Abstract
Background
Fatty liver (hepatic steatosis) is one of the most common diseases globally, with increasing prevalence. The role of alcohol consumption in the development of hepatic steatosis has not been systematically examined.
Methods
We searched Medline, Embase, and ProQuest Dissertations & Theses Global for original data on the relationship between alcohol consumption and hepatic steatosis measured by non-invasive imagery, excluding studies conducted in participants < 18 years, or subgroups related to viral and drug-induced liver disease. We identified 18 articles reporting adjusted data (Japan = 11, other high-income countries = 7). Random-effect categorical meta-analyses (< 20 g/day pure alcohol consumption vs non-drinkers) and dose-response meta-analyses for the whole range of alcohol consumption were conducted.
Results
In total, 99,370 participants and 25,662 cases of hepatic steatosis were included. In Japan, low alcohol consumption was consistently associated with substantially reduced incidence and prevalence of hepatic steatosis compared to non-drinkers (RR for < 20 g pure alcohol/day = 0.75, 95% CI: 0.71–0.79, I2 = 0%). No overall association was found in other countries (RR = 1.05, 95% CI: 0.86–1.30, I2 = 84%). Dose-response analyses in Japan (up to 80 g/day) showed an inverse relationship in men and a J-shape in women.
Conclusions
Alcohol consumption showed a complex association with hepatic steatosis with substantial differences by ethnicity and sex. Low alcohol consumption was beneficial in Japan with good epidemiological evidence, whereas there was no association in other countries. However, heterogeneity was large in countries other than Japan. More and higher quality research in diverse ethnic populations is needed to further clarify this relationship.
Keywords: Non-alcoholic fatty liver, Alcoholic fatty liver, Alcohol drinking, Binge drinking, Prevalence, Incidence, Systematic review, Meta-analysis
Highlights
-
•
The relationship between low alcohol consumption (< 20 g/day) and hepatic steatosis was consistently beneficial in Japan.
-
•
In Japanese men, alcohol consumption up to 80 g/day was inversely associated with hepatic steatosis.
-
•
In Japanese women, the relationship was J-shaped and turned detrimental beyond average alcohol consumption of 20 g/day.
-
•
There was marked heterogeneity across studies in countries other than Japan with no clear relationship.
-
•
More high-quality epidemiological and clinical studies are necessary to confirm these relationships.
Fatty liver (steatosis) is a common disease worldwide. We systematically examined the influence of alcohol consumption on fatty liver using published observational studies. In total, 99370 participants and 25662 cases of fatty liver from 18 studies were included. In comparison to non-drinkers, low alcohol consumption (2–3 drinks per day) showed a beneficial effect (about 25% reduction) in Japan, well adjusted for the most important risk factor for fatty liver (excess body weight). There was no clear relationship in other countries. More research in diverse ethnic populations is needed to further clarify the role of alcohol consumption.
1. Introduction
Liver diseases are prevalent diseases in all regions of the world (Lozano et al., 2012), and associated with marked burden of disease (Byass, 2014). Aside from viral hepatitis, the leading cause for liver disease globally, for all stages of liver disease from hepatic steatosis to liver cirrhosis, there is a basic distinction into alcoholic versus non-alcoholic forms based on the history of alcohol intake with various cut-points (mostly between 20 and 40 g pure alcohol intake per day [g/day]) (Chalasani et al., 2012, LaBrecque et al., 2014, EASL, 2012, Nascimbeni et al., 2013).
The prevalence of non-alcoholic fatty liver disease (NAFLD), now more common than alcoholic liver disease (Sattar et al., 2014), has doubled in both North America and Asia over the past two decades, and it has become one of the most widespread chronic conditions worldwide (about ¼ of the population), with projected further increase (Byrne and Targher, 2015, Vernon et al., 2011, Younossi et al., 2015). It is frequently associated with impaired glucose tolerance, insulin resistance, hypertension, and obesity (Yki-Jarvinen, 2014), and a risk factor for both type 2 diabetes mellitus and the metabolic syndrome (MetS) (Ballestri et al., 2015), all of which are risk factors for cardiovascular diseases and overall mortality. Hepatic steatosis might also be an independent risk factor for cardiovascular disease (Bonci et al., 2015, Lu et al., 2013, Targher et al., 2010, Loria et al., 2014). Aside from other causes of liver disease (such as use of medication or presence of hereditary disorders known to produce hepatic lipidosis, or viral hepatitis B or C), non-alcoholic hepatic steatosis is diagnosed based on hepatic fatty infiltration in the absence of excessive alcohol consumption (Neuman et al., 2014a). However, there are no systematic investigations as to what excessive alcohol consumption means for hepatic steatosis (Nascimbeni et al., 2013), or whether the relationship between alcohol consumption and hepatic steatosis is continuous or characterized by a threshold effect. Furthermore, the role of sex in this relationship is not clear (Vernon et al., 2011).
Several conflicting large-scale population-based studies published recently (e.g. (Lau et al., 2015, Moriya et al., 2015, Tsunoda et al., 2014)) indicated that moderate (or ‘non-alcoholic’) alcohol consumption either has a beneficial or detrimental association with prevalence or development of hepatic steatosis, questioning the distinction into non-alcoholic and alcoholic hepatic steatosis. Furthermore, the impact of irregular heavy (binge) drinking on liver disease among moderate drinkers is currently unknown (Mathurin and Deltenre, 2009, Rehm and Roerecke, 2015). In the US, an episodic heavy drinking pattern is more common than chronic heavy drinking (Naimi et al., 2003, MMWR Morbidity and Mortality Weekly Report 2012, 2010), and the prevalence of heavy episodic drinking is high or on the rise in many countries (Shield et al., 2013).
The objective of this study was to determine the relationship between patterns of alcohol consumption and hepatic steatosis, taking into account confounding from other risk factors for hepatic steatosis. We systematically examined the epidemiological evidence for the relationship between patterns of alcohol consumption (including total average amount and ‘binge’ drinking) and prevalence and incidence of non-alcoholic hepatic steatosis, stratified by sex where possible. Non-linear dose-response analyses were conducted without a distinction between alcoholic and non-alcoholic hepatic steatosis.
2. Material and Methods
2.1. Search Strategy and Selection Criteria
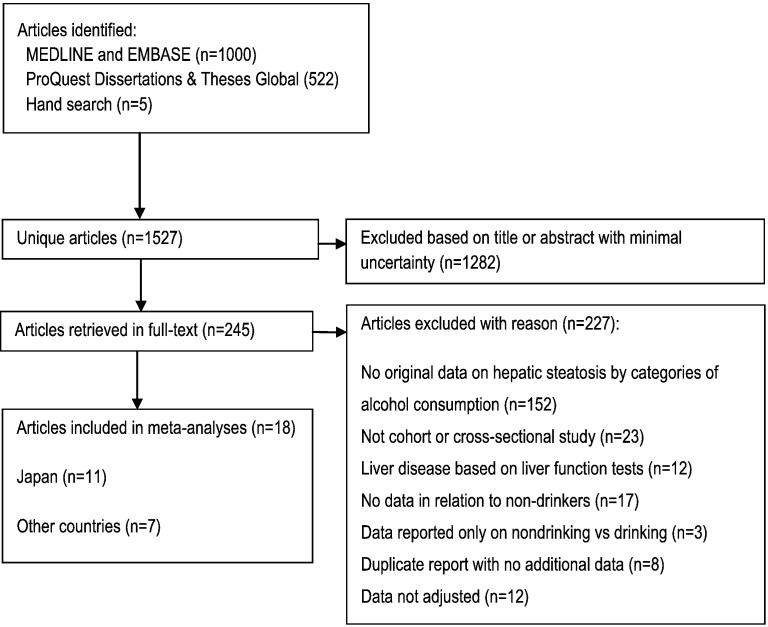
Following the Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies (MOOSE) (Stroup et al., 2000), we conducted a systematic literature search (Fig. 1, Table S1) using Medline, Embase, and ProQuest Dissertations & Theses Global, updated on December 10, 2015 for keywords relating to alcohol consumption and fatty liver disease. Additionally, we searched reference lists of identified articles and published meta-analyses and reviews. Inclusion criteria were: 1) full-text article with original and adjusted population-based data (cross-sectional or cohort design) on the relationship between alcohol consumption and hepatic steatosis in comparison to non-drinkers, 2) hepatic steatosis was diagnosed using non-invasive methods (ultrasound, computed-tomography, magnetic resonance imaging, proton magnetic resonance spectroscopy), 3) results were reported in categories of alcohol consumption in relation to non-drinkers. For categorical meta-analyses, at least one category had to report results for alcohol consumption between 0 and 20 g/day (0–40 g/day in a sensitivity analysis) in comparison to non-drinkers. For a dose-response meta-analysis, results for at least three drinking groups based on average intake in g/day in comparison to non-drinkers had to be reported. We excluded studies with participants or ultrasound examinations were restricted to or based on: a) < 18 years of age, b) viral hepatitis (HBV or HCV), c) drug-induced liver disease, or d) other indications of liver disease (e.g. elevated liver function tests (Bellentani et al., 1997)), e) experimental conditions. No language restrictions were applied. Two reviewers independently excluded articles based on title and abstract on the first pass, articles with unsure eligibility were obtained in full-text and discussed by two authors until consensus was reached. Authors were not contacted.
