Abstract
Two cholesterol recognition/interaction amino-acid consensus peptides, N-acetyl-LWYIKC-amide, and N-acetyl-CLWYIK-amide, have been coupled to exchangeable mimics of Chol (cholesterol) and Phos (1,2-dipalmitoyl-sn-glycerol-3-phospho-(1′rac-glycerol)) via disulfide bond formation. Equilibration between Chol and Phos via thiolate-disulfide interchange reactions has revealed that both peptides favor Chol as a nearest-neighbor in liquid-disordered (ld) bilayers to the same extent. In contrast, no Chol- or Phos-recognition could be detected by these peptides in analogous liquid-ordered (lo) bilayers. Fluorescence measurements of the tryptophan moiety have shown that both peptides favor the membrane-water interface. Taken together, these results provide strong evidence that the recognition behavior of the LWYIK motif is, fundamentally, a surface phenomenon but that partial penetration into the bilayer is also necessary.
Main Text
Understanding how lipids and proteins interact with one another in cell membranes, and defining their time-averaged lateral organization, represent two major challenges presently facing cell biologists, biochemists, and biophysicists (1, 2, 3, 4). Although it is widely believed that the two-dimensional structure of cell membranes plays a key role in the overall functioning of cells, it has proven difficult to characterize membrane organization at the molecular level even in the simplest of model systems.
One popular concept involving lipid-protein interactions that has emerged in recent years is based on the cholesterol recognition/interaction amino-acid consensus (CRAC) hypothesis (5, 6, 7, 8). According to this hypothesis, those segments of an integral membrane protein that lie close to the membrane surface having the sequence (L/V)-X1-5-(Y)-X1-5-(K/R) have a special affinity toward cholesterol. Despite its popularity, experimental evidence in support of this hypothesis has been largely circumstantial.
We have recently begun a bottom-up approach to the study of the CRAC hypothesis, starting with the minimal CRAC peptide, LWYIK. This peptide was selected based on its simplicity and because it appears to play an important role in the fusion protein gp41 found in HIV-1 (7, 8). Specifically, this highly conserved segment has been found to be a determinant for viral infection (7). In addition, there is significant evidence indicating that cholesterol in target cells is required for fusion with HIV virions. This finding strongly suggests that LWYIK plays a critical role in the fusion process through its ability to associate with cholesterol in membranes.
To investigate the cholesterol-recognition properties of LWYIK at the molecular level, we have employed the nearest-neighbor recognition (NNR) method. As discussed elsewhere, NNR measurements afford unique thermodynamic insight into the interactions between membrane components (9). In brief, two membrane components of interest (A and B) are converted into exchangeable, disulfide-based homodimers (AA) and heterodimers (AB) and are allowed to undergo monomer exchange in host liposomes via thiolate-disulfide displacement reactions. Nearest-neighbor recognition is then reflected by an equilibrium constant, K, that differs from 4.0 (random mixing) where K = (AB)2/(AA)(BB).
Recently, we have shown that LWYIK, having Cys attached to its C-terminus (i.e., Pepc), favors association with an exchangeable sterol (Chol, cholesterol) over an exchangeable phospholipid (Phos, 1,2-dipalmitoyl-sn-glycerol-3-phospho-(1′rac-glycerol)) in the liquid-disordered (ld) state (Fig. 1) (10). However, no such recognition by Pepc could be detected in the liquid-ordered (lo) state. Negative control experiments that were carried out with a non-CRAC peptide, VGVAPG (found in elastin), showed no nearest-neighbor preference for Chol or Phos in either the ld or the lo phases (10).
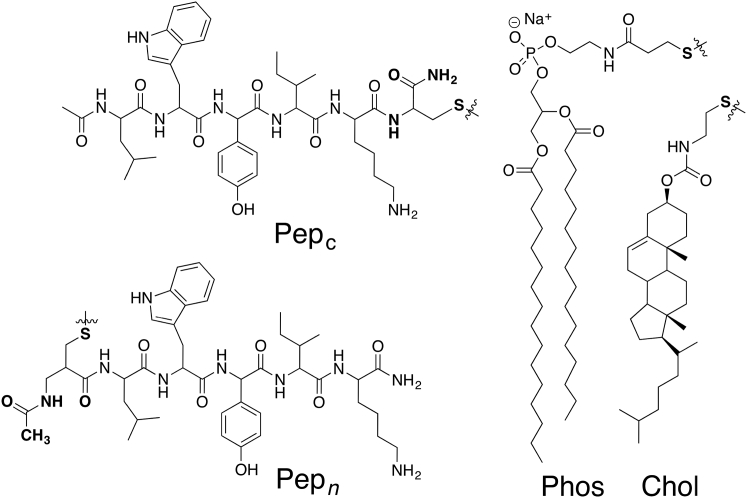
Figure 1.
