Abstract
AIM: To describe the incidence of cardiovascular mortality (CVM) in survivors of major cancers and identify its trends over the past two decades.
METHODS: We used the surveillance, epidemiology and end-results 19 registry to identify young adults (20-49 years), diagnosed with the following major primary cancers: Lung, breast, liver/intrahepatic bile duct, pancreas, prostate, colorectal, and ovarian from 1990 through 2012 and identified the cumulative incidence of CVM after adjusting for confounding factors.
RESULTS: We identified a total of 301923 cancers (breast 173748, lung 38938, colorectal 31722, prostate 22848, ovary 16065, liver 9444, pancreas 9158). A total of 2297 (0.8%) of patients had incident CVM. Lung (10-year cumulative CVM 2.4%) and liver (1.73%) cancers had the highest incidence of CVM, while breast (0.6%) and prostate (1.2%) had the lowest CVM mortality, even after multiple adjustments (P < 0.001). Overall, there was a significant improvement in CVM since 1990 [2005-2012 vs 1990-1994, adjusted HR 0.63 (0.54-0.72), P < 0.001]. This was driven by improvements in CVM in lung cancers (P = 0.02), breast (P < 0.001), and a trend in ovarian cancer (P = 0.097). There was no statistically significant improvement in CVM among survivors of colorectal, pancreatic, liver, or prostate cancers.
CONCLUSION: The risk of CVM differs among different cancers, and is highest among survivors of lung and liver cancers. The incidence of CVM has decreased over the past 2 decades mainly among survivors of lung and breast cancers.
Keywords: Cardiovascular disease, Cancer, Trends, Cardiovascular mortality, Type of cancer
Core tip: Cancers and cardiovascular diseases share many risk factors. Premature cardiovascular mortality (CVM) has been described in cancer survivors. However, the trends of CVM in cancer survivors are largely unknown. Using a large national cancer registry in the United States, we show that CVM has decreased in survivors of breast and lung cancers, but not other cancers. Surprisingly, more than half of all cardiovascular deaths occur before age of 50 years. It is likely that interventions targeted at decreasing CVM in cancer survivors will decrease the overall mortality in those patients.
INTRODUCTION
Cardiovascular diseases and cancers are the leading causes of death in the United States[1]. They often coexist due to similar risk factors (e.g., smoking, advanced age, chronic inflammation). We have previously shown that preexisting cardiovascular diseases are prevalent in patients with cancers and may be undertreated[2].
Patients with cancer may have subclinical cardiac disease even prior to cardiotoxic therapy[3]. In addition, many of cancer therapies (including chemotherapy, radiation therapy, and surgery) can directly or indirectly impact cardiovascular health[4-8]. As a result, patients with different cancers have been shown to have increased cardiovascular morbidity and mortality compared with the general population[6,9-11].
There is wide variability in cardiovascular risk between different cancer populations[2]. Recent advances in cancer and cardiovascular therapies have resulted in overall improved population survival[12,13], however, it is unclear if these advances translate into decreased CVM among cancer survivors. The current study was done to analyze the incidence of CVM after major cancers and report the changes over the past 2 decades in the United States.
MATERIALS AND METHODS
Data source
We used the surveillance, epidemiology and end-results (SEER) 19 database for this study. SEER 19 research data is a program of the national cancer institute and includes incidence and individual-level data collected from 19 cancer registries on patient demographics, histopathology, staging, geographic areas, treatments, follow-up and causes of death on all cancers diagnosed 1973-2012. Data are de-identified and are accessible through an online software (SEER*Stat). Based on November 2014 submission, SEER includes 8689771 cases. Causes of death are reported in broad categories that are coded from a list of International Classification of Diseases (ICDs). SEER data includes public-access deidentified data only, and thus institutional review board approval was not required.
Cohort selection
For this study, we identified young adults (20-49 years at diagnosis), diagnosed with the following major primary cancers using the 3rd edition of the ICDs for Oncology site codes: Lung (C34.0 to C34.9), breast (C50.0 to C50.9), liver/intrahepatic bile duct (C22.0 to C22.1), pancreas (C25.0 to C25.9), prostate (C61.9), colorectal (C18.0 to C18.9; C19.9 to C20.9) and ovarian (C56.9) diagnosed from 1990 through 2012.
