Abstract
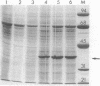
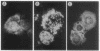
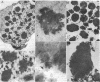
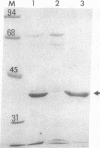
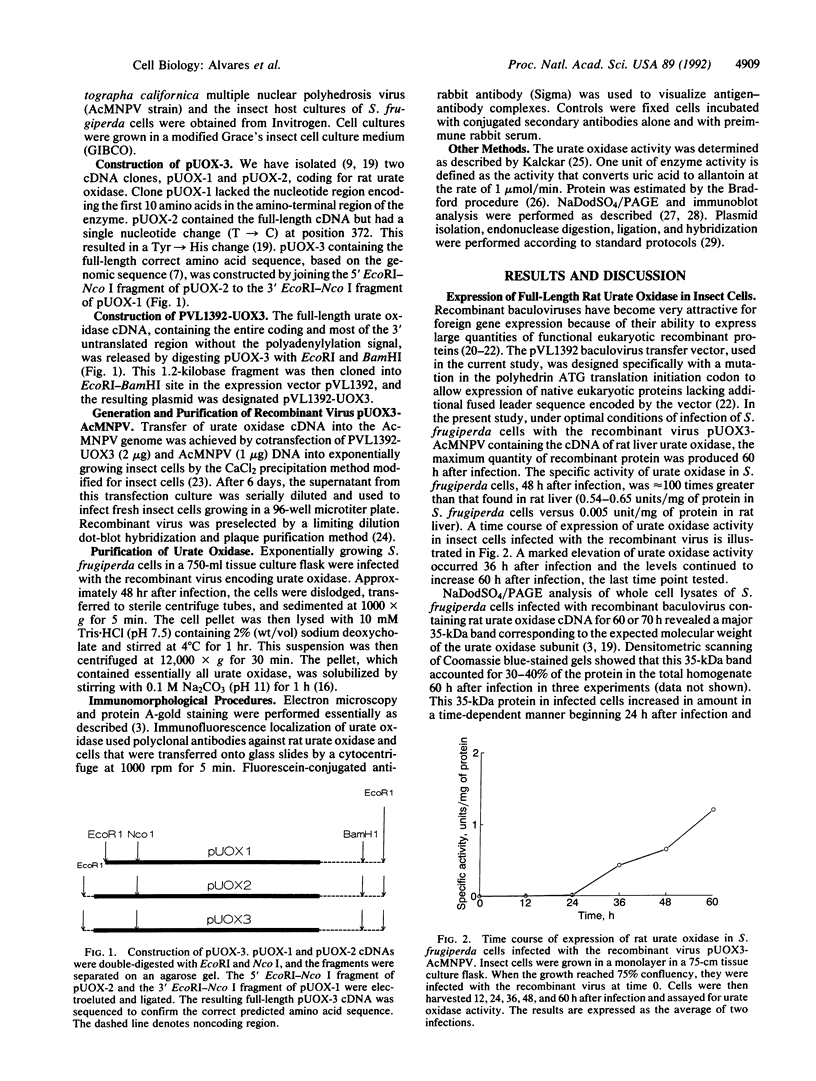
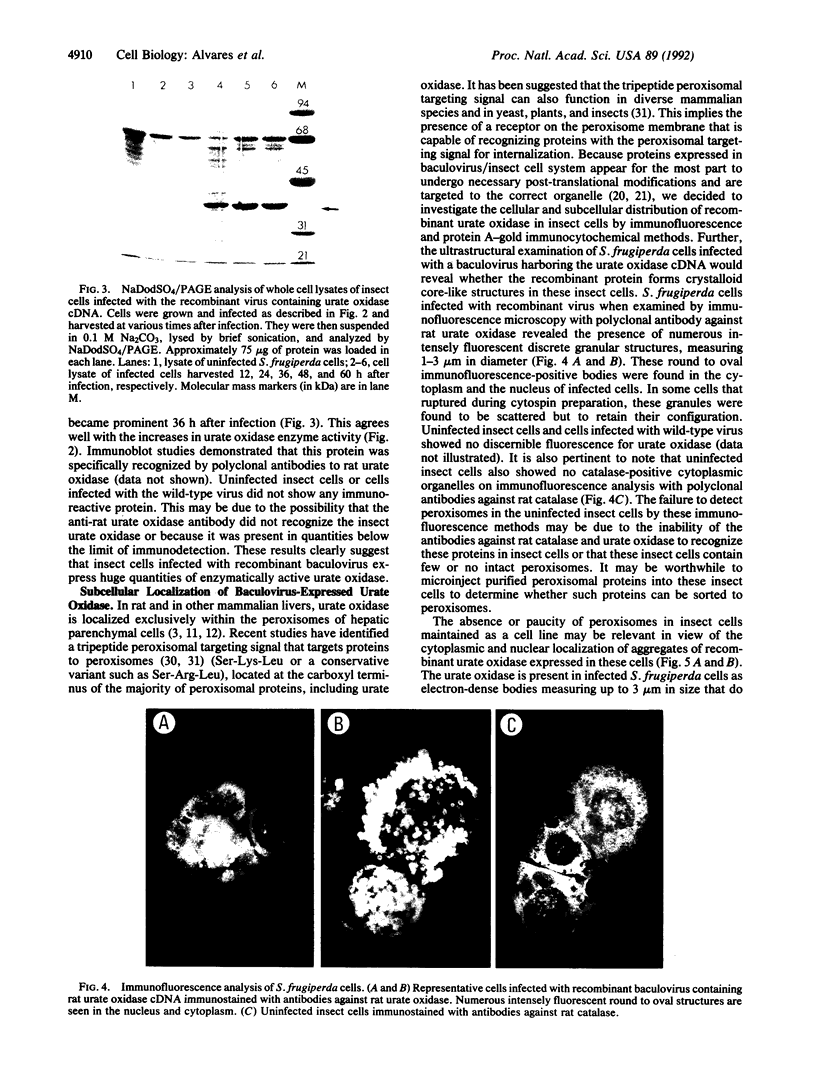
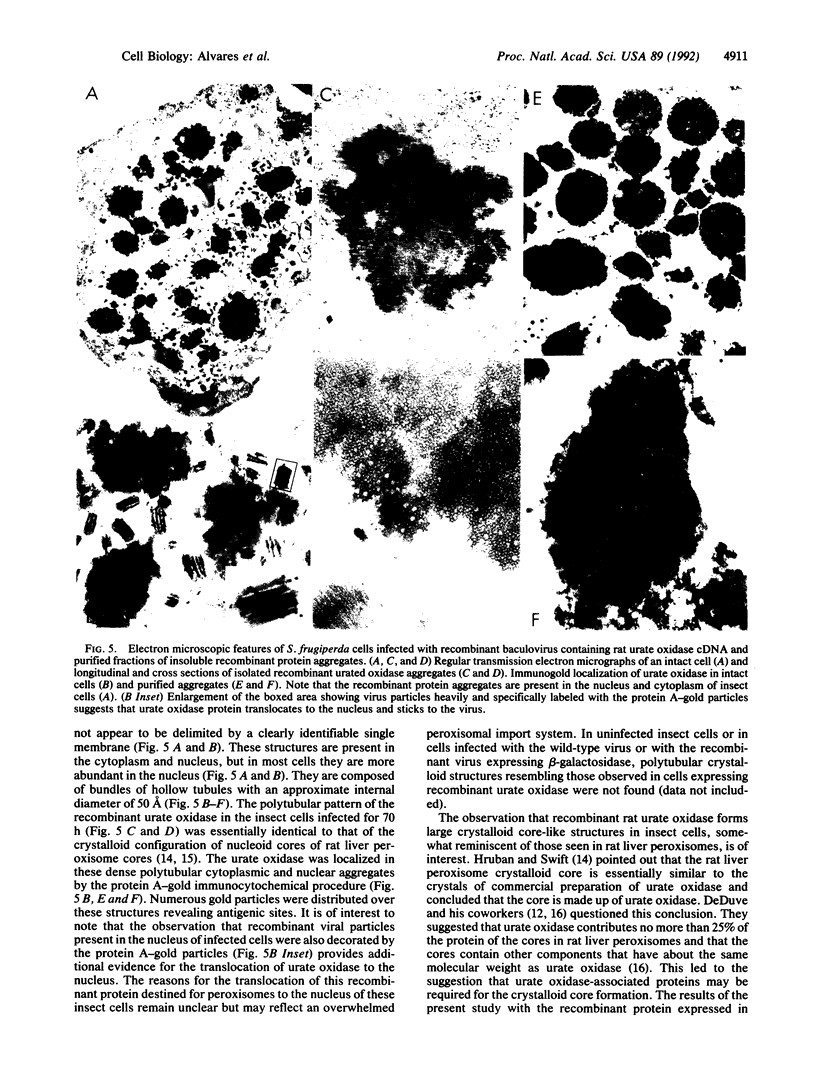
Urate oxidase (EC 1.7.3.3), which catalyzes the oxidation of uric acid to allantoin, is present in most mammals but absent in humans and hominoid primates. In rats and most other mammals that catabolize uric acid to allantoin, this enzyme is localized within the crystalloid cores of peroxisomes present in liver parenchymal cells. To determine whether urate oxidase forms these crystalloid cores or whether core-forming protein(s) exist in association with urate oxidase, a baculovirus expression vector system was used to overproduce the full-length rat urate oxidase in Spodoptera frugiperda cells. Urate oxidase was expressed to a level of approximately 30% of the total protein in this system. Immunoblot analysis demonstrated that the baculovirus-generated protein had electrophoretic and immunologic properties similar to those of urate oxidase expressed in rat liver. Immunofluorescence and electron microscopic examination revealed that the overexpressed recombinant urate oxidase is present in both the cytoplasm and the nucleus of infected insect cells as numerous 1- to 3-microns discrete particles. These insoluble protein aggregates, which were positively stained for urate oxidase by protein A-gold immunocytochemical approach, did not appear to be delimited by a single membrane. They revealed a crystalloid structure reminiscent of rat peroxisomal core consisting of bundles of tubules with an inner diameter of approximately 50 A. The recombinant urate oxidase particles, isolated