Abstract
Diabetic nephropathy (DN) is the leading cause of end-stage renal disease, and its pathogenesis is complex and has not yet been fully elucidated. Abnormal glucose and lipid metabolism is key to understanding the pathogenesis of DN, which can develop in both type 1 and type 2 diabetes. A hallmark of this disease is the accumulation of glucose and lipids in renal cells, resulting in oxidative and endoplasmic reticulum stress, intracellular hypoxia, and inflammation, eventually leading to glomerulosclerosis and interstitial fibrosis. There is a growing body of evidence demonstrating that dysregulation of 5′ adenosine monophosphate–activated protein kinase (AMPK), an enzyme that plays a principal role in cell growth and cellular energy homeostasis, in relevant tissues is a key component of the development of metabolic syndrome and type 2 diabetes mellitus; thus, targeting this enzyme may ameliorate some pathologic features of this disease. AMPK regulates the coordination of anabolic processes, with its activation proven to improve glucose and lipid homeostasis in insulin-resistant animal models, as well as demonstrating mitochondrial biogenesis and antitumor activity. In this review, we discuss new findings regarding the role of AMPK in the pathogenesis of DN and offer suggestions for feasible clinical use and future studies of the role of AMPK activators in this disorder.
Keywords: 5′ Adenosine monophosphate–activated protein kinase, Cellular growth, Cellular metabolism, Diabetic nephropathy, Oxidative stress
Introduction
Diabetes, especially type 2 diabetes, is now a global epidemic that reflects the harmful consequences of modern lifestyles. Consumption of processed, high-calorie foods, along with physical inactivity, has created an imbalance between energy intake and expenditure [1], [2]. This disruption of energy balance is characterized by shifts in lipid and glucose metabolism manifesting as fasting and postprandial hyperglycemia together with dyslipidemia and is further promoted by insulin resistance in a number of tissues [3]. Chronic exposure to glucose overload, free fatty acids, and amino acids can be toxic [4]. Chronic kidney disease (CKD) associated with diabetes is a relatively common manifestation, with both the prevalence and incidence rising in recent years [5]. CKD is the end result of multifactorial processes that mostly stem from deranged glucose and lipid metabolism. This is demonstrated by the increased expression of proteins in relevant signaling pathways and consistent pathologic renal findings. Many studies and experiments have been devised to elucidate the pathogenesis of these metabolic disruptions, although emerging concepts related to such findings remain vast and vague. In an attempt to unravel the core of this disease entity, a growing body of evidence shows that dysregulation of 5′ adenosine monophosphate–activated protein kinase (AMPK) in relevant tissues is crucial to the development of metabolic syndrome and diabetes [6]. Therefore, targeting this enzyme is a matter of great interest, as it may ameliorate some of the pathologic features of the disease. AMPK regulates the coordination of anabolic processes with its activation proven to improve glucose and lipid homeostasis in insulin-resistant animal models [7], [8]. AMPK is strongly expressed in the kidney, where it is involved in diverse physiological and pathologic processes, including ion transport, podocyte function, and diabetic renal hypertrophy. In light of this, we have looked for novel agents that would help to modulate AMPK in an organ-specific manner that targets the diabetic kidney. Instead of providing an exhaustive review of the role of AMPK in these pathologies, this review aims to discuss the mechanisms of AMPK agonists in the context of diabetic nephropathy (DN) and, therefore, shed light on the risks and benefits of currently available and promising future AMPK activators as a treatment option.
AMPK: structure and mechanism of action
AMPK is a metabolic master switch that regulates downstream signals based on shifts in the surrounding energy reservoir [6]. It is expressed in a number of tissues, including the kidney, the liver, the skeletal muscle, the adipose tissue, and the hypothalamus of the brain [9], [10]. It is activated when adenosine triphosphate (ATP) consumption causes an increase in the adenosine monophosphate (AMP)-to-ATP ratio [6]. For example, under such conditions as hypoglycemia, exercise, hypoxia, and ischemia, in which accentuated cellular signaling cascade and intracellular stress arise, consumption of ATP is suppressed while production is spurred [11]. Regulation of AMPK activation can be achieved through either allosteric activation by AMP or stimulation by upstream kinases, including a compound consisting of 3 proteins: STE-related adaptor (STRAD), mouse protein 25, and the tumor-suppressor liver kinase B1 (LKB1) [12]. In addition, other enzymes, such as Ca++/calmodulin-dependent protein kinase kinase β (CaMKKβ) [13] and transforming growth factor (TGF) β-activated kinase [14], also participate in the cellular signaling cascade (Fig. 1). On activation, AMPK signals through its downstream substrates to achieve energy homeostasis by stimulating processes that generate ATP through such actions as fatty acid oxidation and glucose transport, while inhibiting those that use ATP through the opposing actions of fatty acid synthesis and protein synthesis [12]. Thus, the net effect of AMPK activation is an increased cellular energy level via the inhibition of anabolic energy-consuming pathways, as well as the stimulation of catabolic, energy-producing pathways [11].
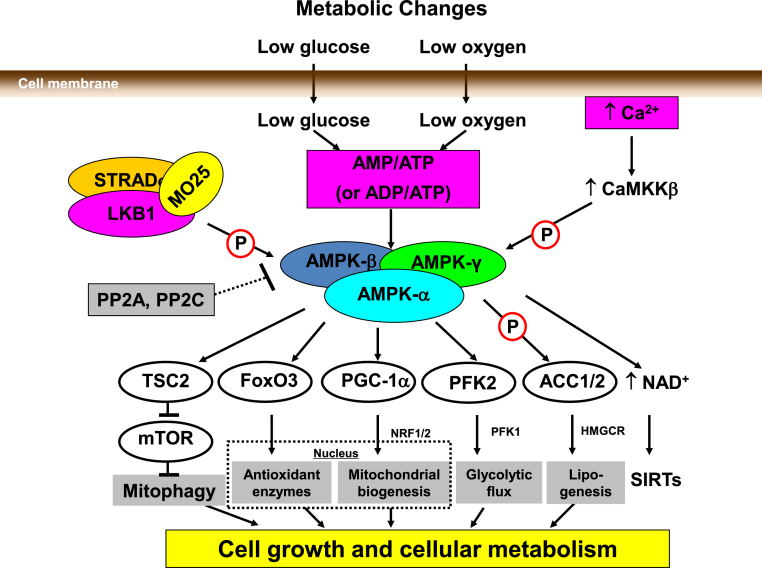
Figure 1.