Fig. 1.
Flowchart of study selection.
2.2. Data Extraction
From all relevant articles we extracted authors' names, year of publication, country, calendar year(s) of baseline examination, follow-up period, setting of the study, assessment of hepatic steatosis status, age (mean or median) at baseline, sex, number of observed hepatic steatosis cases among participants by drinking group, number of total participants by drinking group, specific adjustment or stratification for potential confounders, and adjusted relative risks (RRs) and their confidence intervals (CIs) or standard errors. Estimates by sex and study design were treated as independent samples. As a result, multiple articles and estimates from the same samples and cohorts in Japan (Moriya et al., 2015, Moriya et al., 2013, Moriya et al., 2011, Hamaguchi et al., 2012, Hamaguchi et al., 2005, Hashimoto et al., 2015) were included, but each case of hepatic steatosis was used only once in each of the analyses conducted. If necessary, RRs within studies were re-calculated based on the method described by Hamling and colleagues (Hamling et al., 2008) to contrast several alcohol consumption categories against non-drinkers.
2.3. Outcome Assessment
Histopathology (liver biopsy) is considered the gold standard for diagnosis of NAFLD since it is a direct visualization of the liver, can quantify the amount of lipids and can assess the presence of inflammation and fibrosis. However, liver biopsy is an invasive procedure associated with sampling error and may lead to complications. Because we were interested in population-based risk relationships, hepatic steatosis in our analysis had to be measured by non-invasive imagery. Ultrasound and comparable imaging techniques have very good specificity (94%) and sensitivity (85%) in people who are not morbidly obese (Hernaez et al., 2011).
2.4. Exposure Assessment
Among drinkers, we converted reported average total alcohol intake categories in primary studies into g/day using the midpoints (mean) of reported drinking group categories. For open-ended categories, we added ¾ of the second highest category's range to the lower limit of the open-ended category of drinking. We used reported conversion factors when standard drinks were the unit of measurement. Depending on the country, one standard drink is approximately 10–14 g of pure alcohol (WHO, 2000). Qualitative descriptions, such as ‘social’ or ‘frequent’ drinkers with no clear total alcohol intake were excluded.
2.5. Quality Assessment
Most quality scores are tailored for meta-analyses of randomized trials of interventions (Chalmers et al., 1981, Detsky et al., 1992, Moher et al., 1998) and many criteria do not apply to epidemiological studies examined in this study. Additionally, quality score use in meta-analyses remains controversial (Greenland and O'Rourke, 2001, Herbison et al., 2006). As a result, quality assessment was incorporated by including quality components, such as study design, measurement of alcohol consumption and hepatic steatosis, in the inclusion and exclusion criteria, and further by investigating potential heterogeneity in meta-regression models and several subgroup analyses. We used the most adjusted RR reported and the most comprehensive data available for each analysis, and gave priority to estimates where lifetime abstainers were used as the risk reference group.
2.6. Categorical Analyses
The prevalence (proportion) of hepatic steatosis by drinking group (non-drinkers and drinkers < 20 g/day) across studies included in the meta-analysis was calculated from the raw number of cases and non-cases reported and pooled in random effects models with confidence intervals calculated based on the exact binomial distribution (Newcombe, 1998) and variances stabilized with the Freeman and Tuckey double-arcsine transformation (Freeman and Tukey, 1950).
We conducted categorical meta-analyses (main analysis on alcohol consumption < 20 g/day in comparison to non-drinkers) using reported adjusted data stratified by: 1) country of study origin (Japan vs other countries), 2) sex (men vs women), and 3) study design (prevalence vs incidence data). Because a clear definition of excessive alcohol consumption for hepatic steatosis is missing (Chalasani et al., 2012), though it ranges between 20 and 40 g/day (Nascimbeni et al., 2013, Poli et al., 2013), as a sensitivity analysis, we repeated the main categorical meta-analysis with a cut-point of 40 g/day. RRs were pooled with inverse-variance weighting using DerSimonian-Laird random-effect models to allow for between-study heterogeneity (DerSimonian and Laird, 1986). Publication bias was examined using Egger's regression-based test (Egger et al., 1997). Variation in the effect size because of heterogeneity between-studies was quantified using the I2 statistic (Higgins and Thompson, 2002). Between-study heterogeneity across subgroups defined by sex, study design, and country of study origin was investigated with random-effects meta-regressions (Thompson and Higgins, 2002). All meta-analytical analyses were conducted on the natural log scale in Stata statistical software, version 13.1.
2.7. Dose-response Analyses
Ignoring a distinction between ‘alcoholic’ and ‘non-alcoholic’ hepatic steatosis, we conducted two-stage multivariate meta-regression using restricted cubic spline regression models (3 knots) to calculate continuous non-linear dose-response curves for average alcohol consumption (g/day) in comparison to non-drinkers using the approach described by Orsini and colleagues (Orsini et al., 2006, Orsini et al., 2012). Only adjusted data with ≥ 3 drinking groups in addition to non-drinkers were used in these analyses. Evidence for non-linearity was determined by the significance level of the second meta-regression coefficient. Sex- and design-specific dose-response analyses were conducted where such data were available.
Role of Funding Source
The work was financially supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) at the National Institutes of Health (grant number R21AA023521-01 A1 to MR). The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The authors collected the data, and had full access to all of the data in the study. The authors also had final responsibility for the decision to submit the study results for publication.
3. Results
Of 1527 initial references, 245 were reviewed in full-text (Fig. 1). In total we used data from 18 articles including 25,662 cases of hepatic steatosis among 99,370 participants in the analyses, with sex-specific data from 12,203 men and 4731 women with hepatic steatosis (Table 1). Eleven articles (n = 72.394) were from Japan (Moriya et al., 2015, Tsunoda et al., 2014, Moriya et al., 2013, Moriya et al., 2011, Hamaguchi et al., 2012, Hamaguchi et al., 2005, Hashimoto et al., 2015, Gunji et al., 2009, Hiramine et al., 2011, Doi et al., 2010, Hamabe et al., 2011) and seven (n = 26.976) from other high-income countries (US (Lazo, 2010), Germany (Lau et al., 2015), Finland (Suomela et al., 2015), Italy (Miele et al., 2014), Spain (Caballeria et al., 2010), and China (Peng et al., 2009, Xu et al., 2010, Wong et al., 2012, Liu et al., 2014)). Weighted mean age at baseline was 47.2 years overall (Japan 48.1, other countries 44.9 years) with a range from 42 to 53 years. Five studies from Japan (Moriya et al., 2015, Tsunoda et al., 2014, Hamaguchi et al., 2005, Hashimoto et al., 2015, Hamabe et al., 2011), and one study from China (Xu et al., 2010) provided incidence data. The weighted mean follow-up time in cohort studies was 7 years (range 1 to 10 years). All but one (Moriya et al., 2015) studies were adjusted for age, and all but three (Hamaguchi et al., 2012, Caballeria et al., 2010, Wong et al., 2012) for BMI, visceral fat, or waist circumference. All but five studies (Lau et al., 2015, Moriya et al., 2015, Hashimoto et al., 2015, Suomela et al., 2015, Caballeria et al., 2010) adjusted for multiple components of the MetS.
Table 1.
Study characteristics of 18 adjusted population-based studies on alcohol consumption and hepatic steatosis compared to non-drinkers.