by a single-step procedure, were composed entirely of 35-kDa urate oxidase subunit. These studies indicate that rat urate oxidase is capable of forming insoluble crystalloid core-like structures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvares K., Nemali M. R., Reddy P. G., Wang X. D., Rao M. S., Reddy J. K. The nucleotide sequence of a full length cDNA clone encoding rat liver urate oxidase. Biochem Biophys Res Commun. 1989 Feb 15;158(3):991–995. doi: 10.1016/0006-291x(89)92820-9. [DOI] [PubMed] [Google Scholar]
- Antonenkov V. D., Panchenko L. F. Organization of urate oxidase in peroxisomal nucleoids. FEBS Lett. 1978 Apr 1;88(1):151–154. doi: 10.1016/0014-5793(78)80629-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Conley T. G., Priest D. G. Purification of uricase from mammalian tissue. Prep Biochem. 1979;9(2):197–203. doi: 10.1080/00327487908061683. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Friedman T. B., Polanco G. E., Appold J. C., Mayle J. E. On the loss of uricolytic activity during primate evolution--I. Silencing of urate oxidase in a hominoid ancestor. Comp Biochem Physiol B. 1985;81(3):653–659. doi: 10.1016/0305-0491(85)90381-5. [DOI] [PubMed] [Google Scholar]
- Goswami B. B., Glazer R. I. A simplified method for the production of recombinant baculovirus. Biotechniques. 1991 May;10(5):626–630. [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Hosken N., Wilkinson J., Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989 May;108(5):1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Schneider M., Howell S. H., Garrard L. J., Goodman J. M., Distel B., Tabak H., Subramani S. Peroxisomal protein import is conserved between yeast, plants, insects and mammals. EMBO J. 1990 Jan;9(1):85–90. doi: 10.1002/j.1460-2075.1990.tb08083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRUBAN Z., SWIFT H. URICASE: LOCALIZATION IN HEPATIC MICROBODIES. Science. 