 |
| Duration of dialysis therapy (mo) | 50 (29–83) | 55 (34–86) | 0.377 |
| Systolic BP (mmHg) | 144.0 ± 21.0 | 133.0 ± 22.0 | < 0.001 |
| Diastolic BP (mmHg) | 75.0 ± 14.0 | 80.0 ± 12.0 | < 0.001 |
| Fasting blood glucose (mg/dL) | 146.0 ± 72.0 | 135.0 ± 75.0 | 0.082 |
| BUN (mg/dL) | 66.0 ± 24.0 | 63.0 ± 24.0 | 0.169 |
| Serum creatinine (mg/dL) | 9.0 ± 2.9 | 10.2 ± 3.7 | < 0.001 |
| Hemoglobin (g/dL) | 10.3 ± 1.4 | 10.2 ± 1.5 | 0.373 |
| Serum albumin (g/dL) | 3.7 ± 0.5 | 3.6 ± 0.4 | < 0.001 |
| Serum calcium (mg/dL) | 8.6 ± 1.0 | 8.5 ± 0.9 | 0.326 |
| Serum phosphorus (mg/dL) | 5.0 ± 1.0 | 5.3 ± 1.5 | 0.016 |
| Serum calcium–phosphorus product (mg2/dL2) | 42.7 ± 13.6 | 45.3 ± 13.6 | 0.027 |
| Serum total cholesterol (mg/dL) | 141.0 ± 65.0 | 150.0 ± 75.0 | 0.191 |
| Serum intact PTH (pg/mL) | 147 (65–273) | 222 (110–412) | < 0.001 |
| β2-microglobulin (mg/dL) | 248 (135–414) | 287 (137–569) | 0.043 |
| Serum ferritin (mg/dL) | 62 (45–78) | 67 (52–93) | 0.010 |
| hsCRP (mg/dL) | 0.13 (0.00–1.57) | 0 (0–0.02) | < 0.001 |
| Using high-flux dialysis | 116 (27.3) | ||
| Single-pool Kt/V | 1.6 ± 0.5 | ||
| Total weekly Kt/V | 3.1 ± 1.3 | ||
| Medication | |||
| Calcitriol or vitamin D analogues | 100 (24) | 53 (24) | 0.946 |
| Calcium-containing phosphorus binder | 249 (59) | 116 (52) | 0.109 |
| Non–calcium-containing phosphorus binders | 40 (9) | 16 (7) | 0.336 |
Values for continuous variables are given as mean ± SD and variables without a normal distribution are given as median and interquartile range; values for categorical variables are given as n (%).
BP, blood pressure; BUN, blood urea nitrogen; hsCRP, high-sensitivity C-reactive protein; PTH, parathyroid hormone.
Prevalence and characteristics of uremic pruritus
Table 2 shows the prevalence and characteristics of uremic pruritus according to the dialysis modality. There was a higher prevalence of uremic pruritus throughout the day, and it was higher in the PD patients than in the HD patients (62.6% vs. 48.3%, P = 0.001). Uremic pruritus was more prevalent in PD patients than in HD patients in the morning (62.2% vs. 47.0%, P = 0.002) and afternoon (57.7% vs. 44.3%, P < 0.001).
Table 2.
Prevalence and characteristics of uremic pruritus by dialysis modality
| Characteristics | Hemodialysis (n = 425) | Peritoneal dialysis (n = 223) | P |
|---|---|---|---|
| Prevalence of pruritus (%) | |||
| Morning | 44.3 | 57.7 | 0.002 |
| Afternoon | 47.0 | 62.2 | < 0.001 |
| Throughout the day | 48.3 | 62.6 | 0.001 |
| Characteristics of uremic pruritus | |||
| Detailed score (Pauli-Magnus scoring system) | |||
| Severity | |||
| Morning | 1.32 ± 0.82 | 1.29 ± 0.77 | 0.654 |
| Afternoon | 1.46 ± 0.96 | 1.50 ± 1.02 | 0.288 |
| Distribution | |||
| Morning | 1.17 ± 0.61 | 1.19 ± 0.65 | 0.790 |
| Afternoon | 1.17 ± 0.62 | 1.23 ± 0.68 | 0.272 |
| Sleep disturbance | |||
| Frequency of waking from sleep | 0.24 ± 0.91 | 0.23 ± 0.74 | 0.925 |
| Frequency of scratching during sleep | 0.19 ± 0.96 | 0.12 ± 0.56 | 0.332 |
| Total score by measuring system | 4.54 ± 6.35 | 4.52 ± 5.35 | 0.356 |
| VAS scoring system | |||
| Morning | 1.40 ± 2.10 | 1.73 ± 2.06 | 0.057 |
| Afternoon | 1.90 ± 2.66 | 2.51 ± 2.73 | 0.006 |
| Average | 1.65 ± 2.28 | 2.11 ± 2.32 | 0.013 |
Continuous variables are presented by means ± SD.