Regulation of AMPK by metabolic stress, LKB1, and intracellular Ca++. The AMPK signaling pathway responds to energy stresses that cause fluctuations and imbalances in the AMP/ATP (or ADP/ATP) ratio. As the ATP level falls, there is an increase in the AMP level, which triggers the activation of the AMPK pathway. The upstream regulator of AMPK, LKB1, allows for AMP phosphorylation at a specific site (Thr-172) on the α subunit of AMPK. Two other subunits, STRAD and MO25, form a complex with LKB1 to enhance this process. CaMKK, which is induced by an increase in intracellular Ca++, is also able to activate AMPK. Of the CaMKK proteins, CaMKKβ has the strongest regulatory effect on AMPK. CaMKKβ regulates AMPK in response to changes in surrounding energy stresses.
ACC, acetyl-CoA carboxylase; ADP, adenosine diphosphate; AMP, adenosine monophosphate; AMPK, 5′ adenosine monophosphate–activated protein kinase; ATP, adenosine triphosphate; CaMKKβ, Ca++/calmodulin-dependent protein kinase kinase β; FoxO3, forkhead box O3; HMGCR, 3-hydroxy-3-metylglutaryl-CoA reductase; LKB1, liver kinase B-1; MO25, mouse protein 25; mTOR, mammalian target of rapamycin; NAD, nicotinamide adenine dinucleotide; NRF, nuclear respiratory factor; PGC-1α, peroxisome proliferator–activated receptor-gamma coactivator 1α; PFK2, phosphofructokinase 2; PP2A, protein phosphatase 2A; PP2C, protein phosphatase 2C; SIRTs, sirtuins; STRAD, STE-related adaptor; Thr-172, threonine-172; TSC2, tuberous sclerosis complex 2.
AMPK is a heterotrimer consisting of a catalytic α subunit (α1 and α2) and regulatory β (β1 and β2) and γ subunits (γ1, γ2, and γ3). Its isoforms are tissue specific [15]. The γ subunit includes 4 distinct cystathionine beta synthase domains that perceive shifts in the AMP-to-ATP ratio, conferring the ability to switch AMPK on and off [16]. The α subunit is a catalytic domain with its activating loop at threonine-172 (Thr-172) that switches on as it gets phosphorylated by upstream AMPK [17]. Ser485 of the subunit exerts its inhibitory action via phosphorylation by Akt or protein kinase A or by autophosphorylation [18]. “ST loop,” a serine/threonine–rich insert of C-terminal domains of the α subunit, appears to contain several regulatory phosphorylation sites, which block access to upstream kinases [16]. AMPK activation can be triggered when the following conditions are fulfilled. First, increased levels of AMP binding to the γ subunit incur conformational change that exposes the active site Thr-172 on the α subunit, making it a better substrate for upstream kinases. Second, phosphorylation of the activating loop of the α subunit by an upstream kinase is required. This combination of allosteric activation and phosphorylation of the stimulatory site leads to the accentuation of AMPK activity to levels greater than 1,000-fold [15], [17].
Activated AMPK then phosphorylates its main downstream targets, acetyl-CoA carboxylase (ACC) and hydroxymethylglutaryl CoA reductase (HMGCR), which are primarily involved in the rate-limiting steps of lipid homeostasis. Phosphorylation at serine 79 (Ser79) exerts inhibitory action by preventing the conversion of acetyl-CoA to malonyl-CoA, further encouraging the oxidation of fatty acids. Other downstream targets of AMPK include tuberous sclerosis complex protein-2, which is associated with cell growth and autophagy [19], and peroxisome proliferator–activated receptor-gamma coactivator-1α (PGC-1α), which hinders protein synthesis by inhibiting the mammalian target of rapamycin (mTOR) complex 1 [19], in turn inhibiting cholesterol synthesis and stimulating mitochondrial biogenesis (Fig. 1). Overall, these downstream targets of AMPK are expected to have favorable effects on DN by maintaining metabolic homeostasis.
The role of AMPK beyond energy homeostasis: regulating metabolism
AMPK plays a major role in glucose homeostasis by modulating glucose transport in peripheral tissues [20]. Skeletal muscle, one of the main peripheral tissues involved in glucose uptake and disposal, expresses glucose transporter type 4 (GLUT4). In hyperglycemia, insulin promotes the translocation of intracellular vesicular GLUT4 to the cell membrane, thereby increasing glucose uptake in the muscle [6]. AMPK activation contributes to glucose transport in a similar way as insulin. The result is AMPK-induced glucose uptake stimulation in skeletal cells, with increased expression of enzymes specialized in glucose uptake such as GLUT4 and hexokinase II [6]. Moreover, AMPK directly phosphorylates the GLUT4 enhancer factor that is essential in the regulation of GLUT4 expression. Hexokinase phosphorylates glucose entering the cell, allowing for a structural change that prevents glucose from leaving the cell in the first step of glycolysis. Overall, these sequential alterations in the expression of enzymes involved in glucose uptake are the ultimate result of AMPK activation, which stimulates catabolic processes that counter the deleterious effects of glucose excess and maintains energy homeostasis.
