Abstract
The majority of morbidity and mortality in sickle cell disease is caused by vaso-occlusion: circulatory obstruction leading to tissue ischemia and infarction. The consequences of vaso-occlusion are seen clinically throughout the vascular tree, from the relatively high-oxygen and high-velocity cerebral arteries to the relatively low-oxygen and low-velocity postcapillary venules. Prevailing models of vaso-occlusion propose mechanisms that are relevant only to regions of low oxygen and low velocity, leaving a wide gap in our understanding of the most important pathologic process in sickle cell disease. Progress toward understanding vaso-occlusion is further challenged by the complexity of the multiple processes thought to be involved, including, but not limited to 1) deoxygenation-dependent hemoglobin polymerization leading to impaired rheology, 2) endothelial and leukocyte activation, and 3) altered cellular adhesion. Here, we chose to focus exclusively on deoxygenation-dependent rheologic processes in an effort to quantify their contribution independent of the other processes that are likely involved in vivo. We take advantage of an experimental system that, to our knowledge, uniquely enables the study of pressure-driven blood flow in physiologic-sized tubes at physiologic hematocrit under controlled oxygenation conditions, while excluding the effects of endothelium, leukocyte activation, adhesion, inflammation, and coagulation. We find that deoxygenation-dependent rheologic processes are sufficient to increase apparent viscosity significantly, slowing blood flow velocity at arterial oxygen tension even without additional contributions from inflammation, adhesion, and endothelial and leukocyte activation. We quantify the changes in apparent viscosity and define a set of functional regimes of sickle cell blood flow personalized for each patient that may be important in further dissecting mechanisms of in vivo vaso-occlusion as well as in assessing risk of patient complications, response to transfusion, and the optimization of experimental therapies in development.
Introduction
Sickle cell disease (SCD) afflicts >13 million people worldwide and incurs health care costs in excess of $1 billion per year in the United States (1, 2). Although disease course is typically severe with life expectancy for individuals with SCD in the United States ∼30 years shorter than average (3), there is wide phenotypic heterogeneity, and some patients experience completely benign disease (4, 5). The mechanisms for this phenotypic diversity are largely unknown, and efforts to find useful genotypic or clinical predictors of benign prognosis have been unsuccessful (6, 7). Understanding the mechanisms for this clinical diversity is critical for both the management of patients and the development of new therapies.
The major cause of morbidity and mortality in SCD is impaired blood flow culminating in vaso-occlusion, with occlusive events occurring throughout the vascular tree, from the relatively low-shear and low-oxygen tension postcapillary venules (8, 9) to the relatively high-shear and high-oxygen tension large cerebral arteries (10, 11). The major causes of reduced blood flow are 1) altered sickle cell blood rheology, 2) increased inflammation, and 3) cellular adhesion, with pathologic inflammation and adhesion likely arising at least in part as a result of the underlying altered sickle cell blood rheology (12, 13, 14). The relative importance of these three mechanisms in precipitating vaso-occlusion remains unclear. Additionally, we do not know whether the patients with the mildest disease have benign outcomes because their blood rheologic changes are milder, their inflammatory response is reduced, or because they experience less cellular adhesion. To dissect the mechanisms for this wide clinical heterogeneity, it is therefore essential to quantify phenotypes more precisely by characterizing the rheologic, inflammatory, and adhesive function of individual patients. The current study focuses on understanding the rheologic phenotype.
We previously showed that SCD patient rheologic phenotype might help explain the basis for clinical heterogeneity. We used a simplified in vitro microfluidic model of sickle cell vaso-occlusion and showed that rheologic processes alone were sufficient to cause deoxygenation-dependent cessation of sickle cell blood flow (15) and that the rate of increase in apparent viscosity following complete removal of oxygen correlated more strongly with patient clinical outcomes than any other routinely available measure of disease (16), where apparent viscosity is defined for a given channel geometry as the pressure divided by velocity. Those studies focused only on regimes of very low oxygen tension and thus cannot explain the basis for the most important sickle cell disease complications occurring in the cerebral arterial vasculature and other regions of relatively high oxygen and high shear (17).
Single-red blood cell (RBC) studies decades ago reported that some extreme RBCs demonstrated decreased filterability and deformability even at arterial oxygen tension (18, 19, 20), but the effect of a small minority cell population on overall blood suspension rheology at physiologic hematocrit (HCT) remained unclear. Most recent vaso-occlusion experimental work and predominant models focus on events in low-oxygen and low-velocity postcapillary venules and therefore cannot help explain events occurring in high-velocity or high-oxygen vessels (8, 9). In the absence of local oxygen-dependent effects, reduced arterial blood flow in sickle cell patients has been attributed to chronic cycles of polymerization and depolymerization that damage the RBC cytoskeleton and cause an oxygen-independent reduction in sickle RBC deformability, along with oxygen-independent adhesive and inflammatory responses (8, 14). However, the degree of reduction in oxygen-independent RBC deformability is much more mild, and as has been previously suggested (21, 22), only the dramatic decrease in deformability associated with deoxygenation-induced sickle hemoglobin (HbS) polymerization seems sufficient to explain the severity of sickle cell disease complications found in the cerebral vasculature of sickle cell patients (10). To understand the biophysical basis for these complications, we require a clearer picture of the rheologic behavior of blood under arterial oxygen conditions.
