Abstract
The clinical safety and efficacy of IPI-926 was evaluated in 14 patients with myelofibrosis in a phase II study. Patients received 160-mg IPI-926 orally in continuous 28-day cycles. The median treatment duration was 5.1 months, and all patients had discontinued treatment by 7.5 months. Nine patients discontinued due to lack of response as determined by the treating physician, two after developing acute leukemia and one due to disease progression/loss of response. Twelve patients had slight reductions in spleen size (less than 50% from baseline), but symptoms did not improve consistently. One patient achieved transfusion independence lasting 5 months. Reductions in GLI1 mRNA and protein levels, JAK2V617F allele burden, degree of fibrosis, or cytokine levels were observed in some patients, but were not significant when evaluated for the cohort. Low-grade gastrointestinal/liver abnormalities were the most common toxicities. The results did not support continued evaluation of IPI-926 as a monotherapy in myelofibrosis.
Keywords: IPI-926, Saridegib, Hedgehog inhibitor, Myelofibrosis
INTRODUCTION
Myelofibrosis (MF) is a chronic myeloproliferative neoplasm (MPN) originating from acquired mutations in hematopoietic progenitor cells that is characterized by ineffective hematopoiesis and progressive bone marrow fibrosis. MF can occur de novo or secondary to other related MPNs, including polycythemia vera (post-PV MF) or essential thrombocythemia (post-ET MF).1 The incidence of MF in the United States has been estimated to be ~1 per 100,000.2 Manifestations of MF include varying degrees of reticulin and collagen marrow fibrosis, dysplastic megakaryocyte hyperplasia, ineffective erythropoiesis, and extramedullary hematopoiesis, often associated with marked splenomegaly.1, 3 The disease is progressive and incurable, and up to 30% of cases transform to acute myeloid leukemia.[4] Supportive treatments, such as thalidomide or lenalidomide, interferon-alpha, hydroxyurea and splenectomy have not been shown to alter the natural history of the disease,4–10 and average overall survival is 5-7 years.11 Ruxolitinib, a selective Janus kinase (JAK) 1/JAK2 inhibitor, has recently been proved to reduce spleen size and improve disease-related symptoms and quality of life of many patients with MF. In patients with intermediate-2 and high-risk disease it may prolong patients overall survival.12–16 However, ruxolitinib does not eliminate the disease and further studies and novel agents are clearly needed.
A growing body of evidence implicating the Hedgehog (Hh) pathway in the development of several fibrotic diseases such as biliary and lung fibrosis suggests that inhibition of the Hh pathway may slow or halt the fibrotic process and improve clinical outcomes.17, 18 Recent reports have also demonstrated that aberrant activation of the Hh pathway is associated with many types of cancer, including hematologic malignancies, basal cell carcinoma, small-cell lung cancer, breast cancer, medulloblastoma, pancreatic adenocarcinoma, chondrosarcoma, metastatic prostate cancer, glioma and hepatocellular cancer.19, 20 IPI-926 (saridegib; Infinity Pharmaceuticals, Cambridge, MA) is a potent inhibitor of the Hh pathway that binds to and inhibits the key signaling membrane protein Smoothened (Smo).21, 22. In preclinical studies, IPI-926 has shown activity in a murine model of lung fibrosis (Infinity, unpublished data). IPI-926 reduced the amount of hydroxyproline and collagen in lung fibrotic plaques and reduced the expression of FIZZ1, which regulates myofibroblast differentiation. In addition, preclinical data in a Kras/p53 +/− transgenic murine model of pancreatic cancer showed that inhibiting the Hh pathway with IPI-926 decreased the thickness of the density of the desmoplastic tumor stroma and allowed chemotherapy to more readily access the tumor cells. 23 On the basis of these preclinical results showing a reduction in fibrosis, it was hypothesized that IPI-926 may be an effective treatment for MF. In the first-in-human Phase I study in patients with solid tumors, IPI-926 was well tolerated, with dose-limiting toxicities consisting of reversible elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). 24 Furthermore, preliminary results from a phase I study of the Hh inhibitor PF-04449913 in patients with hematologic malignancies, including six with MF, showed that the drug was well tolerated, with mostly low-grade gastrointestinal toxicities.25 Here, we report the results of a prospective phase II clinical trial of IPI-926 in patients with MF.