| Reference | Sex, Country, Year(s) of baseline | Setting, Age | Design, No. | Participants excluded | Alcohol consumption measurement | Hepatic steatosis measurement | Adjustment |
|---|---|---|---|---|---|---|---|
| Hamaguchi et al. (2005) | M, W, Japan, 2001–2003 | Murakami Memorial Hospital, Health screening, 48 yrs | Cohort, 1093 cases, 3589 non-cases | Positive for hepatitis B antigen or hepatitis C antibody, history of known liver disease or current medication, viral, genetic, autoimmune, and drug-induced liver disease | Non-drinker, < 20 g/day Excluded: > 20 g/day |
Ultrasound The diagnosis of fatty liver was based on the results of abdominal ultrasonography by trained technicians. All ultrasonographic images were stored in the image server. One gastroenterologist reviewed the images and made the diagnosis of fatty liver without reference to any of the participant's other individual data. Of four known criteria (hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring), the participants were required to have hepatorenal contrast and liver brightness to be given a diagnosis of nonalcoholic fatty liver disease. |
Age, MetS, weight gain |
| Gunji et al. (2009) | M, Japan, 2007–2008 | Center for Preventive Medicine, NTT Kanto Medical Center, Health screening, 50.9 yrs | Cross-sectional, 1865 cases, 3702 non-cases | Positive for HBV or HCV, potentially hepatotoxic drug intake, medical treatment for metabolic abnormalities (hypertension, hyperlipidemia, or diabetes mellitus). | Non or minimal drinkers (< 40 g/week), 40–140 g/week Excluded: > 140 g/week |
Ultrasound Details not reported. |
Age, BMI, waist circumference, visceral adipose tissue, subcutaneous adipose tissue, systolic blood pressure, diastolic blood pressure, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride, fasting blood glucose, glycated hemoglobin |
| Xu et al. (2010) | M/W, China, 2006–2009 | Employees of Zhenhai Refining & Chemical Company, Health screening, 44 yrs | Cohort, 813 cases, 6077 non-cases | Fatty liver at baseline, lipid-lowering agents, or hypouricemic agents based on self-reported medical history and medication use, a positive history of known liver disease (such as HBV or HCV) or autoimmune hepatitis, or use of hepatotoxic medications (including hypertensive or antidiabetic agents). | Non-drinkers, < 140 g/week men, < 70 g/week women Excluded: > 140 g/week men, > 70 g/week women |
Ultrasound Abdominal ultrasonography using a Toshiba Nemio 20 sonography machine with a 3.5-MHz probe (Toshiba, Tokyo, Japan). Ultrasound studies were carried out by a trained ultrasonographist who was unaware of the clinical and laboratory data. Hepatic steatosis was diagnosed by characteristic echo patterns according to conventional criteria, such as the evidence of diffuse hyperechogenicity of the liver relative to the kidneys, ultrasound beam attenuation, and poor visualization of intrahepatic structures. |
Age, gender, BMI, waist circumference, systolic blood pressure, diastolic blood pressure, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride, fasting plasma glucose, creatinine, blood urea nitrogen, serum uric acid |
| Caballeria et al. (2010) | M/W, Spain, 2007–2008 | Multicentre (25 primary health care centres), Population-based, 53 yrs | Cross-sectional, 198 cases, 568 non-cases | Chronic liver disease, hepatitis B virus surface antigen or hepatitis C virus antibodies, incapacitating diseases, cognitive deterioration, institutionalized patients, no fixed address | Never drinkers, ≤ 30 g/day men, ≤ 20 g/day women Excluded: > 30 g/day men, > 20 g/day women |
Ultrasound The criteria for the diagnosis of NAFLD included an increase in hepatic echogenicity using renal echogenicity as a reference, the presence of enhancement and a lack of differentiation of periportal and bile duct walls reinforcement because of great hyperechogenicity of the parenchyma. The grade of involvement was standardized using a semiquantitative scale of the grade of hepatic enhancement. |
Age, sex, radiologist |
| Doi et al. (2010) | M/W, Japan, 2008 | Health examinations, Oji General Hospital, Health screening, 51 yrs | Cross-sectional, 1032 cases, 2153 non-cases | HBsAg positive, HCV antibody-positive, autoimmune chronic liver disease, incomplete description of alcohol consumption | Non-drinkers, < 20 g/d, 20- < 40 g/d, 40- < 60 g/d, ≥ 60 g/d Excluded: none |
Ultrasound Details not reported. |
Age, sex, visceral fat obesity, dyslipidemia, high fasting glucose |
| Lazo (2010) | M/W, United States, 1988–1994 | Part of NHANES III (nationwide), Population-based, 43 yrs | Cross-sectional, 2515 cases, 9599 non-cases | None | Never drinkers, < 2 drink/d men, < 1 drink/d women, ≥ 2 drinks/d men, ≥ 1 drink/d women Excluded: none |
Ultrasound Moderate or severe steatosis. Ultrasound examinations on the gallbladder were carried out using a Toshiba (Tustin, CA) SSA-90 A machine using 3.75 and 5.0 MHz transducer. Between 2009 and 2010, 13,856 (96.6%) of the archived videotapes on gallbladder ultrasound examinations originally obtained between 1988 and 1994 were reviewed to ascertain the presence of fat within the hepatic parenchyma. Information was recorded on the presence of liver to kidney contrast, degree of brightness of the liver parenchyma, presence of deep beam attenuation, presence of echogenic walls in the small intrahepatic vessels, and definition of the gallbladder walls. |
Age, sex, race, BMI, diabetes |
| Hamabe et al. (2011) | M/W, Japan, 1998–2008 | Kouseiren Medical Health Care Center, Kagoshima, Health screening, 50 yrs | Cohort, 735 cases, 2854 non-cases | Subjects positive for hepatitis B virus surface antigen (HBsAg) and hepatitis C virus antibody (HCV Ab) and those who did not undergo virus marker measurements | Non-drinker, ≤ 20 g/day Excluded: > 20 g/day |
Ultrasound Fatty liver was diagnosed when hepato-renal echo contrast and liver brightness were observed. The diagnosis of fatty liver was subsequently confirmed by a specialist physician independently without reference to other data. |
Age, sex, obesity, hypertension, dyslipidemia, dysglycemia, smoking |
| Hiramine et al. (2011) | M, Japan, 2000–2007 | Kouseiren Medical Health Care Center, Kagoshima, Health screening, 51 yrs | Cross-sectional, 3816 cases, 6070 non-cases | Positive for HBV surface antigen, HCV antibody | Never drinkers, former drinkers, < 20 g/d, 20–59 g/d, ≥ 60 g/d Excluded: none |
Ultrasound Ultrasonography (SSA-250A and SSA-700A; Toshiba, Tokyo, Japan; Logic 400; GE Healthcare Japan, Tokyo, Japan), from findings of bright liver (increased echogenicity) with liver–kidney contrast (increased echogenicity of the liver in comparison to the right kidney). |
Age, BMI, presence of clinical manifestation (ALT elevation, hypertension, dyslipidemia, diabetes mellitus), smoking status, serum levels of alanine aminotransferase, γ-glutamyl transpeptidase, triglycerides, and high-density lipoprotein cholesterol |
| Moriya et al. (2011) | M, Japan, 2003 | Junpukai Health Maintenance Center, Health screening, 49 yrs | Cross-sectional, 1563 cases, 3394 non-cases | Past or presently treated liver diseases | Non-drinkers, < 70 g/week, 70– < 140 g/week, 140– < 280 g/week, ≥ 280 g/week; Stratified by drinking days per week and amount per drinking day Excluded: none |
Ultrasound Experienced technicians performed real-time ultrasonography to detect vascular blurring, deep attenuation and increased liver echotexture in comparison to the kidneys (liver-kidney contrast) to assess fatty infiltration. Specialists subsequently confirmed the validity of the findings and judged the presence of one or more findings as indicative of a fatty liver. |
Obesity (BMI ≥ 25), atherogenic dyslipidemia, glucose intolerance, hyperuricemia, hypertension and regular exercise |
| Wong et al. (2012) | M/W, China, 2008–2010 | Randomly selected from the census database at Prince of Wales Hospital, Population-based, 48 yrs | Cross-sectional, 264 cases, 658 non-cases | Active malignancy, metallic implants or other contraindications to MRI, positive hepatitis B surface antigen or antibody against hepatitis C virus, secondary causes of fatty liver (e.g., consumption of amiodarone and tamoxifen) and decompensated liver disease (defined as bilirubin > 50 mmol/l, albumin < 35 g/l, platelet count < 1.503,109/l, international normalised ratio > 1.3, or the presence of ascites or varices) | Non-drinkers, ≤ 10 g/d Excluded: > 10 g/d |
Proton-magnetic resonance spectroscopy H-MRS was performed to measure intrahepatic triglyceride (IHTG) content within 8 weeks from the baseline visit. A whole-body 3.0 T scanner with a single voxel point-resolved spectroscopy sequence and an echo time of 40 ms and repetition time of 5000 ms was used. A survey scan was first performed in the abdominal region to help in positioning a volume measuring 20 (AP) 315 (RL) 340 (FH) mm within the liver. The scanner's built-in body coil was used for both signal transmission and reception. A no-water-suppressed spectrum was acquired using 32 signal averages and the data were exported for offline spectral analysis. Water (4.65 ppm) and lipid (1.3 ppm) peak amplitudes were measured to determine vertebral marrow fat content, which was defined as the relative fat signal amplitude in terms of a percentage of the total signal amplitude (water and fat). Correction for relaxation loss was not applied because of the relatively long repetition time and short echo time. An IHTG content of 5% was used to distinguish between patients with and without fatty liver. |