Here, using a simplified in vitro model of sickle cell vaso-occlusion, we present experimental evidence for significant oxygen-dependent decrease in sickle cell blood flow at the higher oxygen tensions found in the arterial circulation of sickle cell patients in vivo. We detect and quantify this blood flow reduction for individual patients, revealing three separate regimes of sickle cell blood flow as a function of oxygen tension: (regime I) an oxygen-independent regime at supraphysiologic oxygen, (regime II) an oxygen-dependent regime that spans oxygen tensions found in the arterial circulation, and (regime III) a second oxygen-independent regime at lower oxygen tensions. We find that sickle cell blood rheology is sensitive to oxygen tension at levels found in the arterial circulation of sickle cell patients in vivo. Our results suggest that oxygen-dependent effects on sickle cell blood rheology may play a dominant role in the most important SCD complications occurring well upstream of the venous circulation.
Materials and Methods
Blood sample collection and storage
Whole sickle cell blood samples were collected from patients at the University of Minnesota Medical Center, Massachusetts General Hospital, and Brigham and Women’s Hospital. Blood sample collection was authorized under protocols approved by Institutional Review Boards at Partners Healthcare and the University of Minnesota. Blood samples were collected from patients with homozygous SS disease, including those who were taking hydroxyurea and those receiving transfusion as noted. Blood was collected in 5 mL EDTA vacutainers and stored at 4°C for between 1 and 5 days. This storage duration, temperature, and anticoagulant do not cause significant changes in blood rheologic properties (23, 24). Our experiments thus minimize the effects of platelets and leukocytes because platelet function is inhibited by EDTA, and leukocyte viability declines upon storage (25). HCT was measured with a Sysmex XE-5000 automated analyzer (Sysmex, Kobe, Japan). Hemoglobin fractions were measured by high-performance liquid chromatography using a Tosoh G7 column (Tosoh Bioscience, San Francisco, CA).
Device design and fabrication
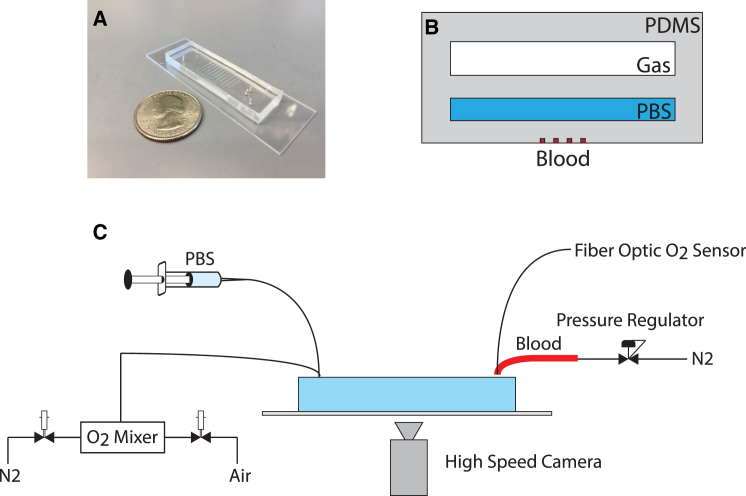
A multilayered polydimethylsiloxane (PDMS)- based microfluidic platform that diffusively couples an oxygen gas reservoir to a blood microchannel was used in this study and has been previously described (16). Briefly, the device (Fig. 1) consists of three separate layers: 1) an arteriole or venule sized blood microchannel with a cross section of 15 μm × 15 μm where whole sickle cell blood flow was analyzed, 2) a 1.5 mm × 100 μm hydration reservoir where phosphate-buffered saline (PBS) prevented evaporation, and 3) a 1.5 mm × 150 μm oxygen gas reservoir enabling diffusive control of oxygen gas tensions in the blood microchannel. Each of the three layers was separated by a 100 μm PDMS membrane.
Figure 1.
Microfluidic platform for high-resolution characterization of sickle cell blood rheologic phenotype. (A) Photograph of the microfluidic device. (B) Schematic of a cross section of the device. Gas with controlled oxygen tension flows in the top Gas channel, phosphate-buffered saline flows in the middle PBS channel, and whole blood flows in the bottom Blood channel. (C) Schematic of the experimental platform, including oxygen control, pressure regulation, and high-speed video capture.
Soft photolithography was used to create master molds for each of the three layers in the device as described previously (16). Blood and PBS layers of the device were created by compression molding of PDMS. PDMS was cast onto the gas layer mold at a 5 mm height with a 10:1 elastomer/curing agent ratio and cured for 2 h at 75°C in a convection oven. Compression molding of the blood microchannel and PBS layers was completed by adhering either a 115-μm-thick glass coverslip (for the blood microchannels) or a 200-μm-thick glass coverslip (for the PBS reservoir) to the edges of the master mold. PDMS with a 10:1 elastomer/curing agent ratio was poured onto the mold and spacers before a large metal weight was placed on top. The setup was left overnight on a 75°C hotplate for the PDMS to cure. The gas, PBS, and blood microchannel layers were plasma bonded together at a power of 100 W, an oxygen flow rate of 100 cc/min, and an exposure time of 45 s. Finally, the device was plasma bonded to a clean microscope slide.