Hyperglycemia is a causative factor in the development of DN through its detrimental effects on glomerular and mesangial cells. Some in vitro studies have demonstrated its association with mesangial cell proliferation and hypertrophy, along with increased matrix production and basement membrane thickening [21]. Moreover, hyperglycemia-induced upregulation of vascular endothelial growth factor expression in podocytes increases vascular permeability [21]. In addition to these changes, classical pathways involving the production of advanced glycosylation end products, activation of protein kinase C, and reinforcement of the aldose reductase pathway contribute to the development of DN, in which oxidative stress appears to be a common theme [22], [23]. In this regard, targeting AMPK in DN could ameliorate these adverse effects by regulating glucose-induced oxidative stress metabolism [4]. There is growing evidence regarding the role of AMPK in mediating intracellular signaling pathways, that is, the alteration of cellular redox state and antioxidant enzyme expression via the AMPK transcriptional activity of class O forkhead box (FoxO) signaling pathway [23]. FoxO proteins are an evolutionary conserved subfamily of transcriptional factors involved in the regulation of energy metabolism. In detail, it increases the expression of antioxidant enzymes, promotes mitochondrial biogenesis, cell survival, and longevity in several tissues, and even participates in tumor suppression [22]. The transcriptional activity of FoxO3a, one of the 4 members of the family consisting of FoxO1, FoxO3, FoxO4, and FoxO6, is further modulated by AMPK in response to metabolic stress. Moreover, FoxO3a is known to shield quiescent cells from reactive oxygen species (ROS) by antagonizing apoptosis through which oxidative stress is reduced by directly increasing their quantities of manganese superoxide dismutase (SOD) messenger RNA and protein [21]. It is well known that the activity of autophatic process is closely related to changes in the production of ROS. Although mild oxidative stress-induced autophagic process is beneficial for cell survival, excessive oxidative damage caused by high ROS would bypass autophagy induction and evoke apoptosis or necrosis, leading to promote cell death [24]. Antioxidant enzymes, including thioredoxin, peroxiredoxin, Mn-SOD, and catalase, are found to be upregulated on activation of the AMPK–FoxO3a signaling pathway [25]. Therefore, on ROS exposure, AMPK, silent information regulator T1 (SIRT1), a well-known FoxO3a coactivator [26], and FoxO3a are expected to intertwine closely to regulate the apoptotic and autophagy crosstalk [22].
AMPK modulates changes in lipid metabolism via the regulation of fatty acid oxidation and cholesterol synthesis in the liver. The key enzymes involved include ACC and HMGCR [27], [28]. Both participate in the rate-limiting steps of fatty acids and cholesterol synthesis and are inactivated on phosphorylation by AMPK. Catabolism is induced by fatty acid beta-oxidation, leading to the production of acetyl-CoA, which then joins the citric acid cycle to generate ATP. On the other hand, fatty acid synthesis is a multistep, energy-consuming, anabolic process that generates fatty acids from acetyl-CoA and malonyl-CoA. Likewise, cholesterol is synthesized from acetyl-CoA to produce 3-hydroxy-3-methylglutaryl-coenzyme A (HMGCoA), which is then converted into mevalonate through an anabolic process that consumes ATP. Hence, in response to energy depletion, the inhibition of ACC by AMPK results in a fall in malonyl-CoA levels and subsequently interrupts fatty acid synthesis, facilitating the acceleration of fatty acid oxidation. Through the activation of malonyl-CoA decarboxylase, AMPK brings about a decrease in malonyl-CoA levels and an increase in fatty acid oxidation. Likewise, HMGCR is inactivated on phosphorylation, which leads to a reduction in cholesterol synthesis. In addition, the expression of such genes as fatty acid synthase, pyruvate kinase, ACC, and sterol regulatory element–binding protein-1 (SREBP-1) is lipogenesis associated and suppressed by AMPK in states of energy deficit [29].
Emerging evidence indicates that renal lipid dysregulation is a major causative factor in the development of CKD, along with DN [30]. Recently, it has been characterized by tubular rather than glomerular lipid accumulation, attributable to decreased lipid uptake, increased renal lipid synthesis, and defective fatty acid utilization in the renal physiology [19]. Systemic and intrarenal alterations in lipid metabolism are reflected by increased triglycerides and low-density lipoprotein levels, decreased high-density lipoprotein levels, increased expression of SREBP-1 and fatty acid synthase, and decreased expression of carnitine palmitoyltransferase I, which is involved in the rate-limiting step of fatty acid oxidation [31]. AMPK inhibits lipogenesis and enhances fatty acid oxidation through targets such as SREBP, ACC, fatty acid synthase, and 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGR) in various tissues. This raises the potential for AMPK activation as a therapeutic target in optimizing lipid metabolism in DN [32].
AMPK perceives shifts in the surrounding energy reservoir and adapts to the nutrient supply status by promoting a cellular response. It participates in protein synthesis by regulating protein translation. Its main targets are cytoplasmic substrates, such as eukaryotic elongation factor 2 and mTOR, which modulate the stimulation of cellular processes that aid cell growth in states of nutritional surplus [33]. On AMPK-dependent phosphorylation of these substrates, repression of protein synthesis ensues. It could be useful to coordinate these processes in DN as they correlate with diminished AMPK activity, resulting in increases in protein synthesis that can cause diabetes-induced renal hypertrophy in hyperglycemic conditions. Thus, specifically stimulating cytoplasmic AMPK could provide a more focused approach in alleviating diabetes-induced renal damage [34].
Managing DN by targeting the AMPK pathway
In parallel with a vast and rapidly growing body of knowledge on the favorable effects of AMPK, of which only a fragment has been discussed, investigators have devised several pharmaceutical agents in the search for naturally occurring compounds that could activate AMPK with minimal toxicity. We will introduce some conventional AMPK activators, along with our data on new therapeutic agents used to experimentally treat diabetic mouse models addressing DN (Figure 2, Figure 3).
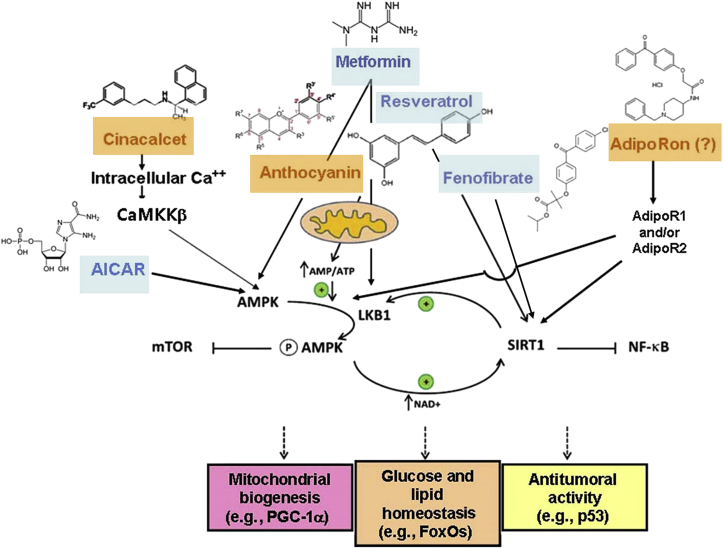
Figure 2.
Representative signaling in the AMPK pathway by various AMPK activators. Schematic diagram of selected physiological effects of AMPK activators on mitochondrial biogenesis, glucose and lipid metabolism, and antitumor activity.