MATERIALS AND METHODS
Patients
Patients aged 18 years or older with a pathologically confirmed diagnosis of primary or secondary (post-PV or post-ET) MF by 2008 World Health Organization criteria26 and with an International Prognostic Scoring System 11 intermediate-1, intermediate-2, or high-risk disease were included in this study. Other eligibility criteria included Eastern Cooperative Oncology Group performance status of 0–2, and life expectancy of at least 3 months. Patients were excluded if they had received any treatment for MF (except blood transfusion) within 2 weeks of study entry or had received prior treatment with a Hh pathway inhibitor. Other exclusion criteria included other invasive malignancies within the last 3 years; inadequate hepatic function (AST and/or ALT > 2.5×upper limit of normal [ULN], direct bilirubin > 1.5×ULN, cirrhotic liver disease, ongoing alcohol abuse, or known chronic active or acute hepatitis); inadequate renal function (serum creatinine > 2×ULN); history of stroke, unstable angina, myocardial infarction, or ventricular arrhythmia; and presence of active infection or systemic use of antibiotics with 72 h of treatment. This study was approved by the institutional review board at each site and conducted according to the Declaration of Helsinki. All patients gave written informed consent before participation in the study. This study was registered at www.clinicaltrials.gov (NCT01371617).
Study Design
We conducted a phase II single-arm study using a Simon two-stage design to determine the efficacy of IPI-926 in patients with MF.27 This design stipulates that efficacy in the first stage must be shown before patients can be enrolled in the second stage. IPI-926 dosing was based on the maximum tolerated dose found in a phase 1 study in patients with solid tumors.24 Patients received 160 mg IPI-926 orally daily (QD) in continuous 28-day cycles. Up to two dose reductions (to 130 mg, then 110 mg) and/or dose interruptions of up to 28 days were permitted for drug-related adverse events (AEs).
Endpoints
The primary endpoint was overall response rate (ORR) defined as a clinical improvement (CI), partial remission (PR) and complete remission (CR) according to the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) criteria.28 Secondary endpoints were incidence and severity of adverse events (AEs) and changes in laboratory test results; change in bone marrow fibrosis; change in the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) score29; and changes in circulating inflammatory cytokines (e.g. interleukin-6 and tumor necrosis factor alpha). AEs were assessed using National Cancer Institute Common Terminology for Adverse Events, version 4.0. The MPN-SAF was administered on Day 1 of cycles 1–3 and every third cycle for one year. Bone marrow (BM) biopsy and aspiration were performed at screening prior and on day 1 of every third cycle for 1 year (Cycles 3, 6, 9 and 12). Exploratory endpoints were the association between safety, efficacy, baseline characteristics and pharmacodynamic or predictive biomarkers, or circulating inflammatory cytokines related to Hh pathway activation.
Statistical Analysis
This single-arm phase II study used a Simon two-stage design 27, with a type I error rate of 0.10 and 90% power. An ORR of ≤ 10% would indicate low activity and ORR ≥ 30% would indicate substantial activity and warrant continuation of the study. Thus, the study would only advance to stage two if ≥ 2 of the first 12 evaluable patients had a response. In the second stage, further clinical evaluation was indicated if ≥ 6 of 35 patients had a response. Because the study was terminated after stage one due to low efficacy, no formal efficacy analysis was performed. Continuous parameters were summarized using measures of central tendency and categorical parameters using frequencies and percentages.