Age, gender, MetS |
| Hamaguchi et al. (2012) | M, W, Japan, 2004–2009 | Murakami Memorial Hospital, Health screening, 47 yrs | Cross-sectional, 6282 cases, 12,290 non-cases | Positive for hepatitis B antigen or hepatitis C antibody, history of known liver disease or current medication, viral, genetic, autoimmune, and drug-induced liver disease. | Non- or minimal drinkers (< 40 g/week), 40–140 g/week ,140–280 g/week, > 280 g/week Excluded: none |
Ultrasound The diagnosis of fatty liver was based on the results of abdominal ultrasonography by trained technicians. All ultrasonographic images were stored in the image server. One gastroenterologist reviewed the images and made the diagnosis of fatty liver without reference to any of the participant's other individual data. Of 4 known criteria (hepato-renal echo contrast, liver brightness, deep attenuation, and vascular blurring), the participants were required to have hepato-renal contrast and liver brightness to be given a diagnosis of non-alcoholic fatty liver disease. |
Age, medication potentially affecting MetS, regular exercise, smoking status |
| Moriya et al. (2013) | W, Japan, 2003–2006 | Junpukai Health Maintenance Center, Health screening, 46 yrs | Cross-sectional, 527 cases, 2876 non-cases | Concurrent liver diseases | Non-drinkers, < 70 g/week, 70- < 140 g/week, ≥ 140 g/week Excluded: none |
Ultrasound Experienced technicians performed real-time ultrasonography to detect vascular blurring, deep attenuation and increased liver echotexture in comparison to the kidneys (liver-kidney contrast) to assess fatty infiltration. Specialists subsequently confirmed the validity of the findings and judged the presence of one or more findings as indicative of a fatty liver. |
Age, obesity, atherogenic dyslipidemia, glucose intolerance, hyperuricemia, hypertension, current smoking |
| Liu et al. (2014) | W, China, 2012 | Department of Medical Examination Center, Peking Union Medical College Hospital, China Academic Medical Science, Health screening, 53 yrs | Cross-sectional, 121 cases, 407 non-cases | Non-obese (BMI < 25) and postmenopausal women were included. SUA levels that were out of the normal reference range, hormone replacement therapy or medication potentially affecting SUA levels, serum creatinine level ≥ 1.2 mg/dl, previous use of steatogenic medications, any evidence of chronic viral liver disease and/or autoimmune liver disease, evidence of liver insufficiency or malignancy. | Non-drinkers, < 10 g/d Excluded: ≥ 10 g/day |
Ultrasound NAFLD was defined as the presence of definite hepatic steatosis on ultrasonography, such as a bright hepatic echo pattern, increased attenuation of the echo beam and loss of intrahepatic architectural detail, without a secondary cause. Ultrasonography of the liver was performed using a 3.5-MHz convex-array probe and a 7.5-MHz linear-array probe (Nemio 30, Toshiba, Japan) by an experienced examiner who was unaware of the laboratory and other results. |
Age, waist circumference, SBP, DBP, GGT, ALT, AST, blood urea nitrogen, creatinine, fasting blood glucose, TC, TG, HDL—C, LDL-C, serum uric acid, C-reactive protein, diabetes, hypertension, MetS, smoking status |
| Tsunoda et al. (2014) | M/W, Japan, 2005–2013 | Meiji Yasuda Shinjuku Medical Center (Meiji Yasuda Longitudinal Study), Health screening, 48 yrs | Cohort, 1775 cases, 8371 non-cases | Histories of liver disease (HBV or HCV, cirrhosis and hepatic hemangioma, drugs associated with hepatic disease or antibodies to HBV or HCV), fatty liver at baseline, could not be followed up for ≥ 1 yr | Non-drinkers, < 23 g/day Excluded: ≥ 23 g/day |
Ultrasound Abdominal ultrasonography machines (EUB-2000, Hitachi, Japan; and SSA-340, 550, 580 and 660, Toshiba, Japan) were used to diagnose fatty liver based on standard criteria, including hepato-renal echo contrast, liver brightness, deep attenuation and vascular blurring. To enhance diagnostic accuracy, the ultrasound images were evaluated in three steps: first, a trained medical technologist performed the ultrasound and provided opinions with images to the doctor; second, the doctor made a diagnosis based on this information and third, a group of medical technologists including the original examiner confirmed the doctor's diagnosis. |
Age, gender, BMI, smoking status, family history of liver disease, alanine aminotransferase, γ-glutamyl transpeptidase, hypertension, diabetes, dyslipidemia, meat intake, vegetable intake, physical activity |
| Lau et al. (2015) | M, W, Germany, 1997–2001 | Study of Health in Pomerania (SHIP), Population-based, 50 yrs | Cross-sectional, 1120 cases, 2638 non-cases | Positive for HBV surface antigen or HCV antibody, known history of liver cirrhosis | Men: non-drinkers, < 40 g/d, 40–60 g/d, > 60 g/d; Women: non-drinkers, < 20 g/d, 20–40 g/d, > 40 g/d; Binge drinking (≥ 5 drinks/day at least once during the last 30 days) among drinkers. Excluded: none |
Ultrasound Sonographic examinations were performed by physicians using a 5 MHz transducer and a high resolution instrument (Vingmed VST Gateway, Santa Clara, CA, USA). The sonographers were unaware of the participants' clinical and laboratory characteristics. Hepatic steatosis was defined as the presence of a hyperechogenic liver pattern, with evident density differences between hepatic and renal parenchyma. |
Age, BMI, HbA1c, menopausal status |
| Moriya et al. (2015) | M/W, Japan, 2003–2006 | Junpukai Health Maintenance Center, Health screening, 48 yrs | Cohort, 449 cases, 3434 non-cases | Presently treated liver diseases | Non-drinkers, < 70 g/week, 70.0– < 140 g/week, 140.0– < 280 g/week, ≥ 280 g/week Excluded: none |
Ultrasound Experienced technicians performed real-time ultrasonography to detect vascular blurring, deep attenuation and increased liver echotexture in comparison to the kidneys (liver-kidney contrast) to assess fatty infiltration. Specialists subsequently confirmed the validity of the findings and judged the presence of one or more findings as indicative of a fatty liver. |
Obesity, smoking status, and regular exercise |
| Suomela et al., (2015) | M/W, Finland, 2011 | Cardiovascular Risk in Young Finns Study, Population-based, 42 yrs | Cross-sectional, 370 cases, 1628 non-cases | None | Non-drinkers, < 1.67 drinks/d Excluded: ≥ 1.67 drinks/d |
Ultrasound Ultrasound imaging of the liver was performed using a validated protocol (Loria et al., 2014) and Sequoia 512 ultrasound mainframes (Acuson, Mountain View, CA, USA) with 4.0 MHz adult abdominal transducers. Evaluation of hepatic steatosis was performed according to liver-to-kidney contrast, parenchymal brightness, deep beam attenuation, and bright vessel walls. According to these criteria the presence of hepatic steatosis was assessed visually from non-blinded images by a trained ultrasonographer. |
Age, sex Stratified by BMI (< 25 and ≥ 25). |
| Hashimoto et al. (2015) | M, W, Japan, 1994–2003 | Murakami Memorial Hospital, Health screening, 43 yrs | Cohort, 1124 cases, 3400 non-cases | Positive for hepatitis B antigen or hepatitis C antibody, history of known liver disease, any current medication, viral, genetic, autoimmune, and drug-induced liver disease | None or minimal (< 40 g/week), 40–140 g/week, 140–280 g/week, > 280 g/week Excluded: none |
Ultrasound Abdominal ultrasonography, which was done by trained technicians with Aloka SSD-650CL (Aloka Co., Ltd, Tokyo, Japan). Gastroenterologists reviewed the images and made the diagnosis of fatty liver without reference to any of the participant's other individual data. Of four known criteria (hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring), the participants were required to have hepatorenal contrast and liver brightness to be given a diagnosis of fatty liver. |
Age, BMI, physical activity, smoking status |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; DBP, diastolic blood pressure; GGT, γ-glutamyl transferase; HBV, hepatitis B; HCV, hepatitis C; HDL-C, high-density cholesterol; LDL-C, low-density cholesterol; MetS, metabolic syndrome; SBP, systolic blood pressure; SUA, serum uric acid; TC, total cholesterol; TG, triglyceride; M, men; W, women; M/W, men and women combined; M,W, men and women stratified.
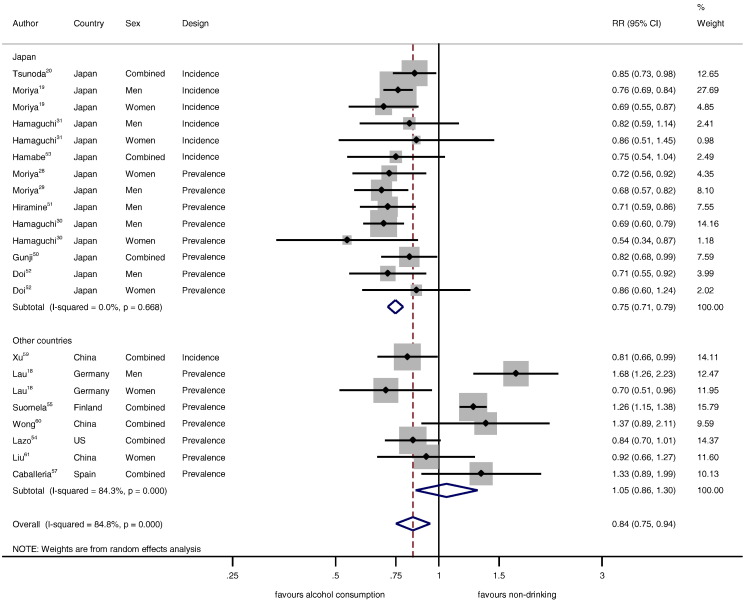
3.1. Categorical Meta-analyses
Table 2 displays pooled adjusted RRs by country, sex, and study design for the relationship between alcohol consumption < 20 g/day and hepatic steatosis in comparison to non-drinkers. In studies from Japan, the relationship was beneficial in all analyses (adjusted prevalence and incidence data RR = 0.75, 95% CI: 0.71–0.79, P < 0.001, Fig. 2, Table 2). Studies from other high-income countries showed no overall association (adjusted prevalence and incidence data RR = 1.05, 95% CI: 0.86–1.30, P = 0.62, Fig. 2, Table 2). Whether or not studies were from Japan explained 64% of the overall between-study variation (RR = 0.71, 95% CI: 0.59–0.85, P = 0.001). There was virtually no change when alcohol consumption < 40 g/day in comparison to non-drinkers was considered in a sensitivity analysis (0.73, 95% CI: 0.68–0.78, and 1.07, 95% CI: 0.86–1.34, respectively). One study from Japan (Hiramine et al., 2011) and two from other countries (Lazo, 2010, Caballeria et al., 2010) had data on lifetime abstainers and showed the same differences in RRs (adjusted RR = 0.71, 95% CI: 0.59–0.86 in Japan, and RR = 1.02, 5% CI: 0.65–1.59 in other countries).