AICAR, 5-aminoimidazole-4-carboxamide ribonucleoside; AMP, adenosine monophosphate; AMPK, 5′ adenosine monophosphate–activated protein kinase; ATP, adenosine triphosphate; CaMKKβ, Ca++/calmodulin-dependent protein kinase kinase β; FoxOs, forkhead transcription factors; LKB1, liver kinase B-1; mTOR, mammalian target of rapamycin; NAD, nicotinamide adenine dinucleotide; NF-κB, nuclear factor kappaB; PGC-1α, peroxisome proliferator–activated receptor-gamma coactivator 1α; SIRT1, silent information regulator T1.
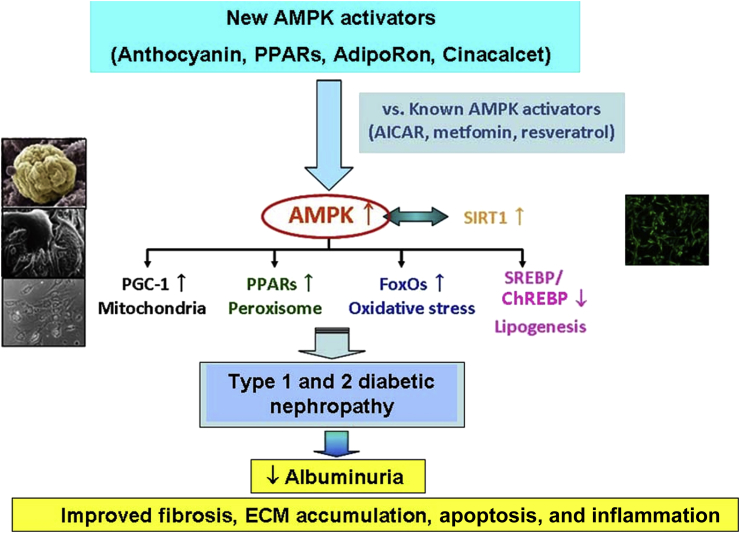
Figure 3.
Proposed renoprotective mechanisms in diabetic nephropathy by known and novel AMPK activators. Treatment with AMPK activators reduces albuminuria, glomerulosclerosis, and tubulointerstitial fibrosis in types 1 and 2 diabetic nephropathy by exerting effects on mitochondrial biogenesis, peroxisomal β-oxidation, oxidative stress, and lipogenesis through the activation of AMPK-SIRT1 signaling.
AICAR, 5-aminoimidazole-4-carboxamide ribonucleoside; AMPK, 5′ adenosine monophosphate–activated protein kinase; ChREBP, carbohydrate regulatory element–binding protein; ECM, extracellular matrix; FoxOs, forkhead transcription factors; PGC-1α, peroxisome proliferator--activated receptorγ coactivator 1α; PPARs, peroxisome proliferator–activated receptors; SIRT1, silent information regulator T1; SREBP, sterol regulatory element–binding protein.
Conventional AMPK activators
5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR) is an adenosine analogue that is frequently demonstrated in animal models as it recapitulates AMP in the course of being metabolized to phosphorylated AICA riboside or AICA ribotide [AICAR 5-monophosphate (ZMP)] (Fig. 2) [35]. ZMP binding to the γ subunit of AMPK directly activates AMPK via 3 mechanisms: accelerating phosphorylation, inhibiting dephosphorylation, and allosterical activation [35], [36]. AICAR upregulates genes associated with oxidative metabolism, angiogenesis, and glucose sparing, thereby improving glucose homeostasis in obese, insulin-resistant rats [37]. Moreover, a recent report on chronic AICAR treatment shows its efficacy in preventing unfavorable functional and morphologic changes in mice raised on high-fat diets, with regard to renal cholesterol and phospholipid accumulation and lysosomal system dysfunction; this demonstrates that AICAR in a viable form may be a promising agent in alleviating the deleterious outcomes of DN [38].
Metformin's pluripotent roles are alluded to in terms of its antitumorigenic, glucose-lowering, and cardioprotective effects, which are due in part to its upregulation of AMPK activity [39], [40]. Metformin's glucose-lowering effect is a result of reduced hepatic gluconeogenesis, whereas improved insulin sensitivity is mediated by the activation of the LKB1–AMPK axis [41]. It is suggested that metformin activates AMPK indirectly by inhibiting relevant cells from entering the mitochondrial respiratory chain, facilitating a switch from aerobic to anaerobic glycolysis in a manner that raises the cellular AMP-to-ATP ratio (Fig. 2) [42]. In addition, mounting evidence indicates that metformin-induced inactivation of mTOR by AMPK is associated with suppressed cell proliferation and increased autophagy, which are thought to lower cancer incidence [43]. Stimulation of AMPK activity with AICAR or metformin also resulted in inhibition of renal hypertrophy due in part to the prevention of glucose-induced protein synthesis. This was associated with decreases in the phosphorylation of Akt, mTOR, p70S6 kinase, and 4E-BP1 in renal cells, suggesting that AMPK regulates the initiation and elongation phases of translation [44].
Adiponectin, an adipose tissue-derived cytokine, is another AMPK activator. Decreases in the plasma level of adiponectin have been correlated with insulin resistance and obesity. In diabetes, reduced AMPK activity was noted to be associated with the renal accumulation of triglyceride and glycogen, which reflects the pathogenesis of diabetic renal hypertrophy [45]. Furthermore, adiponectin knockout mice exhibit increased albuminuria and fusion of the podocyte foot process [46]. In cultured podocytes, both adiponectin and AMPK activation reduced podocyte permeability to albumin, further ameliorating podocyte dysfunction. These effects are mostly due to the reduction of oxidative stress, with subsequent decreases in the level of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 in podocytes [46]. Another study showed that adiponectin receptor 1 and catalytic AMPK subunits α1 and α2 were localized to the glomeruli in endothelial, mesangial, podocyte, and parietal epithelial cells of Bowman capsule. The incubation of freshly isolated glomeruli with adiponectin led to the activation of AMPK via phosphorylation of its catalytic domain [47].
