Abstract
The hyaluronan-rich pericellular matrix (PCM) plays physical and chemical roles in biological processes ranging from brain plasticity, to adhesion-dependent phenomena such as cell migration, to the onset of cancer. This study investigates how the spatial distribution of the large negatively charged bottlebrush proteoglycan, aggrecan, impacts PCM morphology and cell surface access. The highly localized pericellular milieu limits transport of nanoparticles in a size-dependent fashion and sequesters positively charged molecules on the highly sulfated side chains of aggrecan. Both rat chondrocyte and human mesenchymal stem cell PCMs possess many unused binding sites for aggrecan, showing a 2.5x increase in PCM thickness from ∼7 to ∼18 μm when provided exogenous aggrecan. Yet, full extension of the PCM occurs well below aggrecan saturation. Hence, cells equipped with hyaluronan-rich PCM can in principle manipulate surface accessibility or sequestration of molecules by tuning the bottlebrush proteoglycan content to alter PCM porosity and the number of electrostatic binding sites.
Introduction
The cell interface is critical in mediating the integration of cells into tissue via extracellular matrix (ECM) and cell-cell contacts. Cells that maintain hyaluronan-rich sugar coats on their surfaces may have enhanced capabilities to manipulate their very local environment and hence direct their interactions with cells and the ECM (1, 2) (Fig. 1). This is particularly relevant in dynamic tissue environments, where sugar cell coats are involved in the regulation of a wide range of processes that involve cell rearrangements including cell migration (3, 4, 5), division (6), synaptogenesis (7), morphogenesis (8, 9), cancer progression and metastasis (10, 11, 12). The aim of this study is to investigate the physical role that the hyaluronan sugar coat plays in mediating cell surface access.
Figure 1.
(a) Cross-sectional confocal image of pericellular matrix swollen with exogenous aggrecan on RCJ-P cell (cACAN = 333 μg/mL, yellow = aggrecan, white = plasma membrane). The PCM extends in a three-dimensional halo around the cell, with aggrecan intensity visible at distances more than 20 μm from cell surface. (b) Schematic of the PCM. Hyaluronan polymers (green) bind to the plasma membrane via HA-binding membrane proteins (blue). Bottlebrush proteoglycans (black) bind along the chain and stretch the HA into an extended configuration. (c) Erythrocyte particle exclusion assay shows the extent of the cell surface-grafted PCM on aggrecan-saturated mesenchymal stem cell (333 μg/mL for 2 h). (d) Erythrocyte particle exclusion assay shows the extent of the cell surface-grafted PCM on a native RCJ-P cell. To see this figure in color, go online.
The potential significance and impact of the hyaluronan-rich cell coat, also called the pericellular matrix (PCM), is immediately evident when glancing at a cell endowed with a cell coat (Fig. 1). On many cell types, the PCM extends more than 5 μm from the cell surface and on some cell types it extends up to 20 μm (2). Indeed, PCM visualization is traditionally achieved by using one of the important properties of this surface-anchored polymer matrix, i.e., its ability to repel objects from the cell surface (Fig. 1 b). There are three essential components in the cell coat (2): the linear carbohydrate hyaluronan (HA), HA-binding proteins at the cell surface, and HA-binding bottlebrush-shaped proteoglycans, also known as hyalectins (13). In the PCM, HA polymers are anchored to the cell surface by proteins (e.g., CD44, RHAMM) or they remain tethered to the enzyme HA synthase, which directly extrudes HA from the plasma membrane. With lengths ranging from ∼2 to 25 μm (1), cell-surface tethered HA becomes significantly stretched when it aggregates bottlebrushes along its chain. The family of hyalectins, which includes aggrecan, versican, neurocan, and brevican, consists of core proteins that are extremely similar within the N- and C-terminal globular domains and have central domains of variable length with multiple sites for the addition of chondroitin sulfate or dermatan sulfate chains and O-linked oligosaccharides. The hyalectins are present in the ECM and PCM of many tissues, with aggrecan featured in cartilage, brevican and neurocan abundant in the central nervous system, and versican expressed in most soft tissues of the body (14). All hyalectins contain a common HA-binding domain (G1) that allows them to aggregate densely along HA, nearly permanently when reinforced by an additional protein called link protein (15). The key physical characteristics of the bottlebrush proteoglycans stem from a second domain (G2) that contains a dense region of highly sulfated glycosaminoglycans. The third and last domain (G3) is smaller and consists of specific binding sites for various biomolecules. Cell coats can exist without bottlebrushes but unless the HA is grafted so densely that a polymer brush forms (as on some bacteria (16)), the PCM is relatively thin even when consisting of large HA molecules. It is the aggregation of bottlebrush proteoglycans in the PCM that generates its voluminous expansion on most mammalian cells (15, 17), and which dominates the physical and chemical functions of the PCM.
Little work has focused on spatial characterization of bottlebrush molecules in the cell coat or how their distribution and physical properties impact accessibility of the cell surface. Most biophysical studies have focused on structural and biomechanical properties (18, 19, 20, 21). Although chondroitin sulfate proteoglycans are known to have important biochemical roles (14, 22), their unusually large size, their ability to be densely aggregated on HA, and their high negative charge should also strongly influence the physical interaction of proteoglycan-rich PCM (and ECM) with molecules (22), cells (23), and particles (24) including drug delivery vehicles, exosomes, and supermolecular aggregates. This work examines the spatial distribution of bottlebrush proteoglycans in the PCM of living cells. We demonstrate how objects 40 nm and larger are altered in their access to the cell surface; we report that free bottlebrushes diffuse through the PCM, and demonstrate that positively charged molecules become sequestered in the PCM on chondroitin sulfate side chains of bottlebrush proteoglycans. These systematic investigations have direct implications for PCM function and are a first step toward delineating the physical roles of bottlebrush proteoglycans in diverse range of contexts and tissues.
Materials and Methods
All data are reported with two standard errors in text and in plots.
Cell culture and sample preparation
Rat chondrocyte joint (RCJ-P) cells (fetal calvaria, batch 15.01.98; Prochon Biotech, Rehovot, Israel) were cultured under 37°C, 5% CO2 with α-minimum essential medium, 15% fetal bovine serum (FBS), 2% L-glutamine. Mesenchymal stem cells (MSC) (human bone marrow-derived, Texas A&M, College Station, TX) were cultured at 37°C, 5% CO2 with α-minimum essential medium, 16.5% FBS, 2 mM L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin. Cells were seeded on glass coverslips in Teflon sample holders for ∼20 h to achieve 50% confluency of the 0.8 cm2 surface with 2 mL of media. During the imaging, samples were held at 37°C, 5% CO2 and 80% humidity with a stage-top incubator (N.E.-MSI 07-3156; Okolab, Ottaviano, Italy).
Particle exclusion assay
Traditional particle exclusion assay
Fixed sheep erythrocytes (R3378; Sigma Aldrich, St. Louis, MO), were suspended in phosphate buffered saline (PBS) and then added to the sample with a final concentration of 1 mg/mL to achieve monolayer after 10 min. Thickness of the PCM was measured to be the perpendicular distance from the cell plasma membrane (at the flattest location on the cell, usually the middle) to the nearest erythrocyte.