Device operation
Whole blood (EDTA-anticoagulated) from patients was perfused through the blood microchannel of the device, driven by compressed gas at a constant pressure controlled by an electronic pressure regulator (model PCD-15PSIG-D-PCV10, Alicat Scientific, Tucson, Arizona). Temperature was maintained at 37°C with a microscope incubation enclosure (Incubator XLmulti S1, Zeiss, Jena, Germany). PBS was perfused through the PBS layer of the device at a flow rate of 1 mL/h using a syringe pump (NE-500, New Era Pump Systems, Farmingdale, NY). Oxygen gas at specified tensions was pushed into the gas layer of the device. Discrete oxygen gas tensions were created by mixing 0 mmHg oxygen (5% CO2, balance N2) and 159.6 mmHg oxygen (21% O2, 5% CO2, balance N2) in a periodic manner as described previously (26). Briefly, solenoid valves were cycled between fully deoxygenated gas and fully oxygenated gas at preset duty cycles, resulting in discrete intermediate oxygen gas tensions. As blood flowed through the microchannels of the device, oxygen gas tension was decreased from 159.6 to 114 mmHg. Oxygen gas tension was further decreased in 19 mmHg intervals until a gas tension of 0 mmHg was reached, or until an occlusive event was seen. Each oxygen gas tension was held for a minimum of 5 min to allow velocities time to stabilize. The timescales were not intended to mimic in vivo timescales, where absolute changes in oxygen tension and diffusion distances are typically much smaller, allowing steady states to be reached more quickly. Oxygen gas tension was measured at the outlet of the gas reservoir on the microfluidic device using a fiber optic sensor (NeoFox-GT, Ocean Optics, Dunedin, FL) (Fig. 1 C). We do not directly measure hemoglobin oxygen saturation, and saturation levels can be inferred from reported oxygen tensions using standard hemoglobin oxygen dissociation curves (27). See Fig. S1 in the Supporting Material for more detail.
Blood rheology and conductance
Rheological behavior was determined by tracking the movement of blood cells in the microchannels using high frame-rate imaging and computational video processing as described previously (16, 28). Briefly, cells in each movie frame were identified computationally on the basis of morphologic criteria. Cell locations in subsequent frames were linked to form trajectories using heuristics and machine learning techniques. The velocity at each point in time was defined as the median cell velocity calculated over a 32-frame movie captured at ∼300 frames per second. Steady-state velocities for each constant oxygen interval were determined by calculating the median of the velocities measured during the 50-80% portion of the time interval to exclude any transients related to diffusion or polymerization delay times. See Figs. S2 and S3 for more detail.
For each patient blood sample, we determined a confidence interval for the high-oxygen threshold at which sickle cell blood apparent viscosity changed (regime I–II boundary, as defined in Results). The lower limit of the confidence interval was the highest oxygen tension meeting the following two conditions: 1) the steady-state blood flow velocity at that oxygen tension was lower than the velocity measured when full oxygenation was restored, and 2) the steady-state blood velocity at that oxygen tension was significantly lower than the velocity at the next highest oxygen tension, with significance determined by a Kruskal-Wallis test followed by a pairwise comparison of individual groups using a Mann-Whitney U test and Bonferroni corrected p-values. The upper bound of the confidence interval for the regime I–II boundary was the highest oxygen tension whose steady- state blood flow velocity was significantly different from the flow velocity at full oxygenation, with significance determined by the Kruskal-Wallis test followed by pairwise Mann-Whitney U tests with Bonferroni corrected p-values.
Results
Effect of oxygen tension on the flow velocity of sickle cell blood driven in a physiologic-sized tube by a pressure gradient at physiologic HCT and physiologic temperature
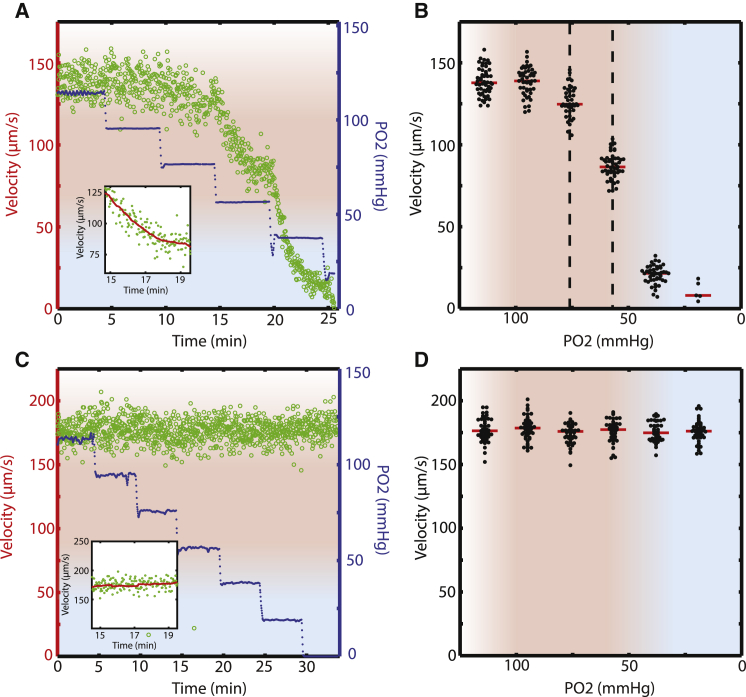
We measured flow velocity over a range of oxygen tensions for blood samples from 15 patients with SCD, with unaltered HCT and at physiologic temperature. In our experimental system, blood flow was driven by a pressure head through a 15 μm × 15 μm cross section tube, as is the case in vivo. Oxygen tension was serially decreased from 114 mmHg (15%) to 19 mmHg (2.5%) in steps of 19 mmHg (2.5%), and steady-state flow velocity was calculated at each oxygen tension, as described in Materials and Methods. Fig. 2 A shows the results for a representative blood sample from a SCD patient. The blood flow velocity declined slightly when the oxygen tension was lowered from 95 to 76 mmHg and then slowed dramatically when the oxygen was reduced from 76 to 57 mmHg. Fig. 2 B shows median velocities calculated at each oxygen tension level. The flow properties of this blood sample are oxygen-dependent at oxygen tensions at least as high as 57 mmHg and likely higher than 76 mmHg.