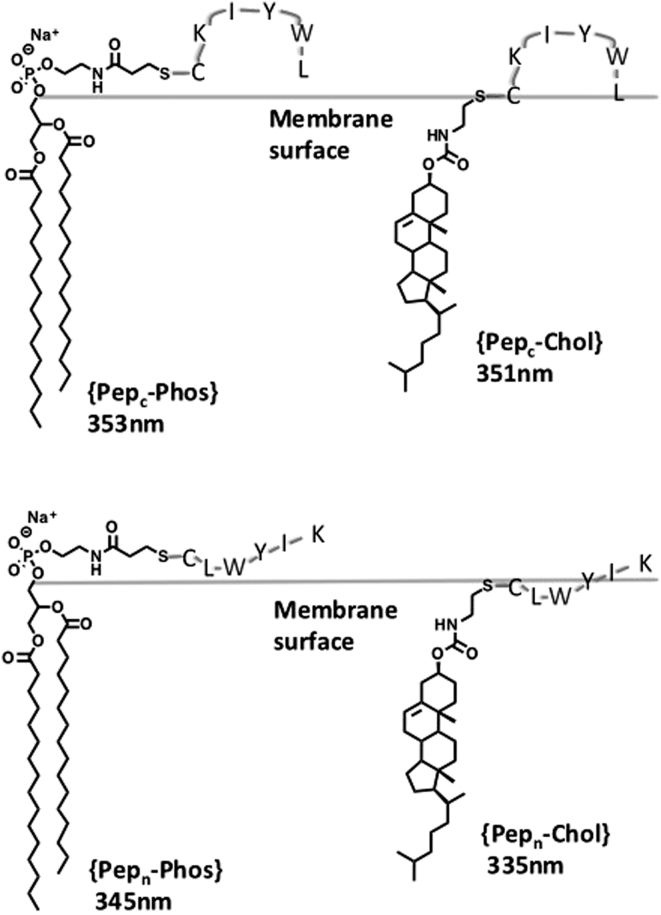
Structures of exchangeable lipids and peptides.
Here, we sought to test the hypothesis that the recognition behavior of LWYIK is largely confined to the membrane surface. In principle, if such recognition were a surface phenomenon where the CRAC motif wraps around the A-ring of the sterol nucleus, as has been previously proposed, then a similar degree of recognition would be expected when the peptide is attached via its N-terminus (Pepn) as compared with this C terminus (Pepc) (8). For both modes of attachment, the peptide’s hydrophobic- and hydrogen bond-interactions with the A-ring should be similar. However, if the peptide were to extend into the bilayer, then different amino acids would be interacting with the same segments of a neighboring sterol nucleus, and, a different degree of recognition is expected.
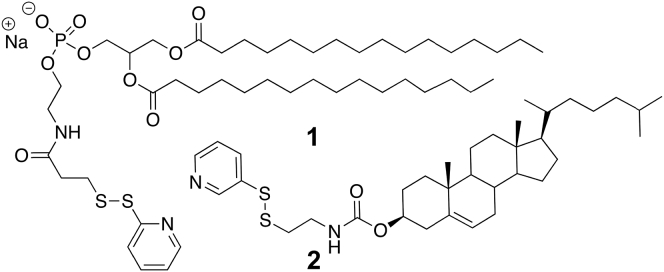
To probe this question, we synthesized four lipid conjugates of LWYIK: i.e., {Pepc-Phos}, {Pepc-Chol}, {Pepn-Phos}, and {Pepn-Chol}, where each dimer bears a disulfide linkage (Fig. 1). These conjugates were readily prepared via thiolate-disulfide interchange between N-acetyl-LWYIKC-amide and N-acetyl-CLWYIK-amide with the activated lipids, 1 and 2 (Fig. 2).
Figure 2.
Activated forms of Phos and Chol.