Quantitative PEA
Fluorescent polystyrene spheres (FluoSpheres; Invitrogen, Waltham, MA) of different sizes were passivated before use in quantitative particle exclusion assay (qPEA). Larger particles (>200 nm) were reversibly swollen with toluene to enable physical entanglement of Pluronic F127 as previously described (25). Smaller particles were covalently modified with methoxypolyergylene glycol amine (Fluka, St. Louis, MO) (26). For qPEA, 22 μL of the microspheres (5% solid) were added to 178 μL media and allowed to settle for 10 min. To facilitate image analysis, the cell surface was fluorescently labeled with wheat germ agglutinin (WGA-Alexa Fluor 633 conjugate; Sigma), which was added to the sample (125 μg/mL) 5 min before measurements were obtained. The samples were imaged with a confocal microscope (FV1000; Olympus, Tokyo, Japan). Microsphere distribution was measured by taking the fluorescence intensity perpendicular to the cell surface at 3 μm above the glass surface and averaged over 2 μm. The effective thickness, deff, identified to be the distance between cell surface and the plateau in the average bead intensity.
Bacterial PEA
Cells were intentionally exposed to nonsterile environment in lab and allowed to grow resultant bacterial infection.
Time-dependent swelling of PCM
PCM of RCJ-P cells were visualized with PEA. 333 μg/mL aggrecan was then added to the sample. The PEA is then used to monitor the aggrecan-driven extension of the cell coat versus time.
Fluorescent protein labeling
Bovine articulate aggrecan (A1960; >2500 kDa, Sigma Aldrich) was dissolved in PBS to reach 2 mg/mL. Histone type II-A (H9250; ∼20 kDa, Sigma Aldrich) was dissolve in PBS to reach 1 mg/mL. Similar procedures used for bovine serum albumin (66 kDa, AP-4510-60; SeraCare Life Sciences, Milford, MA). Fluorescent-labeling was achieved by incubating the protein with ATTO NHS ester dye at 2 mg/mL for 1 h followed by a Zeba desalting column (89882; Thermo Scientific, Waltham, MA) to remove excess dye.
Optical force probe assays
Optical force probe assays were performed by using a static calibrated optical trap (OT) and a programmable stage (ProScan H117; Prior Scientific, Rockland, MA). In a typical experiment, an OT holding a 3 μm passivated microsphere was positioned 20 μm outside of the PCM, where it could be translated toward and away (orthogonally) to the cell surface at 8 μm/s. The OT was paused for 5 s at the cell surface (always at a distance of 3 μm) and outside of the matrix. The forces on the probe were extracted by using a standard subpixel particle-tracking algorithm (27) to find the bead position in the OT. The bead height above the coverslip was ∼5 μm.
Fluorescent aggrecan exchange assay
Cells were incubated with the fluorescent aggrecan (333 μg/ml concentration) for 2 h at 37°C and 5% CO2. Media was replaced with fresh media without aggrecan before imaging the initial fluorescent aggrecan distribution with a confocal microscope (FV1000; Olympus, Tokyo, Japan). The media was then replaced (within 1 min) with nonfluorescently labeled aggrecan solution (333 μg/ml concentration), and then imaged over time to monitor the fluorescent intensity of the aggrecan in the PCM. All fluorescent intensity profiles were taken perpendicular to the center of the cells at 3 μm height and averaged over 2 μm.
GFPn labeling of hyaluronan
Fluorescent labeling of HA was achieved using neurocan-G1 EGFP fusion protein (GFPn) expressed by HEK 293 EBNA cells, which were cultured at 37°C and 5% CO2 in media (50% Dulbecco’s modified eagle medium and 50% F12), with 10% FBS, 1% L-glutamine with 0.1% puromycin (EMD Biosciences, San Diego, CA) (28, 29). The cells were a gift from Uwe Rauch (Dept. Experimental Medical Science, Biomedical Center, Lund University, Sweden). Cells were grown to confluence, at which time serum was removed from culture media and the cells were then incubated for 48 h under serum-free conditions. 50 mL of conditioned media was collected from a total of 5 culture flasks (250 mL) and concentrated to ∼2 mL by centrifugal ultrafiltration with a membrane cut-off of 30 kDa (Millipore, Billerica, MA). The His-tagged fusion protein was purified on a HisPur Cobalt spin column (Thermo Scientific, Rockford, IL). During HA labeling, 10 μL of GFPn solution was added to 90 μL of media covering the cells for 15 min incubation.
Chondroitin sulfate digestion assay
Chondroitinase ABC (ChABC) from Proteus vulgaris (C2905; Sigma Aldrich) was used to digest the chondroitin sulfate side chains of aggrecan. ChABC was dissolved in PBS and then directly added to cell sample to achieve a final concentration of 0.27 units/mL.
Results and Discussion
PCM swells with increasing aggrecan concentration
The bottlebrush proteoglycans largely determine the extent of the PCM (together with HA length). The PCM can be removed from the cell surface by digestion of the HA with enzymes; or it can be collapsed to a thin layer at the cell surface by digestion of the chondroitin sulfate side chains on the proteoglycans. Treatment of RCJ-P chondrocyte cells and human MSC cells with ChABC nearly eliminates the native 7 μm thick PCM on both cell types (Fig. S1 in the Supporting Material). Induction of versican expression increases the thickness of PCM on smooth muscle cells (30), whereas work in our lab confirms that downregulation of aggrecan expression with si-RNA in RCJ-P cells eliminates the PCM detectable by particle exclusion assay or optical force probe assays (data not shown). Interestingly, Knudson et al. showed that both digested and displaced pericellular matrices can be reconstructed by the addition of exogenous HA and aggrecan, where aggrecan is an essential component for the detectable matrix by particle exclusion assays (15, 17).
Following a similar approach to that of Knudson but without first digesting the cell coat, we examined how the equilibrium thickness of the PCM varies with the solution concentration of exogenous aggrecan. For RCJ-P and MSC cells, the PCM width increases until it plateaus at a value 250% larger than its original thickness (7.0 ± 0.9 μm; 6.6 ± 1.6 μm; NRCJP = 114; NMSC = 72). For the RCJ-P cells, the PCM thickness reaches a maximum size of 17.8 ± 1.2 μm at cACAN = 180 μg/mL (N = 73). For the MSC cells, the critical concentration to achieve maximal extension is slightly higher, cACAN = 260 μg/mL, with a similarly large extent of 18.0 ± 1.0 μm (N = 75). These data were obtained by image analysis of traditional erythrocyte PEA (Fig. 1 c) of cells after incubation with exogenous aggrecan for 2 h (31). We performed time-dependent studies of the swelling of the PCM (cACAN = 333 μg/mL) to confirm that 2 h is sufficient to reach equilibrium (Fig. 2 b). This is consistent with Knudson et al., who waited 30 min and 2 h, respectively, to reconstitute cell coats on hyaluronidase-treated chondrocytes (17) and other cell types (15) using much higher concentrations of aggrecan (2 mg/mL) together with exogenous HA (cHA = 1 mg/mL).
Figure 2.
(a) PCM thickness measured perpendicular to the cell surface on human bone marrow-derived MSC and RCJ-P versus exogenous aggrecan concentration. (b) Time-dependent swelling of the PCM after addition of aggrecan shows that reaching equilibrium extension requires 1 h (cACAN = 333 μg/mL). Data is an average for N = 10 cells with two standard errors reported. To see this figure in color, go online.