Exploratory Analyses
BM aspirates and biopsy were collected from all patients at baseline and after 3 and 6 months on therapy. GLI1 mRNA levels were measured in BM aspirates after RNA was isolated from cell pellets using the Ambion RNA 4-PCR isolation kit according to the manufacturer’s guidance, and reverse transcription-polymerase chain reaction (PCR) was performed for human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and GLI1 using an ABI-7300 system with ABI primer/probes. Relative quantification of the expression data was obtained using the ΔΔCT method as described by Applied Biosystems. GLI1 protein levels in nuclei were detected by immunohistochemistry using GLI1 monoclonal antibodies purchased from Cell Signaling Technologies as previously described[24] and quantitated using Definiens software. Serum cytokine levels were measured at baseline and every 3 months after starting treatment using Myriad RBM HumanMAP v1.6 panels. JAK2V617F mutant allele burden was measured in DNA isolated from peripheral blood cells (Qiacube) after PCR amplification of JAK2 mutation specific target sequences and next generation sequencing on an Ion Torrent platform (GeneWiz). The degree of BM fibrosis was assessed in reticulin stained paraffin embedded pre- and post-treatment bone marrow biopsies by: (1) quantitative image analysis for fiber length density or (2) pathologist manual evaluation (both in conjunction with Flagship Biosciences, LLC). For fiber length density analysis, 10 regions of interest were selected randomly on each slide within the marrow space using a systematic uniform random sampling algorithm (Flagship Biosciences) after excluding bone, large interstitial spaces, and histology and whole slide scanning artifact areas (ImageScope; Aperio, Vista, CA). A length density stereology estimator known as a Merz guide was used to evaluate length density, counting the number of fibers that crossed the semicircular lines. 30 Ten counts were performed in randomly selected areas and length density calculated as the average of the 10 counts for length density, and the average of the cross-sectional area estimate, along with the standard deviations and coefficient of variations for each measurement. For pathologist evaluation, blinded reticulin stained cases were scored according to the European consensus system by a trained hematopathologist. 31
RESULTS
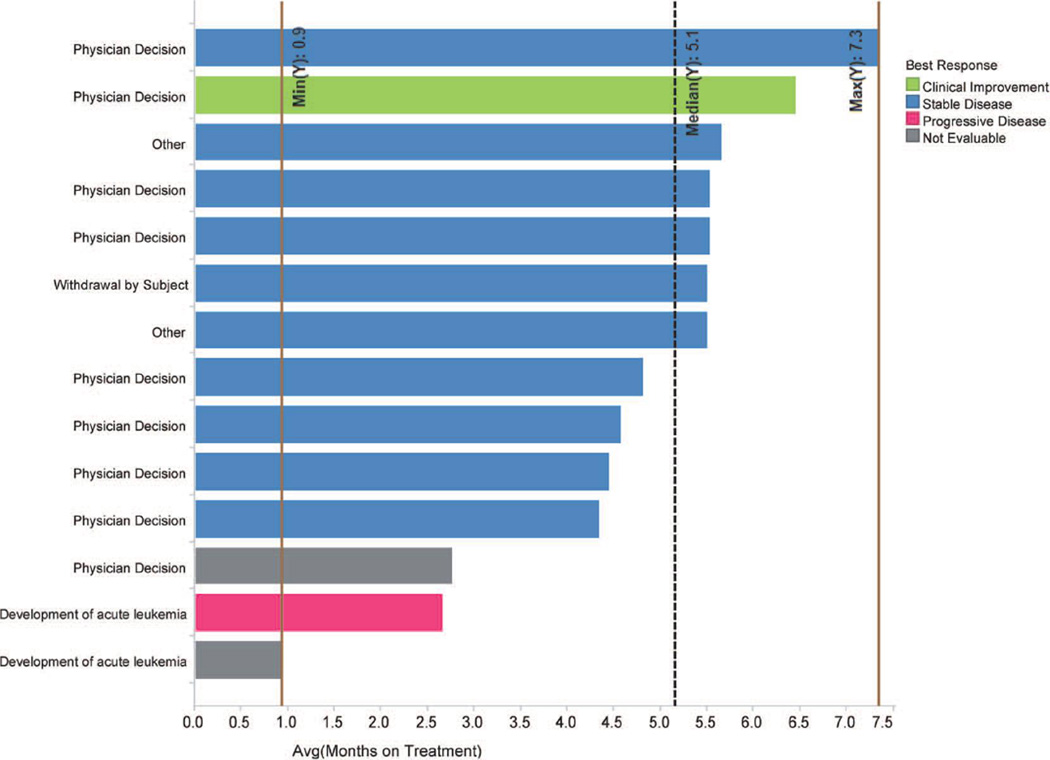
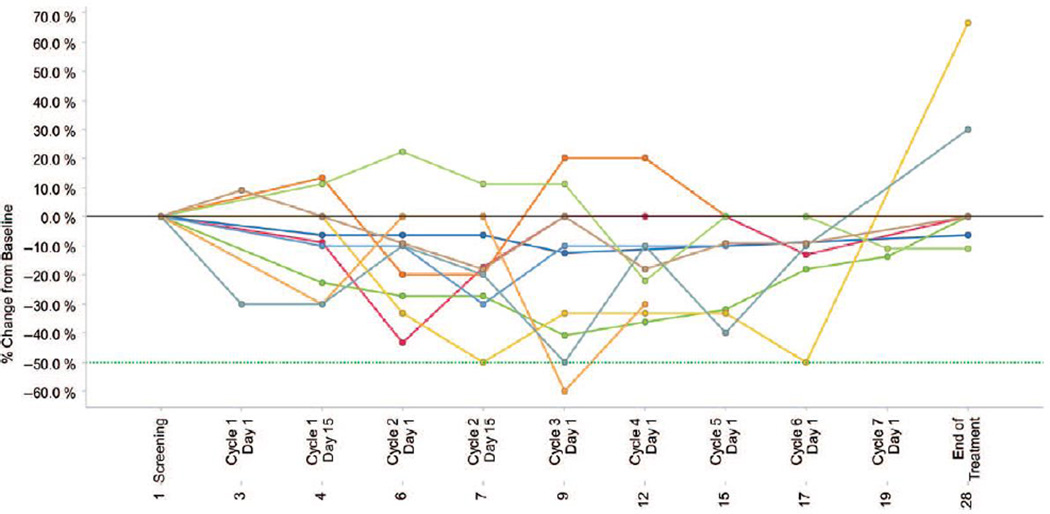