Outcomes
Outcomes include cardiovascular mortality (CVM) stratified by type of cancer and by era of diagnosis. We defined CVM to include the following ICD codes: ICD 9 (1979 to 1998): 390 to 398, 402, 404, 410 to 429; and ICD 10 (1999þ): I00 to I09, I11, I13, I20 to I513.
Statistical analysis
Continuous variables are presented as mean ± SD and compared using t-test. Categorical variables are presented as numbers and percentages and compared using χ2 test. Cox-proportional hazard models were used for survival adjusting for age, gender, race, year of diagnosis, surgery, radiation, SEER stage, and cancer site; censoring for loss to follow-up or death from other causes. All test were two sided and P < 0.05 was considered significant.
RESULTS
We identified a total of 301923 cancers (breast 173748, lung 38938, colorectal 31722, prostate 22848, ovary 16065, liver 9444, pancreas 9158). Mean age at cancer diagnosis for the entire cohort was 43 ± 5.6 years, 24.4% were male, and 74.3% were white; 45.3% had local disease, 78.8% had surgery, and 35.4% had beam radiation. Table 1 shows the characteristics of study population by cancer type.
Table 1.
Characteristics of patients by cancer type
| Breast | Colorectal | Liver | Lung | Ovary | Pancreas | Prostate | All | |
| Age (yr) | 42.6 ± 5.3 | 42.2 ± 6.2 | 43.4 ± 6.1 | 44.0 ± 5.1 | 40.5 ± 7.5 | 43.5 ± 5.4 | 46.4 ± 2.8 | 43.0 ± 5.6 |
| Sex | ||||||||
| Female | 99.6% | 48.3% | 22.2% | 46.0% | 100.0% | 42.3% | 0.0% | 75.6% |
| Male | 0.4% | 51.7% | 77.8% | 54.0% | 0.0% | 57.7% | 100.0% | 24.4% |
| Race | ||||||||
| White | 75.8% | 72.7% | 61.0% | 73.8% | 77.9% | 74.5% | 68.4% | 74.3% |
| Black | 12.9% | 16.3% | 14.9% | 18.4% | 9.4% | 16.6% | 25.9% | 15.0% |
| Other | 10.5% | 10.0% | 23.6% | 7.5% | 12.0% | 8.5% | 2.9% | 9.9% |
| Unknown | 0.8% | 0.9% | 0.5% | 0.3% | 0.7% | 0.4% | 2.8% | 0.9% |
| Year of diagnosis | ||||||||
| 1990-1994 | 9.8% | 8.9% | 7.9% | 13.1% | 12.1% | 9.1% | 3.6% | 9.7% |
| 1995-1999 | 12.7% | 11.4% | 13.5% | 13.9% | 13.6% | 12.4% | 9.2% | 12.5% |