It is unlikely that the PCM swelling is due to increased HA synthesis or elongation of HA synthase-bound HA strands. HA synthesis is regulated through transcriptional activation of HA synthase by growth factors and cytokines or regulated by posttranscriptional events like phosphorylation (32). Furthermore, in their reconstituted PCM assays, Knudson et al. performed controls comparing the final thickness of the PCM on living and fixed cells, demonstrating the same outcome (15, 17). Instead, we infer that the PCM swells as aggrecan binds to free sites on the HA, further stretching out of the polymer in an aggrecan concentration-dependent manner (33, 34). Swelling might also be partly driven by the formation of a polymer brush type scenario, because overlap of the grafted HA strands might increase with exogenous aggrecan binding (35, 36). This is consistent with our observation that bottlebrush-free (via digestion) PCMs are thin, suggesting they have low grafting densities that put them in the mushroom rather than polymer brush regime.
The significant increase in PCM thickness provides the somewhat surprising insight that many of the bottlebrush binding sites in the PCM remain available despite the already naturally swollen coats of RCJ-P and MSC cells (∼7 μm). Furthermore, for the PCM to expand to such large sizes, particularly on the RCJ-P cells, which we have shown has no measurable cross-linking in the PCM (19), the HA strands must be at least 18 μm in length (∼7.5 MDa, 1 disaccharide∼1 nm ∼400 Da (37)) or it requires that the PCM is comprised in part by free HA strands entangled with surface bound strands (38). This is unlikely, however, as several studies of biomimetic HA-aggrecan systems suggest that such uncross-linked multilayers do not occur (39, 40). Finally, we infer differences between the MSC and RCJ-P cell PCMs by comparing the difference in exogenous aggrecan concentration to reach maximal PCM. Although the final size is similar, higher aggrecan concentration is needed for the MSC, suggesting more free HA binding sites than in the RCP-P cell coat. In the next sections, we present data on the distribution of the available binding sites in the PCM of RCJ-P cells, and explore how tuning aggrecan concentration can affect cell interactions with the surroundings.
PCM maximal extension occurs before bottlebrush saturation
The spatial distribution of PCM-tethered bottlebrush molecules likely dominates access to the cell surface by molecules, nanoparticles, exosomes, and the protrusions/surfaces of other cells. Yet, few quantitative data support this hypothesis (41). For example, there is no accurate spatial map of the endogenous aggregating proteoglycans in the PCM on single living cells. In most studies, the natural PCM structure is destroyed by fixation before labeling for bottlebrush proteoglycans (38, 42). Indeed, despite our own efforts, we have been unable to reliably label endogenous aggrecan on living RCJ-P cells using standard methods.
In an alternative approach, we use fluorescently labeled exogenous aggrecan to study the spatial distribution of aggrecan in the PCM. Figs. 1 a and 3 a present typical examples of a cell coat enriched with aggrecan (333 and 39 μg/mL, 2 h). Profiles of the variation of aggrecan fluorescence versus distance to the cell surfaces were extracted from the same position of the PCM as used for PEA thickness. Confocal images of the PCM taken at a fixed height (3 μm) above the coverslip show that at all solution concentrations, the bound aggrecan intensity peaks close to the cell surface and then decays rapidly (Fig. 3 b). The curves in Fig. 3 b show the average intensity distribution at different concentrations (cACAN = 39, 260, 333, 460 μg/mL, N∼20 for each). Fits to the averaged data reveal an exponentially decaying curve for all aggrecan concentrations. At higher concentrations, the intensity decreases less rapidly, possibly due to the stretched out HA and its more uniform coverage by aggrecan. At distances beyond the edge of the PCM detected by PEA, there is a nonnegligible background fluorescence.
Figure 3.
(a) Fluorescently labeled exogenous aggrecan at low concentration (39 μg/mL) swells the PCM. (b) Profiles of the aggrecan distribution versus distance to the cell surface at four concentrations. To see this figure in color, go online.
The results indicate that many of the available binding sites in the PCM are located within a few microns of the cell surface. The majority of the incorporated exogenous aggrecan appears within 6 μm of the plasma membrane (67%, 70%, 50%, 56%, 50%, 55% for concentrations cACAN = 39/95/180/260/333/460 μg/mL).
For concentrations of 180 μg/mL and above, >50% of the aggrecan are incorporated within the inner 35% of the PCM. This is due to a higher density of HA strands in this range, whereas a lower number of long HA strands are available to stretch from the cell (18). This is visible, for example in Fig. 1 a. The PCM of these cells extends to an average of ∼18 μm under these conditions according to PEAs and is visible out to ∼12 μm using fluorescent aggrecan profiles. Speckles representing fluorescent aggrecan are apparent in the region beyond about ∼10 μm. In a related work, we use this observation to study the motion of individually bound aggrecan molecules as a function of distance to the cell surface.
Little difference is apparent between the fluorescent profiles of cells treated with cACAN = 333 vs. cACAN = 460 μg/mL aggrecan. This indicates that the free binding sites saturate at values above 333 μg/mL. Interestingly, the saturation value is much higher than the solution concentration needed to fully stretch out the PCM (cACAN = 180 μg/mL). These data provide evidence that the HA strands stretch to near full extension and then the remaining binding sites along the HA are filled by additional bottlebrush molecules.
Fluorescent aggrecan assay reveals free binding sites in PCM
The equilibrium distribution of exogenous aggrecan shown in Fig. 3 b depends in part on the binding affinity of the endogenous aggrecan. Previous studies suggest that in vitro and in vivo endogenous aggrecan is very strongly bound to HA due to its reinforcement by link protein, which simultaneously binds both to the aggrecan and HA (14). Knudson et al. state that the link protein stabilized binding of aggrecan is nondissociating and nondisplaceable under physiological conditions (43). Our data are consistent with the endogenous aggrecan stability in the PCM of RCJ-P cells. For example, exchange of media does not significantly alter the PCM size suggesting that the majority of endogenous aggrecan are stably bound and unaffected by shifts in aggrecan solution concentration (Fig. S2). We therefore estimate that the majority of endogenous aggrecan in the cell coats remains localized during the treatments for our studies.
Thus, the profiles of exogenous aggrecan at saturation (>260 μg/mL) provide a rough map of the available binding sites in the PCM. This distribution should not be confused, however, with the availability of binding sites along a single HA strand, since the data are weighted by the natural density variation of HA in the PCM. Fluorescent labeling of the HA shows that the RCJ-P cell coat has a higher density of HA near the cell surface, which decays exponentially in the absence of exogenous aggrecan (18). Such spatial concentration variation arises in part from the physics of adsorbed HA strands (44), but also is likely due to HA polydispersity. As expected, when aggrecan-saturated PCMs are labeled for HA, the aggrecan and hyaluronan have nearly identical profiles (Fig. S3).
The remarkable implication from these observations is that RCJ-P cells have access to a sizable parameter space, cACAN = 180 to 333 μg/mL, over which they can maintain a maximized PCM thickness, while leaving the possibility to tune the aggrecan concentration and hence the binding sites for molecular sequestration (specific and nonspecific sites on the bottlebrushes) and the PCM permeability to nanoparticles, extracellular matrix, and cell protrusions.