Resveratrol is a naturally occurring plant polyphenol that may target aging and obesity-related chronic diseases by preventing inflammation and oxidative stress [48]. It activates SIRT1, a nicotinamide adenine dinucleotide–dependent protein deacetylase, and AMPK (Fig. 2). Resveratrol's subsequent activation of downstream targets is expected to have some renoprotective effects involving changes in catabolic metabolism, mitochondrial activation, and angiogenesis, as well as enhanced cell survival [49], [50], [51]. Resveratrol's renoprotective effects with regard to oxidative stress and proinflammatory reactions are demonstrated in the attenuation of diabetes-induced superoxide anion, protein carbonyl levels, and cytokines interleukin-1β, tumor necrosis factor-α, and interleukin-6, which are associated with increased AMPK phosphorylation in diabetic renal tissues [52]. Moreover, in our previous experiment, db/db mice treated with resveratrol demonstrated improvements in not only renal functional parameters but also pathologic features. This manifestation was consistent with increases in the phosphorylation of AMPK, activation of SIRT1–PGC-1α signaling, and activation of the key downstream effector, PPARα–estrogen-related receptor (ERR)-1α–SREBP-1c. In addition, resveratrol reduced the activity of phosphatidylinositol-3 kinase-Akt phosphorylation and FoxO3a phosphorylation, which further caused a decrease in B-cell leukemia/lymphoma 2 (BCL-2)–associated X protein and increases in BCL-2, SOD1 and SOD2 production. In cultured mesangial cells, resveratrol attenuated renal apoptotic changes further, preventing high-glucose-induced oxidative stress and apoptosis through the phosphorylation of AMPK and activation of SIRT1--PGC-1α signaling and the downstream effector, PPARα--ERR-1α--SREBP1c. The results suggest that resveratrol prevents DN in db/db mice through the phosphorylation of AMPK and activation of SIRT1–PGC-1α signaling, which seem to prevent lipotoxicity-related apoptosis and oxidative stress in the kidney [8].
New AMPK activators
Fenofibrate is a drug of the fibrate class used for the treatment of dyslipidemia [53] but is also an agent that could alleviate DN-induced alterations through AMPK activation (Fig. 2). Streptozotocin-induced diabetic rats treated with fenofibrate improved in not only renal functional parameters but also hyperglycemia-induced oxidative damage, as indicated by significantly increased levels of glutathione and catalase together with a noticeable decrease in lipid peroxidation. Fenofibrate's protective role in DN is in ameliorating endothelial dysfunction via increased modulation of AMPK, LKB1, and endothelial nitric oxide synthase (eNOS) expression [54]. In parallel with this, our previous experiments on the diabetic mouse model have demonstrated that fenofibrate can ameliorate albuminuria, glomerular matrix expansion, and inflammatory cell infiltration in the glomeruli and inhibit the accumulation of intrarenal free fatty acids and triglycerides. These results are compatible with the increased expression of PPARα and the activation of AMPK, PGC1α-ERR1α-pACC, as well as the suppression of SREBP-1c and carbohydrate regulatory element–binding protein-1, which constitutes the main downstream effector in lipid metabolism. Fenofibrate decreased the phosphorylation of phosphatidylinositol-3 kinase-Akt and FoxO3a in the kidneys, resulting in the demonstrated BCL-2-to-BCL-2–associated X protein ratio and SOD1 levels. Such findings were reproducible in cultured mesangial cells as reflected by the prevention of high-glucose–induced apoptosis and oxidative stress via AMPK phosphorylation, PGC-1α-ERR-1α activation, and SREBP-1c and carbohydrate regulatory element–binding protein-1 suppression. Our results suggest that fenofibrate's potential as a therapeutic modality for DN lies in its ability to improve lipotoxicity by stimulating AMPK-PGC-1α-ERR-1α-FoxO3a signaling [55].
Anthocyanin, a flavonoid in the polyphenol class, is a major constituent of food color and has been reported to possess both antidiabetic and antioxidant properties [56]. As its beneficial effects on lipid metabolism are dependent on AMPK activation (Fig. 2) [57], we were prompted to investigate whether an anthocyanin-rich Seoritae extract could ameliorate DN in db/db mice. Indeed, anthocyanin ameliorated intrarenal lipid accumulation with attenuations of mesangial expansion and glomerular inflammation; these effects appear to be attributable to the increased phosphorylation of AMPK, activation of PPARα and peroxisome proliferator–activated receptor-gamma, and inhibitory activity of ACC and SREBP-1c, leading to a reduction in lipotoxicity in the kidney. In cultured human glomerular endothelial cells, anthocyanin prevented high-glucose–induced oxidative stress and apoptosis via activation of AMPK in the same manner. The results indicate that anthocyanin could help alleviate some of the pathologic features of DN as it prevents lipotoxicity-related apoptosis and oxidative stress in the diabetic kidney through the activation of AMPK and its downstream effectors [58].
Cinacalcet is a calcimimetic that positively modulates the calcium-sensing receptor (CaSR). It is mainly used in the treatment of secondary hyperparathyroidism in CKD patients as it both diminishes the inhibitory effect of parathyroid hormone on renal phosphate reabsorption and inhibits renal calcium excretion [59]. However, some recent reports have demonstrated that CaSRs are expressed in human endothelial cells and that their stimulation by cinacalcet induces nitric oxide (NO) production, resulting in vasorelaxation [60]. Moreover, calcimimetics significantly increased intracellular Ca++ levels, which in turn augmented NO release via a time- and Ca++-dependent increase in eNOS-ser1177 phosphorylation activity [61]. Based on these findings, we proposed that DN-induced endothelial dysfunction could be ameliorated by the activation of eNOS–NO as a result of cinacalcet administration (unpublished observations). Our preliminary work shows that cinacalcet reduced albuminuria without influencing either blood glucose or Ca++ concentration and ameliorated mesangial expansion and inflammatory cell infiltration in the glomerulus of db/db mice. In the renal cortex, cinacalcet increased the expression of CaSR and the phosphorylation of CaMKKβ and LKB1. Subsequent activation of AMPK was followed by the activation of PGC-1α and phospho-Ser1177 eNOS–NO (Fig. 2). In vitro studies were consistent with the fact that cinacalcet decreases oxidative stress and apoptosis; this effect is attributable to increases in intracellular Ca++ and the phosphorylation of CaMMKβ, LKB1, and AMPK. All these changes were associated with an increase in the phosphorylation of eNOS. Overall, our results suggest that cinacalcet increases intracellular Ca++, subsequently activating the CaMKKβ–LKB1–AMPK signaling pathway in the kidney. This increase and activation of relevant molecules ameliorates renal damage, potentially providing a therapeutic modality for DN.