Figure 2.
Rheologic phenotype of sickle cell blood is altered by deoxygenation at arterial oxygen tension. (A) The flow velocity (green dots, left side y axis) of a sickle cell patient’s blood sample depends on oxygen tension. Oxygen tension (blue dots, right side y axis) was decreased in a stepwise manner while blood velocity was measured. The red shaded region corresponds to oxygen tensions found in the arterial circulation (>60 mmHg) in sickle cell patients (see the main text), and the blue shaded region corresponds to oxygen tensions generally found in the venous circulation (<30 mmHg). The blue to red gradient region spans oxygen tensions between 30 and 60 mmHg, and the red to white gradient region represents supraphysiological oxygen tensions above 100 mmHg. Driving pressure is constant. Inset shows that flow velocity stabilizes by the end of each oxygen step. (B) Steady-state flow velocity of a sickle cell patient’s blood sample depends on oxygen tension. Each collection of black dots represents flow velocity measurements at each oxygen tension shown in (A). Median velocity for each oxygen tension is represented by a red horizontal line. See Materials and Methods for more detail. This sickle cell patient’s blood shows a dramatic decrease in flow velocity as oxygen is lowered within the arterial oxygen tension range (red shaded region) and below (blue shaded region). Driving pressure is constant. (C) The flow velocity of a normal blood sample (HbA) does not depend on oxygen tension. Inset shows that flow velocity is stable. (D) The steady-state flow velocity of a normal blood sample does not depend on oxygen tension.
Qualitatively, the flow of blood from this patient (Fig. 2 B) displays three distinct regimes:
-
I)
At very high oxygen tensions (supraphysiologic), velocity is oxygen independent and comparable to that of normal (HbA) blood (Fig. 2, C and D).
-
II)
At intermediate sampled oxygen tensions, velocity is very sensitive to oxygen tension, decreasing monotonically but not linearly, with the same step in oxygen tension leading to varying changes in velocity.
-
III)
At low oxygen tensions, blood flow velocity is again oxygen independent but at a much lower velocity (sometimes zero) than at high oxygen tensions.
We use the term regime to describe qualitative flow behaviors that can be distinguished subject to the limits of our experimental system. For sufficiently low oxygen tensions, blood samples from most patients (11/15) stopped flowing, completely occluding the in vitro device. As we have previously shown (15, 16), in vitro occlusions were completely reversible by raising the oxygen tension. See Movie S1 and Fig. S1.
Flow velocity of sickle cell blood is routinely sensitive to oxygen at high oxygen tension
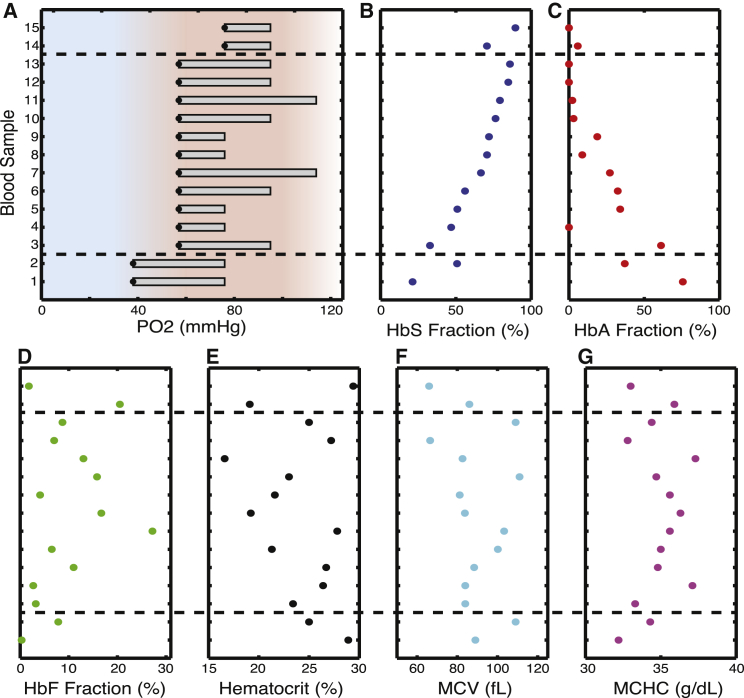
To determine whether these qualitative features of the oxygen-flow velocity relationship were common among patients with SCD, we performed similar studies on blood samples from 14 additional SCD patients. For all blood samples, we first measured the flow velocity at various oxygen tensions. We then determined a confidence interval for the oxygen threshold at which blood flow velocity first began to decrease: the boundary between regimes I and II as defined previously. Fig. 3 A shows confidence intervals for the regime I–II boundaries for all 15 patients. For example, the blood sample shown in Fig. 2 B has a regime I–II boundary that is at least as high as 57 mmHg (black dot in Fig. 3 A) and possibly as high as 95 mmHg. All blood samples show evidence of oxygen-dependent behavior at oxygen tensions well above mixed venous oxygen tension (40 mmHg) (20). Many show evidence of oxygen-dependent behavior at 80 mmHg and above. Previous studies have measured oxygen tension in sickle cell patients in vivo and have repeatedly found arterial oxygen tensions below 65 mmHg under steady-state conditions, with occasional reports of readings even below 60 mmHg (20, 29, 30, 31). Our results therefore suggest that it is not unusual for the arterial oxygen tension of sickle cell patients to reach levels where the apparent viscosity of blood is significantly increased as a result of oxygen-dependent effects alone, without the need for any cellular adhesion, activation, or inflammation. In terms of the rheologic regime space defined previously, for most SCD patients it is not unusual for arterial blood flow to be in regime II. Understanding the biological and physical factors controlling the location of this regime I/II boundary will likely reveal important disease mechanisms and opportunities for intervention.
