Abstract
Solitary fibrous tumor (SFT) is a mesenchymal tumor typically located in the pleura, but can also be found as an asymptomatic mass in other areas, including the liver, peritoneum, kidney and salivary glands. However, SFT rarely locates in the pancreas. We present such a case of pancreatic SFT, along with a review of all reported cases. A 55-year-old man was treated surgically for an asymptomatic pancreatic mass after a rigorous preoperative control. Histologic examination of the resected specimen showed characteristics of an SFT. As only 15 cases of pancreatic SFT have been reported so far, an attempt to compare the cases was considered intriguing. We found that patients with pancreatic SFT were mainly women (81.25%), with a median age of 54 years at the time of diagnosis and a median tumor size of 5.83 cm. Pancreatic SFTs were revealed incidentally in 50% of cases, and all of them showed an enhancement through arterial computed tomography. All tumors were positive for CD34, ten were positive for Bcl-2, and twelve were negative for S100. The diagnosis of this pancreatic tumor is established by a combination of clinical suspicion, imaging procedures and histological findings, and is confirmed by immunohistochemical staining. Although the behavior of SFTs is rather benign, close clinical follow-up is recommended due to a potentially malignant nature.
Keywords: Solitary fibrous tumor, Pancreas, Mesenchymal tumors, Differential diagnosis, Solitary fibrous tumor treatment
Core tip: Solitary fibrous tumor (SFT) is a mesenchymal tumor that rarely locates in the pancreas. We present the case of a 55-year-old man with a pancreatic lesion that proved to be an SFT after thorough pre- and post-operative control. The characteristics of this case and the other fifteen cases of pancreatic SFT reported in the literature are described.
INTRODUCTION
Solitary fibrous tumor (SFT) was described as a distinct type of mesenchymal tumor for the first time at 1931[1]. SFT can be benign or malignant and is typically located in the pleura, but the tumor can also be found as an asymptomatic mass in other areas such as the liver, peritoneum, kidney and salivary glands[2-4]. SFTs often occur in middle age (40-70 years), especially in the fifth decade of life, and metastases can be detected in 10% to 15% of the cases[4-6]. This type of tumor appears very rarely in the pancreas, as only 15 cases have been reported so far.
CASE REPORT
A 55-year-old man was admitted in our surgical department for further evaluation of an asymptomatic pancreatic mass that had been found incidentally by upper abdomen ultrasonography. No specific symptoms were mentioned except vague abdominal pain. Clinical examination and laboratory results were unremarkable, and the levels of tumor markers (CEA, CA 19-9, CA 15-3) were within normal ranges. The patient’s medical history included coronary heart disease (one myocardial infarction and coronary artery bypass surgery seven years ago), open cholecystectomy (27 years ago), choledocholithiasis treated with endoscopic retrograde cholangiopancreatography (5 years ago), dyslipidemia and hypertension under medication.
During ultrasound examination of the abdomen for non-specific pain, a hypoechoic solid mass of 2.8 cm × 3.1 cm on the pancreatic body (Figure 1) and a possible dilation of the common bile duct were found. Further imaging was performed. Computed tomography (CT) scans revealed a 3.9-cm-diameter exophytic mass arising from the pancreatic body that was enhanced from the arterial to portal venous phase (Figure 2). T1-weighted magnetic resonance imaging (MRI) scans showed a hypointense mass. Regional lymphadenopathy was not found on the CT or MRI images. Magnetic resonance cholangiopancreatography imaging revealed a pancreatic body cystic portion with heterogeneous internal echogenicities of mixed substantial and necrotic parts. Although the main pancreatic duct was not dilated, the common bile duct displayed a filling deficit. The upper digestive tract endoscopy showed no abnormalities.
Figure 1.
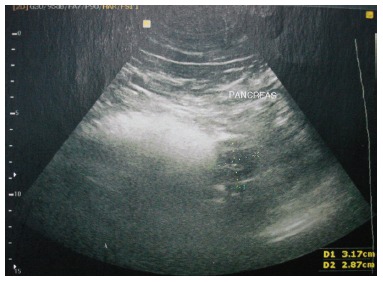
Ultrasound echo showing a 2.8 cm × 3.1 cm hypoechoic solid mass located on the pancreatic body.