1964 Dec 4;146(3649):1316–1318. doi: 10.1126/science.146.3649.1316. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Taya K., Suga T., Niinobe S. Studies on peroxisomes. VI. Relationship between the peroxisomal core and urate oxidase. J Biochem. 1976 May;79(5):1029–1034. doi: 10.1093/oxfordjournals.jbchem.a131143. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leighton F., Poole B., Lazarow P. B., De Duve C. The synthesis and turnover of rat liver peroxisomes. I. Fractionation of peroxisome proteins. J Cell Biol. 1969 May;41(2):521–535. doi: 10.1083/jcb.41.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHLER H. R., HUBSCHER G., BAUM R. Studies on uricase. I. Preparation, purification, and properties of a cuproprotein. J Biol Chem. 1955 Oct;216(2):625–641. [PubMed] [Google Scholar]
- Paul J. I., Tavaré J., Denton R. M., Steiner D. F. Baculovirus-directed expression of the human insulin receptor and an insulin-binding ectodomain. J Biol Chem. 1990 Aug 5;265(22):13074–13083. [PubMed] [Google Scholar]
- Reddy P. G., Nemali M. R., Reddy M. K., Reddy M. N., Yuan P. M., Yuen S., Laffler T. G., Shiroza T., Kuramitsu H. K., Usuda N. Isolation and sequence determination of a cDNA clone for rat peroxisomal urate oxidase: liver-specific expression in the rat. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9081–9085. doi: 10.1073/pnas.85.23.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitka T. K. Comparative ultrastructure of hepatic microbodies in some mammals and birds in relation to species differences in uricase activity. J Ultrastruct Res. 1966 Dec;16(5):598–625. doi: 10.1016/s0022-5320(66)80009-6. [DOI] [PubMed] [Google Scholar]
