Abstract
Liquid-liquid phase separation in giant unilamellar vesicles (GUVs) leads to the formation of intramembrane domains. To mimic charged biological membranes, we studied phase separation and domain formation in GUVs of ternary lipid mixtures composed of egg sphingomyelin, cholesterol, and the negatively charged lipid dioleoylphosphatidylglycerol. The GUVs were exposed to solutions of sucrose and high-saline buffer. The phase diagram was determined using epifluorescence microscopy for vesicle populations with symmetric and asymmetric solution compositions across the membranes. Trans-membrane solution asymmetry was found to affect the membrane phase state. Furthermore, compared to the case of salt-free conditions, the phase diagram in the presence of high-saline buffer (both symmetrically or asymmetrically present across the membrane) was found to exhibit a significantly extended region of liquid-ordered and liquid-disordered coexistence. These observations were confirmed on single GUVs using microfluidics and confocal microscopy. Moreover, we found that the miscibility temperatures markedly increased for vesicles in the presence of symmetric and asymmetric salt solutions. Our results demonstrate a substantial effect of salt and solution asymmetry on the phase behavior of charged membranes, which has direct implications for protein adsorption onto these membranes and for the repartitioning of proteins within the membrane domains.
Main Text
In the fluid-mosaic model of Singer and Nicolson, the plasma membrane is viewed as a more or less homogeneous lipid bilayer decorated by membrane-anchored proteins. This view has been challenged with the extraction of detergent-resistant membrane domains from biological cells, a discovery that led to the lipid raft hypothesis proposing the existence of lipid domains that are rich in sphingomyelin and cholesterol (1, 2). Even though this hypothesis has now been pursued for almost two decades, it is still a matter of ongoing debate (3, 4, 5). The different experimental techniques used to search for rafts in biological membranes have been recently reviewed (6). One important result of this activity was the identification of ternary lipid mixtures, which undergo phase separation into liquid-disordered (Ld) and liquid-ordered (Lo) phases and can be directly observed in giant unilamellar vesicles (GUVs) (7) using fluorescence microscopy (8).
This type of phase separation has now been examined in numerous studies in which the GUVs were prepared in nonphysiological buffers such as pure water, sucrose, or low-saline solution (9, 10, 11, 12, 13). Here, we studied the phase behavior of lipid bilayers under high-saline physiological conditions. Because different GUV dispersion media can significantly alter physical membrane properties such as the bending rigidity (14, 15), we addressed the question of how high-saline buffers influence the lipid phase state of GUVs. Similar investigations with charged vesicles in low-saline buffers resulted in rather small effects (11, 12, 16). On the other hand, neutral membranes in salt-free conditions (17) and exposed to saline buffers in the vesicle exterior (18) seem to differ in their phase behavior.
Here, we first compared the phase states of charged GUVs with membranes symmetrically exposed (inside and outside) to sucrose solution or high-saline buffer. We also investigated GUVs exposed to asymmetric conditions with sucrose solution and high-saline buffer on either side of the membrane. Solution asymmetry across plasma membranes is not only biologically relevant; implementing asymmetric solution conditions is also important in GUV-based studies. Examples include establishing, e.g., optical contrast or sucrose/glucose density gradients (11, 13) and mimicking the native environment for proteins (13, 19, 20).
We analyzed the phase state of charged multicomponent GUVs via fluorescence microscopy. Vesicles were prepared from negatively charged DOPG (dioleoylphosphatidylglycerol), eSM (egg sphingomyelin), and Chol (cholesterol), in either sucrose (210 mOsm/kg) or high-saline buffer (100 mM NaCl, 10 mM Tris, pH 7.5, 210 mOsm/kg) using spontaneous swelling; see the Supporting Material for GUV preparation and visualization. As a fluorescent marker, GUVs additionally contained 0.1 mol% of DiIC18, which is known to preferentially partition into the Ld phase and to be excluded from the Lo and solid (S) phases (21).