Bottlebrush-enriched PCM has reduced pore size and permeability to nanoparticles
The transport of molecules and nanoparticles to the cell surface is broadly relevant for understanding cell-cell communications, drug delivery, and tissue homeostasis. The local physicochemical environment associated with the PCM is an additional layer worth investigating independently from the ECM that will impact these processes. Previous work has examined the permeability of the cell coat to nanoparticles (18, 19, 41, 45, 46, 47), the free diffusion of particles in the PCM (18, 45, 46, 47), and quantified the variations in porosity of the PCM using qPEA and optical force probe microscopy (19, 41). Here, we use qPEAs to characterize changes in PCM permeability and porosity after it has been saturated with exogenous aggrecan (cag = 333 μg/mL).
In earlier work, we showed that inert nanoparticles, unlike erythrocytes, are able to penetrate into the RCJ-P cell coat in a size-dependent fashion (Fig. 4 a). For particles small enough to penetrate, gradients in the particle concentration are established in the PCM, where the spatial distribution depends on the particle size (Fig. 4 b). We estimate that the local pore size roughly equals a given particle size at the location, deff, where the particle distribution plateaus (19). Thus, we can generate a map of pore size versus distance to the cell surface. The porosity decreases in a roughly linear fashion toward the RCJ-P cell surface (Fig. 5). Particles with diameters 500 nm or larger rarely pass into the PCM of RCJ-P and MSC cells. This is consistent with our observation that the PCM prevents bacteria from reaching the cell surface (Fig. 5 a), raising the question of what role the PCM may play in controlling bacterial and viral infections (48).
Figure 4.
(a) qPEA on RCJ-P cells using 100 nm fluorescent beads incubated for 2 h with 333 μg/mL aggrecan. The visible gradient in red intensity shows how the average local concentration of beads decreases toward the cell surface. (b) Typical profiles of nanoparticle distributions in RCJ-P cells swollen with 333 μg/mL aggrecan. To see this figure in color, go online.
Figure 5.
(a) Bacteria grown with samples of RCJ-P cells show slowed infection due to the PCM, which acts as a barrier. The size of the bacteria is larger than the threshold maximum pore size found for native RCJ-P cells using qPEAs (500 nm). (b) Pore size versus distance to the cell surface: the porosity decreases toward the surface. For swollen PCMs (cACAN = 333 μg/mL), red curve, the porosity is reduced and it changes more slowly throughout the PCM. Maximum pore size for native and exogenous aggrecan RCJ-P swollen coats: 500 nm, 300 nm. To see this figure in color, go online.
The PCM microstructure is dramatically altered by increasing the aggrecan content (cACAN = 333 μg/mL). Particles 300 nm and larger are unable to enter the PCM (500 nm was the previous cut-off), a result consistent with characterization of mesothelial cells by Heldin and co-workers (24). The position where 100 nm particles achieve uniform access, e.g., deff, more than doubles from 3.5 to 8 μm. The sharp gradient of particles in the PCM demonstrates that few particles (>=100 nm) reach the surface (Fig. 4 b). The percentage of 40 nm particles that reach the cell surface appears unaffected by the aggrecan increase (73.2 ± 10.8% vs. 70.3 ± 15.4% relative intensity N = 20 (27) and N = 11 (18), where the second number is the number of total measurements made on N cells). However, the percentage of 100 nm particles that reach the surface decreases from 38.8 ± 7.8% to 25.7 ± 6.2% (N = 32 (45) and N = 14 (24)). Meanwhile, 200 nm diameter particles do not reach the cell surface in native or aggrecan-treated cells. Their average distance from the cell surface is 3.7 μm in both cases (N = 24 (34), N = 15 (19)), with low relative intensities of 12.2 ± 1.8% and 17.2 ± 2.6% (N = 24 (33) and N = 12 (18)). This blockage may represent the limit to approach the cell surface due to microvilli on the RCJ-P surface, which we estimate extend ∼1–3 μm (18). As with the native cells, the pore size in the aggrecan-enriched PCM decreases linearly toward the cell surface, but more slowly due to the limited dynamic range in porosity (Fig. 5 b).
From these studies, we conclude that cells apparently have the possibility to control their very local environment and the accessibility of the cell surface by tuning the bottlebrush content in the surface anchored polymer matrix. Although the openings in the PCM are large compared to molecules, it may be small enough to affect the transport of exosomes (49, 50, 51) and supermolecular assemblies, including bottlebrush molecules themselves. For example, aggrecan is ∼300 nm × 80 nm (52). Increasing bottlebrush density also likely controls interactions with other cells (53) or adhesion to the surrounding extracellular matrix (4, 54, 55). Furthermore, the high negative charge densities established by chondroitin sulfate side chains provide another venue for cells to control passage of positively charged molecules to the cell surface, such as growth factors (22). Theoretical and experimental studies of the passage of proteins through and adsorption of proteins to poly(ethylene glycol) brushes also provide a context to think about the physical interactions described here (56, 57, 58, 59). This possibility is explored below.
Increasing bottlebrush concentration drives PCM microstructure toward spatial uniformity
Another way to estimate the openings in the PCM is via optical tweezers based measurement (19, 60). These mechanical measurements relate the equilibrium force, , in the PCM to an estimate of the correlation length, ξ, of the polymer matrix, using the relationship, , where z is perpendicular distance to the cell surface. The correlation length is expected to roughly reflect the changing matrix openings discovered with qPEA.
In our previous work (19), we demonstrated that with optical tweezers, we can position 3 μm diameter passivated spheres within the PCM with little matrix compression (the HA-bottle brush strands relax around the sphere). The microsphere in the PCM, even when held statically, experiences an ejection force that would push the bead out of the PCM if it were not held with the optical trap. This is called the equilibrium force (because the bead is static) and is hypothesized to arise from an osmotic pressure gradient in the PCM across the bead. Using this insight, we can estimate the spatially varying correlation length of the PCM by relating the osmotic pressure of a polymer solution to the measured equilibrium force. The dependence of osmotic pressure on correlation length, , provides the connection with the equilibrium force needed to extract a value. Indeed, it was this analysis of optical tweezers data that first inspired our development of the qPEA to confirm the prediction of varying openings in the PCM.
In this study, we measured the equilibrium force at multiple positions throughout the PCM of RCJ-P cells with and without incubation with aggrecan. The two force curves in Fig. 6 a show little statistical difference in the regions closest to the cell surface (error bars are two standard errors), however at further distances the two are distinct, and even more so when the correlation length is extracted using the theory (Fig. 6 b). We measured the equilibrium force at four different aggrecan concentrations (0, 39, 95, and 333 μg/mL). The results show little difference between 0 and 39 μg/mL, but a significant change at 95 μg/mL. At 333 μg/mL, a value where both PEA and exogenous aggrecan experiments show evidence of PCM saturation, is nearly constant throughout the PCM, barely changing from ∼100 to 200 nm (between 3 and 8 μm). The data is qualitatively consistent with qPEA data, which also shows a linear decrease in porosity toward the cell surface. Both assays indicate that as the PCM becomes saturated with aggrecan, the openings in the PCM become much smaller, reduced to values below 200 nm. Furthermore, the variation in openings decreases, becoming more uniform.
Figure 6.
