Abstract
AIM: To examine the results of orthotopic liver transplantation (OLT) for hepatocellular carcinoma (HCC) in Ireland over a 14-year period.
METHODS: Cases of HCC receiving OLT between January 1995 and September 2009 in the Irish Liver Transplant Unit were reviewed from a prospectively maintained database. Outcome measures included overall and recurrence free survival, alpha-fetoprotein (AFP) and tumour pathological features.
RESULTS: On explant pathology, 57 patients had HCC. The median follow-up time was 42.7 mo. The overall 1, 3 and 5 years survival was 87.7%, 72.1% and 72.4%. There was no difference in survival when compared to patients undergoing OLT without malignancy. The tumour recurrence rate was 14%. The Milan criteria were exceeded in 32% of cases but this did not predict overall survival or recurrence. On multivariate analysis pre-operative AFP > 100 ng/mL was an independent risk factor for recurrence (RR = 5.2, CI: 1.1-24.3, P = 0.036).
CONCLUSION: Patients undergoing OLT for HCC had excellent survival even when conventional listing criteria were exceeded. Pre-operative AFP predicts recurrence independent of tumour size and its role in selection criteria should be investigated in larger studies.
Keywords: Liver transplantation, Alpha-fetoprotein, Hepatocellular carcinoma, Transplantation selection criteria, Liver cirrhosis
Core tip: We have shown good survival from a medium volume transplant centre in a small cohort of patients exceeding Milan criteria. We show an association between a pre-operative alpha-fetoprotein (AFP) > 100 and hepatocellular carcinoma (HCC) recurrence, independent of tumour size. Our study supports other single centre experience on survival after transplant for HCC with low AFP and indicates that AFP needs to be interrogated in large, multi-centre studies to see if it can be included in transplant listing criteria to augment the current radiology based dimensional criteria.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the 5th most common cancer and 3rd leading cause of cancer-related death worldwide[1]. The incidence and related mortality are increasing, particularly in Western countries[2]. It is now well accepted that the optimal treatment for small HCC in the setting of cirrhosis is orthotopic liver transplantation (OLT)[3]. Since the publication and adaptation of the Milan criteria[4] the outcomes have improved dramatically compared to results from the era prior to established selection criteria[5]. Patients undergoing OLT for HCC within the Milan criteria achieve outcomes comparable to non-malignant transplant cohorts. However, recurrence is the most important cause of post-transplant death[6]. Appropriate patient selection is crucial as patients with large or biologically unfavourable tumours have unacceptable recurrence and overall survival rates[7]. It is essential that centres which provide OLT for HCC audit their results to ensure outcomes compare to international survival rates thus enabling appropriate patient prioritisation and organ allocation.
The aim of the current study was to determine the outcomes of OLT for HCC in a single, national institution over a 14-year period. Overall and recurrence free survival rates were compared to clinical and pathological factors using multivariate analysis to identify independent predictors of recurrence.
MATERIALS AND METHODS
All patients undergoing OLT with HCC proven on explant pathology between January 1995 and September 2009 were included in the study. All OLT in the Republic of Ireland are carried out in the Liver Unit of St Vincent’s University Hospital. The Liver Unit maintains a prospective database containing patients’ clinical details. Tumour characteristics are recorded on a computerised pathology database. A retrospective review of this data was performed. Patient characteristics recorded included age at OLT, sex, aetiology of underlying liver disease, pre-operative alpha-fetoprotein (AFP), survival, and recurrence status. Tumour data recorded included size and number of tumours, compliance with the Milan and University of California San Francisco (UCSF) criteria and microvascular invasion. The study was approved by the St Vincent’s University Hospital ethics review board.
Patient selection and transplant protocol
All patients listed were classified clinically as cirrhotic. HCC diagnosis was based on a combination of ultrasound, computed tomography (CT) and double-contrast magnetic resonance imaging (MRI). After 1996 patients were listed for OLT if they met Milan criteria on pre-operative imaging. Patients in Ireland listed for OLT for HCC receive an adjusted Model for End-Stage Liver Disease (MELD) score[8]. All OLT were from deceased donor transplants and organs were retrieved before cardiac death. Patients were followed up at a dedicated transplant clinic every three months. In general, post-transplant immunosuppression consisted of a reducing dose of corticosteroids and a calcineurin inhibitor (tacrolimus) or azothioprine. Follow up included annual abdominal ultrasound and CT where appropriate.
Survival and recurrence
Overall patient survival was determined from date of OLT until the most recently attended clinic. HCC recurrence free survival was determined by the date of the most recently available radiological imaging. Deaths from recurrence were prospectively recorded in the database. Patients without recurrence that died were documented free of recurrence only if the most recent available imaging or post-mortem report excluded recurrence. Two investigators (O’Connor DB and Cooney A) independently reviewed the database to ensure accuracy of the survival and recurrence data. Patients were censored in September 2009 to ensure a minimum of 5-year follow-up.