AdipoRon is an orally active, synthetic small molecule that activates adiponectin receptor (AdipoR). It mimics the antidiabetic effects of adiponectin, exhibiting its effect through the activation of AMPK and PPARα pathways via AdipoR1 and AdipoR2, respectively (Fig. 2) [62]. AdipoR activation has recently become a promising treatment for diabetes, nonalcoholic fatty liver disease, and cardiovascular disease, demonstrating anti-inflammatory actions in macrophages and cytoprotective effects on pancreatic β-cells [63]. However, mice overexpressing adiponectin are found to have reduced bone density [64], heart damage (left ventricular hypertrophy) [65], increased angiogenesis and adipogenesis [65], and infertility [66]. Despite these concerns, AdipoRon appears to be weight neutral and actually prolongs the life span in db/db mice [62]. Our unpublished data suggest that AdipoRon-treated db/db mice exhibit improvements in DN associated with increased expression of AdipoR1 and AdipoR2 in the glomerulus and consistent upregulation of phosphorylated AMPK, PPARα, and their downstream target molecules (unpublished observations). With respect to renal ultrastructure, AdipoRon treatment is associated with a noticeable reversal of diabetes-induced glomerular basement membrane (GBM) thickening, foot process widening, and slit diaphragm space narrowing; furthermore, it decreased glomerular matrix expansion and inflammation. AdipoRon may control oxidative stress in the glomerulus through AMPK- and PPARα-activated pathways, helping to prevent the deterioration of renal function. The protective role of AdipoRon against the development of albuminuria appears to occur through direct action on podocytes, independent of the systemic effects of adiponectin. Its reduction of oxidative stress confers protection against albuminuria and podocyte damage, ameliorating endothelial dysfunction in DN. Therefore, AdipoRon is a novel, promising therapeutic agent in preventing and ameliorating DN, particularly in type 2 diabetes.
Conclusion and future perspectives
Despite progress in the field of pharmacologic therapies that aims to prevent or delay the progression of DN, the number of end-stage renal disease patients is rising steadily, especially in Korea [5], [67]. Therefore, the development of new therapeutic agents is of crucial concern to public health. Mounting evidence suggests that AMPK activation in various tissues can help achieve metabolic homeostasis, with subsequent improvements in glucose and lipid profiles. We have summarized the role of AMPK activation in the context of several known and new activators that can treat DN by preventing renal fibrosis, extracellular matrix accumulation, apoptosis, and inflammation in the kidney (Fig. 3). However, a major pitfall in the use of AMPK activators is their as-yet-unknown adverse effects, which may counter the therapeutic effects we are aiming for. Such off-target effects involve AMPK's possible role in the stimulation of food intake through the activation of AMPK expressed in certain nuclei of the hypothalamus [68]. This provides a clear example of the need for both target and tissue specificity. Pharmacologic activation of AMPK needs to be achieved tissue specifically, and the isoforms of AMPK expressed in different tissues need to be identified [69]. In addition, we must determine the effective dose with least toxicity that can still produce detectable AMPK activation to achieve adequate metabolic regulation. The evidence of the metabolic effects of AMPK signifies just the beginning of the field, with significant challenges lying ahead in the translation of these data into feasible therapeutics for DN and obesity-related metabolic disease [5].
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea funded by the Research Fund of Seoul St. Mary's Hospital, the Catholic University of Korea (C.W.P.).
References
- 1.Willett W.C. Dietary fat plays a major role in obesity: no. Obes Rev. 2002;3:59–68. doi: 10.1046/j.1467-789x.2002.00060.x. [DOI] [PubMed] [Google Scholar]
- 2.Gross L.S., Li L., Ford E.S., Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79:774–779. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- 3.Viollet B., Lantier L., Devin-Leclerc J., Hebrard S., Amouyal C., Mounier R., Foretz M., Andreelli F. Targeting the AMPK pathway for the treatment of type 2 diabetes. Front Biosci (Landmark Ed) 2009;14:3380–3400. doi: 10.2741/3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dronavalli S., Duka I., Bakris G.L. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–452. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- 5.Park C.W. Diabetic kidney disease: from epidemiology to clinical perspectives. Diabetes Metab J. 2014;38:252–260. doi: 10.4093/dmj.2014.38.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallows K.R., Mount P.F., Pastor-Soler N.M., Power D.A. Role of the energy sensor AMP-activated protein kinase in renal physiology and disease. Am J Physiol Renal Physiol. 2010;298:F1067–F1077. doi: 10.1152/ajprenal.00005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim M.Y., Lim J.H., Youn H.H., Hong Y.A., Yang K.S., Park H.S., Chung S., Ko S.H., Shin S.J., Choi B.S., Kim H.W., Kim Y.S., Lee J.H., Chang Y.S., Park C.W. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Diabetologia. 2013;56:204–217. doi: 10.1007/s00125-012-2747-2. [DOI] [PubMed] [Google Scholar]