Figure 3.
Threshold oxygen tensions for diminished blood flow. (A) Confidence intervals for threshold oxygen tensions at which blood velocity becomes oxygen dependent. Gray boxes show confidence intervals for each of 15 patients. Black dots mark the lower limit of each confidence interval. For example, blood sample #10 is the sample shown in Fig. 2, A and B, and its apparent viscosity becomes oxygen-dependent above 57 mmHg and possibly as high as 95 mmHg. The red shaded region corresponds to oxygen tensions found in the arterial circulation (60–100 mmHg) in sickle cell patients (see the main text), and the blue shaded region corresponds to oxygen tensions found in the venous circulation (0–30 mmHg). The blue to red gradient region spans oxygen tensions between 30 and 60 mmHg, and the red to white gradient region represents supraphysiological oxygen tensions above 100 mmHg. The gray box thus represents a confidence interval for the location of the regime I-regime II boundary, as described in Results, and this confidence interval includes oxygen tensions found in the arterial circulation for all patients studied. Patient blood samples are ordered by the lower limits of their confidence intervals. Within groups with identical lower limits, patient blood samples are ordered by HbS fraction (B). Commonly measured clinical laboratory characteristics have little impact on the location of the regime I-regime II boundaries: (B–G) HbS fraction, HbA fraction, fetal hemoglobin (HbF) fraction, HCT, mean RBC volume (MCV), and mean RBC hemoglobin concentration (MCHC) for each blood sample. Horizontal dashed lines separate the blood samples by threshold lower limits.
Threshold for oxygen sensitivity of blood flow velocity is only weakly dependent on HbS%, HbF%, HCT, and other blood cell characteristics
Fig. 3 A shows mild variation in the location of the regime I–II boundaries from one patient sample to the next. Two of 15 samples have boundary confidence intervals extending below 57 mmHg, and another two have boundaries extending above 95 mmHg. To investigate potential determinants of a blood sample’s regime I–II boundary location, we compared boundary intervals to basic blood sample characteristics, including the HbS fraction (Fig. 3 B), the HbA fraction (Fig. 3 C), the HbF fraction (Fig. 3 D), HCT (Fig. 3 E), the mean cell volume (MCV, Fig. 3 F), and the mean corpuscular hemoglobin concentration (MCHC, Fig. 3 G). We found no statistically significant pairwise correlation between any of these factors and the location of the regime I/II boundary. There is a weak apparent trend for HbS%, with the lowest HbS% samples generally having the lowest boundaries and the highest HbS% samples having the highest boundaries, but the magnitude of this possible effect of HbS% on the threshold is quite modest. Even if it represents more than statistical artifact, it is unlikely to explain a meaningful amount of the clinical variation between nontransfused patients. In our study population, only an extreme drop in the HbS fraction from 80% to 20% is associated with a noticeable effect on the location of threshold. It is important to note that the variation in HbS% occurs both because of biological variation in the composition of hemoglobin isoforms in each patient’s native RBCs and also because some of these patients had received transfusions, which decrease average HbS% by adding HbA cells containing 0% HbS. It is surprising that even blood samples from repeatedly transfused patients whose HbS fraction drops below 20% show oxygen-dependent rheologic change at arterial or near-arterial oxygen tension. Only a small minority of the RBCs in the samples with 20% HbS contains any HbS, and yet slight decreases in oxygen even within the arterial range will increase the apparent viscosity and decrease the flow of blood. (See Fig. S4 for further detail.) Overall, although there appears to be a weak relationship between HbS% and the location of the regime I–II boundary, the other hematologic characteristics have no apparent effect on the location of the regime I–II boundary.