Table 2.
Pooled relative risks for the relationship between alcohol consumption (< 20 g/day) and hepatic steatosis compared to non-drinkers.
| All countries |
Japan |
Other countries |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Population | Estimates, casesb (No.) | RR (95% CI) | I2 (%) | Estimates, casesb (No.) | RR (95% CI) | I2 (%) | Estimates, casesb (No.) | RR (95% CI) | I2 (%) | Pa |
| All studies | 22 (18,682) | 0.85 (0.75–0.96) | 86 | 14 (13,335) | 0.75 (0.71–0.79) | 0 | 8 (5346) | 1.05 (0.86–1.30) | 84 | 0.001 |
| Men | 7 (7546) | 0.80 (0.68–0.95) | 82 | 6 (6949) | 0.73 (0.68–0.78) | 0 | 1 (597) | 1.68 (1.26–2.23) | NA | 0.002 |
| Women | 7 (4554) | 0.74 (0.66–0.83) | 0 | 5 (3714) | 0.72 (0.62–0.83) | 0 | 2 (840) | 0.80 (0.61–1.04) | 28 | 0.49 |
| Cohort studies | 7 (3146) | 0.78 (0.73–0.84) | 6 (2333) | 0.78 (0.72–0.84) | 1 (813) | 0.81 (0.66–0.99) | ||||
| Men | NA | NA | NA | 2 (634) | 0.77 (0.70–0.84) | 0 | NA | NA | NA | NA |
| Women | NA | NA | NA | 2 (178) | 0.72 (0.58–0.89) | 0 | NA | NA | NA | NA |
NA, not applicable.
For difference Japan vs other countries.
Cases of hepatic steatosis.
Fig. 2.
Hepatic steatosis in alcohol drinkers < 20 g/day in comparison to non-drinkers (relative risk on the log scale).
There was no statistical heterogeneity in studies from Japan no matter whether prevalence, incidence, or sex-specific data were considered (Table 2). Similarly, clinical heterogeneity was also small because most studies from Japan used a similar methodology (Table 1). Incidence-only data from Japan (adjusted at least for age, BMI, and smoking), showed the same relationships as the main analysis. Studies from Japan were mostly well adjusted including multiple components of the MetS. The RR for men versus women in Japan was 0.99 (95% CI: 0.83–1.18, P = 0.89). Heterogeneity was large across other countries and most data were not sex-specific. There was no statistical evidence for publication bias (P = 0.72 in Japan, P = 0.49 in other countries), and sequential exclusion of each estimate one at a time showed similarly little impact on the pooled estimates (Figs. S1–S4).
Among cross-sectional studies included in our analyses, the unadjusted pooled prevalence of hepatic steatosis was 31% (95% CI: 23–39%) among non-drinkers in Japan and 26% (95% CI: 20–33%) among drinkers < 20 g/day. In countries other than Japan, the prevalence was 28% (95% CI: 23–32%) among non-drinkers and 28% (95% CI: 21–36%) among drinkers < 20 g/day average alcohol consumption. The prevalence of hepatic steatosis in women was about half compared to men in Japan (17% [95% CI: 6–33%], and 33% [95% CI: 28–38%], respectively), few studies provided sex-specific data in other countries. In meta-regression models, overall prevalence rates of hepatic steatosis did not explain any heterogeneity among all countries (adjusted RR = 1.00, 95% CI: 0.99–1.01, P = 0.79) or among Japanese studies (adjusted RR = 1.00, 95% CI: 0.99–1.00, P = 0.24); however, in other countries there was a borderline significant relationship with a 3% increase in RR per 1% increase in baseline prevalence of hepatic steatosis (adjusted RR = 1.03, 95% CI: 0.997–1.058, P = 0.064). Mean age did not show any effects on the pooled RRs when all studies (adjusted RR = 1.00, 95% CI: 0.96–1.04, P = 0.85), Japanese studies (adjusted RR = 1.01, 95% CI: 0.97–1.06, P = 0.49), or countries other than Japan (adjusted RR = 1.01, 95% CI: 0.94–1.08, P = 0.71) were considered.
Studies that adjusted for multiple components of the MetS including BMI showed the same relationships with regard to alcohol consumption and hepatic steatosis in Japan (adjusted RR = 0.77, 95% CI: 0.72–0.83, P = < 0.001) (Tsunoda et al., 2014, Moriya et al., 2013, Moriya et al., 2011, Hamaguchi et al., 2005, Gunji et al., 2009, Hiramine et al., 2011, Doi et al., 2010, Hamabe et al., 2011). Only two studies from China (Xu et al., 2010, Liu et al., 2014) were available for such an analysis from other countries (adjusted RR = 0.84, 95% CI: 0.71–0.996, P = 0.045).
A study from Germany (Lau et al., 2015) showed interaction effects between BMI and both average and binge alcohol consumption. However, participants with hepatic steatosis in this study were also 9 (men) and 16 (women) years older than participants without hepatic steatosis. A Finnish study (Suomela et al., 2015) showed detrimental associations for < 20 g/day in both obese and non-obese participants. A Japanese study (Tsunoda et al., 2014) showed that physical activity may have differential effects on the development of hepatic steatosis in heavy and non-heavy drinkers.
Binge drinking (defined as ≥ 5 standard drinks per day on at least one occasion in the last 30 days) in comparison to other drinkers (adjusted for age, BMI, HbA1c, average daily alcohol consumption, and menopausal status in women) was detrimentally associated with hepatic steatosis in men but not in women in one study from Germany (Lau et al., 2015). Two other studies from Japan (Moriya et al., 2015, Hiramine et al., 2011) showed a similar detrimental relationship in men, but the data were not adjusted for any potential confounders.
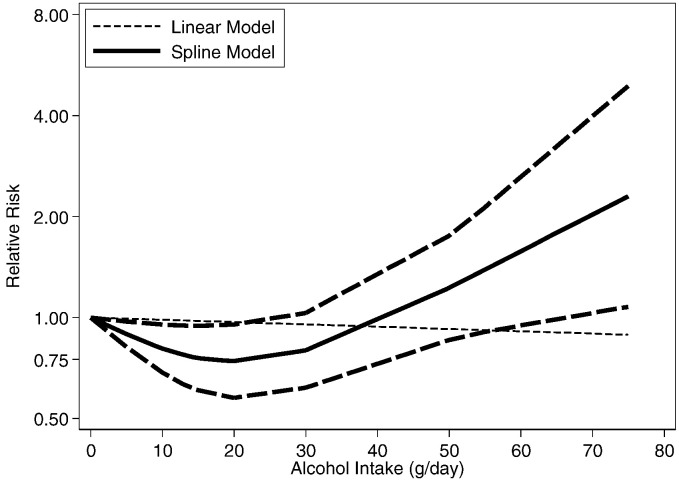
3.2. Dose Response Meta-analyses
Adjusted non-linear dose-response RR relationships (data available up to 80 g/day) in comparison to non-drinkers in Japan (Moriya et al., 2015, Moriya et al., 2013, Moriya et al., 2011, Hamaguchi et al., 2012, Hashimoto et al., 2015, Hiramine et al., 2011) are shown in Figs. 3 (men) and 4 (women). There was strong statistical evidence for a non-linear relationship for both men and women, and in cohort studies (Table 3). The dose-response risk relationship in men was non-linear and inverse with no evidence for a detrimental effect (Fig. 3). The risk curve in women was J-shaped and turned to a detrimental association with increasing alcohol consumption; a statistical significant beneficial effect was seen below 20 g/day (Fig. 4). Aside from Japanese studies, only one German study (Lau et al., 2015) had dose-response data beyond 20 g/day. The reported relationships were increasing with no beneficial effects in men (data available for up to 80 g/day), and non-linear and inverse in women (data available for up to 40 g/day) (Lau et al., 2015).
Fig. 3.
Dose-response relationships between alcohol consumption and hepatic steatosis in Japan, men (n = 6 estimates, relative risk on the log scale).
Fig. 4.
Dose-response relationships between alcohol consumption and hepatic steatosis in Japan, women (n = 5 estimates, relative risk on the log scale).
Table 3.
Spline regression coefficients for the non-linear relationship between alcohol consumption and hepatic steatosis compared to non-drinkers in Japan.
| Sex | Estimates No. | Casesa No. | Coefficient 1 |
Coefficient 2 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| beta | SE | P | I2(%) | beta | SE | P | I2(%) | |||
| All studies | ||||||||||
| Both sexes | 11 | 15,728 | − 0.0224 | 0.0021 | < 0.001 | 6 | 0.0371 | 0.0049 | < 0.001 | 16 |
| Men | 6 | 11,692 | − 0.0242 | 0.0024 | < 0.001 | 0 | 0.0338 | 0.0045 | < 0.001 | 0 |
| Women | 5 | 4036 | − 0.0234 | 0.0091 | 0.010 | 47 | 0.0539 | 0.0193 | 0.005 | 40 |
| Cohort studies | ||||||||||
| Both sexes | 4 | 2310 | − 0.0230 | 0.0035 | < 0.001 | 0 | 0.0217 | 0.0043 | < 0.001 | 0 |
SE, standard error
Cases of hepatic steatosis.