- 9.Andersson U., Filipsson K., Abbott C.R., Woods A., Smith K., Bloom S.R., Carling D., Small C.J. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 10.Kubota N., Yano W., Kubota T., Yamauchi T., Itoh S., Kumagai H., Kozono H., Takamoto I., Okamoto S., Shiuchi T., Suzuki R., Satoh H., Tsuchida A., Moroi M., Sugi K., Noda T., Ebinuma H., Ueta Y., Kondo T., Araki E., Ezaki O., Nagai R., Tobe K., Terauchi Y., Ueki K., Minokoshi Y., Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Hardie D.G., Scott J.W., Pan D.A., Hudson E.R. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 12.Hawley S.A., Boudeau J., Reid J.L., Mustard K.J., Udd L., Makela T.P., Alessi D.R., Hardie D.G. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawley S.A., Selbert M.A., Goldstein E.G., Edelman A.M., Carling D., Hardie D.G. 5'-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem. 1995;270:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- 14.Momcilovic M., Hong S.P., Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 15.Hardie D.G., Ashford M.L. AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda) 2014;29:99–107. doi: 10.1152/physiol.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao B., Heath R., Saiu P., Leiper F.C., Leone P., Jing C., Walker P.A., Haire L., Eccleston J.F., Davis C.T., Martin S.R., Carling D., Gamblin S.J. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 17.Gowans G.J., Hawley S.A., Ross F.A., Hardie D.G. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18:556–566. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horman S., Vertommen D., Heath R., Neumann D., Mouton V., Woods A., Schlattner U., Wallimann T., Carling D., Hue L., Rider M.H. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 19.Sanders M.J., Ali Z.S., Hegarty B.D., Heath R., Snowden M.A., Carling D. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem. 2007;282:32539–32548. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- 20.Jensen T.E., Sylow L., Rose A.J., Madsen A.B., Angin Y., Maarbjerg S.J., Richter E.A. Contraction-stimulated glucose transport in muscle is controlled by AMPK and mechanical stress but not sarcoplasmatic reticulum Ca2+ release. Mol Metab. 2014;3:742–753. doi: 10.1016/j.molmet.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kops G.J., Dansen T.B., Polderman P.E., Saarloos I., Wirtz K.W., Coffer P.J., Huang T.T., Bos J.L., Medema R.H., Burgering B.M. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 22.Wu S.B., Wu Y.T., Wu T.P., Wei Y.H. Role of AMPK-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress. Biochim Biophys Acta. 2014;1840:1331–1344. doi: 10.1016/j.bbagen.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez A.M., Csibi A., Raibon A., Cornille K., Gay S., Bernardi H., Candau R. AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. J Cell Biochem. 2012;113:695–710. doi: 10.1002/jcb.23399. [DOI] [PubMed] [Google Scholar]
- 24.Filomeni G., Desideri E., Cardaci S., Rotilio G., Ciriolo M.R. Under the ROS...thiol network is the principal suspect for autophagy commitment. Autophagy. 2010;6:999–1005. doi: 10.4161/auto.6.7.12754. [DOI] [PubMed] [Google Scholar]
- 25.Zrelli H., Matsuoka M., Kitazaki S., Zarrouk M., Miyazaki H. Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK-FOXO3a pathway. Eur J Pharmacol. 2011;660:275–282. doi: 10.1016/j.ejphar.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 26.Canto C., Gerhart-Hines Z., Feige J.N., Lagouge M., Noriega L., Milne J.C., Elliott P.J., Puigserver P., Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi T., Hirshman M.F., Kurth E.J., Winder W.W., Goodyear L.J. Evidence for 5' AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 28.Ouchi N., Shibata R., Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 29.Li Y., Xu S., Mihaylova M.M., Zheng B., Hou X., Jiang B., Park O., Luo Z., Lefai E., Shyy J.Y., Gao B., Wierzbicki M., Verbeuren T.J., Shaw R.J., Cohen R.A., Zang M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberg J.M. Lipotoxicity. Kidney Int. 2006;70:1560–1566. doi: 10.1038/sj.ki.5001834. [DOI] [PubMed] [Google Scholar]
- 31.Ginsberg H.N. Diabetic dyslipidemia: basic mechanisms underlying the common hypertriglyceridemia and low HDL cholesterol levels. Diabetes. 1996;45(Suppl 3):S27–S30. doi: 10.2337/diab.45.3.s27. [DOI] [PubMed] [Google Scholar]
- 32.Stadler K., Goldberg I.J., Susztak K. The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr Diab Rep. 2015;15:40. doi: 10.1007/s11892-015-0611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horman S., Browne G., Krause U., Patel J., Vertommen D., Bertrand L., Lavoinne A., Hue L., Proud C., Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- 34.Kodiha M., Stochaj U. Targeting AMPK for therapeutic intervention in type 2 diabetes. In: Croniger C., editor. Medical Complications of Type 2 Diabetes. InTech; Rijeka (Croatia): 2011. doi: 10.5772/20930. Available from: http://www.intechopen.com/books/medical-complications-of-type-2-diabetes/targeting-ampk-for-therapeutic-intervention-in-type-2-diabetes. [Google Scholar]
- 35.Corton J.M., Gillespie J.G., Hawley S.A., Hardie D.G. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 36.Hardie D.G., Ross F.A., Hawley S.A. AMP-activated protein kinase: a target for drugs both ancient and modern. Chem Biol. 2012;19:1222–1236. doi: 10.1016/j.chembiol.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narkar V.A., Downes M., Yu R.T., Embler E., Wang Y.X., Banayo E., Mihaylova M.M., Nelson M.C., Zou Y., Juguilon H., Kang H., Shaw R.J., Evans R.M. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Decléves A.E., Zolkipli Z., Satriano J., Wang L., Nakayama T., Rogac M., Le T.P., Nortier J.L., Farquhar M.G., Naviaux R.K., Sharma K. Regulation of lipid accumulation by AMP-activated kinase [corrected] in high fat diet-induced kidney injury. Kidney Int. 2014;85:611–623. doi: 10.1038/ki.2013.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buzzai M., Jones R.G., Amaravadi R.K., Lum J.J., DeBerardinis R.J., Zhao F., Viollet B., Thompson C.B. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 40.El Messaoudi S., Rongen G.A., Riksen N.P. Metformin therapy in diabetes: the role of cardioprotection. Curr Atheroscler Rep. 2013;15:314. doi: 10.1007/s11883-013-0314-z. [DOI] [PubMed] [Google Scholar]
- 41.Viollet B., Guigas B., Sanz Garcia N., Leclerc J., Foretz M., Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122:253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Mir M.Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 43.Vazquez-Martin A., Oliveras-Ferraros C., Cufí S., Martin-Castillo B., Menendez J.A. Metformin and energy metabolism in breast cancer: from insulin physiology to tumour-initiating stem cells. Curr Mol Med. 2010;10:674–691. doi: 10.2174/156652410792630625. [DOI] [PubMed] [Google Scholar]