From October 2011 to January 2012, 14 patients were enrolled at 3 sites in the United States. Patient characteristics are listed in Table I. Two patients were not evaluable because of discontinuation before the first response assessment at 3 months. Patients received treatment for a median of 5.1 months (range, 0.9 – 7.3 months). All patients had discontinued treatment by 7.5 months. Nine patients discontinued due to lack of response as determined by the treating physician, two after developing acute leukemia, and one due to disease progression/loss of response (Figure 1). Nine patients had slight reductions in spleen size (less than 50% from baseline) at some point in the study (Figure 2); however, MPN-SAF scores did not improve consistently after IPI-926 administration. One patient achieved transfusion independence after one month of therapy but lost the response after 6 months of therapy when dose reduction was required due to abnormal liver function tests.
Table I.
Patient Characteristics
| Characteristic | N = 14 |
|---|---|
| Age (y), median (range) | 71 (55–82) |
| Gender, n (%) | |
| Male | 6 (43) |
| Female | 8 (57) |
| Palpable splenomegaly, (%) | 4 (29) |
| Spleen size (cm) in 10 patients, median (range) | 10.5 (6–23) |
| Prior transfusions, n (%) | 6 (42.9) |
| Prior regimens, median (range) | 2 (1–4) |
| Prior treatment with JAK2 inhibitor, (%) | 4 (28.6) |
| Years since myelofibrosis diagnosis (year), median (range) | 5.8 (0.2–25.4) |
| Diagnosis at screening | |
| Primary myelofibrosis | 8 (57) |
| Post-essential thrombocythemia myelofibrosis | 4 (29) |
| Post-polycythemia vera myelofibrosis | 2 (14) |
| IPSS scorea | |
| High | 5 (36) |
| Intermediate-2 | 3 (21) |
| Leukocytes (109/L), median (range) | 12.7 (1.1–117.3) |
| Absolute neutrophil count (109/L), median (range) | 9.4 (0.5–84.5) |
| Hemoglobin (g/dL), median (range) | 8.9 (7.6–13.6) |
| Platelet counts (109/L), median (range) | 78.5 (8–1600) |
Six patients who had either post-polycythemia vera or post-essential thrombocythemia myelofibrosis were not graded by IPSS score.
IPSS, International Prognostic Scoring System.
Figure 1.
Best response and time on treatment for each patient enrolled in the study. Reasons for discontinuation are shown on the left-hand side. Median duration of treatment and range are shown as vertical lines.
Figure 2.
Changes in spleen size over time. Percent change in palpable spleen size from baseline while on treatment. Lines depict changes in spleen for individual patients.