Tumour characteristics
All explants were examined by a histopathologist experienced in HCC pathology (Nolan N). Tumour size, number of lesions, presence of macro or microvascular invasion, and condition of the non-tumour bearing liver were recorded. Tumours were graded as well, moderate or poorly differentiated. Compliance with Milan or UCSF criteria was based on size and number of lesions and was determined by explant pathology rather than pre-operative imaging.
Statistical analysis
Patients were divided into groups based on meeting or exceeding listing criteria, presence or absence of vascular invasion, tumour grade, and pre-operative AFP levels to determine impact on overall overall and recurrence free survival. Data is presented as median (interquartile range). Factors affecting survival were determined by a Cox Proportional Hazard Model and significant factors were incorporated into a multivariate analysis. Kaplan-Meier analysis and the log-rank test were used to illustrate differences between recurrence free and overall survival according to clinical factors. Comparisons between the HCC and control cohort were made using Fisher’s Exact test. All calculations were done using SPSS version 12.0 (SPSS, Inc., Chicago, IL). P < 0.050 was set as the threshold for statistical significance.
RESULTS
During the 14-year study period 57 patients underwent OLT for HCC confirmed on explant pathology. One patient received OLT in 1995 and 56 patients were transplanted between 1998 and 2009. This represented 11.3% of the 504 patients undergoing OLT in the Liver Unit during that time. HCC was diagnosed radiologically in 52 cases pre-operatively and 5 cases were incidental findings in cirrhotic patients. HCC was absent on explant pathology in 4 additional patients transplanted for presumed HCC, representing false positives who were excluded from the analysis. Pre-operative AFP, tumour histopathology and clinical follow up data were available for all 57 patients. Median follow up was 42.7 (14.6-67.6) mo.
The median age at OLT was 59 years. The most common underlying causes of cirrhosis were alcoholic liver disease (30%), hepatitis C (30%) and Haemochromotosis (23%). The Milan criteria were exceeded in 16 (28%) and 8 patients (14%) exceeded UCSF criteria. Median largest tumour size was 3 (2.5-4.5) cm. Micro-vascular invasion was present in 24 (42%) tumours. The mean time to OLT following diagnosis was 3 mo. Bridging therapy was not routinely used. Only 4 patients underwent trans-arterial chemo-embolization and this was not included in statistical analysis. Patient and tumour characteristics are outlined in Table 1.
Table 1.
Patient demographics
| Male:female | 44:13 |
| Age, median (IQR) | 59.1 (53.5-63.6) |
| Aetiology of HCC | |
| Alcoholic liver disease | 17 (29.8%) |
| Hepatitis C | 17 (29.8%) |
| Haemochromatosis | 13 (22.8%) |
| α-1-antitrypsin deficiency | 3 (5.3%) |
| Primary sclerosing cholangitis | 2 (3.5%) |
| Primary biliary cirrhosis | 2 (3.5%) |
| Autoimmune hepatitis | 2 (3.5%) |
| Hepatitis B | 2 (3.5%) |
| Cryptogenic | 2 (3.5%) |
| Cystic fibrosis | 1 (1.8%) |
| Sarcoidosis | 1 (1.8%) |
| Nash | 1 (1.8%) |
| Pre-operative α-fetoprotein, median (IQR) | 8.8 (3.3-29.2) |
| Compliant with Milan criteria | 41 (71.9%) |
| Compliant with UCSF criteria | 49 (86.0%) |
| Largest lesion, median (IQR) | 3 (2.5-4.5) |
| Cirrhosis | 53 (93.0%) |
| Steatosis | 2 (3.5%) |
| Multifocal lesions | 24 (42.1%) |
| Micro-vascular invasion | 24 (42.1%) |
| Tumour differentiation | |
| Well | 24 (42.1%) |
| Moderate | 28 (49.1%) |
| Poor | 5 (8.8%) |
| Incidental lesions | 5 (8.8%) |
HCC: Hepatocellular carcinoma; UCSF: University of California San Francisco; IQR: Interquartile range.
Survival
Overall survival at 1, 3 and 5 years was 87.7% (50/57), 72.1% (31/43) and 72.4% (21/29) respectively. The HCC transplant group were compared to a cohort of 313 patients undergoing OLT between 1998 and 2008 who underwent their primary, non-emergent, transplant during that period. There was no statistical difference between the HCC and control cohort in 1 (87.7% vs 89.1%, P = 0.450), 3 (72.1% vs 84.2%, P = 0.050) and 5 years (72.4% vs 80.9%, P = 0.211) overall survival rates. No clinical or pathological variable significantly affected overall survival in those undergoing OLT for HCC (Table 2). Overall survival was not affected by patients exceeding the Milan (Figure 1A) or UCSF (Figure 1B).