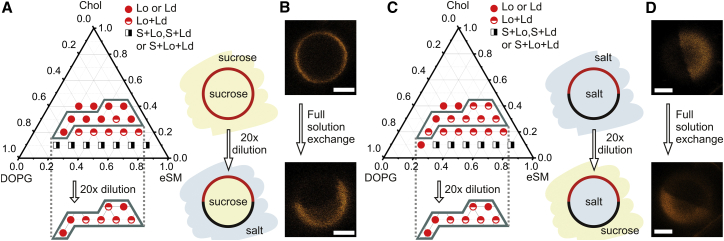
The phase diagram of GUVs symmetrically exposed to sucrose solution or salt buffer at ∼23°C contains one-phase fluid, liquid-liquid, and solid-liquid coexistence regions (Fig. 1, A and C). Membrane domains always appeared to be in registration, i.e., the domains in the outer and inner leaflets were spatially matched. Differences from observations with the same ternary system (11) may be ascribed to a different method of vesicle preparation as well as to the differences in solution compositions (12). Notably, compared to symmetric sucrose conditions, the Lo/Ld coexistence region of GUVs is increased for symmetric salt conditions (compare upper parts of Fig. 1, A and C).
Figure 1.
Effect of trans-membrane solution asymmetry on the phase behavior of DOPG/eSM/Chol. (A) Phase diagram for GUVs with symmetric sucrose/sucrose conditions; the lower polygonal section corresponds to asymmetric sucrose/salt (in/out) conditions after 20-fold dilution of the vesicle suspension with high-saline buffer. The cartoons illustrate the solution conditions and the dominant domain pattern within the highlighted section. (B) Confocal images of a single GUV (DOPG/eSM/Chol 30:40:30) captured within a microfluidic device before (top) and after (bottom) full exchange of the external solution from symmetric sucrose/sucrose to sucrose/salt (in/out). (C) Phase diagram for symmetric salt/salt conditions; the lower polygonal section is obtained for asymmetric salt/sucrose (in/out) conditions after 20-fold dilution with sucrose solution. As in (A), the cartoons illustrate solution conditions and the dominant domain pattern within the highlighted section. (D) Confocal images of a single GUV (DOPG/eSM/Chol 30:40:30) before (top) and after (bottom) the full exchange of the external solution from symmetric salt/salt to asymmetric salt/sucrose (in/out). Scale bars, 3 μm.
The region of lipid compositions showing different phase behaviors under symmetric conditions in either sucrose solution or salt buffer were reinvestigated with asymmetrically distributed solutions on either side of the membrane. GUVs initially grown in the internal solution were 20-fold diluted with the desired external solution (corresponding to a 95% external solution exchange). Phase states remained the same for all but two of the examined compositions going from symmetric salt buffer to salt buffer inside and sucrose solution outside the vesicle (Fig. 1 C). In the opposite case, however, the exchange from symmetric sucrose solution to sucrose solution inside and salt buffer outside changed the GUV phase state to that of the symmetric salt buffer conditions for most compositions (Fig. 1 A). Note that the same phase behavior of asymmetrically exposed membranes was found for both salt/sugar and sugar/salt in/out asymmetry, as expected.
These bulk observations were corroborated by studies on single GUVs trapped in a microfluidic device (22). This approach not only allows us to track individual GUVs but also to fully exchange the external solution. Vesicles consisting of 30:40:30 DOPG/eSM/Chol (mol %) prepared in either sucrose solution or salt buffer were captured by microposts (see the Supporting Material for fabrication) and observed by confocal microscopy before a full external solution exchange was performed. As in the bulk, these vesicles were in the single-liquid state when prepared in symmetric sucrose solutions (Fig. 1 B, top) and exhibited Lo/Ld coexistence when prepared in symmetric salt buffer (Fig. 1 D, top). Observations after a full external solution exchange showed that vesicles prepared in symmetric sucrose solutions had a uniform lipid composition but underwent Lo/Ld phase separation when exposed to salt buffer outside (Fig. 1 B, bottom). On the other hand, vesicles prepared in salt buffer and exposed to sucrose solution outside remained phase-separated as for symmetric salt conditions (Fig. 1 D, bottom). It was also possible to reach a homogeneous state with GUVs, which initially exhibited Lo/Ld coexistence (Fig. S1).