4. Discussion
Using high-quality epidemiological evidence, the association between alcohol consumption as a risk factor for hepatic steatosis was complex and non-linear, showing marked differences by ethnicity. At moderate alcohol intake (up to 20 g/day), studies from Japan showed a beneficial association, whereas studies from other countries, including China, Europe and North America, showed no overall association. The difference between studies from Japan and other countries was consistent and independent of the adjustment for confounding for metabolic risk factors, baseline prevalence of hepatic steatosis, and mean age at baseline. This questions the usefulness of terms such as ‘alcoholic’ or ‘non-alcoholic’, at least for hepatic steatosis, and highlights the need and importance of evidence from multiple populations of diverse drinking patterns and ethnic backgrounds. Others have noted that thresholds for non-alcoholic and alcoholic steatosis are arbitrary and a distinction between the two seems impossible (Nascimbeni et al., 2013, Volzke, 2012). Our results support this view. Nevertheless, there was a lack of data for high alcohol intake in countries other than Japan to draw firm conclusions about a dose-response relationship beyond moderate alcohol intake.
Sex was an important effect modifier when the whole range of alcohol consumption was considered. In general, consequences of heavy alcohol use appear to be more negative for women than for men based on higher blood alcohol levels because of smaller body size, lower body water content, and lower gastric alcohol dehydrogenase activity (Lieber, 2004, Zimmerman, 1999).
Across countries, binge drinking in moderate drinkers in comparison to non-binge drinkers was consistently associated with higher risk for hepatic steatosis in men, though evidence was scarce and mostly unadjusted. A potential explanation of the detrimental association in men may be that in high doses, as the doses ingested in binge-drinking, there is a dose dependent alcohol-induced hepatotoxicity (Lieber, 2004, Zimmerman, 1999, Zimmerman and KG, 1955). The one study reporting results for women did not show evidence for an association; however, binge drinking is usually less prevalent among women. Higher prevalence and volume of binge drinking in men might explain the sex discrepancy; however, few data were available for such analyses and conclusions need to be treated with caution.
4.1. Limitations
It should be noted that some subgroup analyses investigating potential effect modification by study characteristics were subject to low power because of the small number of studies in several subgroups. Furthermore, we cannot derive any conclusions on the shape of the risk curve > 80 g/day because of the lack of data. People with very heavy alcohol intake, such as people with alcohol use disorders, are usually missed in studies included in our review, and rates of liver diseases are certainly much higher (Roerecke and Rehm, 2014a). Although self-reported alcohol consumption seems to be reasonably valid (Greenfield, 2000), some drinkers and non-drinkers may change their alcohol consumption over time (Kerr et al., 2002, Rehm et al., 2008), leading to over- or under-estimation of alcohol intake. Thus, all drinking groups we have identified may have been subject to misclassification bias, as they were in the primary studies.
Most studies from Japan were from popular free health screenings associated with the workplace and may constitute a different population compared to studies from other countries. However, baseline prevalence rates in Japanese studies were very similar to what is known about the prevalence of hepatic steatosis in the general population. In fact, the relation between alcohol consumption and hepatic steatosis in Japan was so consistent that there was no heterogeneity to be explained. Nevertheless, we cannot draw conclusions for countries other those included in our analysis. Furthermore, because all data were from high-income countries, malnutrition seems unlikely to explain our findings.
Most of the data outside of Japan was based on cross-sectional studies making it impossible to determine a temporal exposure-disease relationship. However, five studies from Japan provided well-adjusted incidence data with no difference in comparison to prevalence data, similar to comparisons by sex and adjustment, all showing a substantial beneficial effect of alcohol consumption on hepatic steatosis. Despite the variation in baseline prevalence rates of hepatic steatosis by sex, there was no heterogeneity by sex among studies from Japan in terms of risk relationships between alcohol and hepatic steatosis. Data from other countries were more heterogeneous, showing no overall association, but a potential beneficial association in women. There were not enough data to systematically investigate observed heterogeneity in studies from other countries, though the baseline prevalence of hepatic steatosis may play a role. The larger heterogeneity in studies from other countries compared to studies from Japan may be explained by the fact that in an ethnically homogeneous population lives in Japan, while the rest of the studies comprise of ethnically more heterogeneous populations, including drinking cultures and diets. Because of the lack of incidence data and adjustment for other risk factors for hepatic steatosis in countries other than Japan, we rate the quality of the evidence as rather poor outside of Japan.
Potential explanations for the observed differences in risk for hepatic steatosis from alcohol consumption in Japan compared to other countries are speculative at this point. Although excessive alcohol consumption leads to morphological changes, which can occur despite an adequate nutritional state (Zimmerman and KG, 1955, Neuman et al., 2014b), the relationship with moderate alcohol consumption may be different. Differences in nutrition and microbiota can affect immune cells in the liver, which in turn release various inflammatory cytokines and recruit neutrophils, leading to the activation of macrophages (Kupffer cells) and to the inflammatory cascade (Neuman et al., 2014b). This phenomenon may be an additional explanation of the results seen in this meta-analysis (Grove et al., 1997, Neuman, 2003). The liver is involved in critical steps of lipid metabolism, including de novo synthesis and uptake of free fatty acids from diet as well as from lipolysis of the adipose tissue. Additionally, utilization of the free fatty acids, synthesis of triglycerides and their release from hepatocytes is dependent on several steps that can differ in genetically different population (Neuman et al., 2014b). We were unable to investigate genetic characteristics of the individual participants or the use of specific diets in our analysis because of the lack of data.
Although smoking status was adjusted for in most of the studies from Japan, including cohort studies, the effect of tobacco use on hepatic steatosis may be larger than is captured by a dichotomous variable such as status of smoking. Tobacco smoke creates a state of chronic inflammation leading to pro-inflammatory cytokine activation, impaired antioxidant metabolism and change in lipid profile of the individuals as well as potentiating insulin resistance syndrome and diabetes, all of which are risk factors for NAFLD (Attvall et al., 1993, Will et al., 2001, Zein, 2010).
Studies from Japan and other countries have shown a strong association of BMI with NAFLD (Li et al., 2016a, Matsuura et al., 2013) and other more severe forms of liver disease in studies of people with alcohol use disorders with much more alcohol intake than we observed in our analysis (Naveau et al., 2013, Naveau et al., 2009, Raynard et al., 2002). Almost all studies in our analysis were adjusted for BMI, visceral fat, or waist circumference, and these potential confounders did not explain the differential relationships between Japan and other countries. However, an interaction effect between BMI and alcohol intake was shown in two European studies (Lau et al., 2015, Bellentani et al., 2000). One Japanese study showed that alcohol intake was beneficial in both obese (BMI ≥ 25) and non-obese (BMI < 25) participants using unadjusted data (Nishioji et al., 2015). More research taking into account a possible interaction effect of BMI, other metabolic risk factors, and alcohol intake is needed to further elucidate the complex interplay of risk factors in the development of hepatic steatosis.
4.2. Implications
Having examined one of the most prevalent risk factors for non-communicable diseases in relation to the most prevalent liver disease globally, we found a complicated and non-linear relationship between the two with unclear but plausible pathways. There are plausible explanations for a non-linear association (i.e. a beneficial and detrimental effect depending on the dose and pattern of intake) of alcohol consumption on hepatic steatosis based on short-term biochemical evidence. Systematic evidence from trials for biomarkers related to fat and insulin metabolism showed a positive relationship with alcohol in relation to high density lipoproteins, an inverse relationship with low density lipoproteins, and no association with triglycerides (Brien et al., 2011). A reduction in HbA1c and fasting insulin with alcohol consumption, but not for insulin sensitivity or fasting glucose was reported in another meta-analysis of trial data (Schrieks et al., 2015). We found some evidence for effect modification from binge drinking even when average alcohol intake is moderate in men in our analysis. Similarly complicated and non-linear associations were reported using observational evidence on alcohol and ischemic heart disease (Roerecke and Rehm, 2014b), diabetes (Knott et al., 2015, Li et al., 2016b), and MetS (Loria et al., 2014, Sun et al., 2014). Although we analyzed observational studies, we rate the evidence for a beneficial effect of low alcohol consumption in Japan as relatively high because of its quality, magnitude, consistency, and precision. Evidence for an association of alcohol on hepatic steatosis from other countries in our analysis was mixed with large heterogeneity. More research on the role of alcohol consumption patterns and other risk factors in the development of hepatic steatosis and sex differences in countries other than Japan is needed as there were too few studies to derive meaningful conclusions. Though confounding did not seem to explain the findings of differential relations between Japan and other countries, interaction effects may play an important role. Interaction effects among risk factors such as alcohol consumption, BMI, diet, physical activity, and smoking should be routinely considered in sample size calculations for future studies and analyses using non-linear models for current studies in order to make progress in the understanding of combined and individual contributions of each risk factor.
5. Conclusions
The etiology of liver diseases is multi-factorial and given the results of this meta-analysis, alcohol's effects on liver steatosis are complex and non-linear. There was good evidence that alcohol consumption < 20 g/day has a beneficial effect on the development of hepatic steatosis in Japan, in contrast to other high-income countries. At least sex, ethnicity, BMI, and possibly drinking patterns seem to modify the relationship between alcohol consumption and hepatic steatosis. Large heterogeneity outside Japan precludes us from making firm judgements. More and higher quality research in diverse ethnic populations is needed to further clarify this relationship.