| 2000-2004 | 28.8% | 28.8% | 31.4% | 31.4% | 29.0% | 28.7% | 29.6% | 29.3% |
| 2005-2012 | 48.8% | 50.8% | 47.3% | 41.6% | 45.4% | 49.7% | 57.6% | 48.5% |
| Surgery | ||||||||
| No | 3.8% | 7.8% | 63.5% | 63.1% | 5.4% | 61.8% | 23.4% | 17.0% |
| Yes | 94.2% | 89.7% | 23.5% | 26.9% | 92.1% | 28.2% | 68.2% | 78.8% |
| Unknown | 2.1% | 2.4% | 13.0% | 10.0% | 2.5% | 9.9% | 8.4% | 4.2% |
| Stage | ||||||||
| Local | 53.3% | 29.2% | 33.9% | 11.6% | 35.6% | 8.8% | 90.7% | 45.3% |
| Regional | 38.7% | 37.2% | 27.2% | 22.8% | 8.5% | 26.3% | 0.0% | 31.2% |
| Distant | 6.1% | 29.1% | 21.5% | 58.7% | 50.6% | 57.3% | 3.4% | 19.5% |
| Unstaged | 2.0% | 4.5% | 17.3% | 6.9% | 5.3% | 7.7% | 5.9% | 4.0% |
| Radiation | ||||||||
| None | 47.8% | 94.0% | 91.0% | 46.7% | 97.1% | 76.1% | 79.3% | 59.7% |
| Beam | 47.0% | 4.1% | 4.6% | 48.9% | 1.6% | 20.3% | 10.5% | 35.4% |
| Other | 0.7% | 0.1% | 1.0% | 0.4% | 0.2% | 0.2% | 8.0% | 1.1% |
| Unknown | 4.4% | 1.9% | 3.4% | 4.0% | 1.1% | 3.4% | 2.3% | 3.7% |
A total of 2297 (0.8%) of patients had incident CVM. Majority were females (60.4%), white (64.7%), with mean age at cancer diagnosis of 44.7 ± 4.6 years. Majority were survivors of breast cancer (40%), followed by lung (25%), colorectal (11.8%), prostate (11.4%), liver and ovary (4.3% each), and pancreas (3.2%). CVM occurred at a mean 5.3 ± 5.2 years after diagnosis of cancer. Mean age at cardiovascular death was 50.1 ± 6.8 years (range 20-70 years). The distribution of age at death is shown in Figure 1.
Figure 1.
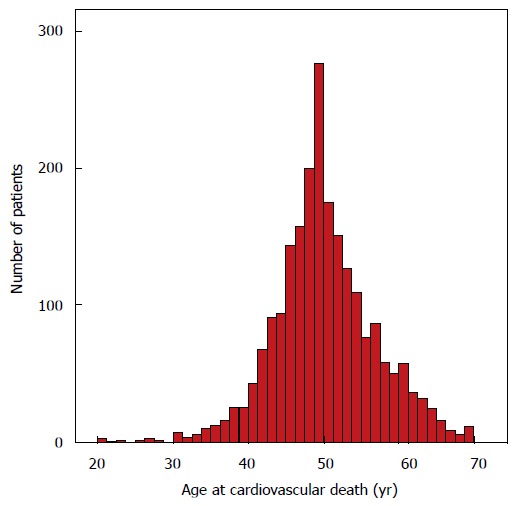
Age at cardiovascular death for all patients (n = 2297).
Cumulative CVM varied by cancer type: Lung (10 year cumulative CVM 2.4%) and liver (1.73%) cancers had the highest incidence of CVM, while breast (0.6%) and prostate (1.2%) had the lowest CVM mortality, even after multiple adjustments (P < 0.001, Figure 2).
Figure 2.
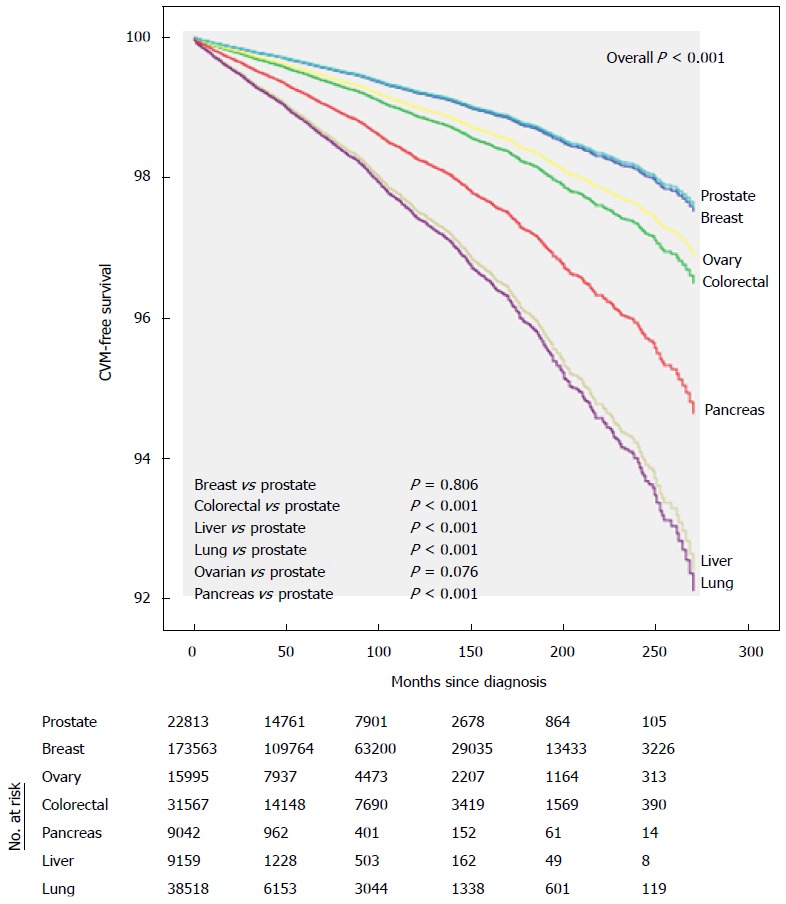
Adjusted cumulative cardiovascular mortality by cancer site.