(a) Optical tweezers measured equilibrium force in PCM on native and aggrecan-saturated RCJ-P cells (cACAN = 333 μg/mL, 2 h). (b) Estimate of PCM porosity using polymer theory and equilibrium force measurement at four different aggrecan concentrations. The pore size decreases with increasing aggrecan and becomes more uniform. All error bars are two standard errors. To see this figure in color, go online.
Unbound aggrecan diffuses swiftly through the PCM
Next, we begin to address the question of how molecular diffusion is affected by the presence of the PCM. Both steric hindrance and electrostatic effects are expected to be relevant, depending on the size and charge of the diffusing molecules. Yet the openings in the aggrecan saturated cell coat are still quite large relative to molecular scales. Indeed bovine serum albumin (Fig. S4) uniformly penetrates the PCM of RCJ-P cells.
We focus on two classes of physically and biologically relevant molecules, bottlebrush proteoglycans and positively charged analogs for growth factors. First, we examine the diffusion of aggrecan, whose large size is similar to the 200 nm cut-off for penetration into the PCM. Studying how aggrecan diffuses into the PCM is of great interest both in the context of PCM synthesis, as well as PCM and ECM transformation in processes like development (61, 62), wound healing (63), central nervous system injury and repair (64), brain plasticity (65), and disease (66).
We monitor the diffusion of label-free aggrecan into the PCM by quantifying the time-dependent turnover of fluorescently labeled aggrecan. As in the assays reported in Fig. 3, RCJ-P cells were incubated for 2 h with fluorescent exogenous aggrecan (333 μg/mL). The media was then gently exchanged with solution containing the same concentration of dark aggrecan, e.g., molecules with no fluorescent labeling. The local average intensity dropped precipitously within the first minute (Fig. 7, a–c), after the exchange. Plotting the integrated intensity at each time step shows an initial rapid decrease in intensity of ∼23% (N = 17, 3 experiments), which is followed by a slower, linear decrease in intensity (Fig. 7 b). Controls involving the exchange of the media with aggrecan-free media showed no significant drop in the aggrecan intensity within the first minute (N = 15, 3 experiments), whereas the sharp drop was reproducible.
Figure 7.
Time-dependent replacement of PCM bound fluorescent aggrecan with unlabeled aggrecan. (a) Fluorescent profiles of the aggrecan distribution versus time. The first curve marked “Before” is taken after the fluorescent-aggrecan is removed and replaced with media (no aggrecan). The buffer is then exchanged with a media containing the same concentration of aggrecan but with no fluorescent label (cACAN = 333 μg/mL). (b) Integrated aggrecan intensity (over entire PCM) versus time. A reproducible drop occurs in the first step and then the aggrecan turnover proceeds linearly. (c) Monitoring aggrecan change at fixed positions in the PCM reveals that after the first time step, only locations 7.5 μm and closer to the cell continue to significantly change. To see this figure in color, go online.
In the first minute, the aggrecan turnover occurs uniformly at distances from 2 to 12 μm (Fig. 7, a and c). Thereafter, at distances 7.5 μm and greater, little apparent change occurs. The majority of the remaining dynamics takes place at the inner, tighter portion of the cell coat, where according to the qPEA assays, the average porosity is <100 nm. Hence, we observe that the initial replacement and diffusion of dark-aggrecan into the PCM is rapid and penetrating to the cell surface. However, complete replacement of the aggrecan requires more than 1 h.
In these experiments, the dark-aggrecan molecules competitively bind with the fluorescently labeled aggrecan. In the presence of high concentrations of dark-aggrecan, the turnover of the fluorescent population is expected to be quite fast. The dissociation constant and off rate of the G1-module of aggrecan is kd = 20 nM (67) and koff = 2.5E-3 s−1 (68). The slower turnover in the inner regions of the PCM likely arises from a combination of factors that impact the kinetics. First, in these tighter spaces where the average opening is less than the average dimension of the aggrecan, diffusion inward will be slowed. Like the 100 nm particles that have a reduced concentration in the inner regions, the aggrecan accessibility may also be reduced. Second, when the fluorescent aggrecan is released in regions where there is a high concentration of possible binding sites, there is a higher probability that it will rebind, slowing the loss of fluorescence.
The fact that aggrecan is able to diffuse quickly into the aggrecan-enriched PCM is significant, considering its reported extended size is on the order of ∼300 × 80 nm or larger. In solution, aggrecan forms a globule, which according to dynamic light scattering has a hydrodynamic radius of ∼200 nm (69). Yet, we have shown that 200 nm particles are mostly localized to the edge of the PCM at ∼11.5 μm from the cell surface in aggrecan-treated cells (333 μg/mL) and significantly hindered from entering the PCM of the native RCJ-P cells at distances closer than 6 μm to the cell surface. It is possible that the asymmetry of aggrecan as well as its flexible, globular properties enables freer passage through the cell coat.
Electrostatic sequestration of positively charged ∼20 kDa molecules in the PCM
In this final section, we study the interaction of small positively charged molecules with the high negatively charged landscape generated by the chondroitin sulfate-rich PCM. Looking for visual evidence of spatial localization of the positive molecules in the PCM is directly relevant to questions regarding the passage of growth factors, cytokines, and chemokines through hyaluronan-aggregate-rich PCM and ECM (22). Small molecules should not be sterically hindered by the PCM because it still maintains >100 nm openings even during bottlebrush saturation. Yet, there is evidence that PCM can sequester molecules via nonspecific and specific binding to the sulfated chains in bottlebrush (22). Here, we focus on the possible role of electrostatic interactions.
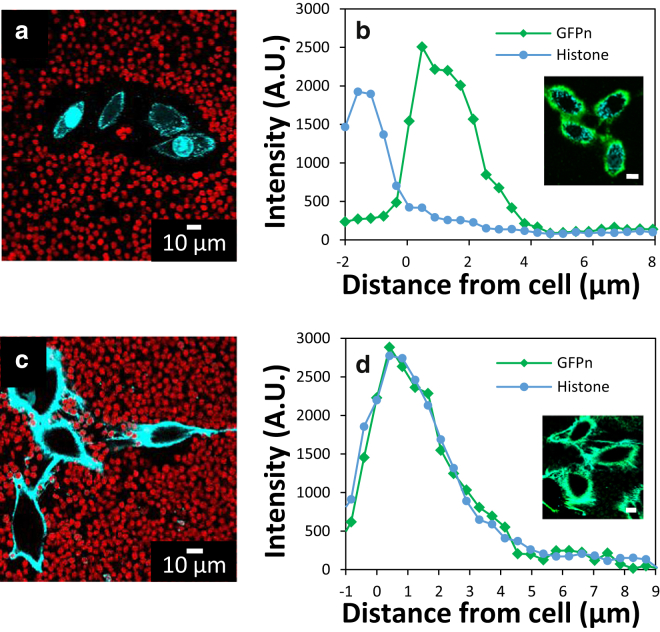
For this exploratory study, we use an inexpensive surrogate for growth factors, histone proteins, which have similar charge and size to growth factors (70). Fig. 8 a shows an image of RCJ-P cells incubated with fluorescently labeled histones (cH = 232 μg/mL, 20 min). Very little of the fluorescent histone (Fig. 8 a) is visible outside of the cell. For most cells, no labeling outside the cell was visible, whereas uptake onto the plasma membrane and nucleus was significant. To distinguish histones, which are bound close to the cell surface versus those taken inside of the cell, we fluorescently labeled the PCM’s hyaluronan using GFPn (Fig. 8 b). The majority of the histone appears inside of the cell or close to cell surface. A very small signal appears in the PCM region from ∼2–6 μm.