Acknowledgements
Conflict of Interest
MR and JR report grants from National Institutes of Health (NIH), National Institute on Alcohol Abuse and Alcoholism (NIAAA), during the conduct of the study. MN and RN report no conflicts of interest.
Financial Support
The work was financially supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) at the National Institutes of Health (grant number R21AA023521-01A1 to MR). The sponsor of the study (NIAAA) had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The authors collected the data, and had full access to all of the data in the study. The authors also had final responsibility for the decision to submit the study results for publication.
Author's Contributions
MR designed the study, and oversaw and conducted the literature review, data extraction, statistical analysis, data interpretation, article preparation, article review, and correspondence. JR contributed to design and data interpretation, article preparation, and article review. RN contributed to the literature review, article preparation, and article review. MN contributed to the design, literature review, data interpretation, article preparation, and article review. All authors contributed to the final article and approved the final version.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.04.023.
Appendix A. Supplementary data
Supplementary material.
References
- Attvall S., Fowelin J., Lager I., Von Schenck H., Smith U. Smoking induces insulin resistance–a potential link with the insulin resistance syndrome. J. Intern. Med. 1993;233(4):327–332. doi: 10.1111/j.1365-2796.1993.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Ballestri S., Zona S., Targher G. Evidence from a systematic review and meta-analysis; Journal of Gastroenterology and Hepatology: 2015. Nonalcoholic Fatty Liver Disease Is Associated with an almost two-Fold Increased Risk of Incident Type 2 Diabetes and Metabolic Syndrome. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Bellentani S., Saccoccio G., Costa G. Drinking habits as cofactors of risk for alcohol induced liver damage. Dionysos Study Group. Gut. 1997;41(6):845–850. doi: 10.1136/gut.41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellentani S., Saccoccio G., Masutti F. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann. Intern. Med. 2000;132(2):112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- Bonci E., Chiesa C., Versacci P., Anania C., Silvestri L., Pacifico L. Association of nonalcoholic fatty liver disease with subclinical cardiovascular changes: a systematic review and meta-analysis. BioMed Res. Int. 2015;2015:213737. doi: 10.1155/2015/213737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien S.E., Ronksley P.E., Turner B.J., Mukamal K.J., Ghali W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byass P. The global burden of liver disease: a challenge for methods and for public health. BMC Med. 2014;12:159. doi: 10.1186/s12916-014-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C.D., Targher G. NAFLD: a multisystem disease. J. Hepatol. 2015;62(1 Suppl):S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Caballeria L., Pera G., Auladell M.A. Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. Eur. J. Gastroenterol. Hepatol. 2010;22(1):24–32. doi: 10.1097/MEG.0b013e32832fcdf0. [DOI] [PubMed] [Google Scholar]
- Chalasani N., Younossi Z., Lavine J.E. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- Chalmers T.C., Smith H., Jr., Blackburn B. A method for assessing the quality of a randomized control trial. Control. Clin. Trials. 1981;2(1):31–49. doi: 10.1016/0197-2456(81)90056-8. [DOI] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Detsky A.S., Naylor C.D., O'Rourke K., McGeer A.J., L'Abbe K.A. Incorporating variations in the quality of individual randomized trials into meta-analysis. J. Clin. Epidemiol. 1992;45(3):255–265. doi: 10.1016/0895-4356(92)90085-2. [DOI] [PubMed] [Google Scholar]
- Doi T., Tanaka S., Sato Y. Effect of alcohol consumption on the prevalence of fatty liver disease. Acta Hepato. Jpn. 2010;51(9):501–507. [Google Scholar]
- EASL Clinical practical guidelines: management of alcoholic liver disease. J. Hepatol. 2012;57(2):399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M.F., Tukey J.W. Transformations related to the angular and the square root. Ann. Math. Stat. 1950;21(4):607–611. [Google Scholar]
- Greenfield T.K. Ways of measuring drinking patterns and the difference they make: experience with graduated frequencies. J. Subst. Abus. 2000;12(1–2):33–49. doi: 10.1016/s0899-3289(00)00039-0. [DOI] [PubMed] [Google Scholar]
- Greenland S., O'Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics. 2001;2(4):463–471. doi: 10.1093/biostatistics/2.4.463. [DOI] [PubMed] [Google Scholar]
- Grove J., Daly A.K., Bassendine M.F., Day C.P. Association of a tumor necrosis factor promoter polymorphism with susceptibility to alcoholic steatohepatitis. Hepatology. 1997;26(1):143–146. doi: 10.1002/hep.510260119. [DOI] [PubMed] [Google Scholar]
- Gunji T., Matsuhashi N., Sato H. Light and moderate alcohol consumption significantly reduces the prevalence of fatty liver in the Japanese male population. Am. J. Gastroenterol. 2009;104(9):2189–2195. doi: 10.1038/ajg.2009.361. [DOI] [PubMed] [Google Scholar]
- Hamabe A., Uto H., Imamura Y. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J. Gastroenterol. 2011;46(6):769–778. doi: 10.1007/s00535-011-0376-z. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M., Kojima T., Takeda N. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. Intern. Med. 2005;143(10):722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M., Kojima T., Ohbora A., Takeda N., Fukui M., Kato T. Protective effect of alcohol consumption for fatty liver but not metabolic syndrome. World J. Gastroenterol. 2012;18(2):156–167. doi: 10.3748/wjg.v18.i2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamling J., Lee P., Weitkunat R., Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat. Med. 2008;27(7):954–970. doi: 10.1002/sim.3013. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Hamaguchi M., Kojima T. Modest alcohol consumption reduces the incidence of fatty liver in men: a population-based large-scale cohort study. J. Gastroenterol. Hepatol. 2015;30(3):546–552. doi: 10.1111/jgh.12786. [DOI] [PubMed] [Google Scholar]
- Herbison P., Hay-Smith J., Gillespie W.J. Adjustment of meta-analyses on the basis of quality scores should be abandoned. J. Clin. Epidemiol. 2006;59(12):1249–1256. doi: 10.1016/j.jclinepi.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Hernaez R., Lazo M., Bonekamp S. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54(3):1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hiramine Y., Imamura Y., Uto H. Alcohol drinking patterns and the risk of fatty liver in Japanese men. J. Gastroenterol. 2011;46(4):519–528. doi: 10.1007/s00535-010-0336-z. [DOI] [PubMed] [Google Scholar]
- Kerr W.C., Fillmore K.M., Bostrom A. Stability of alcohol consumption over time: evidence from three longitudinal surveys from the United States. J. Stud. Alcohol. 2002;63(3):325–333. doi: 10.15288/jsa.2002.63.325. [DOI] [PubMed] [Google Scholar]
- Knott C., Bell S., Britton A. Alcohol consumption and the risk of type 2 diabetes: a systematic review and dose-response Meta-analysis of > 1.9 million individuals from 38 observational studies. Diabetes Care. 2015;38(9):1804–1812. doi: 10.2337/dc15-0710. [DOI] [PubMed] [Google Scholar]
- LaBrecque D.R., Abbas Z., Anania F. World gastroenterology organisation global guidelines: nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2014;48(6):467–473. doi: 10.1097/MCG.0000000000000116. [DOI] [PubMed] [Google Scholar]
- Lau K., Baumeister S.E., Lieb W. The combined effects of alcohol consumption and body mass index on hepatic steatosis in a general population sample of European men and women. Aliment. Pharmacol. Ther. 2015;41(5):467–476. doi: 10.1111/apt.13067. [DOI] [PubMed] [Google Scholar]
- Lazo Elizondo M. John Hopkins University; Baltimore, MD: 2010. Epidemiology of Nonalcoholic Fatty Liver Disease in the United States: Prevalence, Correlates and Mortality. [Google Scholar]
- Li L., Liu D.W., Yan H.Y., Wang Z.Y., Zhao S.H., Wang B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes. Rev.: Off J. Int. Assoc Study Obes. 2016 doi: 10.1111/obr.12407. [DOI] [PubMed] [Google Scholar]
- Li X.H., Yu F.F., Zhou Y.H., He J. Association between alcohol consumption and the risk of incident type 2 diabetes: a systematic review and dose-response meta-analysis. Am. J. Clin. Nutr. 2016 doi: 10.3945/ajcn.115.114389. [DOI] [PubMed] [Google Scholar]
- Lieber C.S. CYP2E1: from ASH to NASH. Hepatol. Res. 2004;28(1):1–11. doi: 10.1016/j.hepres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Liu P.J., Ma F., Lou H.P., Zhu Y.N., Chen Y. Relationship between serum uric acid levels and hepatic steatosis in non-obese postmenopausal women. Climacteric. 2014;17(6):692–699. doi: 10.3109/13697137.2014.926323. [DOI] [PubMed] [Google Scholar]
- Loria P., Marchesini G., Nascimbeni F. Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology. Atherosclerosis. 2014;232(1):99–109. doi: 10.1016/j.atherosclerosis.2013.10.030. [DOI] [PubMed] [Google Scholar]