- 44.Lee M.J., Feliers D., Mariappan M.M., Sataranatarajan K., Mahimainathan L., Musi N., Foretz M., Viollet B., Weinberg J.M., Choudhury G.G., Kasinath B.S. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007;292:F617–F627. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- 45.Bamri-Ezzine S., Ao Z.J., Londoño I., Gingras D., Bendayan M. Apoptosis of tubular epithelial cells in glycogen nephrosis during diabetes. Lab Invest. 2003;83:1069–1080. doi: 10.1097/01.lab.0000078687.21634.69. [DOI] [PubMed] [Google Scholar]
- 46.Sharma K., Ramachandrarao S., Qiu G., Usui H.K., Zhu Y., Dunn S.R., Ouedraogo R., Hough K., McCue P., Chan L., Falkner B., Goldstein B.J. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cammisotto P.G., Bendayan M. Adiponectin stimulates phosphorylation of AMP-activated protein kinase alpha in renal glomeruli. J Mol Histol. 2008;39:579–584. doi: 10.1007/s10735-008-9198-6. [DOI] [PubMed] [Google Scholar]
- 48.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 49.Brunet A., Sweeney L.B., Sturgill J.F., Chua K.F., Greer P.L., Lin Y., Tran H., Ross S.E., Mostoslavsky R., Cohen H.Y., Hu L.S., Cheng H.L., Jedrychowski M.P., Gygi S.P., Sinclair D.A., Alt F.W., Greenberg M.E. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 50.Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 51.Yeung F., Hoberg J.E., Ramsey C.S., Keller M.D., Jones D.R., Frye R.A., Mayo M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang C.C., Chang C.Y., Wu Y.T., Huang J.P., Yen T.H., Hung L.M. Resveratrol retards progression of diabetic nephropathy through modulations of oxidative stress, proinflammatory cytokines, and AMP-activated protein kinase. J Biomed Sci. 2011;18:47. doi: 10.1186/1423-0127-18-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang L.P., Keating G.M. Fenofibric acid: in combination therapy in the treatment of mixed dyslipidemia. Am J Cardiovasc Drugs. 2009;9:401–409. doi: 10.2165/11203920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 54.Al-Rasheed N.M., Al-Rasheed N.M., Attia H.A., Al-Amin M.A., Al-Ajmi H.N., Hasan I.H., Mohamad R.A., Sinjilawi N.A. Renoprotective effects of fenofibrate via modulation of LKB1/AMPK mRNA expression and endothelial dysfunction in a rat model of diabetic nephropathy. Pharmacology. 2015;95:229–239. doi: 10.1159/000381190. [DOI] [PubMed] [Google Scholar]
- 55.Hong Y.A., Lim J.H., Kim M.Y., Kim T.W., Kim Y., Yang K.S., Park H.S., Choi S.R., Chung S., Kim H.W., Kim H.W., Choi B.S., Chang Y.S., Park C.W. Fenofibrate improves renal lipotoxicity through activation of AMPK-PGC-1alpha in db/db mice. PLoS One. 2014;9:e96147. doi: 10.1371/journal.pone.0096147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y., Li D., Zhang Y., Sun R., Xia M. Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. Am J Physiol Endocrinol Metab. 2014;306:E975–E988. doi: 10.1152/ajpendo.00699.2013. [DOI] [PubMed] [Google Scholar]
- 57.Valenti L., Riso P., Mazzocchi A., Porrini M., Fargion S., Agostoni C. Dietary anthocyanins as nutritional therapy for nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2013;2013:145421. doi: 10.1155/2013/145421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koh E.S., Lim J.H., Kim M.Y., Chung S., Shin S.J., Choi B.S., Kim H.W., Hwang S.Y., Kim S.W., Park C.W., Chang Y.S. Anthocyanin-rich Seoritae extract ameliorates renal lipotoxicity via activation of AMP-activated protein kinase in diabetic mice. J Transl Med. 2015;13:203. doi: 10.1186/s12967-015-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Massy Z.A., Henaut L., Larsson T.E., Vervloet M.G. Calcium-sensing receptor activation in chronic kidney disease: effects beyond parathyroid hormone control. Semin Nephrol. 2014;34:648–659. doi: 10.1016/j.semnephrol.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 60.Thakore P., Ho W.S. Vascular actions of calcimimetics: role of Ca(2)(+) -sensing receptors versus Ca(2)(+) influx through L-type Ca(2)(+) channels. Br J Pharmacol. 2011;162:749–762. doi: 10.1111/j.1476-5381.2010.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonomini M., Giardinelli A., Morabito C., Di Silvestre S., Di Cesare M., Di Pietro N., Sirolli V., Formoso G., Amoroso L., Mariggió M.A., Pandolfi A. Calcimimetic R-568 and its enantiomer S-568 increase nitric oxide release in human endothelial cells. PLoS One. 2012;7:e30682. doi: 10.1371/journal.pone.0030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okada-Iwabu M., Yamauchi T., Iwabu M., Honma T., Hamagami K., Matsuda K., Yamaguchi M., Tanabe H., Kimura-Someya T., Shirouzu M., Ogata H., Tokuyama K., Ueki K., Nagano T., Tanaka A., Yokoyama S., Kadowaki T. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493–499. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- 63.Holland W.L., Scherer P.E. Cell biology. Ronning after the adiponectin receptors. Science. 2013;342:1460–1461. doi: 10.1126/science.1249077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ealey K.N., Kaludjerovic J., Archer M.C., Ward W.E. Adiponectin is a negative regulator of bone mineral and bone strength in growing mice. Exp Biol Med (Maywood) 2008;233:1546–1553. doi: 10.3181/0806-RM-192. [DOI] [PubMed] [Google Scholar]
- 65.Yamauchi T., Kamon J., Waki H., Terauchi Y., Kubota N., Hara K., Mori Y., Ide T., Murakami K., Tsuboyama-Kasaoka N., Ezaki O., Akanuma Y., Gavrilova O., Vinson C., Reitman M.L., Kagechika H., Shudo K., Yoda M., Nakano Y., Tobe K., Nagai R., Kimura S., Tomita M., Froguel P., Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 66.Combs T.P., Pajvani U.B., Berg A.H., Lin Y., Jelicks L.A., Laplante M., Nawrocki A.R., Rajala M.W., Parlow A.F., Cheeseboro L., Ding Y.Y., Russell R.G., Lindemann D., Hartley A., Baker G.R., Obici S., Deshaies Y., Ludgate M., Rossetti L., Scherer P.E. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 67.Jin D.C., Ha I.S., Kim N.H., Lee S.W., Lee J.S., Yoon S.R., Kim B.S. Brief report: renal replacement therapy in Korea, 2010. Kidney Res Clin Pract. 2012;31:62–71. doi: 10.1016/j.krcp.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Minokoshi Y., Alquier T., Furukawa N., Kim Y.B., Lee A., Xue B., Mu J., Foufelle F., Ferré P., Birnbaum M.J., Stuck B.J., Kahn B.B. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 69.Burkewitz K., Zhang Y., Mair W.B. AMPK at the nexus of energetics and aging. Cell Metab. 2014;20:10–25. doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]