Figure 2.
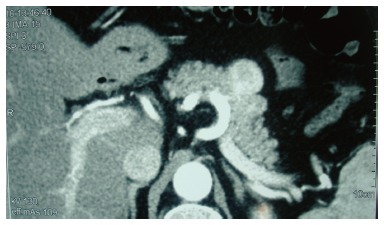
Computed tomography scan revealing a 3.9 cm exophytic mass, arising from the pancreatic body, which was enhanced from the arterial to portal venous phase.
Based on the above findings, we considered that the differential diagnosis of the mass should include exophytic non-functioning islet tumor, solid pseudopapillary mass of the pancreas, gastrointestinal stromal tumor (GIST), neuroendocrine tumor and hemangiopericytoma of the pancreas.
The surgical removal of the mass was considered necessary and the patient underwent a pancreatectomy including body and tail, whereas the spleen was maintained. The resected mass measured 3.6 cm in its greatest diameter and had an ovoid shape with well-capsulated, smooth margins and a yellowish-grey hue (Figures 3 and 4). The abdominal exploration did not show any signs of metastasis or tumor infiltration into neighboring organs.
Figure 3.
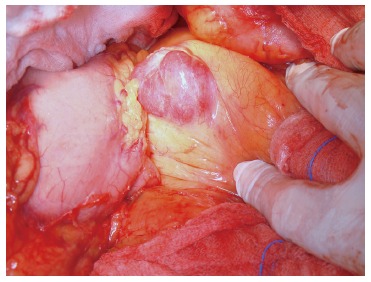
Intra-operative appearance of the pancreatic mass.
Figure 4.
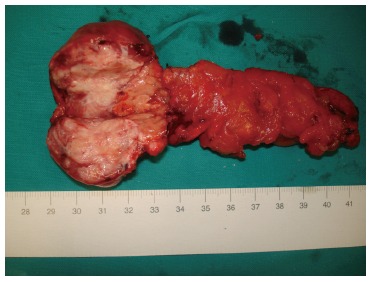
Pancreatic mass specimen.
On histopathological examination, the mass showed characteristics of a neoplasm, including spindle-shaped tumor cells with elongated nuclei. Mitoses were scarce. The neoplasm presented a storiform pattern with alternating hypocellular and hypercellular areas, and some areas showed myxoid degeneration. There were a large number of blood vessels within the stroma (Figure 5).
Figure 5.
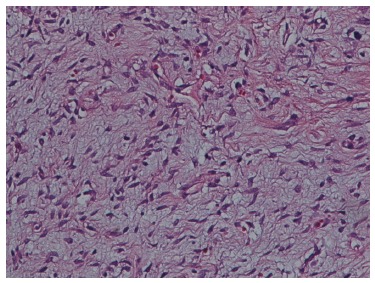
Solitary fibrous tumor with a hyalinized area, HE stain. Magnification: × 400 .
Immunohistochemically, the tumor cells were positive for CD34, CD99, Bcl-2 (Figure 6) and vimentin, and exhibited focal positivity for S-100 protein. Only a small number of cells were positive for the cell proliferation marker Ki-67/MIB-1. The tumor cells were negative for smooth muscle actin (SMA), keratin, cytokeratin (AE1/AE3), CD117, epithelial membrane antigen and desmin. The final diagnosis was pancreatic SFT with a low grade of malignancy.
Figure 6.