Overall, there was a significant improvement in CVM between era 4 and era 1 [2005-2012 vs 1990-1994, adjusted HR 0.63 (0.54-0.72), P < 0.001], era 4 vs era 2 [2005-2012 vs 1995-1999, adjusted HR 0.67 (0.58-0.79), P < 0.001] and era 4 vs era 3 [2005-2012 vs 2000-2004, adjusted HR 0.79 (0.70-0.90), P < 0.001] (Table 2 and Figure 3). When taken as a continuous variable, there was an average decrease in CVM of 3% per year [adjusted HR 0.97 (0.96-0.98) per year, P < 0.001]. This was driven by improvements in CVM in lung cancers (2005-2012 vs 1990-1994, adjusted HR 0.69, P = 0.02), breast (2005-2012 vs 1990-1994, adjusted HR 0.58, P < 0.001), and a trend in ovarian cancer (2005-2012 vs 1990-1994, adjusted HR 0.46, P = 0.097). There was no statistically significant improvement in CVM among survivors of colorectal (P = 0.331), pancreatic (P = 0.119), liver (P = 0.696), or prostate cancers (P = 0.148), Figure 4.
Table 2.
Multivariable model for cardiovascular mortality
| HR | 2.5th-ile | 97.5th-ile | P-value | |
| Cancer type | ||||
| Breast vs prostate | 1.026 | 0.837 | 1.258 | 0.806 |
| Colorectal vs prostate | 1.456 | 1.196 | 1.773 | < 0.001 |
| Liver vs prostate | 3.221 | 2.495 | 4.158 | < 0.001 |
| Lung vs prostate | 3.348 | 2.797 | 4.007 | < 0.001 |
| Ovarian vs prostate | 1.298 | 0.973 | 1.731 | 0.076 |
| Pancreas vs prostate | 2.248 | 1.675 | 3.016 | < 0.001 |
| Demographics | ||||
| Age at diagnosis (per year) | 1.078 | 1.068 | 1.089 | < 0.001 |
| Black vs white | 2.397 | 2.18 | 2.635 | < 0.001 |
| Other vs white | 0.79 | 0.662 | 0.942 | 0.009 |
| Unknown vs white | 0.428 | 0.203 | 0.903 | 0.026 |
| Female vs male | 0.678 | 0.595 | 0.773 | < 0.001 |
| Year of diagnosis (per year) | 0.97 | 0.962 | 0.978 | < 0.001 |
| Radiation | ||||
| Beam radiation vs no radiation | 0.814 | 0.737 | 0.9 | < 0.001 |
| Other radiation vs no radiation | 0.463 | 0.304 | 0.703 | < 0.001 |
| Unknown vs no radiation | 0.867 | 0.673 | 1.119 | 0.273 |
| Surgery | ||||
| Surgery vs no surgery | 0.424 | 0.368 | 0.489 | < 0.001 |
| Unknown vs no surgery | 0.937 | 0.78 | 1.127 | 0.491 |
| Stage | ||||
| Regional vs local | 1.394 | 1.254 | 1.548 | < 0.001 |
| Distant vs local | 1.705 | 1.472 | 1.976 | < 0.001 |
| Unstaged vs local | 1.304 | 1.093 | 1.555 | 0.003 |
Figure 3.