Figure 8.
(a) Fluorescent histones (cyan) are barely detectable within the PCM of native RCJ-P cells (cH = 232 μg/mL for 20 min). (b) The majority of histones bind very close to the surface and are largely ingested by the cells. Labeling of the HA in the PCM with GFPn confirms as expected the PCM extends beyond the region where histones bind. (c) In aggrecan-enriched PCMs (cACAN = 333 μg/mL), the histones bind strongly to the PCM, whose presence is confirmed by PEA (cH = 232 μg/mL for 20 min). The PCM is thinner than histone-free measurements, indicating a collapse driven by cross-linking (see Figs. S5 and S6). (d) GFP and histones colocalize in the aggrecan-enriched PCM. To see this figure in color, go online.
We repeated the same experiment in aggrecan-enhanced PCMs (333 μg/mL, 2 h) followed by exposure to histones (cH = 232 μg/mL, 20 min). Strong binding between the exogenous aggrecan and histone is reflected by the brightly labeled PCM (Fig. 8 c). Far less of the histones are taken up by the cells, showing that an aggrecan-rich PCM sequesters positively charged molecules and reduces their uptake by the cell.
There is a direct correlation between the fluorescent aggrecan, the HA (Fig. 8 d), and the histone distributions (Fig. S5) in aggrecan-enriched samples (333 μg/mL). Most samples showed evidence of HA cross-linking and resultant PCM shrinkage in the presence of histones (Figs. 8 c and S5). Analysis of aggrecan fluorescence versus distance to the cell (Fig. 3 b vs. Figs. 8 d and S5) suggests that the PCM is collapsed from an average is reduced from ∼12 to ∼6 μm. Evidently the positively charged histones can act as cross-linkers between the aggrecan molecules. Similar effects have been shown by cross-linkers in PCM model systems (71, 72, 73).
Interestingly, in control experiments to further demonstrate that the histones localize to the chondroitin sulfate side chains on aggrecan, we attempted to remove the histones by their degradation with ChABC. Instead, we found that histone-enriched PCMs could no longer be fully digested (see Fig. S7). This result confirms that the histones bind to the chondroitin sulfate chains so strongly that they block the enzymatic activity of ChABC. Repeating these experiments on the native RCJ-P cells incubated with histones showed similar results: the PCM remained intact despite minimal visual evidence of histone sequestration (Fig. S8).
This is compelling evidence that the chondroitin sulfates on native endogenous aggrecan and exogenous aggrecan in the PCM sequester positively charged histones, and at high bottlebrush concentrations, significant reduction in histone uptake is a consequence.
Conclusions
These studies provide quantitative illustrations of how changes in bottlebrush proteoglycan concentration in the PCM can affect the transport of nanoparticles to the cell surface as well as the sequestration of positively charged molecules. Diffusion of the bottlebrushes themselves still occurs through saturated pericellular matrix despite the dimensions of the molecule and its negative charge. Furthermore, several experiments independently imply that the native PCM of RCJ-P and MSC cells in vitro are sparsely populated by endogenous bottlebrushes.
The sparse population of bottlebrushes points to the possibility for cells to use surface-tethered proteoglycans to native cell surface accessibility or to concentrate and sequester biomolecules like growth factors. Mechanics could be altered this way as well. For the cell types studied here, a special regime exists where the PCM appears fully stretched, but where the porosity and molecular interactions can be tuned significantly by varying the solution concentration between 180 and 333 μg/mL.
Although these studies do not recapitulate in vivo conditions, where the PCM is likely compressed by surrounding tissue, they provide compelling data that modulation of bottlebrush proteoglycan distribution in the PCM and indeed in the ECM can affect tissues physical properties dramatically.
There are many important directions to pursue in future work. Immediately relevant are follow-up studies examining growth factor sequestration to the PCM, investigation of its release, and its possible effects on cell. For example, looking at whether BMP-2 is sequestered and concentrated to high enough concentrations to drive differentiation of stem cells would be an excellent example of how PCM can be used by cells as a molecular reservoir. Furthermore, given the observation that PCM can significantly reduce the molecular uptake of histone proteins suggests the need to investigate possible filtration effects that might eliminate harmful molecules and/or interfere with proper cell function by removing necessary molecules. More generally, our studies demonstrate how important it is for researchers to be cognizant of possible PCM effects in studies of drug delivery vehicles, exosomes, or any study looking at size-dependent endocytosis or phagocytosis. In the longer term, the ability of the PCM to dynamically transform is relevant for understanding cell-cell and cell-ECM interactions, for example in migration and proliferation. Finally, interesting strategies for the bioengineering of scaffolds or tissues could be taken from the studies here. For example, generic seeder cells that produce PCM could be planted in matrices and used to control local production and concentration of growth factors. To conclude, the pericellular matrix is often broadly neglected; yet its presence adds another dimension of control and tunability for cells that likely play an elegant role in cell control and integration.
Author Contributions
P.C. and L.M. designed research, performed research, and analyzed data; R.F. performed research and analyzed data; J.S. provided research tool and discussed data; J.T. provided research material and provided research idea; A.G. performed research, provided research tool, and analyzed data; J.C. designed research, analyzed data, and wrote the article.
Acknowledgments
The authors thank Anton Souslov, Paul Goldbart, Todd McDevitt, and Paul Weigel for insightful conversations.
The human MSCs employed in these studies were provided by T.M. and J.T. via the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White through a grant from the National Center for Research Resources (NCRR) of the National Institutes of Health (NIH), grant No. P40RR017447. J.E.C. gratefully acknowledges support for this work by the National Science Foundation CAREER Award under Division of Materials Research (DMR) grant No. 0955811.
Editor: Cecile Sykes.
Footnotes
Eight figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30352-6.