Discussion
We find that the flow velocity of sickle cell blood is sensitive to slight reductions in oxygen even at the higher oxygen tensions found in the arterial circulation of SCD patients (29, 32, 33). We used an experimental platform that focuses on oxygen-dependent rheologic processes and excludes some other physiologic conditions thought to predispose patients to vaso-occlusion in vivo. Our system lacks endothelium or functional platelets and leukocytes, yet even after excluding the processes of cellular adhesion, activation, inflammation, and coagulation, we found significantly diminished blood flow in response to deoxygenation at high oxygen tension under physiologic conditions of temperature, HCT, vessel-geometry, while also replicating the pressure-driven nature of in vivo blood flow. The adhesive processes absent from our system would only amplify the effects we have seen, as has been shown under conditions of constant high oxygen tension (9, 34). Analogous dissection of adhesive or inflammatory contributions to vaso-occlusion would help establish the relative importance of these different processes in vivo, but performing those controlled experiments will likely be challenging. Our experiments involve a wider absolute range of oxygen tension than is typically found in vivo, and the diffusion distances are also much longer in our experiments. The timescales to reach steady states are thus longer in our experiments (minutes) than in vivo where individual RBCs typically traverse a full range of oxygen concentrations in less than a minute. Despite the differences between our system and in vivo, we expect that the viscosities induced by the relatively steady oxygen tensions in the various parts of the vasculature should be comparable to the steady-state viscosities measured here. Future work will be required to determine the patient-specific relationship between the rheologic changes we describe here and clinical progression.
Most recent studies of sickle cell blood flow have focused on low-velocity and low-oxygen conditions relevant only to the venous circulation, with the most prominent models of vaso-occlusion describing events occurring only in the postcapillary venules (8, 9, 12). To understand the mechanism for the vast majority of clinical vaso-occlusive events occurring in SCD patients at other sites, including cerebral arteries, we need other models that incorporate the rheologic processes described here.
Studies of single RBCs decades ago found that some extreme RBCs show decreased deformability at oxygen tensions found in the arterial circulation (18, 19, 20). These studies raised an important question: what is the effect of a small minority cell population with extreme oxygen-dependent mechanical properties on sickle cell blood in a physiologic pressure-driven flow in a tube at physiologic HCT? We provide a clear answer to this question for the first time, to our knowledge. Apparent viscosity of blood samples from all SCD patients tested show significant elevation, and the oxygen threshold for increase in apparent viscosity (60–80 mmHg) was somewhat below the threshold at which the most extreme RBCs showed reduced filterability (100–120 mmHg) (18, 19, 20). Although blood samples from all patients demonstrated oxygen-dependent rheology, the magnitude of elevation in apparent viscosity caused by a given decrease in oxygen varied from sample to sample and depended on the initial oxygen tension. Future study is needed to determine if interpatient variation in oxygen tension or in the magnitude of apparent viscosity increase corresponding to a fixed oxygen change may help explain interpatient variation in clinical outcomes.
Our study demonstrates the high sensitivity of bulk blood rheologic properties to the single-cell mechanical properties of even a small minority fraction of cells with extreme oxygen-dependent stiffness. For example, in the blood sample from a patient with multiple transfusions to reduce the HbS fraction below 20% and increase the HbA fraction above 80%, at least 80% of the RBCs are from healthy donors and contain no HbS and will remain soft at all oxygen concentrations. The presence of a 20% fraction of RBCs containing any HbS is thus sufficient to cause a measurable increase in apparent viscosity as a function of decreasing oxygen in the range of arterial oxygen tension. Our study does not find a consistent effect of HbF%. HbF% averaged over groups of patients has consistently been shown to correlate with better clinical outcomes (35, 36), though there is wide variation in the effect on individual patients. It is possible that our sample size is too small to demonstrate the modest average effect or that the HbF% effect is confounded by simultaneous variation of HbS% in our study samples. Future work is needed to elucidate the role of HbF% and F-cells on oxygen-dependent rheology. It will also be important to correlate interpatient differences in the distribution of single-RBC oxygen-dependent mechanical properties with bulk rheologic properties, as well as patient-specific differences in the effects of hydroxyurea or transfusion on these properties. Interpatient variation in in vivo oxygen tension or local gradients may also be important but will be challenging to measure.
Our previous work found that the rate of deceleration following a drop in oxygen from 160 to 0 mmHg at room temperature correlated strongly with patient clinical outcomes, more so than any other available clinical marker (16). That finding may reflect patient-specific differences in regime boundaries, differences in the nature of the oxygen-dependence in regime II, or a combination of the two effects. It seems likely based on prior single-cell work (18, 19, 20) that the patient-specific relationship between oxygen tension and apparent viscosity in regime II reflects the distribution of single-cell polymer concentrations at a given oxygen concentration across the population of flowing cells. Other factors may include the rates of change in polymer concentration following oxygen change, the intracellular morphology of polymer domains, which is likely affected by rates of oxygen change as well, and variation between RBCs in hemoglobin-oxygen dissociation curves. We also observe that the cell-free layer known to be present in vivo is greatly reduced, and patient-specific cellular or plasma factors affecting the rate of reduction in this layer may also be important and warrant future investigation.
Our study may have important implications for the development of novel treatments. Although modulation of the oxygen-independent effects exacerbating rheology (i.e., the concentration of irreversibly sickled cells) requires transfusion, the oxygen-dependent effects we show to be significant can be alleviated by increasing hemoglobin oxygen affinity, perhaps even slightly, something that can be achieved with small molecules (16). The exquisite sensitivity of bulk sickle cell blood apparent viscosity demonstrated by blood samples from patients with ∼20% HbS suggests that it will be difficult to achieve complete abrogation of oxygen dependence, but even partial modulation may reduce the magnitude of apparent viscosity change and decrease the risk of vaso-occlusive crises. Our experimental system’s ability to measure the location of the regime space boundaries may be important in optimizing drug development or monitoring patient response to existing or experimental treatments.