VAS, visual analog scale.
We also assessed the clinical characteristics of uremic pruritus according to the dialysis modality. There were no significant differences in pruritus intensity, scratching activity, pruritus distribution, or frequency of pruritus-related sleep disturbances between PD and HD patients.
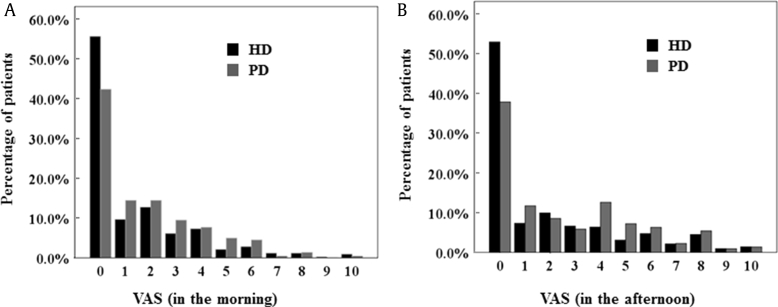
The VAS score was significantly higher in PD patients than it was in HD patients, especially in the afternoon (average, PD 2.11 ± 2.32 vs. HD 1.65 ± 2.28, P = 0.013; afternoon, PD 2.51 ± 2.73 vs. HD 1.90 ± 2.66, P = 0.006; Table 2). Fig. 1 shows the distribution of patients with each range of VAS scores in the patients with HD and PD. There are few PD and HD patients with severe uremic pruritus (VAS score > 7) in the morning. PD patients with moderate-to-severe uremic pruritus (VAS score ≥ 4) were more common in the afternoon compared with HD patients (Fig. 1B).
Figure 1.
Distribution of patients in each range of VAS scores in HD and PD patients. VAS in the morning (A) and VAS in the afternoon (B) are shown. HD, hemodialysis; PD, peritoneal dialysis; VAS, visual analog scale.
Determinants of prevalence and intensity of uremic pruritus
First, we evaluated the clinical parameters to predict the prevalence of uremic pruritus. Table 3 shows the clinical and laboratory risk factors that influence the prevalence of uremic pruritus in the entire patient cohort. In the univariate logistic analysis, PD treatment [odds ratio (OR), 1.79; 95% confidence interval (CI), 1.28–2.49; P = 0.001] and BMI (OR, 1.07; 95% CI, 1.02–1.12; P = 0.006) significantly influenced the prevalence of uremic pruritus. In multivariable logistic analysis, PD treatment and BMI were significant independent risk factors for the prevalence of uremic pruritus (OR, 1.76; 95% CI, 1.20–2.57; P = 0.004 and OR, 1.06; 95% CI, 1.01–1.12; P = 0.017, respectively) after adjustment for age, sex, BMI, dialysis modality, DM, and serum albumin level.
Table 3.