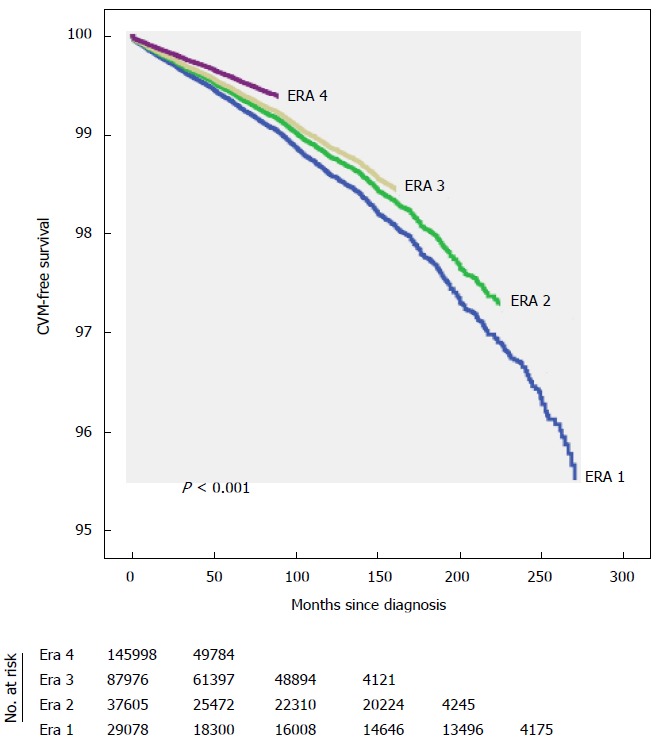
Adjusted overall cardiovascular mortality-free survival across eras.
Figure 4.
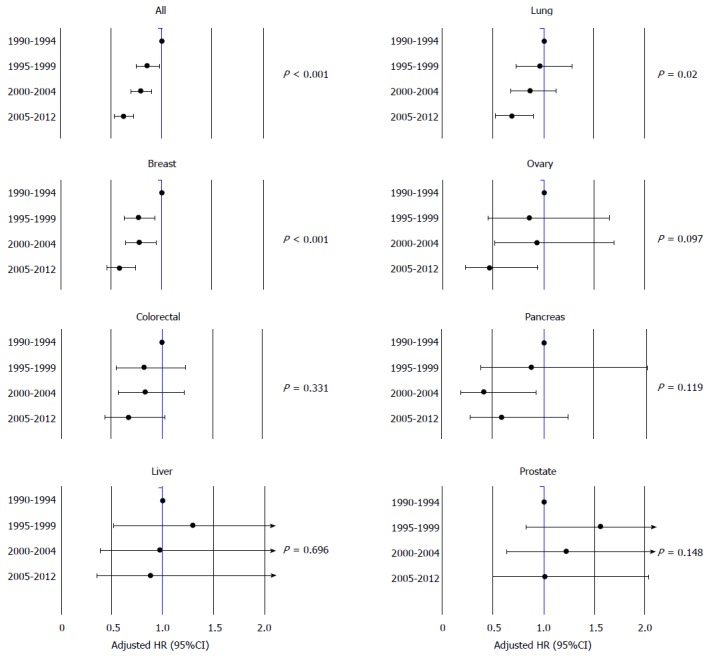
Adjusted HR of cardiovascular mortality by year of cancer diagnosis.
DISCUSSION
Our findings suggest that young adults remain at high risk for CVM following cancer diagnosis and this risk varies by type of cancer. Half of all cardiovascular deaths occur before age of 50; however, the incidence of CVM has decreased over the last 2 decades, mainly among survivors of lung and breast cancers.