Conclusions
The consequences of vaso-occlusion are seen in patients throughout the vascular tree, from the relatively high-oxygen and high-velocity cerebral arteries (10, 11) to the relatively low-oxygen and low-velocity postcapillary venules (8, 9). Prevailing models of sickle cell vaso-occlusion propose mechanisms that are relevant only to regions of low oxygen and low velocity (8, 9), leaving a wide gap in our understanding of the most important pathologic process in sickle cell disease. We find that deoxygenation-dependent rheologic processes are sufficient to increase sickle cell blood apparent viscosity and slow blood flow velocity at arterial oxygen tension even without additional contributions from inflammation, adhesion, and endothelial and leukocyte activation. We quantify the changes in apparent viscosity and define a regime space of sickle cell blood flow personalized for each patient that may be important in further dissecting mechanisms of in vivo vaso-occlusion as well as in assessing risk of patient complications, response to transfusion, and the optimization of experimental therapies in development.
Author Contributions
X.L. designed experiments, performed experiments, analyzed data, and wrote the article. D.K.W. designed experiments, analyzed data, and wrote the article. J.M.H. designed experiments, analyzed data, and wrote the article.
Acknowledgments
The authors thank Anwesha Chaudhury, Frank Bunn, and Bob Hebbel for helpful discussions. For assistance with blood sample identification, collection, testing, and transport, the authors thank Judith Oakley, Kerry Breen, Sofia Shaikh, John Yablonski, Nivisha Naik, Rosy Jaromin, Alex Barrasso, Joanna Witkin, Alicia Soriano, Colin Gorman, Francine Molay, Maureen Hames-English, Linda Ardisson, Paula Carbone, and other members of the MGH Clinical Laboratories and the MGH Clinical Research Program. All video processing was performed on the Harvard Medical School Orchestra Computing Cluster. The authors thank the Minnesota Nanofabrication Center for device fabrication support.
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) grant R21HL130818. J.M.H. was also supported by NHLBI grant HL114476, and a National Institutes of Health (NIH) Director’s New Innovator Award (DP2DK098087). D.K.W. was also supported by the American Heart Association under grant 13SDG6450000.
Editor: Jochen Guck.
Footnotes
Four figures and one movie are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30283-1.
Contributor Information
David K. Wood, Email: dkwood@umn.edu.
John M. Higgins, Email: john_higgins@hms.harvard.edu.
Supporting Material
References
- 1.Weatherall D.J. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115:4331–4336. doi: 10.1182/blood-2010-01-251348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballas S.K. The cost of health care for patients with sickle cell disease. Am. J. Hematol. 2009;84:320–322. doi: 10.1002/ajh.21443. [DOI] [PubMed] [Google Scholar]
- 3.Yawn B.P., Buchanan G.R., John-Sowah J. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- 4.Beutler E. Discrepancies between genotype and phenotype in hematology: an important frontier. Blood. 2001;98:2597–2602. doi: 10.1182/blood.v98.9.2597. [DOI] [PubMed] [Google Scholar]
- 5.Serjeant G.R., Higgs D.R., Hambleton I.R. Elderly survivors with homozygous sickle cell disease. N. Engl. J. Med. 2007;356:642–643. doi: 10.1056/NEJMc066547. [DOI] [PubMed] [Google Scholar]
- 6.Weatherall M.W., Higgs D.R., Serjeant G.R. Phenotype/genotype relationships in sickle cell disease: a pilot twin study. Clin. Lab. Haematol. 2005;27:384–390. doi: 10.1111/j.1365-2257.2005.00731.x. [DOI] [PubMed] [Google Scholar]
- 7.Thomas P.W., Higgs D.R., Serjeant G.R. Benign clinical course in homozygous sickle cell disease: a search for predictors. J. Clin. Epidemiol. 1997;50:121–126. doi: 10.1016/s0895-4356(96)00320-4. [DOI] [PubMed] [Google Scholar]
- 8.Frenette P.S. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr. Opin. Hematol. 2002;9:101–106. doi: 10.1097/00062752-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Kaul D.K., Fabry M.E., Nagel R.L. Microvascular sites and characteristics of sickle cell adhesion to vascular endothelium in shear flow conditions: pathophysiological implications. Proc. Natl. Acad. Sci. USA. 1989;86:3356–3360. doi: 10.1073/pnas.86.9.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connes P., Verlhac S., Bernaudin F. Advances in understanding the pathogenesis of cerebrovascular vasculopathy in sickle cell anaemia. Br. J. Haematol. 2013;161:484–498. doi: 10.1111/bjh.12300. [DOI] [PubMed] [Google Scholar]
- 11.Switzer J.A., Hess D.C., Adams R.J. Pathophysiology and treatment of stroke in sickle-cell disease: present and future. Lancet Neurol. 2006;5:501–512. doi: 10.1016/S1474-4422(06)70469-0. [DOI] [PubMed] [Google Scholar]
- 12.Barabino G.A., Platt M.O., Kaul D.K. Sickle cell biomechanics. In: Yarmush M.L., Duncan J.S., Gray M.L., editors. Vol. 12. Annual Reviews; Palo Alto, CA: 2010. pp. 345–367. (Annual Review of Biomedical Engineering). [DOI] [PubMed] [Google Scholar]