Adverse Events
The most common low-grade toxicities (grade 1 or 2 drug-related and -unrelated) included elevations in AST (11 patients), ALT (10 patients) and conjugated bilirubin four patients), nausea (eight patients), decreased appetite (six patients), constipation (five patients), and vomiting (five patients) (Table II). One patient who had elevated conjugated bilirubin (grade 3) and elevated ALT required a dose reduction. An additional 4 patients had dose interruptions, two due to grade 3 elevations in conjugated bilirubin.
Table II.
All Drug-Related AEs
| Adverse Event | Any grade, n (%) | Grade 3 or 4, n (%) |
|---|---|---|
| Aspartate aminotransferase elevation | 11 (78.6) | 0 |
| Alanine aminotransferase elevation | 10 (71.4) | 0 |
| Bilirubin increased | 8 (57) | 3 (21.4) |
| Nausea | 3 (21.4) | 0 |
| Vomiting | 2 (14.3) | 0 |
| Depressed level of consciousness | 1 (7.1) | 0 |
| Diarrhea | 1 (7.1) | 0 |
| Dysphagia | 1 (7.1) | 1 (7.1) |
| Dysgeusia | 1 (7.1) | 0 |
| Exacerbation of COPD | 1 (7.1) | 0 |
| Elevated alkaline phosphatase | 1 (7.1) | 0 |
| Hypoaesthesia | 1 (7.1) | 0 |
| Pollakiuria | 1 (7.1) | 0 |
COPD, chronic obstructive pulmonary disease
Correlative Studies
The effect of IPI-926 on BM fibrosis was assessed in BM biopsies from 10 patients. Four patients (40%) had minimal to modest decreases in fiber-length density scores after 3 months of treatment, and two of these had a further reduction after 6 months. The other six patients showed increases in fiber length density. Pathologist-based evaluations of reticulin levels showed two (20%) patients with a one-grade reduction in BM fibrosis after 3 months of treatment, and one patient who showed a one-grade decline in fibrosis after 6 months of treatment.
Pharmacodynamic markers of Hh inhibition, including inhibition of GLI1 mRNA expression and nuclear GLI1 protein localization were also assessed. GLI1 mRNA levels were evaluated in BM samples from eight patients after 3 months on therapy. Two patients (25%) had a minor reduction in GLI1 mRNA levels, one at the end of cycle 4 and the other at the end of study. The other six patients had either stable or increased GLI1 mRNA levels. GLI1 protein expression was evaluated by immunohistochemical analysis in bone marrow biopsy samples from 10 patients. Compared to baseline biopsy levels, three patients (30%) showed reductions in GLI1 at any point during treatment; however, this reduction was most meaningful and prolonged (progressive reduction in GLI levels at 3 and 6 months) in only one patient. JAK2-mutant allele burden was evaluated in peripheral blood samples of eight patients who had the JAK2V617F mutation. Only one patient (13%) showed a minor decrease in mutant allele burden at the end of study, while the other seven showed either stable or increasing allele burden. Serum cytokine levels after one cycle of treatment were also assessed: individual cytokines did not show any trends toward increasing or decreasing levels after IPI-926 treatment.
DISCUSSION
This study is the first phase II clinical trial to evaluate the efficacy and safety of a Hh inhibitor in patients with MF. Although the pre-specified efficacy criteria for study expansion were not met, limited clinical responses and improvements in correlative parameters were observed in some patients. Together these findings did not support the continued development of IPI-926 monotherapy in MF, but combination studies of Hh inhibitors with other novel agents may be considered.
The toxicity profile of IPI-926 in patients with MF was similar to that seen in a phase I trial in patients with solid tumors.24 Predominantly gastrointestinal/liver abnormalities precluded continuous delivery of therapy at the desired dose, which may have contributed to the lack of efficacy seen in some patients. However, no myelosuppression related to IPI-926 administration was observed. Most patients (79%) were discontinued due to lack of response as judged by the treating physician, while experiencing relatively frequent low-grade side-effects, sometimes requiring dose adjustment or interruptions.