We provide the first evidence that CVM has been decreasing over the past decades in lung and breast cancers, but not others. We have previously shown that these trends were also seen among young adults with early stage Hodgkin lymphoma[14], and were also recently reported in survivors of childhood cancers[15]. This is likely due to recognition of cardiovascular disease in cancer survivors, improvements in cardiovascular screening and treatment options, in addition to better, less cardiotoxic cancer treatment. Oncocardiology, a field of cardiovascular disease management and assessment in cancer patients, has played a role in comprehensive assessment and follow-up in patients with cancer[1,4,16]. The availability of newer imaging techniques in detecting subclinical myocardial dysfunction (e.g., strain imaging, cardiac magnetic resonance imaging), helped identify patients earlier, and thus provide opportunities for treatment or prevention, especially in patients receiving anthracyclines and HER2 antagonists[17,18].
It is important to note, however, that there has been no significant reduction in CVM among patients with prostate, liver, colorectal, and pancreas. It is likely that this is due to high utilization of non-anthracycline chemotherapy, whose cardiotoxic effects have not been well studied. It is also possible that these patients have higher prevalence of comorbidities not accounted for in this analysis. These findings are hypothesis generating and require further investigation.
Our multivariable model suggests that patients with advanced cancers (regional or metastatic) have and those inoperable cancers have higher rates of CVM. The reasons for this finding remain speculative, but it is possible that patients with advanced diseases may receive more cardiotoxic chemotherapy and/or radiation, which have been shown to impact long-term survival. These findings were also observed in young adults with Hodgkin lymphoma[14].
It is surprising that half of all cardiovascular deaths occurred before age of 50 years, suggesting premature cardiac death. The implication of this finding is that improving cardiovascular health with early monitoring and prevention strategies may significantly decrease the overall mortality in patients with cancer. This can be accomplished through development of non-cardiotoxic targeted therapies, reduced heart radiation-dose, and modulation of cardiovascular risk factors before, during and after treatment.
This report highlights the need for intensive management of cardiovascular risk factors and cardiovascular disease in patients with cancer diagnosis, particularly lung and liver. Early involvement of cardiologists, through oncocardiology practices, may prove helpful in patients at high risk, especially those with preexisting heart disease or those undergoing cardiotoxic therapies. Future studies should focus on the impact of cardiovascular disease management on long-term outcomes in these cancers.
While this is a very large cohort of patients, this study has limitations that need to be acknowledged. First, we do not have data on cardiovascular risk factors in patients (such as smoking, diabetes, hypertension) and cardiovascular medications. Second, we don’t have data on cardiotoxic chemotherapy, and radiation doses which may impact the development of cardiovascular disease. Hence, we were unable to ascertain the etiology of CVM. Also, we did not have granular data on the exact causes of death. Therefore, it is imperative to study these factors in a prospective fashion.
CVM is highest among survivors of lung and liver cancers and lowest among prostate and breast cancer survivors. The incidence of CVM has significantly decreased over the past 2 decades mainly among survivors of lung and breast cancers.
COMMENTS
Background
Cancers and cardiovascular diseases share many risk factors. Premature cardiovascular mortality (CVM) has been described in cancer survivors. However, the applicability of improved CVM in the general population to cancer survivors is largely unknown.
Research frontiers
The impact of preexisting cardiovascular disease on overall survival in cancer survivors need to be investigated. In addition, the role of primary and secondary prevention for cardiovascular disease in this cohort needs to be studied.
Innovations and breakthroughs
The authors show, for the first time, that survivors of cancers of breast and lung, but not others, have a decreasing risk of CVM over the past 2 decades.
Applications
The implications of the current study help raise awareness about the cardiovascular disease in cancer survivors. Efforts should be focused on decreasing cardiovascular disease in patients with cancers of liver, pancreas, colorectal, and ovarian cancers.
Terminology
CVM is death due to any cardiovascular disease which include but not limited to: Ischemic heart disease, heart failure, stroke, thrombosis. Cardiotoxic chemotherapy is any chemotherapy (mainly anthracyclines and HER2 antagonists) that has a negative direct or indirect effect on the myocardium.
Peer-review
The authors present here a nice paper on CVM and cancer. The manuscript is well written and pretty interesting, even with its (recognized) inherent limitations.
Footnotes
Institutional review board statement: This study included only deidentified data and was exempt from institutional review board approval at University Hospitals Case Medical Center.
Informed consent statement: This study used deidentified data and did not require informed consent.
Conflict-of-interest statement: Both authors have no conflict of interest pertinent to this study.