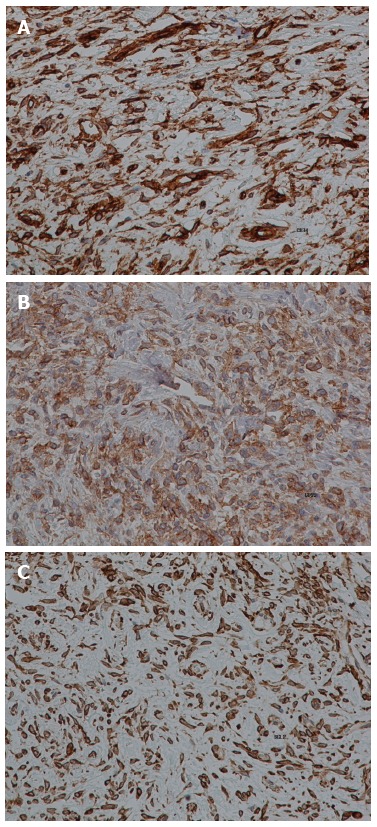
Immunohistochemical staining (brown) for CD34 (A), CD99 (B) and Bcl-2 (C). Magnification: × 400.
After surgery, the patient was led to the intensive care unit. His post-operative course was uneventful and he was discharged on the 9th postoperative day with the recommendation to follow a non-fat diet. Chemotherapy was not considered necessary. In the 6- and 12-mo follow-up periods the patient was asymptomatic and in good health.
DISCUSSION
Mesenchymal tumors of the pancreas include leiomyosarcoma, peripheral nerve tumors, fibro-histiocytic tumors and vascular tumors[7-9]. Differential diagnosis is based on histology and immunohistochemistry findings and, therefore, diagnosis is often very difficult before surgery.
On the other hand, pancreatic SFTs are very rare, as only 16 cases have been reported in the literature[1,3-16], including our case. The patients were mainly women (13/16, 81.25%), with a median age of 54 years at the time of diagnosis (range: 24-78 years, median age: 50 years for males, 55.46 years for females) and a median tumor size of 5.83 cm (range: 1.5-18.5 cm). In six cases the tumor was located in the pancreatic body (37%) and in seven cases the tumor was located in the pancreatic head (43.75%) (Table 1). The tumor was revealed incidentally in half the cases; however, in the other half, the main symptom was pain (jaundice was presented in only one case). Regarding imaging, all tumors showed an enhancement through arterial and portal CT. The results of MRI imaging were described for seven cases: These tumors were hypointense in T1-weighted images and hyperintense in T2-weighted images (Table 2).
Table 1.
Characteristics of patients with solitary fibrous tumor of the pancreas
| Ref. | Sex | Age | Symptoms | Size (cm) | Location |
| Lüttges et al[4] | Female | 50 | Incidental | 5.5 | Body |
| Chatti et al[8] | Male | 41 | Pain | 13 | Body |
| Gardini et al[10] | Female | 62 | Pain | 3 | Head |
| Miyamoto et al[9] | Female | 41 | Pain | 2 | Head-body |
| Srinivasan et al[5] | Female | 78 | Pain, weight loss | 5 | Body |
| Kwon et al[7] | Male | 54 | Incidental | 4.5 | Body |
| Ishiwatari et al[11] | Female | 58 | Incidental | 3 | Head |
| Chetty et al[3] | Female | 67 | Incidental | 2.6 | Body |
| Sugawara et al[6] | Female | 55 | Incidental | 7 | Head |
| Santos et al[12] | Female | 40 | Incidental | 3 | Body |
| Tasdemir et al[1] | Female | 24 | Incidental | 18.5 | Head |
| van der Vorst et al[13] | Female | 67 | Pain | 2.8 | Uncinate process |
| Chen et al[14] | Female | 49 | Pain, distension, appetite loss | 13 | Head |
| Hwang et al[15] | Female | 53 | Incidental | 5.2 | Head |
| Hee Han et al[16] | Female | 77 | Jaundice | 1.5 | Head |
| Paramythiotis et al | Male | 55 | Pain | 3.6 | Body |
Table 2.