- 13.Usami S., Chien S., Bertles J.F. Effect of deoxygenation on blood rheology in sickle cell disease. Microvasc. Res. 1975;9:324–334. doi: 10.1016/0026-2862(75)90069-2. [DOI] [PubMed] [Google Scholar]
- 14.Embury S.H. The not-so-simple process of sickle cell vasoocclusion. Microcirculation. 2004;11:101–113. doi: 10.1080/10739680490278277. [DOI] [PubMed] [Google Scholar]
- 15.Higgins J.M., Eddington D.T., Mahadevan L. Sickle cell vasoocclusion and rescue in a microfluidic device. Proc. Natl. Acad. Sci. USA. 2007;104:20496–20500. doi: 10.1073/pnas.0707122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood D.K., Soriano A., Bhatia S.N. A biophysical indicator of vaso-occlusive risk in sickle cell disease. Sci. Transl. Med. 2012;4:123ra126. doi: 10.1126/scitranslmed.3002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeBaun M.R., Gordon M., Casella J.F. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N. Engl. J. Med. 2014;371:699–710. doi: 10.1056/NEJMoa1401731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green M.A., Noguchi C.T., Stuart J. Polymerization of sickle cell hemoglobin at arterial oxygen saturation impairs erythrocyte deformability. J. Clin. Invest. 1988;81:1669–1674. doi: 10.1172/JCI113504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh T., Chien S., Usami S. Effects of hemoglobin concentration on deformability of individual sickle cells after deoxygenation. Blood. 1995;85:2245–2253. [PubMed] [Google Scholar]
- 20.Klug P.P., Lessin L.S., Radice P. Rheological aspects of sickle cell disease. Arch. Intern. Med. 1974;133:577–590. [PubMed] [Google Scholar]
- 21.Nash G.B. Red cell mechanics: what changes are needed to adversely affect in vivo circulation. Biorheology. 1991;28:231–239. doi: 10.3233/bir-1991-283-414. [DOI] [PubMed] [Google Scholar]
- 22.Nash G.B., Johnson C.S., Meiselman H.J. Influence of oxygen tension on the viscoelastic behavior of red blood cells in sickle cell disease. Blood. 1986;67:110–118. [PubMed] [Google Scholar]
- 23.Reinhart S.A., Schulzki T., Reinhart W.H. Studies on metabolically depleted erythrocytes. Clin. Hemorheol. Microcirc. 2014;56:161–173. doi: 10.3233/CH-131682. [DOI] [PubMed] [Google Scholar]
- 24.Henkelman S., Dijkstra-Tiekstra M.J., van Oeveren W. Is red blood cell rheology preserved during routine blood bank storage? Transfusion. 2010;50:941–948. doi: 10.1111/j.1537-2995.2009.02521.x. [DOI] [PubMed] [Google Scholar]
- 25.Hedberg P., Lehto T. Aging stability of complete blood count and white blood cell differential parameters analyzed by Abbott CELL-DYN Sapphire hematology analyzer. Int. J. Lab. Hematol. 2009;31:87–96. doi: 10.1111/j.1751-553X.2007.01009.x. [DOI] [PubMed] [Google Scholar]
- 26.Adler M., Polinkovsky M., Groisman A. Generation of oxygen gradients with arbitrary shapes in a microfluidic device. Lab Chip. 2010;10:388–391. doi: 10.1039/b920401f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall J.E., Guyton A.C. Saunders/Elsevier; Philadelphia, Pa: 2011. Guyton and Hall Textbook of Medical Physiology. [Google Scholar]
- 28.Higgins J.M., Eddington D.T., Mahadevan L. Statistical dynamics of flowing red blood cells by morphological image processing. PLOS Comput. Biol. 2009;5:e1000288. doi: 10.1371/journal.pcbi.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Needleman J.P., Setty B.N., Allen J.L. Measurement of hemoglobin saturation by oxygen in children and adolescents with sickle cell disease. Pediatr. Pulmonol. 1999;28:423–428. doi: 10.1002/(sici)1099-0496(199912)28:6<423::aid-ppul7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 30.Fowler N.O., Smith O., Greenfield J.C. Arterial blood oxygenation in sickle cell anemia. Am. J. Med. Sci. 1957;234:449–458. doi: 10.1097/00000441-195710000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Uchida K., Rackoff W.R., Asakura T. Effect of erythrocytapheresis on arterial oxygen saturation and hemoglobin oxygen affinity in patients with sickle cell disease. Am. J. Hematol. 1998;59:5–8. doi: 10.1002/(sici)1096-8652(199809)59:1<5::aid-ajh2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 32.Jones S.W. Pulse oximetry in sickle cell disease. Arch. Dis. Child. 1994;70:71. doi: 10.1136/adc.70.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wall M.A., Platt O.S., Strieder D.J. Lung function in children with sickle cell anemia. Am. Rev. Respir. Dis. 1979;120:210–214. doi: 10.1164/arrd.1979.120.1.210. [DOI] [PubMed] [Google Scholar]
- 34.Tsai M., Kita A., Lam W.A. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J. Clin. Invest. 2012;122:408–418. doi: 10.1172/JCI58753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Platt O.S., Brambilla D.J., Klug P.P. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N. Engl. J. Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 36.Platt O.S., Thorington B.D., Kinney T.R. Pain in sickle cell disease. Rates and risk factors. N. Engl. J. Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.