Logistic regression analysis for predicting prevalence of uremic pruritus
| Risk factors | Univariate |
Multivariate |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| PD (vs. HD) | 1.79 (1.28–2.49) | 0.001 | 1.76 (1.20–2.57) | 0.004 |
| Age (per 10 y) | 1.01 (0.99–1.02) | 0.429 | 1.01 (0.99–1.02) | 0.412 |
| Sex (male vs. female) | 1.05 (0.77–1.44) | 0.746 | 1.00 (0.71–1.41) | 0.989 |
| Body mass index (per 1 kg/m2) | 1.07 (1.02–1.12) | 0.006 | 1.06 (1.01–1.12) | 0.017 |
| DM (vs. non-DM) | 1.11 (0.81–1.53) | 0.512 | 1.07 (0.76–1.52) | 0.695 |
| Duration of dialysis (per 1 mo) | ||||
| Systolic BP (per 10 mmHg) | 1.01 (1.00–1.01) | 0.211 | ||
| Fasting blood glucose (mg/dL) | 1.00 (1.00–1.00) | 0.221 | ||
| HbA1c in patients with DM (per 1%) | 1.03 (0.99–1.08) | 0.155 | ||
| BUN (per 1 mg/dL) | 1.00 (0.99–1.01) | 0.607 | ||
| Serum creatinine (per 1 mg/dL) | ||||
| Hemoglobin (per 1 g/dL) | 1.06 (0.96–1.19) | 0.263 | ||
| Serum albumin (every 1 mg/dL) | 0.69 (0.48–1.00) | 0.050 | 0.77 (0.52–1.14) | 0.190 |
| Serum calcium (per 1 mg/dL) | 0.98 (0.82–1.16) | 0.779 | ||
| Serum phosphorus (per 1 mg/dL) | 1.02 (0.92–1.13) | 0.708 | ||
| Serum calcium–phosphorus product (per 1 mg2/dL2) | 1.00 (0.99–1.01) | 0.862 | ||
| Serum alkaline phosphatase (per 1 mg/dL) | ||||
| Total cholesterol (per 1 mg/dL) | 1.00 (1.00–1.01) | 0.236 | ||
| iPTH (per 100 pg/mL) | 1.00 (0.98–1.03) | 0.769 | ||
| β2-microglobulin (per 1 mg/dL) | 1.00 (1.00–1.00) | 0.448 | ||
| Serum ferritin (per 1 mg/dL) | 1.00 (0.99–1.01) | 0.917 | ||
| hsCRP (mg/dL) | 1.00 (0.99–1.01) | 0.795 | ||
| spKt/V < 1.2 in HD patients (vs. spKt/V ≥ 1.2) | 1.07 (0.41–2.76) | 0.892 | ||
| Total weekly Kt/V < 1.7 in PD patients (vs. total weekly Kt/V ≥ 1.7) | 1.89 (0.44–8.16) | 0.396 | ||
| Use of calcitriol or vitamin D analogues (vs. nonuser) | 1.17 (0.81–1.68) | 0.401 | ||
| Use of calcium-containing phosphorus binder (vs. nonuser) | 1.11 (0.81–1.52) | 0.508 | ||
| Use of non–calcium-containing phosphorus binders (vs. nonuser) | 1.10 (0.63–1.90) | 0.741 | ||
BP, blood pressure; BUN, blood urea nitrogen; CI, confidence interval; DM, diabetes mellitus; HbA1c, hemoglobin A1c; HD, hemodialysis; hsCRP, high-sensitivity C-reactive protein; iPTH, intact parathyroid hormone; PD, peritoneal dialysis; spKt/V, single-pool Kt/V.
Next, we determined the clinical parameters associated with the intensity of uremic pruritus according to dialysis modality. Table 4 shows the Spearman correlation between the intensity of uremic pruritus and clinical parameters in HD and PD patients. The intensity of uremic pruritus was negatively correlated with serum albumin levels in HD patients. In PD patients, the intensity of uremic pruritus was negatively correlated with total weekly Kt/V and positively correlated with duration of dialysis, systolic BP, and serum total cholesterol levels.
Table 4.