Imaging features of pancreatic Solitary fibrous tumors
| Ref. | Arterial CT | Venous CT | MRI T1 | MRI T2 |
| Lüttges et al[4] | Enhanced | Enhanced | ||
| Chatti et al[8] | Enhanced | Enhanced | Hypointense | Hyperintense |
| Gardini et al[10] | Enhanced | Enhanced | ||
| Miyamoto et al[9] | Enhanced | Enhanced | ||
| Srinivasan et al[5] | Hyper-enhanced | Enhanced | ||
| Kwon et al[7] | Enhanced | Enhanced | Hypointense | Hyperintense |
| Ishiwatari et al[11] | Enhanced | Enhanced | Hypointense | Hyperintense |
| Chetty et al[3] | Enhanced | Enhanced | ||
| Sugawara et al[6] | Enhanced | Enhanced | Hypointense | Hyperintense |
| Santos et al[12] | ||||
| Tasdemir et al[1] | Enhanced | Enhanced | ||
| van der Vorst et al[13] | Enhanced | Enhanced | ||
| Chen et al[14] | Enhanced | Enhanced | ||
| Hwang et al[15] | Enhanced | Enhanced | Hypointense | Hyperintense |
| Hee Han et al[16] | Enhanced | Enhanced | Hypointense | Hyperintense |
| Paramythiotis et al | Enhanced | Enhanced | Hypointense | Hyperintense |
CT: Computed tomography.
All cases were treated surgically except for one case which was diagnosed after biopsy. By immunohistochemistry, all tumors were positive for CD34, ten tumors were positive for Bcl-2, and only one tumor was positive for S100. Many tumors were positive for keratin types. Controls for CD117 (1 positive, 10 negatives), CD19 (11 positives, 1 negative) and SMA (4 positives, 5 negatives) didn’t show the same results in all cases (Table 3).
Table 3.
Treatment and immunohistochemistry findings
| Ref. | Surgery | Immunohistochemistry |
| Lüttges et al[4] | Distal pancreatectomy | Positive: CD34, CD99, Bcl-2, vimentin Negative: SMA, S100 |
| Chatti et al[8] | Enuclation | Positive: CD34, CD99 (focal), Bcl-2, SMA (focal), CD117 (focal) Negative: EMA, cytokeratin, S100 |
| Gardini et al[10] | Traverso-longmire | Positive: CD34, CD99, Bcl-2, vimentin, smooth muscle actin (focal) Negative: Desmin, CD117, S100 |
| Miyamoto et al[9] | Head-body junction | Positive: CD34, Bcl-2 Negative: cytokeratin, CAM 5.2, SMA, desmin, S100, CD117 |
| Srinivasan et al[5] | Distal pancreatectomy | Positive: CD34 (focal), CD99, Bcl-2, vimentin Negative: CAM 5.2, SMA, desmin, CD117, CD10, chromogranin, S100 |
| Kwon et al[7] | Median segmentectomy | Positive: CD34 , CD99 Negative: CD117, S100 |
| Ishiwatari et al[11] | Pancreaticoduodenectomy | Positive: CD34, Bcl-2 Negative: S100 |
| Chetty et al[3] | Whipple | Positive: CD34, CD99, Bcl-2 |
| Sugawara et al[6] | Pancreaticoduodenectomy | Positive: CD34 Negative: SMA, S100, CD117, ALK, cytokeratin |
| Santos et al[12] | Partial pancreatectomy | Positive: CD34, b-catenin Negative: C-kit |
| Tasdemir et al[1] | Enuclation | Positive: CD34, vimentin, SMA (focal) Negative: S100, CD117, desmin, cytokeratin |
| van der Vorst et al[13] | Enuclation | Positive: CD34, CD99, Bcl-2 Negative: CD117, b-catenin |
| Chen et al[14] | Whipple/child | Positive: CD34, CD68, MSA, Bcl-2, Ki-67 Negative: SMA, cytokeratin, desmin, CD117, CD99, S100 |
| Hwang et al[15] | Partial head resection | Positive: CD34, CD99, Bcl-2, SMA, CD10, ER, PR Negative: CD117, DOG-1, caldemon, desmin, EMA, S100, cytokeratin |
| Hee Han et al[16] | Echo-guided needle biopsy | Positive: CD34, CD99 Negative: C-kit, S100 |
| Paramythiotis et al | Distal pancreatectomy | Positive: CD34, CD99, Bcl-2, vimentin, S100 (focal) Negative: SMA, keratin, cytokeratin, CD117, EMA and desmin |
SMA: Smooth muscle actin; EMA: Epithelial membrane antigen; CAM: Anti-cytokeratin mouse monoclonal primary antibody; ALK: Anaplastic lymphoma kinase; MSA: Muscle specific actin; DOG-1: Anoctamin-1.