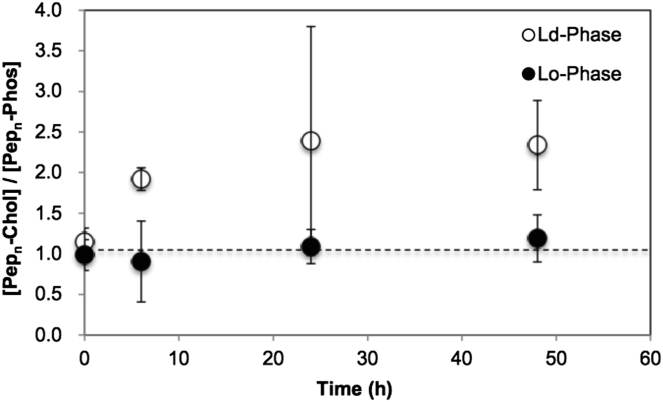
To determine whether Pepn is capable of favoring Chol over Phos, we carried out NNR measurements both in host membranes made from DPPC (1,2-dipalmitoyl-sn-glycerol-3-phosphocholine) and in ones made from DPPC that were rich in cholesterol, at 45°C. The former were in the ld phase and the latter were in the lo phase (11). The progress of the interchange was monitored by following the molar ratio of {Pepn-Chol}/{Pepn-Phos}, and also the formation of {Phos-Chol} as a function of time (Fig. 3). The latter confirms that monomer exchange has, in fact, occurred in the lo phase (see the Supporting Material).
Figure 3.
Plot of the molar ratio, {Pepn-Chol}/{Pepn-Phos}, as a function of time at 45°C in (●) cholesterol-rich and (○) cholesterol-poor bilayers.
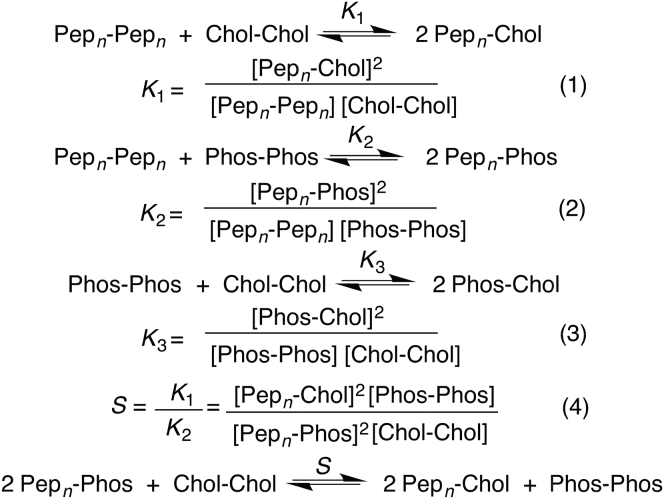
As discussed elsewhere, when three exchangeable monomers are involved in a NNR experiment (e.g., Pepn, Phos, and Chol), three equilibria exist that are governed by Eqs. 1–3 (Fig. 4) (10). Here, the ratio K1/K2 (or selectivity, S) is a measure of the peptide’s preference for associating with Chol (10). Our principal findings are summarized in Table 1. Based on its selectivity (S) values, the preference that Pepn has in becoming a nearest-neighbor of Chol in the ld phase is virtually the same as that found with Pepc. In addition, Pepn shows the same lack of selectivity that Pepc exhibits in the lo phase. Thus, turning around the peptide has no significant influence on its selectivity properties.
Figure 4.
Dimer equilibria.
Table 1.
Recognition of Chol by Pepn and Pepc
| Phase | Peptide | K1 | K2 | K3 | Ra | S |
|---|---|---|---|---|---|---|
| ld | Pepn | 0.68 ± 0.25 | 0.10 ± 0.04 | 2.39 ± 0.83 | 2.34 ± 0.55 | 7.01 ± 3.99 |
| lo | Pepn | 0.67 ± 0.25 | 0.85 ± 0.49 | 9.23 ± 4.22 | 1.19 ± 0.29 | 0.79 ± 0.54 |
| ldb | Pepc | 2.08 ± 0.71 | 0.23 ± 0.07 | 3.05 ± 1.15 | 2.50 ± 0.37 | 8.89 ± 4.08 |
| lob | Pepc | 0.21 ± 0.12 | 0.26 ± 0.07 | 7.40 ± 1.55 | 0.95 ± 0.45 | 0.81 ± 0.78 |
Molar ratio of Pepn-Chol/Pepn-Phos or Pepc-Chol/Pepc-Phos.
Data taken from Mukai et al. (10).
To gain insight into the favored location of the LYWIK motif in the ld phase, where Chol recognition was found, we examined the fluorescence of the tryptophan moiety. As previously shown, the λmax value of W is very sensitive to the polarity of its microenvironment; i.e., aqueous, interfacial, and hydrophobic microenvironments are reflected by λmax values of near 355, 345, and 335 nm, respectively (12).