Spearman's correlation between the intensity of uremic pruritus using VAS and clinical parameters in HD and PD patients
| Clinical parameters | HD |
PD |
||
|---|---|---|---|---|
| ρ | P | ρ | P | |
| Age (per 10 y) | 0.032 | 0.506 | 0.096 | 0.156 |
| Duration of dialysis (per 1 mo) | –0.023 | 0.632 | 0.139 | 0.040 |
| Body mass index (per 1 kg/m2) | 0.070 | 0.172 | 0.063 | 0.356 |
| Systolic BP (per 10 mmHg) | 0.070 | 0.170 | 0.137 | 0.047 |
| Fasting blood glucose (mg/dL) | –0.015 | 0.763 | –0.043 | 0.523 |
| HbA1c in patients with DM (per 1%) | 0.074 | 0.172 | –0.072 | 0.287 |
| BUN (per 1 mg/dL) | 0.004 | 0.931 | –0.022 | 0.753 |
| Serum creatinine (per 1 mg/dL) | 0.030 | 0.557 | 0.088 | 0.198 |
| Hemoglobin (per 1 g/dL) | –0.032 | 0.525 | 0.106 | 0.116 |
| Serum albumin (every 1 mg/dL) | –0.156 | 0.002 | –0.022 | 0.754 |
| Serum calcium (per 1 mg/dL) | –0.081 | 0.111 | 0.096 | 0.173 |
| Serum phosphorus (per 1 mg/dL) | 0.022 | 0.665 | 0.014 | 0.841 |
| Total cholesterol (per 1 mg/dL) | –0.014 | 0.783 | 0.153 | 0.036 |
| iPTH (per 100 pg/mL) | 0.168 | 0.143 | 0.114 | 0.514 |
| β2-microglobulin (per 1 mg/dL) | 0.008 | 0.876 | –0.091 | 0.321 |
| Serum ferritin (per 1 mg/dL) | –0.011 | 0.837 | –0.096 | 0.247 |
| hsCRP (mg/dL) | 0.032 | 0.517 | –0.096 | 0.307 |
| spKt/V in HD patients | –0.048 | 0.376 | ||
| Total weekly Kt/V in PD patients | –0.206 | 0.002 | ||
BP, blood pressure; BUN, blood urea nitrogen; DM, diabetic mellitus; HbA1c; hemoglobin A1c; HD, hemodialysis; hsCRP, high-sensitivity C-reactive protein; PD, peritoneal dialysis; PTH, intact parathyroid hormone; spKt/V, single-pool Kt/V; VAS, visual analog scale.
To determine the independent predictor for the intensity of uremic pruritus, we performed multiple linear regression analysis using the model including age, sex, DM, and all univariate correlates of the intensity of uremic pruritus (Table 5). In HD patients, serum albumin levels (β = –0.143, P = 0.006) maintained an independent relationship with the intensity of uremic pruritus, whereas in PD patients, total weekly Kt/V (β = –0.176, P = 0.028) was independently associated with the subjective intensity of uremic pruritus.
Table 5.
Multivariate linear regression analyses for intensity of uremic pruritus using VAS in HD and PD patients
| Clinical parameters | HD |
PD |
||
|---|---|---|---|---|
| β coefficient | P | β coefficient | P | |
| Age (per 10 y) | –0.062 | 0.244 | 0.108 | 0.172 |
| Sex (male) | –0.003 | 0.950 | 0.086 | 0.274 |
| Body mass index (per 1 kg/m2) | 0.030 | 0.562 | 0.093 | 0.246 |
| DM | –0.097 | 0.071 | –0.041 | 0.620 |
| Duration of dialysis (per 1 mo) | 0.010 | 0.845 | –0.026 | 0.755 |
| Systolic BP (per 10 mmHg) | 0.005 | 0.924 | 0.093 | 0.231 |
| Fasting blood glucose, mg/dL | –0.102 | 0.069 | –0.039 | 0.666 |
| BUN (per 1 mg/dL) | 0.030 | 0.562 | 0.051 | 0.545 |
| Serum creatinine (per 1 mg/dL) | 0.014 | 0.785 | 0.219 | 0.026 |
| Hemoglobin (per 1 g/dL) | 0.044 | 0.440 | 0.102 | 0.205 |
| Serum albumin (every 1 mg/dL) | –0.143 | 0.006 | –0.064 | 0.443 |
| Serum calcium (per 1 mg/dL) | –0.006 | 0.921 | 0.056 | 0.523 |
| Serum phosphorus (per 1 mg/dL) | 0.037 | 0.482 | 0.120 | 0.163 |
| Total cholesterol (per 1 mg/dL) | 0.012 | 0.819 | 0.027 | 0.732 |
| iPTH (per 100 pg/mL) | 0.095 | 0.436 | –0.447 | 0.181 |
| β2-microglobulin (per 1 mg/dL) | –0.060 | 0.269 | 0.001 | 0.996 |
| Serum ferritin (per 1 mg/dL) | 0.017 | 0.758 | –0.046 | 0.629 |
| hsCRP (mg/dL) | –0.029 | 0.589 | 0.054 | 0.481 |
| spKt/V in HD patients | –0.021 | 0.701 | ||
| Total weekly Kt/V in PD patients | –0.176 | 0.028 | ||
Adjusted for age, sex, DM, systolic BP, and serum albumin levels in HD patients.
Adjusted for age, sex, DM, duration of dialysis, systolic BP, serum total cholesterol levels, and total weekly Kt/V in PD patients.