In this population of pretreated patients, MPN-SAF scores were not improved after IPI-926 administration, although most patients had a mild reduction in spleen size (less than 50%). Only one patient (7%) showed clinical improvement by becoming transfusion independent. Interestingly, this patient also had the most significant reduction in GLI1 protein levels after 3 months on therapy. GLI1 expression has been previously used to determine changes in Hh pathway activity in studies of Smo inhibitors in patients with other tumor types, as the Hh pathway activates GLI proteins (a family of transcription factors that regulate the transcription of several genes involved in cell growth and development).32 Activation of the Hh pathway leads to nuclear translocation of GLI1 and the induction of Hh target gene transcription, including GLI1 itself. Interestingly, GLI1 expression was shown to be significantly increased in granulocytes from patients with myeloproliferative neoplasms.33 Therefore, GLI1 mRNA and nuclear protein levels were assessed as a marker of Hh pathway activation in this study. At a dose of 160 mg daily, neither GLI1 mRNA nor GLI1 protein levels were consistently reduced, but three out of 10 patients assessed showed reductions in GLI1 at any point during treatment, with progressive reduction in GLI1 levels at 3 and 6 months in just one patient. The limited decreases in GLI1 RNA or protein is of considerable interest. In the phase 1 study of IPI-926 in adult patients with solid tumors, IPI-926 pharmacokinetics were characterized by prolonged absorption, slow clearance relative to most small molecule therapeutics and an extended elimination half-life, which was projected to provide sustained pathway abrogation[24]. In addition, normal skin biopsies in 48 of 65 patients (74%) demonstrated a drop in GLI1 transcript level after 21 days of treatment with IPI-926[24]. Furthermore, immunohistochemical staining for nuclear GLI1 protein showed decreases in the stromal cells of a rectal cancer after 22 days of treatment [24]. Given the excellent pharmacokinetic properties of IPI-926 and the previously described pharmacodynamic effect on GLI1 [24], the limited decreases in GLI1 RNA or protein in this study are surprising. The assessments to make these determinations were taken at a later time period (≥ 3 months) compared to the phase 1 trial; therefore, it is possible that Smo pathway inhibition as measured by GLI1 at this timepoint is not maintained in myelofibrosis. Since other direct measures of Smo activity beyond GLI1 quantitation are very limited and not employed in this trial, it also indicates that orthogonal approaches to assess Smo inhibition may be needed in future trials.
Beyond GLI1 quantitation, other biological assessments of response to IPI-926 included an assessment of bone marrow fibrosis, JAK2 mutant allele burden and evaluation of systemic cytokine production. The degree of bone marrow fibrosis was mildly improved in selected patients, while JAK2 mutant allele burden did not decrease significantly in any patient. In addition, a serum cytokine panel that was shown to be significantly altered after treatment with ruxolitinib [13] was assayed in IPI-926-treated patients in this trial after 3 months of treatment. Significant changes in cytokine levels from baseline were not observed, suggesting that the extent of Smo inhibition achieved in these patients did not alter the overall systemic inflammatory profile.
Although IPI-926 as a single agent was not effective in this study, Bhagwat and colleagues recently reported that the combination of a JAK2 inhibitor and a Hh inhibitor was more effective than either agent alone in a murine model of MF.33 The combination was shown to reduce mutant allele burden and bone marrow fibrosis, a result which was not observed with either treatment alone. These data suggest that combining Hh and JAK inhibitors may be an effective strategy for treating MF. A phase I/II study of sonidegib (LDE225), another Hh inhibitor, and ruxolitinib (a JAK inhibitor) is currently enrolling patients and other studies are being planned.
In conclusion, the clinical activity of IPI-926 did not support its continued evaluation as a monotherapy in MF. However, studies of Hh-pathway inhibitors in combination with other novel agents in MF may be considered.