Most pancreatic SFTs presented as well-circumscribed, often partially-encapsulated masses[9] with a multinodular, whitish and firm appearance. On microscope sections, the tumors showed a patternless architecture characterized by alternating hypocellular and hypercellular areas separated by thick bands of collagen and branching hemangiopericytoma-like vessels[6]. The tumor cells were round-to-spindle-shaped and the mitoses were scarce. Tumor necrosis and infiltrative margins were observed in malignant neoplasms occurring in other locations.
The differential diagnosis of SFT includes leiomyosarcoma, liposarcoma, fibrosarcoma, fibrous histiocytoma, hemangioendothelioma, hemangiopericytoma, hemangioma, lymphangioma, islet cell tumor, paraganglioma, neuroectodermal tumor, and schwannoma[12]. The main diagnostic consideration based on imaging findings is thought to be endocrine neoplasms[3,5]. These are composed of round cells with granular, eosinophilic cytoplasm and are positive for synaptophysin and chromogranin. As leiomyosarcomas are considered to be more frequent than SFTs in the pancreas, the former must be differentiated by their nuclear atypia and increased mitotic activity[1,5]. Moreover, GISTs are positive for CD34, vimentin and CD117 but SFTs are negative for CD117 [1,5,9].
In our case, preoperative CT and MRI scans strongly showed the typical pattern of SFTs[15]. Histopathological examination of the resected tumor revealed that it was covered with a fibrous capsule and contained necrotic tissue, favoring the diagnosis of SFT. Immunohistochemistry findings confirmed the diagnosis.
The diagnosis of pancreatic SFT is established from a combination of clinical suspicion (mainly women of middle age, with abdominal pain and nonspecific symptoms), imaging procedures (ultrasound, CT and MRI) and histological findings, and is confirmed by immunohistochemistry. Although SFTs are usually benign, clinical follow-up is recommended because these tumors can become malignant[1,5].
COMMENTS
Case characteristics
Asymptomatic 55-year-old male.
Clinical diagnosis
Abdominal pain.
Differential diagnosis
Upper abdomen tumors.
Imaging diagnosis
Exophytic mass of the pancreatic body.
Pathologic diagnosis
Pancreatic solitary fibrous tumor (SFT).
Treatment
Pancreatectomy (body and tail).
Related reports
Fifteen case reports of this rare condition.
Term explanation
Mesenchymal tumors arise from mesodermally-derived tissues, and can be either benign or malignant.
Experiences and lessons
This report discusses the basic characteristics of pancreatic SFTs, based on all the cases reported so far.
Peer-review
The authors report a case of a rare pancreatic tumor and compare it to cases in the existing bibliography.
Footnotes
Institutional review board statement: This case report was approved by the A.H.E.P.A. University Hospital Institutional Review Board.
Informed consent statement: The patient provided informed consent prior to the review.