BP, blood pressure; BUN, blood urea nitrogen; DM, diabetic mellitus; HbA1c, hemoglobin A1c; HD, hemodialysis; hsCRP, high-sensitivity C-reactive protein; iPTH, intact parathyroid hormone; PD, peritoneal dialysis; spKt/V, single-pool Kt/V; VAS, visual analogue scale.
Uremic pruritus and all-cause mortality
To determine the clinical impact of uremic pruritus in HD and PD patients, we performed the survival analysis. We divided the patients into 4 groups according to the intensity of uremic pruritus by using VAS as follows: group 1, patients with no pruritus (n = 304); group 2, mild pruritus with VAS scores < 4.0 (n = 207); group 3, moderate pruritus with VAS score 4.0–6.9 (n = 106); and group 4, severe pruritus with VAS score ≥ 7 (n = 31). The Kaplan–Meier survival analysis showed that there was no significant difference in all-cause mortality among the groups in whole cohort including HD and PD patients (P = 0.249, log-rank test) as well as in HD patients (P = 0.055, log-rank test) and PD patients (P = 0.922, log-rank test).
Discussion
In this study, we showed that there was a higher prevalence of uremic pruritus in PD patients than there was in HD patients. Furthermore, the subjective intensity of itchiness (assessed by VAS) was higher in PD patients than it was in HD patients. These findings indicate that prevalence and clinical characteristics of uremic pruritus differ according to the dialysis modality.
The prevalence of uremic pruritus in HD patients has been previously reported. However, there are limited data regarding uremic pruritus in PD patients. In their study of 113 continuous ambulatory peritoneal dialysis patients and 76 HD patients, Balaskas et al [11] found that the prevalence of uremic pruritus was 62% in continuous ambulatory peritoneal dialysis and 54% in HD. In this study, the prevalence of uremic pruritus over the course of the whole day was 62.6% in PD patients and 48.3% in HD patients. The PD patients had approximately 1.8-fold increased risk of uremic pruritus than did the HD patients.
Another interesting finding of this study is the different relationship between the intensity of uremic pruritus and clinical parameters according to dialysis modality. In this study, the intensity of uremic pruritus was negatively associated with serum albumin levels in HD patients, which suggests the impact of the serum albumin as a negative acute-phase protein on uremic pruritus in HD patients [8]. Interestingly, in this study, single-pool Kt/V was not related with the intensity of uremic pruritus in HD patients, whereas total weekly Kt/V was independently associated with the intensity of uremic pruritus in PD patients. These findings are consistent with previous studies [4], [15]. Pisoni et al [15] reported that Kt/V did not show any significant relationship with pruritus in HD patients in Dialysis Outcomes and Practice Patterns Study. Liakopoulos et al [4] demonstrated the beneficial effect of increased dialyzate volume on uremic symptoms in chronic PD patients. These findings support the idea that adequate dialysis may be critically important in reducing the intensity of uremic pruritus in PD patients.
One strength of this study was its relatively large sample size and multicenter design, which enhances the evidence of prevalence of uremic pruritus.
Another strength of this study is that we assessed the uremic pruritus by using a pruritus scoring system modified by Pauli-Magnus [12] and the VAS. The detailed characteristics of pruritus including intensity, scratching activity, pruritus distribution, and the frequency of pruritus-related sleep disturbances were assessed by Pauli-Magnus scoring system. The subjective intensity of itchiness was assessed by VAS scoring system. In this study, scratching activity, pruritus distribution, and the frequency of pruritus-related sleep disturbances were not significantly different according to dialysis modality. Interestingly, the VAS score was significantly higher in PD patients than it was in HD patients, especially in the afternoon. This finding suggests that PD patients have greater subjective itch intensity than do HD patients (Table 2). However, the intensity measured by VAS was not severe in general, and the difference of VAS score between HD and PD patients was relatively small (HD: 1.65 ± 2.28 vs. PD: 2.11 ± 2.32). Therefore, clinical significance of these findings needed to be cautiously interpreted.