Supporting Material
References
- 1.Toole B.P. Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 2.Evanko S.P., Tammi M.I., Wight T.N. Hyaluronan-dependent pericellular matrix. Adv. Drug Deliv. Rev. 2007;59:1351–1365. doi: 10.1016/j.addr.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricciardelli C., Russell D.L., Horsfall D.J. Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J. Biol. Chem. 2007;282:10814–10825. doi: 10.1074/jbc.M606991200. [DOI] [PubMed] [Google Scholar]
- 4.Itano N., Atsumi F., Kimata K. Abnormal accumulation of hyaluronan matrix diminishes contact inhibition of cell growth and promotes cell migration. Proc. Natl. Acad. Sci. USA. 2002;99:3609–3614. doi: 10.1073/pnas.052026799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evanko S.P., Angello J.C., Wight T.N. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb. Vasc. Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- 6.Röck K., Fischer K., Fischer J.W. Hyaluronan used for intradermal injections is incorporated into the pericellular matrix and promotes proliferation in human skin fibroblasts in vitro. Dermatology (Basel) 2010;221:219–228. doi: 10.1159/000318905. [DOI] [PubMed] [Google Scholar]
- 7.Wang D., Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012;349:147–160. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- 8.Knudson C.B., Toole B.P. Changes in the pericellular matrix during differentiation of limb bud mesoderm. Dev. Biol. 1985;112:308–318. doi: 10.1016/0012-1606(85)90401-4. [DOI] [PubMed] [Google Scholar]
- 9.Toole B.P. Hyaluronan in morphogenesis. J. Intern. Med. 1997;242:35–40. doi: 10.1046/j.1365-2796.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- 10.Auvinen P., Tammi R., Kosma V.M. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am. J. Pathol. 2000;156:529–536. doi: 10.1016/S0002-9440(10)64757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimata K., Honma Y., Suzuki S. Increased synthesis of hyaluronic acid by mouse mammary carcinoma cell variants with high metastatic potential. Cancer Res. 1983;43:1347–1354. [PubMed] [Google Scholar]
- 12.Bernert B., Porsch H., Heldin P. Hyaluronan synthase 2 (HAS2) promotes breast cancer cell invasion by suppression of tissue metalloproteinase inhibitor 1 (TIMP-1) J. Biol. Chem. 2011;286:42349–42359. doi: 10.1074/jbc.M111.278598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wight T.N. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr. Opin. Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 14.Wight T.N., Toole B.P., Hascall V.C. Hyaluronan and the aggregating proteoglycans. In: Mecham P.R., editor. The Extracellular Matrix: An Overview. Springer; Berlin, Heidelberg: 2011. pp. 147–195. [Google Scholar]
- 15.Knudson W., Knudson C.B. Assembly of a chondrocyte-like pericellular matrix on non-chondrogenic cells. Role of the cell surface hyaluronan receptors in the assembly of a pericellular matrix. J. Cell Sci. 1991;99:227–235. doi: 10.1242/jcs.99.2.227. [DOI] [PubMed] [Google Scholar]
- 16.Weigel, P. H. 1998. Bacterial hyaluronan synthases. Science Hyaluronan Today. Available at: http://www.GlycoForum.gr.ip. Accessed May 27, 2016.
- 17.Knudson C.B. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J. Cell Biol. 1993;120:825–834. doi: 10.1083/jcb.120.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boehm H., Mundinger T. a. Mapping the mechanics and macromolecular organization of hyaluronan-rich cell coats. Soft Matter. 2009;5:4331–4337. [Google Scholar]
- 19.McLane L.T., Chang P., Curtis J.E. Spatial organization and mechanical properties of the pericellular matrix on chondrocytes. Biophys. J. 2013;104:986–996. doi: 10.1016/j.bpj.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iyer S., Gaikwad R.M., Sokolov I. Atomic force microscopy detects differences in the surface brush of normal and cancerous cells. Nat. Nanotechnol. 2009;4:389–393. doi: 10.1038/nnano.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon M., Dokukin M., Staii C. Load rate and temperature dependent mechanical properties of the cortical neuron and its pericellular layer measured by atomic force microscopy. Langmuir. 2016;32:1111–1119. doi: 10.1021/acs.langmuir.5b04317. [DOI] [PubMed] [Google Scholar]
- 22.Macri L., Silverstein D., Clark R.A.F. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv. Drug Deliv. Rev. 2007;59:1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Pyka M., Wetzel C., Faissner A. Chondroitin sulfate proteoglycans regulate astrocyte-dependent synaptogenesis and modulate synaptic activity in primary embryonic hippocampal neurons. Eur. J. Neurosci. 2011;33:2187–2202. doi: 10.1111/j.1460-9568.2011.07690.x. [DOI] [PubMed] [Google Scholar]
- 24.Heldin P., Pertoft H. Synthesis and assembly of the hyaluronan-containing coats around normal human mesothelial cells. Exp. Cell Res. 1993;208:422–429. doi: 10.1006/excr.1993.1264. [DOI] [PubMed] [Google Scholar]
- 25.Kim A.J., Manoharan V.N., Crocker J.C. Swelling-based method for preparing stable, functionalized polymer colloids. J. Am. Chem. Soc. 2005;127:1592–1593. doi: 10.1021/ja0450051. [DOI] [PubMed] [Google Scholar]
- 26.Valentine M.T., Perlman Z.E., Weitz D.A. Colloid surface chemistry critically affects multiple particle tracking measurements of biomaterials. Biophys. J. 2004;86:4004–4014. doi: 10.1529/biophysj.103.037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crocker J.C., Grier D.G. Methods of digital video microscopy for colloidal studies. J. Colloid Interface Sci. 1996;179:298–310. [Google Scholar]
- 28.Zhang H., Baader S.L., Rauch U. Neurocan-GFP fusion protein: a new approach to detect hyaluronan on tissue sections and living cells. J. Histochem. Cytochem. 2004;52:915–922. doi: 10.1369/jhc.3A6221.2004. [DOI] [PubMed] [Google Scholar]
- 29.Scrimgeour J., Cho J.K., Breedveld V., Curtis J.E. Microfluidic dialysis cell for characterization of macromolecule interactions. Soft Matter. 2011;7:4762. [Google Scholar]
- 30.Evanko S.P., Johnson P.Y., Wight T.N. Platelet-derived growth factor stimulates the formation of versican-hyaluronan aggregates and pericellular matrix expansion in arterial smooth muscle cells. Arch. Biochem. Biophys. 2001;394:29–38. doi: 10.1006/abbi.2001.2507. [DOI] [PubMed] [Google Scholar]
- 31.Clarris B.J., Fraser J.R.E. On the pericellular zone of some mammalian cells in vitro. Exp. Cell Res. 1968;49:181–193. doi: 10.1016/0014-4827(68)90530-2. [DOI] [PubMed] [Google Scholar]
- 32.Kultti A., Pasonen-Seppänen S., Tammi M.I. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp. Cell Res. 2009;315:1914–1923. doi: 10.1016/j.yexcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Attili S., Richter R.P. Self-assembly and elasticity of hierarchical proteoglycan–hyaluronan brushes. Soft Matter. 2013;9:10473–10483. [Google Scholar]
- 34.Souslov A., Curtis J.E., Goldbart P.M. Beads on a string: structure of bound aggregates of globular particles and long polymer chains. Soft Matter. 2015;11:8092–8099. doi: 10.1039/c5sm01316j. [DOI] [PubMed] [Google Scholar]
- 35.Milner S.T., Witten T.A., Cates M.E. Theory of the grafted polymer brush. Macromolecules. 1988;21:2610–2619. [Google Scholar]
- 36.Milner S.T. Polymer brushes. Science. 1991;251:905–914. doi: 10.1126/science.251.4996.905. [DOI] [PubMed] [Google Scholar]