Conflict-of-interest statement: All authors declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 11, 2015
First decision: August 24, 2015
Article in press: March 18, 2016
P- Reviewer: Kai K, Sakata N S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
References
- 1.Tasdemir A, Soyuer I, Yurci A, Karahanli I, Akyildiz H. A huge solitary fibrous tumor localized in the pancreas: a young women. JOP. 2012;13:304–307. [PubMed] [Google Scholar]
- 2.Ginat DT, Bokhari A, Bhatt S, Dogra V. Imaging features of solitary fibrous tumors. AJR Am J Roentgenol. 2011;196:487–495. doi: 10.2214/AJR.10.4948. [DOI] [PubMed] [Google Scholar]
- 3.Chetty R, Jain R, Serra S. Solitary fibrous tumor of the pancreas. Ann Diagn Pathol. 2009;13:339–343. doi: 10.1016/j.anndiagpath.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Lüttges J, Mentzel T, Hübner G, Klöppel G. Solitary fibrous tumour of the pancreas: a new member of the small group of mesenchymal pancreatic tumours. Virchows Arch. 1999;435:37–42. doi: 10.1007/s004280050392. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan VD, Wayne JD, Rao MS, Zynger DL. Solitary fibrous tumor of the pancreas: case report with cytologic and surgical pathology correlation and review of the literature. JOP. 2008;9:526–530. [PubMed] [Google Scholar]
- 6.Sugawara Y, Sakai S, Aono S, Takahashi T, Inoue T, Ohta K, Tanada M, Teramoto N. Solitary fibrous tumor of the pancreas. Jpn J Radiol. 2010;28:479–482. doi: 10.1007/s11604-010-0453-x. [DOI] [PubMed] [Google Scholar]
- 7.Kwon HJ, Byun JH, Kang J, Park SH, Lee MG. Solitary fibrous tumor of the pancreas: imaging findings. Korean J Radiol. 2008;9 Suppl:S48–S51. doi: 10.3348/kjr.2008.9.s.s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatti K, Nouira K, Ben Reguigua M, Bedioui H, Oueslati S, Laabidi B, Alaya M, Ben Abdallah N. [Solitary fibrous tumor of the pancreas. A case report] Gastroenterol Clin Biol. 2006;30:317–319. doi: 10.1016/s0399-8320(06)73174-8. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto H, Molena DA, Schoeniger LO. Solitary fibrous tumor of the pancreas: a case report. Int J Surg Pathol. 2007;15:311–314. doi: 10.1177/1066896907302419. [DOI] [PubMed] [Google Scholar]
- 10.Gardini A, Dubini A, Saragoni L, Padovani F, Garcea D. [Benign solitary fibrous tumor of the pancreas: a rare location of extra-pleural fibrous tumor. Single case report and review of the literature] Pathologica. 2007;99:15–18. [PubMed] [Google Scholar]
- 11.Ishiwatari H, Hayashi T, Yoshida M, Kuroiwa G, Sato Y, Kobune M, Takimoto R, Kimura Y, Hasegawa T, Hirata K, et al. [A case of solitary fibrous tumor of the pancreas] Nihon Shokakibyo Gakkai Zasshi. 2009;106:1078–1085. [PubMed] [Google Scholar]
- 12.Santos LA, Santos VM, Oliveira OC, De Marco M. Solitary fibrous tumour of the pancreas: a case report. An Sist Sanit Navar. 2012;35:133–136. doi: 10.4321/s1137-66272012000100013. [DOI] [PubMed] [Google Scholar]
- 13.van der Vorst JR, Vahrmeijer AL, Hutteman M, Bosse T, Smit VT, van de Velde CJ, Frangioni JV, Bonsing BA. Near-infrared fluorescence imaging of a solitary fibrous tumor of the pancreas using methylene blue. World J Gastrointest Surg. 2012;4:180–184. doi: 10.4240/wjgs.v4.i7.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JW, Lü T, Liu HB, Tong SX, Ai ZL, Suo T, Ji Y. A solitary fibrous tumor in the pancreas. Chin Med J (Engl) 2013;126:1388–1389. [PubMed] [Google Scholar]
- 15.Hwang JD, Kim JW, Chang JC. Imaging findings of a solitary fibrous tumor in pancreas: a case report. J Korean Soc Radiol. 2014;70:53–57. [Google Scholar]
- 16.Hee Han S, Hyun Baek Y, Han SY, Wook Lee S, Sook Jeong J, Han Cho J, Kwon HJ. Solitary fibrous tumor of the pancreas: A case report and review of the literature. Korean J Med. 2015;88:293–298. [Google Scholar]