It is unclear why PD patients had a higher prevalence and subjective intensity of uremic pruritus than did HD patients. However, there are several potential explanations. Uremic pruritus has been associated with the accumulation of middle molecular weight uremic toxins such as β2-microglobulin [2], [9]. Dialysis clearance of β2-microglobulin with HD treatment using a high-flux membrane has been reported to be much higher than with PD treatment [16], [17], [18]. High-flux dialyzers are now widely used in clinical practice. The present study included approximately 30% of HD patients using the high-flux dialyzer. In concordance with this idea, PD patients had significantly higher serum levels of β2-microglobulin than did HD patients in this study. Therefore, it is possible that increased accumulation of uremic toxins in PD patients may contribute to the higher prevalence and intensity of uremic pruritus compared to that in HD patients.
For the association between uremic pruritus and clinical outcomes in ESRD patients, Narita et al [3] reported that severe uremic pruritus (VAS score > 7) is associated with clinical poor outcomes including mortality in chronic HD patients. However, in this study, the presence of uremic pruritus was not associated with all-cause mortality not only in HD patients but also in PD patients. It may be due to the differences in the study population. In this study, the percentage of patients with severe uremic pruritus was much lower (4.5% in HD patients and 5.4% in PD patients) than that of the previous study (25.6% in HD patients) [3], which may have resulted in the discrepancy between the studies. Despite the nonsignificant association between uremic pruritus and all-cause mortality in this study, uremic pruritus might be considered as an important risk factor for poor clinical outcomes in consideration of the relationship between uremic pruritus and quality of life or morality in ESRD patients from previous studies [3], [6].
Our study has several limitations. First, the design of our study was not a randomized controlled study but rather was an observational study. The choice of dialysis modality may have been influenced by clinicians and patients. Therefore, it is difficult to infer causality between dialysis modality and uremic pruritus. In addition, despite the study's multicenter nature, all patients were of Asian descent. Therefore, our results may not be generalizable to other ethnic groups with ESRD. Second, dry skin may be one of the major factors to exacerbate pruritus [19]. Unfortunately, it was not assessed in this study. Third, chronic inflammation had significant impact on uremic pruritus and mortality in dialysis patients [3]. To clarify the relationship between uremic pruritus and mortality, the patients with chronic inflammatory diseases may need to be excluded. However, they were not excluded at the time of enrollment in this study.
In conclusion, our data demonstrate the differences in prevalence, intensity, and risk factors of uremic pruritus between HD and PD patients. These findings suggest that careful consideration for uremic pruritus might be needed in ESRD patients according to the dialysis modality.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
The authors thank the study coordinators Hye Young Lim, Nam Hee Kim, Mi Joung Moon, Hwa Young Lee, Mi Joung Kwon, Su Yeon An, Su Joung Oh, and Hye Young Kwak for contribution to this study.
This work was supported by a grant of the Korea Healthcare Technology R&D Project (HI10C2020), Ministry of Health and Welfare, Korea (HI10C2020).
References
- 1.Mettang T., Kremer A.E. Uremic pruritus. Kidney Int. 2015;87:685–691. doi: 10.1038/ki.2013.454. [DOI] [PubMed] [Google Scholar]
- 2.Urbonas A., Schwartz R.A., Szepietowski J.C. Uremic pruritus–an update. Am J Nephrol. 2001;21:343–350. doi: 10.1159/000046272. [DOI] [PubMed] [Google Scholar]
- 3.Narita I., Alchi B., Omori K., Sato F., Ajiro J., Saga D., Kondo D., Skatsume M., Maruyama S., Kazama J.J., Akazawa K., Gejyo F. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69:1626–1632. doi: 10.1038/sj.ki.5000251. [DOI] [PubMed] [Google Scholar]