References
- 1.Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT) Leukemia research. 2007;31(6):737–740. doi: 10.1016/j.leukres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leukemia & lymphoma. 2013:1–6. doi: 10.3109/10428194.2013.813500. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342(17):1255–1265. doi: 10.1056/NEJM200004273421706. [DOI] [PubMed] [Google Scholar]
- 4.Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(6):761–770. doi: 10.1200/JCO.2010.31.8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesa RA, Nagorney DS, Schwager S, Allred J, Tefferi A. Palliative goals, patient selection, and perioperative platelet management: outcomes and lessons from 3 decades of splenectomy for myelofibrosis with myeloid metaplasia at the Mayo Clinic. Cancer. 2006;107(2):361–370. doi: 10.1002/cncr.22021. [DOI] [PubMed] [Google Scholar]
- 6.Kiladjian JJ, Chomienne C, Fenaux P. Interferon-alpha therapy in bcr-abl-negative myeloproliferative neoplasms. Leukemia. 2008;22(11):1990–1998. doi: 10.1038/leu.2008.280. [DOI] [PubMed] [Google Scholar]
- 7.Thomas DA, Giles FJ, Albitar M, et al. Thalidomide therapy for myelofibrosis with myeloid metaplasia. Cancer. 2006;106(9):1974–1984. doi: 10.1002/cncr.21827. [DOI] [PubMed] [Google Scholar]
- 8.Mesa RA, Yao X, Cripe LD, et al. Lenalidomide and prednisone for myelofibrosis: Eastern Cooperative Oncology Group (ECOG) phase 2 trial E4903. Blood. 2010;116(22):4436–4438. doi: 10.1182/blood-2010-05-287417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quintas-Cardama A, Kantarjian HM, Manshouri T, et al. Lenalidomide plus prednisone results in durable clinical, histopathologic, and molecular responses in patients with myelofibrosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(28):4760–4766. doi: 10.1200/JCO.2009.22.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manoharan A. Management of myelofibrosis with intermittent hydroxyurea. Br J Haematol. 1991;77(2):252–254. doi: 10.1111/j.1365-2141.1991.tb07989.x. [DOI] [PubMed] [Google Scholar]
- 11.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 12.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 13.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety and survival with ruxolitinib in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Haematologica. 2013;98(12):1865–1871. doi: 10.3324/haematol.2013.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verstovsek S, Kantarjian HM, Estrov Z, et al. Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: survival advantage in comparison to matched historical controls. Blood. 2012;120(6):1202–1209. doi: 10.1182/blood-2012-02-414631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cervantes F, Vannucchi AM, Kiladjian JJ, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122(25):4047–4053. doi: 10.1182/blood-2013-02-485888. [DOI] [PubMed] [Google Scholar]
- 17.McGowan SE, McCoy DM. Platelet-derived growth factor-A and sonic hedgehog signaling direct lung fibroblast precursors during alveolar septal formation. American journal of physiology Lung cellular and molecular physiology. 2013;305(3):L229–L239. doi: 10.1152/ajplung.00011.2013. [DOI] [PubMed] [Google Scholar]
- 18.Greenbaum LE. Hedgehog signaling in biliary fibrosis. The Journal of clinical investigation. 2008;118(10):3263–3265. doi: 10.1172/JCI37189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nature reviews Cancer. 2003;3(12):903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nature reviews Cancer. 2002;2(5):361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- 21.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes & development. 2002;16(21):2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taipale J, Chen JK, Cooper MK, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406(6799):1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 23.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science (New York, NY) 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jimeno A, Weiss GJ, Miller WH, Jr, et al. Phase I study of the Hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(10):2766–2774. doi: 10.1158/1078-0432.CCR-12-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamieson C, Cortes JE, Oehler V, et al. Phase 1 Dose-Escalation Study of PF-04449913, An Oral Hedgehog (Hh) Inhibitor, in Patients with Select Hematologic Malignancies. ASH Annual Meeting Abstracts. 2011;118(21) 424- [Google Scholar]
- 26.Swerdlow SH International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 27.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled clinical trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 28.Tefferi A, Barosi G, Mesa RA, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT) Blood. 2006;108(5):1497–1503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- 29.Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–408. doi: 10.1182/blood-2011-01-328955. [DOI] [PubMed] [Google Scholar]
- 30.Calhoun ME, Mouton PR. Length measurement: new developments in neurostereology and 3D imagery. Journal of chemical neuroanatomy. 2000;20(1):61–69. doi: 10.1016/s0891-0618(00)00074-0. [DOI] [PubMed] [Google Scholar]
- 31.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90(8):1128–1132. [PubMed] [Google Scholar]
- 32.Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes & development. 2010;24(7):670–682. doi: 10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhagwat N, Keller MD, Rampal RK, et al. Improved Efficacy Of Combination Of JAK2 and Hedgehog Inhibitors In Myelofibrosis. Blood. 2013;122(21):666. [Google Scholar]