- 37.Johnson M., Kamm R., Pedley T. Scaling laws and the effects of concentration polarization on the permeability of hyaluronic acid. Physicochem. Hydrodyn. 1987;9:427–444. [Google Scholar]
- 38.Cohen M., Klein E., Addadi L. Organization and adhesive properties of the hyaluronan pericellular coat of chondrocytes and epithelial cells. Biophys. J. 2003;85:1996–2005. doi: 10.1016/S0006-3495(03)74627-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attili S., Richter R.P. Combining colloidal probe atomic force and reflection interference contrast microscopy to study the compressive mechanics of hyaluronan brushes. Langmuir. 2012;28:3206–3216. doi: 10.1021/la204602n. [DOI] [PubMed] [Google Scholar]
- 40.Seror J., Merkher Y., Klein J. Articular cartilage proteoglycans as boundary lubricants: structure and frictional interaction of surface-attached hyaluronan and hyaluronan--aggrecan complexes. Biomacromolecules. 2011;12:3432–3443. doi: 10.1021/bm2004912. [DOI] [PubMed] [Google Scholar]
- 41.Heldin P., Suzuki M., Pertoft H. Chondroitin sulfate proteoglycan modulates the permeability of hyaluronan-containing coats around normal human mesothelial cells. J. Cell. Physiol. 1995;165:54–61. doi: 10.1002/jcp.1041650107. [DOI] [PubMed] [Google Scholar]
- 42.Knudson W., Aguiar D.J., Knudson C.B. CD44-anchored hyaluronan-rich pericellular matrices: an ultrastructural and biochemical analysis. Exp. Cell Res. 1996;228:216–228. doi: 10.1006/excr.1996.0320. [DOI] [PubMed] [Google Scholar]
- 43.Knudson C.B., Knudson W. Cartilage proteoglycans. Semin. Cell Dev. Biol. 2001;12:69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 44.de Gennes P.G. Conformations of polymers attached to an interface. Macromolecules. 1980;13:1069–1075. [Google Scholar]
- 45.Zhou R., Zhou H., Yeung E.S. Pericellular matrix enhances retention and cellular uptake of nanoparticles. J. Am. Chem. Soc. 2012;134:13404–13409. doi: 10.1021/ja304119w. [DOI] [PubMed] [Google Scholar]
- 46.Zhou R., Xiong B., Yeung E.S. Slowed diffusion of single nanoparticles in the extracellular microenvironment of living cells revealed by darkfield microscopy. Anal. Bioanal. Chem. 2011;399:353–359. doi: 10.1007/s00216-010-4340-1. [DOI] [PubMed] [Google Scholar]
- 47.Xu R., Xiong B., He Y. Pericellular matrix plays an active role in retention and cellular uptake of large-sized nanoparticles. Anal. Bioanal. Chem. 2014;406:5031–5037. doi: 10.1007/s00216-014-7877-6. [DOI] [PubMed] [Google Scholar]
- 48.Yaron M., Yaron I., Herzberg M. Hyaluronic acid produced by human synovial fibroblasts. Effect of polyinosinic-polycytidylic acid (poly I:C) and interferon. Arthritis Rheum. 1976;19:1315–1320. doi: 10.1002/art.1780190612. [DOI] [PubMed] [Google Scholar]
- 49.Record M., Carayon K., Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim. Biophys. Acta. 2014;1841:108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian T., Wang Y., Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J. Cell. Biochem. 2010;111:488–496. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- 52.Ng L., Grodzinsky A.J., Ortiz C. Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy. J. Struct. Biol. 2003;143:242–257. doi: 10.1016/j.jsb.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Pizzorusso T., Medini P., Berardi N., Chierzi S., Fawcett J.W. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 54.Brecht M., Mayer U., Prehm P. Increased hyaluronate synthesis is required for fibroblast detachment and mitosis. Biochem. J. 1986;239:445–450. doi: 10.1042/bj2390445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paszek M.J., DuFort C.C., Weaver V.M. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 2014;511:319–325. doi: 10.1038/nature13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halperin A. Polymer brushes that resist adsorption of model proteins: design parameters. Langmuir. 1999;15:2525–2533. [Google Scholar]
- 57.Halperin A., Fragneto G., Sferrazza M. Primary versus ternary adsorption of proteins onto PEG brushes. Langmuir. 2007;23:10603–10617. doi: 10.1021/la701007j. [DOI] [PubMed] [Google Scholar]
- 58.Halperin A., Kröger M. Ternary protein adsorption onto brushes: strong versus weak. Langmuir. 2009;25:11621–11634. doi: 10.1021/la9008569. [DOI] [PubMed] [Google Scholar]
- 59.Schneck E., Schollier A., Fragneto G. Neutron reflectometry elucidates density profiles of deuterated proteins adsorbed onto surfaces displaying poly(ethylene glycol) brushes: evidence for primary adsorption. Langmuir. 2013;29:14178–14187. doi: 10.1021/la403355r. [DOI] [PubMed] [Google Scholar]
- 60.van Oosten A.S., Janmey P.A. Extremely charged and incredibly soft: physical characterization of the pericellular matrix. Biophys. J. 2013;104:961–963. doi: 10.1016/j.bpj.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carulli D., Laabs T., Fawcett J.W. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr. Opin. Neurobiol. 2005;15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 62.Mark M.P., Baker J.R., Ruch J.-V. Regulated changes in chondroitin sulfation during embryogenesis: an immunohistochemical approach. Int. J. Dev. Biol. 1990;34:191–204. [PubMed] [Google Scholar]
- 63.Burd D.A.R., Greco R.M., Garg H.G. Hyaluronan and wound healing: a new perspective. Br. J. Plast. Surg. 1991;44:579–584. doi: 10.1016/0007-1226(91)90093-y. [DOI] [PubMed] [Google Scholar]
- 64.Morgenstern D.A., Asher R.A., Fawcett J.W. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 2002;137:313–332. doi: 10.1016/s0079-6123(02)37024-9. [DOI] [PubMed] [Google Scholar]
- 65.Galtrey C.M., Fawcett J.W. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Brain Res. Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Knudson C.B., Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993;7:1233–1241. [PubMed] [Google Scholar]
- 67.Hardingham T. Proteoglycans: their structure, interactions and molecular organization in cartilage. Biochem. Soc. Trans. 1981;9:489–497. doi: 10.1042/bst0090489. [DOI] [PubMed] [Google Scholar]
- 68.Shi S., Grothe S., Mort J.S. Link protein has greater affinity for versican than aggrecan. J. Biol. Chem. 2004;279:12060–12066. doi: 10.1074/jbc.M310091200. [DOI] [PubMed] [Google Scholar]
- 69.Papagiannopoulos A., Waigh T.A., Heinrich M. Solution structure and dynamics of cartilage aggrecan. Biomacromolecules. 2006;7:2162–2172. doi: 10.1021/bm060287d. [DOI] [PubMed] [Google Scholar]
- 70.Lei J., McLane L.T., Temenoff J.S. Characterization of a multilayer heparin coating for biomolecule presentation to human mesenchymal stem cell spheroids. Biomater. Sci. 2014;2:666–673. doi: 10.1039/C3BM60271K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baranova N.S., Nilebäck E., Richter R.P. The inflammation-associated protein TSG-6 cross-links hyaluronan via hyaluronan-induced TSG-6 oligomers. J. Biol. Chem. 2011;286:25675–25686. doi: 10.1074/jbc.M111.247395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baranova N.S., Foulcer S.J., Richter R.P. Inter-α-inhibitor impairs TSG-6-induced hyaluronan cross-linking. J. Biol. Chem. 2013;288:29642–29653. doi: 10.1074/jbc.M113.477422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baranova N.S., Inforzato A., Richter R.P. Incorporation of pentraxin 3 into hyaluronan matrices is tightly regulated and promotes matrix cross-linking. J. Biol. Chem. 2014;289:30481–30498. doi: 10.1074/jbc.M114.568154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.