- 4.Liakopoulos V., Krishnan M., Stefanidis I., Savaj S., Ghareeb S., Musso C., Vas S., Bargman J.M., Jassal S.V., Oreopoulos D.G. Improvement in uremic symptoms after increasing daily dialysate volume in patients on chronic peritoneal dialysis with declining renal function. Int Urol Nephrol. 2004;36:437–443. doi: 10.1007/s11255-004-8788-9. [DOI] [PubMed] [Google Scholar]
- 5.Narita I., Iguchi S., Omori K., Gejyo F. Uremic pruritus in chronic hemodialysis patients. J Nephrol. 2008;21:161–165. [PubMed] [Google Scholar]
- 6.Mathur V.S., Lindberg J., Germain M., Block G., Tumlin J., Smith M., Grewal M., McGuire D. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1410–1419. doi: 10.2215/CJN.00100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duque M.I., Thevarajah S., Chan Y.H., Tuttle A.B., Freedman B.I., Yosipovitch G. Uremic pruritus is associated with higher kt/V and serum calcium concentration. Clin Nephrol. 2006;66:184–191. doi: 10.5414/cnp66184. [DOI] [PubMed] [Google Scholar]
- 8.Melo N.C., Elias R.M., Castro M.C., Romao J.E., Jr., Abensur H. Pruritus in hemodialysis patients: the problem remains. Hemodial Int. 2009;13:38–42. doi: 10.1111/j.1542-4758.2009.00346.x. [DOI] [PubMed] [Google Scholar]
- 9.Lin H.H., Liu Y.L., Liu J.H., Chou C.Y., Yang Y.F., Kuo H.L., Huang C.C. Uremic pruritus, cytokines, and polymethylmethacrylate artificial kidney. Artif Organs. 2008;32:468–472. doi: 10.1111/j.1525-1594.2008.00568.x. [DOI] [PubMed] [Google Scholar]
- 10.Capusa C., Stoian I., Rus E., Lixandru D., Barbulescu C., Mircescu G. Does dialysis modality influence the oxidative stress of uremic patients? Kidney Blood Press Res. 2012;35:220–225. doi: 10.1159/000331560. [DOI] [PubMed] [Google Scholar]
- 11.Balaskas E.V., Chu M., Uldall R.P., Gupta A., Oreopoulos D.G. Pruritus in continuous ambulatory peritoneal dialysis and hemodialysis patients. Perit Dial Int. 1993;13(Suppl 2):S527–S532. [PubMed] [Google Scholar]
- 12.Pauli-Magnus C., Mikus G., Alscher D.M., Kirschner T., Nagel W., Gugeler N., Risler T., Berger E.D., Kuhlmann U., Mettang T. Naltrexone does not relieve uremic pruritus: results of a randomized, double-blind, placebo-controlled crossover study. J Am Soc Nephrol. 2000;11:514–519. doi: 10.1681/ASN.V113514. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y.C., Chiu W.T., Wu M.S. Therapeutic effect of topical gamma-linolenic acid on refractory uremic pruritus. Am J Kidney Dis. 2006;48:69–76. doi: 10.1053/j.ajkd.2006.03.082. [DOI] [PubMed] [Google Scholar]
- 14.Ashmore S.D., Jones C.H., Newstead C.G., Daly M.J., Chrystyn H. Ondansetron therapy for uremic pruritus in hemodialysis patients. Am J Kidney Dis. 2000;35:827–831. doi: 10.1016/s0272-6386(00)70251-4. [DOI] [PubMed] [Google Scholar]
- 15.Pisoni R.L., Wikström B., Elder S.J., Akizawa T., Asano Y., Keen M.L., Saran R., Mendelssohn D.C., Young E.W., Port F.K. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2006;21:3495–3505. doi: 10.1093/ndt/gfl461. [DOI] [PubMed] [Google Scholar]
- 16.Evenepoel P., Bammens B., Verbeke K., Vanrenterghem Y. Superior dialytic clearance of beta(2)-microglobulin and p-cresol by high-flux hemodialysis as compared to peritoneal dialysis. Kidney Int. 2006;70:794–799. doi: 10.1038/sj.ki.5001640. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita A.C. A kinetic model for peritoneal dialysis and its application for complementary dialysis therapy. Contrib Nephrol. 2012;177:3–12. doi: 10.1159/000336927. [DOI] [PubMed] [Google Scholar]
- 18.Ko M.J., Wu H.Y., Chen H.Y., Chiu Y.L., Hsu S.P., Pai M.F., Ju Y., Lai C.F., Lu H.M., Huang S.C., Yang S.Y., Wen S.Y., Chiu H.C., Hu F.C., Peng Y.S., Jee S.H. Uremic pruritus, dialysis adequacy, and metabolic profiles in hemodialysis patients: a prospective 5-year cohort study. PLoS One. 2013;8:e71404. doi: 10.1371/journal.pone.0071404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zucker I., Yosipovitch G., David M., Gafter U., Boner G. Prevalence and characterization of uremic pruritus in patients undergoing hemodialysis: uremic pruritus is still a major problem for patients with end-stage renal disease. J Am Acad Dermatol. 2003;49:842–846. doi: 10.1016/s0190-9622(03)02478-2. [DOI] [PubMed] [Google Scholar]