Using host liposomes made from DPPC that were maintained at 45°C, the λmax values for {Pepc-Phos}, {Pepc-Chol}, {Pepn-Phos}, and {Pepn-Chol} were 353 ± 0.09, 351 ± 0.01, 345 ± 0.77, and 335 ± 0.44 nm, respectively. These results reveal a floppy LWYIK segment that favors the membrane-water interface. Thus, the very wet W in {Pepc-Phos} and {Pepc-Chol} can be accounted for by a polar lysine group that separates the hydrophobic IYWL segment from the hydrocarbon interior of the membrane, thereby reducing hydrophobic interactions between them (Fig. 5) (13). The drier W groups that are found in {Pepn-Phos} and {Pepn-Chol} have their lysine group distal from the headgroup of the lipids. This positioning can allow for greater hydrophobic interactions between IYWL and the membrane interior; here K can hang out in the aqueous phase. Finally, the driest W that was found in {Pepn-Chol} is a likely result of having a distal lysine group and having the peptide attached to Chol, which can penetrate deeper into the membrane.
Figure 5.
A stylized illustration showing four lipid-peptide conjugates at a water/membrane interface with hypothetical peptide conformations.
The fact that turning around LWYIK does not alter its selectivity and that it favors the membrane surface constitutes strong evidence that its recognition behavior is, fundamentally, a surface phenomenon. The fact that such recognition occurs only in the ld phase further implies that partial penetration into the bilayer is necessary. From a biological standpoint, these results provide a firm basis for believing that CRAC motifs, typically found in the juxtamembrane region of proteins, play an important role in defining the protein’s lateral organization.
Author Contributions
M.M. carried out all NNR studies; K.J.G. recorded fluorescence measurements; and S.L.R. designed the research and wrote the article.
Acknowledgments
We are grateful to Dr. Maxim Chudaev for valuable technical assistance.
We are also grateful to the National Science Foundation (grant No. CHE-1145500 to S.L.R.) and the National Institutes of Health (grant No. R01-GM093258-01A1 to K.J.G.) for financial support.
Editor: Kalina Hristova.
Footnotes
Supporting Materials and Methods, one scheme, seven figures, and six tables, are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30284-3.
Supporting Material
References
- 1.Nicolson G.L. The fluid-mosaic model of membrane structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta. 2014;1838:1451–1466. doi: 10.1016/j.bbamem.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Goñi F.M. The basic structure and dynamics of cell membranes: an update of the Singer-Nicolson model. Biochim. Biophys. Acta. 2014;1838:1467–1476. doi: 10.1016/j.bbamem.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Brown D.A., London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 4.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 5.Li H., Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998;139:4991–4997. doi: 10.1210/endo.139.12.6390. [DOI] [PubMed] [Google Scholar]
- 6.Fantini J., Barrantes F.J. How cholesterol interacts with membrane proteins: an exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 2013;4:31. doi: 10.3389/fphys.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S.S., Yang P., Huang S.-C. Identification of the LWYIK motif located in the human immunodeficiency virus type 1 transmembrane gp41 protein as a distinct determinant for viral infection. J. Virol. 2009;83:870–883. doi: 10.1128/JVI.01088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epand R.F., Thomas A., Epand R.M. Juxtamembrane protein segments that contribute to recruitment of cholesterol into domains. Biochemistry. 2006;45:6105–6114. doi: 10.1021/bi060245+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause M.R., Regen S.L. The structural role of cholesterol in cell membranes: from condensed bilayers to lipid rafts. Acc. Chem. Res. 2014;47:3512–3521. doi: 10.1021/ar500260t. [DOI] [PubMed] [Google Scholar]
- 10.Mukai M., Krause M.R., Regen S.L. Peptide recognition of cholesterol in fluid phospholipid bilayers. J. Am. Chem. Soc. 2015;137:12518–12520. doi: 10.1021/jacs.5b08132. [DOI] [PubMed] [Google Scholar]
- 11.Sankaram M.B., Thompson T.E. Cholesterol-induced fluid-phase immiscibility in membranes. Proc. Natl. Acad. Sci. USA. 1991;88:8686–8690. doi: 10.1073/pnas.88.19.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladokhin A.S., Jayasinghe S., White S.H. How to measure and analyze tryptophan fluorescence in membranes properly, and why bother? Anal. Biochem. 2000;285:235–245. doi: 10.1006/abio.2000.4773. [DOI] [PubMed] [Google Scholar]
- 13.de Planque M.R.R., Kruijtzer J.A.W., Killian J.A. Different membrane anchoring positions of tryptophan and lysine in synthetic transmembrane α-helical peptides. J. Biol. Chem. 1999;274:20839–20846. doi: 10.1074/jbc.274.30.20839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.