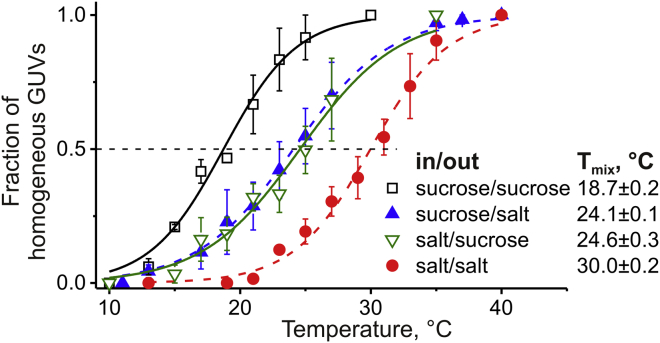
The increased Lo/Ld coexistence region in the presence of salt (Fig. 1) implies the stabilization of Ld domains with an increased fraction of the charged DOPG lipid. This was further tested by measuring the miscibility transition temperatures of GUVs prepared from 40:30:30 DOPG/eSM/Chol under different solution conditions (see Supporting Materials and Methods). In bulk observations at ∼23°C, GUVs of this composition had a uniform lipid composition under symmetric sucrose conditions and exhibited Lo/Ld coexistence under symmetric salt conditions. Fig. 2 summarizes the miscibility transition temperatures, Tmix, for all symmetric and asymmetric conditions. While Tmix is lowest for symmetric sucrose, it increases with salt present on one side of the membrane and is highest for salt symmetrically distributed on both sides of the membrane. We showed that both symmetric and asymmetric high-saline conditions for the two leaflets of charged GUV membranes have a substantial effect on their phase behavior. Together with the expanded Lo/Ld coexistence region of the GUV phase diagrams, the increase of the miscibility transition temperatures implies that salt leads to the stabilization of domains with an increased fraction of DOPG.
Figure 2.
Lo/Ld miscibility transition curves for 40:30:30 DOPG/eSM/Chol vesicles under different solution conditions. Data were fitted using the Boltzmann model (see the Supporting Material), where Tmix was deduced from the half-maximum indicated by the dashed line. Error bars represent the standard error of the mean of three independent experiments. To see this figure in color, go online.
Previously, the presence of salt was shown to alter DOPG mobility in membranes because of screening of the headgroup charges (23). The effect of asymmetric ionic solutions on the phase diagram of binary lipid mixtures has also been studied using Poisson-Boltzmann theory (24). High salt concentrations decrease the Debye length, thereby reducing the energetic cost for the dense packing of charges. This allows for Ld domains enriched in DOPG to form, thus expanding the Lo/Ld coexistence region and raising the miscibility temperature. This explanation for the observed effect of salt cannot be fully applied to the results obtained with asymmetric conditions, for which only one leaflet is exposed to salt. In principle, the salt-induced phase separation in this leaflet could propagate to the other leaflet, but another factor to consider is the spontaneous curvature generated in the membrane by the asymmetric solutions (25). Thus, it is important to study the interplay of spontaneous curvature and phase separation for multicomponent membranes under asymmetric solution conditions. Our study emphasizes that the precise buffer composition and trans-membrane solution asymmetry influence the membrane phase behavior, and should not be ignored when comparing available data. Furthermore, the effect of salt and solution asymmetry on the phase behavior of charged membranes might have direct implications for protein adsorption and domain repartitioning.
Author Contributions
B.K., T.R., R.L., and R.D. designed the experiments; B.K. and T.R. performed the experiments and analyzed the results; and all authors wrote and edited the article.
Acknowledgments
This work is part of the MaxSynBio consortium, which is jointly funded by the Federal Ministry of Education and Research of Germany and the Max Planck Society.
Editor: Tobias Baumgart.
Footnotes
Supporting Materials and Methods and one figure are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30353-8.