- Lozano R., Naghavi M., Foreman K. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Liu H., Hu F., Zou L., Luo S., Sun L. Independent association between nonalcoholic fatty liver disease and cardiovascular disease: a systematic review and meta-analysis. Int. J. Endocrinol. 2013;2013:124958. doi: 10.1155/2013/124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathurin P., Deltenre P. Effect of binge drinking on the liver: an alarming public health issue? Gut. 2009;58(5):613–617. doi: 10.1136/gut.2007.145573. [DOI] [PubMed] [Google Scholar]
- Matsuura B., Nunoi H., Miyake T., Hiasa Y., Onji M. Obesity and gastrointestinal liver disorders in Japan. J. Gastroenterol. Hepatol. 2013;28(Suppl. 4):48–53. doi: 10.1111/jgh.12238. [DOI] [PubMed] [Google Scholar]
- Miele L., Dall'armi V., Cefalo C. A case-control study on the effect of metabolic gene polymorphisms, nutrition, and their interaction on the risk of non-alcoholic fatty liver disease. Genes Nutr. 2014;9(2):383. doi: 10.1007/s12263-013-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital signs: Binge drinking prevalence, frequency, and intensity among adults — United StatesMMWR Morb. Mortal. Wkly Rep. 2012. 2010;61(1):14–19. [PubMed] [Google Scholar]
- Moher D., Pham B., Jones A. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609–613. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- Moriya A., Iwasaki Y., Ohguchi S. Alcohol consumption appears to protect against non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2011;33(3):378–388. doi: 10.1111/j.1365-2036.2010.04520.x. [DOI] [PubMed] [Google Scholar]
- Moriya A., Iwasaki Y., Ohguchi S. Roles of alcohol drinking pattern in fatty liver in Japanese women. Hepatol. Int. 2013;7(3):859–868. doi: 10.1007/s12072-013-9449-9. [DOI] [PubMed] [Google Scholar]
- Moriya A., Iwasaki Y., Ohguchi S. Roles of alcohol consumption in fatty liver: a longitudinal study. J. Hepatol. 2015;62(4):921–927. doi: 10.1016/j.jhep.2014.11.025. [DOI] [PubMed] [Google Scholar]
- Naimi T.S., Brewer R.D., Mokdad A., Denny C., Serdula M.K., Marks J.S. Binge drinking among US adults. JAMA. 2003;289(1):70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- Nascimbeni F., Pais R., Bellentani S. From NAFLD in clinical practice to answers from guidelines. J. Hepatol. 2013;59(4):859–871. doi: 10.1016/j.jhep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- Naveau S., Thaury J., Barri-Ova N. Predictive factors for pure steatosis in alcoholic patients. Alcohol. Clin. Exp. Res. 2009;33(6):1104–1110. doi: 10.1111/j.1530-0277.2009.00932.x. [DOI] [PubMed] [Google Scholar]
- Naveau S., Dobrin A.S., Balian A. Body fat distribution and risk factors for fibrosis in patients with alcoholic liver disease. Alcohol. Clin. Exp. Res. 2013;37(2):332–338. doi: 10.1111/j.1530-0277.2012.01927.x. [DOI] [PubMed] [Google Scholar]
- Neuman M.G. Cytokines–central factors in alcoholic liver disease. Alcohol Res. Health. 2003;27(4):307–316. [PMC free article] [PubMed] [Google Scholar]
- Neuman M.G., Cohen L.B., Nanau R.M. Biomarkers in nonalcoholic fatty liver disease. Can. J. Gastroenterol. Hepatol. 2014;28(11):607–618. doi: 10.1155/2014/757929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman M.G., French S.W., French B.A. Alcoholic and non-alcoholic steatohepatitis. Exp. Mol. Pathol. 2014;97(3):492–510. doi: 10.1016/j.yexmp.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe R.G. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat. Med. 1998;17(8):857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Nishioji K., Sumida Y., Kamaguchi M. Prevalence of and risk factors for non-alcoholic fatty liver disease in a non-obese Japanese population, 2011-2012. J. Gastroenterol. 2015;50(1):95–108. doi: 10.1007/s00535-014-0948-9. [DOI] [PubMed] [Google Scholar]
- Orsini N., Bellocco R., Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6(1):40–57. [Google Scholar]
- Orsini N., Li R., Wolk A., Khudyakov P., Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X.E., Lai Z.S., Lu Q.Q., Lin J.Y., Lin X. A hospital-based case-control study on influencing factors of nonalcoholic fatty liver disease. Chin. J. Hepatol. 2009;17(7):535–539. [PubMed] [Google Scholar]
- Poli A., Marangoni F., Avogaro A. Moderate alcohol use and health: a consensus document. Nutr. Metab. Cardiovasc. Dis. 2013;23(6):487–504. doi: 10.1016/j.numecd.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Raynard B., Balian A., Fallik D. Risk Factors of Fibrosis in Alcohol-Induces Liver Diseases. Hepatology. 2002;35(3):635–638. doi: 10.1053/jhep.2002.31782. Baltimore, Md. [DOI] [PubMed] [Google Scholar]
- Rehm J., Roerecke M. Patterns of drinking and liver cirrhosis — what do we know and where do we go? J. Hepatol. 2015;62(5):1000–1001. doi: 10.1016/j.jhep.2015.01.027. [DOI] [PubMed] [Google Scholar]
- Rehm J., Irving H., Ye Y., Kerr W.C., Bond J., Greenfield T.K. Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. Am. J. Epidemiol. 2008;168(8):866–871. doi: 10.1093/aje/kwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerecke M., Rehm J. Cause-specific mortality risk in alcohol use disorder treatment patients: a systematic review and meta-analysis. Int. J. Epidemiol. 2014;43(3):906–919. doi: 10.1093/ije/dyu018. [DOI] [PubMed] [Google Scholar]
- Roerecke M., Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014;12:182. doi: 10.1186/s12916-014-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N., Forrest E., Preiss D. Non-alcoholic fatty liver disease. BMJ. 2014;349:g4596. doi: 10.1136/bmj.g4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrieks I.C., Heil A.L., Hendriks H.F., Mukamal K.J., Beulens J.W. The effect of alcohol consumption on insulin sensitivity and glycemic status: a systematic review and meta-analysis of intervention studies. Diabetes Care. 2015;38(4):723–732. doi: 10.2337/dc14-1556. [DOI] [PubMed] [Google Scholar]
- Shield K.D., Rylett M., Gmel G., Gmel G., Kehoe-Chan T.A., Rehm J. Global alcohol exposure estimates by country, territory and region for 2005–a contribution to the comparative risk assessment for the 2010 global burden of disease study. Addiction. 2013;108(5):912–922. doi: 10.1111/add.12112. [DOI] [PubMed] [Google Scholar]
- Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Sun K., Ren M., Liu D., Wang C., Yang C., Yan L. Alcohol consumption and risk of metabolic syndrome: a meta-analysis of prospective studies. Clin. Nutr. 2014;33(4):596–602. doi: 10.1016/j.clnu.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Suomela E., Oikonen M., Virtanen J. Prevalence and determinants of fatty liver in normal-weight and overweight young adults. The cardiovascular risk in young Finns study. Ann. Med. 2015;47(1):40–46. doi: 10.3109/07853890.2014.966752. [DOI] [PubMed] [Google Scholar]
- Targher G., Day C.P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010;363(14):1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- Thompson S.G., Higgins J.P. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002;21(11):1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- Tsunoda K., Kai Y., Uchida K., Kuchiki T., Nagamatsu T. Physical activity and risk of fatty liver in people with different levels of alcohol consumption: a prospective cohort study. BMJ Open. 2014;4(8) doi: 10.1136/bmjopen-2014-005824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon G., Baranova A., Younossi Z.M. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34(3):274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- Volzke H. Multicausality in fatty liver disease: is there a rationale to distinguish between alcoholic and non-alcoholic origin? World J. Gastroenterol. 2012;18(27):3492–3501. doi: 10.3748/wjg.v18.i27.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2000. International Guide for Monitoring Alcohol Consumption and Related Harm. Geneva, Switzerland. [Google Scholar]
- Will J.C., Galuska D.A., Ford E.S., Mokdad A., Calle E.E. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int. J. Epidemiol. 2001;30(3):540–546. doi: 10.1093/ije/30.3.540. [DOI] [PubMed] [Google Scholar]
- Wong V.W., Chu W.C., Wong G.L. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61(3):409–415. doi: 10.1136/gutjnl-2011-300342. [DOI] [PubMed] [Google Scholar]
- Xu C., Yu C., Xu L., Miao M., Li Y. High serum uric acid increases the risk for nonalcoholic fatty liver disease: a prospective observational study. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diab. Endocrinol. 2014;2(11):901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of non-alcoholic fatty liver disease-Meta-analytic assessment of prevalence. Incidence Outcomes. Hepatology. 2015 doi: 10.1002/hep.28431. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Zein C.O. Clearing the smoke in chronic liver diseases. Hepatology. 2010;51(5):1487–1490. doi: 10.1002/hep.23694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman H. second ed. Lippincott-Williams-Wilkins; Philadelphia: 1999. Adverse Effects of Drugs and Other Chemicals on the Liver. [Google Scholar]
- Zimmerman H.J., Ishak K.G. Non-alcoholic steatohepatitis and other forms of pseudoalcoholic liver disease. In: Hall P., editor. Alcoholic Liver Disease Pathology and Pathogenesis. second ed. Edward Arnold; London: 1955. pp. 165–198. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.