Data sharing statement: Data used for this analysis are available from SEER program (seer.cancer.gov).
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 29, 2016
First decision: March 22, 2016
Article in press: April 22, 2016
P- Reviewer: Nishio K, Nunez-Gil IJ, Pauliks L, Sicari R, Tan XR S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
References
- 1.Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc. 2014;89:1287–1306. doi: 10.1016/j.mayocp.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Kindi SG, Oliveira GH. Prevalence of Preexisting Cardiovascular Disease in Patients With Different Types of Cancer: The Unmet Need for Onco-Cardiology. Mayo Clin Proc. 2016;91:81–83. doi: 10.1016/j.mayocp.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Pavo N, Raderer M, Hülsmann M, Neuhold S, Adlbrecht C, Strunk G, Goliasch G, Gisslinger H, Steger GG, Hejna M, et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101:1874–1880. doi: 10.1136/heartjnl-2015-307848. [DOI] [PubMed] [Google Scholar]
- 4.Al-Kindi S, Younes A, Qattan M, Oliveira GH. Preemptive Cardioprotective Strategies in Patients Receiving Chemotherapy. Curr Cardiovasc Risk Rep. 2014;8:1–14. [Google Scholar]
- 5.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, van ‘t Veer MB, Baaijens MH, de Boer JP, Hart AA, Klokman WJ, Kuenen MA, Ouwens GM, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 6.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinou M, Gaya A. Cardiac complications after radical radiotherapy. Semin Oncol. 2013;40:178–185. doi: 10.1053/j.seminoncol.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Weaver KE, Foraker RE, Alfano CM, Rowland JH, Arora NK, Bellizzi KM, Hamilton AS, Oakley-Girvan I, Keel G, Aziz NM. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv. 2013;7:253–261. doi: 10.1007/s11764-013-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung C, Fossa SD, Milano MT, Sahasrabudhe DM, Peterson DR, Travis LB. Cardiovascular Disease Mortality After Chemotherapy or Surgery for Testicular Nonseminoma: A Population-Based Study. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.60.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang F, Keating NL, Mucci LA, Adami HO, Stampfer MJ, Valdimarsdóttir U, Fall K. Immediate risk of suicide and cardiovascular death after a prostate cancer diagnosis: cohort study in the United States. J Natl Cancer Inst. 2010;102:307–314. doi: 10.1093/jnci/djp537. [DOI] [PubMed] [Google Scholar]
- 11.Tukenova M, Guibout C, Oberlin O, Doyon F, Mousannif A, Haddy N, Guérin S, Pacquement H, Aouba A, Hawkins M, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28:1308–1315. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. QuickStats: Age-Adjusted Death Rates for Heart Disease and Cancer, by Sex - United States, 1980-2011. Morbidity and Mortality Weekly Report (MMWR), 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Kindi SG, Abu-Zeinah GF, Kim CH, Hejjaji V, William BM, Caimi PF, Oliveira GH. Trends and Disparities in Cardiovascular Mortality Among Survivors of Hodgkin Lymphoma. Clin Lymphoma Myeloma Leuk. 2015;15:748–752. doi: 10.1016/j.clml.2015.07.638. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, Stovall M, Oeffinger KC, Bhatia S, Krull KR, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med. 2016;374:833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lunning MA, Kutty S, Rome ET, Li L, Padiyath A, Loberiza F, Bociek RG, Bierman PJ, Vose JM, Armitage JO, et al. Cardiac magnetic resonance imaging for the assessment of the myocardium after doxorubicin-based chemotherapy. Am J Clin Oncol. 2015;38:377–381. doi: 10.1097/COC.0b013e31829e19be. [DOI] [PubMed] [Google Scholar]
- 17.Thavendiranathan P, Wintersperger BJ, Flamm SD, Marwick TH. Cardiac MRI in the assessment of cardiac injury and toxicity from cancer chemotherapy: a systematic review. Circ Cardiovasc Imaging. 2013;6:1080–1091. doi: 10.1161/CIRCIMAGING.113.000899. [DOI] [PubMed] [Google Scholar]
- 18.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]