- Stout J. T., Caskey C. T. The Lesch-Nyhan syndrome: clinical, molecular and genetic aspects. Trends Genet. 1988 Jun;4(6):175–178. doi: 10.1016/0168-9525(88)90024-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada H., Mochizuki Y., Fujiwara S. The nucleoids of rat liver cell microbodies. Fine structure and enzymes. J Cell Biol. 1966 Mar;28(3):449–460. doi: 10.1083/jcb.28.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda N., Reddy M. K., Hashimoto T., Rao M. S., Reddy J. K. Tissue specificity and species differences in the distribution of urate oxidase in peroxisomes. Lab Invest. 1988 Jan;58(1):100–111. [PubMed] [Google Scholar]
- Wang X. D., Kawano H., Alvares K., Reddy P. G., Getto H., Rao M. S., Reddy J. K. Rat urate oxidase: cloning and structural analysis of the gene and 5'-flanking region. Gene. 1991 Jan 15;97(2):223–229. doi: 10.1016/0378-1119(91)90055-g. [DOI] [PubMed] [Google Scholar]
- Wu X. W., Lee C. C., Muzny D. M., Caskey C. T. Urate oxidase: primary structure and evolutionary implications. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9412–9416. doi: 10.1073/pnas.86.23.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeldandi A. V., Wang X. D., Alvares K., Kumar S., Rao M. S., Reddy J. K. Human urate oxidase gene: cloning and partial sequence analysis reveal a stop codon within the fifth exon. Biochem Biophys Res Commun. 1990 Sep 14;171(2):641–646. doi: 10.1016/0006-291x(90)91194-w. [DOI] [PubMed] [Google Scholar]
- Yeldandi A. V., Yeldandi V., Kumar S., Murthy C. V., Wang X. D., Alvares K., Rao M. S., Reddy J. K. Molecular evolution of the urate oxidase-encoding gene in hominoid primates: nonsense mutations. Gene. 1991 Dec 30;109(2):281–284. doi: 10.1016/0378-1119(91)90622-i. [DOI] [PubMed] [Google Scholar]