Supporting Material
References
- 1.Brown D.A., London E. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem. Biophys. Res. Commun. 1997;240:1–7. doi: 10.1006/bbrc.1997.7575. [DOI] [PubMed] [Google Scholar]
- 2.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 3.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 4.Owen D.M., Magenau A., Gaus K. The lipid raft hypothesis revisited—new insights on raft composition and function from super-resolution fluorescence microscopy. BioEssays. 2012;34:739–747. doi: 10.1002/bies.201200044. [DOI] [PubMed] [Google Scholar]
- 5.Goñi F.M. The basic structure and dynamics of cell membranes: an update of the Singer-Nicolson model. Biochim. Biophys. Acta. 2014;1838:1467–1476. doi: 10.1016/j.bbamem.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Klotzsch E., Schutz G.J. A critical survey of methods to detect plasma membrane rafts. Philos. Trans. Roy. Soc. B. 2013;368:20120033. doi: 10.1098/rstb.2012.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimova R., Aranda S., Lipowsky R. A practical guide to giant vesicles. Probing the membrane nanoregime via optical microscopy. J. Phys. Condens. Matter. 2006;18:S1151–S1176. doi: 10.1088/0953-8984/18/28/S04. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich C., Bagatolli L.A., Gratton E. Lipid rafts reconstituted in model membranes. Biophys. J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumgart T., Hess S.T., Webb W.W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821–824. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]
- 10.Bacia K., Schwille P., Kurzchalia T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl. Acad. Sci. USA. 2005;102:3272–3277. doi: 10.1073/pnas.0408215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vequi-Suplicy C.C., Riske K.A., Dimova R. Vesicles with charged domains. Biochim. Biophys. Acta. 2010;1798:1338–1347. doi: 10.1016/j.bbamem.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Blosser M.C., Starr J.B., Keller S.L. Minimal effect of lipid charge on membrane miscibility phase behavior in three ternary systems. Biophys. J. 2013;104:2629–2638. doi: 10.1016/j.bpj.2013.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pataraia S., Liu Y.G., Dimova R. Effect of cytochrome c on the phase behavior of charged multicomponent lipid membranes. Biochim. Biophys. Acta. 2014;1838:2036–2045. doi: 10.1016/j.bbamem.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Dimova R. Recent developments in the field of bending rigidity measurements on membranes. Adv. Colloid Interface Sci. 2014;208:225–234. doi: 10.1016/j.cis.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Bouvrais H., Duelund L., Ipsen J.H. Buffers affect the bending rigidity of model lipid membranes. Langmuir. 2014;30:13–16. doi: 10.1021/la403565f. [DOI] [PubMed] [Google Scholar]
- 16.Shimokawa N., Hishida M., Yoshikawa K. Phase separation of a mixture of charged and neutral lipids on a giant vesicle induced by small cations. Chem. Phys. Lett. 2010;496:59–63. [Google Scholar]
- 17.Bezlyepkina N., Gracià R.S., Dimova R. Phase diagram and tie-line determination for the ternary mixture DOPC/eSM/cholesterol. Biophys. J. 2013;104:1456–1464. doi: 10.1016/j.bpj.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carravilla P., Nieva J.L., Huarte N. Two-photon Laurdan studies of the ternary lipid mixture DOPC:SM:cholesterol reveal a single liquid phase at sphingomyelin:cholesterol ratios lower than 1. Langmuir. 2015;31:2808–2817. doi: 10.1021/la504251u. [DOI] [PubMed] [Google Scholar]
- 19.Manneville J.B., Casella J.F., Goud B. COPI coat assembly occurs on liquid-disordered domains and the associated membrane deformations are limited by membrane tension. Proc. Natl. Acad. Sci. USA. 2008;105:16946–16951. doi: 10.1073/pnas.0807102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wollert T., Wunder C., Hurley J.H. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahya N., Scherfeld D., Schwille P. Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J. Biol. Chem. 2003;278:28109–28115. doi: 10.1074/jbc.M302969200. [DOI] [PubMed] [Google Scholar]
- 22.Robinson T., Kuhn P., Dittrich P.S. Microfluidic trapping of giant unilamellar vesicles to study transport through a membrane pore. Biomicrofluidics. 2013;7:44105. doi: 10.1063/1.4816712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filippov A., Orädd G., Lindblom G. Effect of NaCl and CaCl2 on the lateral diffusion of zwitterionic and anionic lipids in bilayers. Chem. Phys. Lipids. 2009;159:81–87. doi: 10.1016/j.chemphyslip.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Shimokawa N., Komura S., Andelman D. Charged bilayer membranes in asymmetric ionic solutions: phase diagrams and critical behavior. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2011;84:031919. doi: 10.1103/PhysRevE.84.031919. [DOI] [PubMed] [Google Scholar]
- 25.Lipowsky R. Spontaneous tubulation of membranes and vesicles reveals membrane tension generated by spontaneous curvature. Faraday Discuss. 2013;161:305–331. doi: 10.1039/c2fd20105d. discussion 419–459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.