Table 2.
Univariate analysis of factors affecting overall survival
| HR | CI | P-value | |
| Male sex | 0.786 | 0.301-2.055 | 0.623 |
| Age | 1.001 | 0.954-1.051 | 0.952 |
| Aetiology of HCC | |||
| Alcoholic liver disease | 0.523 | 0.175-1.567 | 0.247 |
| Hepatitis C | 2.098 | 0.849-5.183 | 0.108 |
| Haemochromatosis | 0.715 | 0.239-2.143 | 0.549 |
| Other | 1.198 | 0.459-3.126 | 0.712 |
| Pre-operative α-fetoprotein > 100 ng/mL | 1.502 | 0.437-5.165 | 0.519 |
| Compliant with Milan criteria | 0.994 | 0.381-2.590 | 0.989 |
| Compliant with UCSF criteria | 0.871 | 0.290-2.618 | 0.805 |
| Largest lesion | 1.207 | 0.963-1.513 | 0.102 |
| Cirrhosis | 23.309 | 0.024-224.813 | 0.369 |
| Steatosis | 0.044 | 0.000-187.285 | 0.465 |
| Multi-focal lesions | 1.201 | 0.499-2.890 | 0.683 |
| Micro-vascular invasion | 1.489 | 0.619-3.578 | 0.374 |
| Tumour differentiation | |||
| Well | 0.862 | 0.349-2.131 | 0.748 |
| Moderate | 1.100 | 0.448-2.698 | 0.835 |
| Poor | 1.159 | 0.268-5.022 | 0.843 |
| Incidental lesions | 0.450 | 0.060-3.391 | 0.438 |
HCC: Hepatocellular carcinoma; UCSF: University of California San Francisco.
Figure 1.
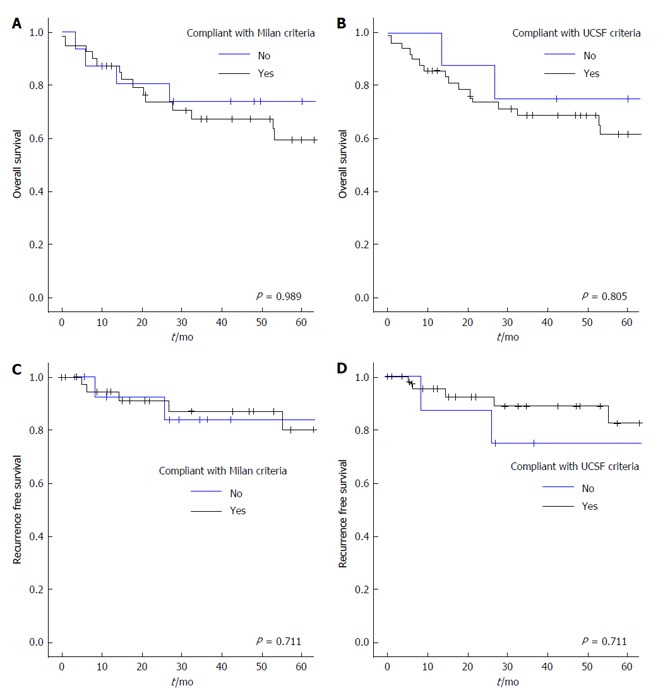
Kaplan-Meier estimates of overall survival (A and B) and recurrence free survival (C and D) in relation to compliance with the Milan and University of California San Francisco criteria. UCSF: University of California San Francisco.
Recurrence
Recurrence free survival was 86%, 69.7% and 69.5% at 1, 3 and 5 years respectively. There were 8 recurrences in total (14%) and 5 patients died from recurrence. Recurrence occurred within 1 year in 3 patients, within 2 years in 3 and beyond 3 and 5 years in one patient each. The location of recurrent disease was hepatic in 3 (including 2 patients with additional extra-hepatic metastases), porta-hepatis lymph nodes in 2, and in one patient multiple recurrence occurred in lung, omentum and sacrum. Hepatic recurrences were diagnosed on CT and extra hepatic disease was confirmed by biopsy. Recurrence free survival was similar between patients meeting or exceeding the Milan (Figure 1C) and the UCSF criteria (Figure 1D). Underlying liver disease, tumour size or vascular invasion did not affect recurrence free survival. On univariate analysis only poorly differentiated tumours and AFP levels > 100 ng/mL were associated with reduced disease free survival (Table 3) and a shorter time to recurrence (Figure 2). On multivariate analysis, pre-operative AFP > 100 ng/mL remained an independent predictor of recurrence free survival (HR = 5.2, P = 0.036).
Table 3.
Univariate analysis of factors affecting recurrence free survival
| HR | CI | P-value | |
| Male sex | 0.681 | 0.156-2.971 | 0.609 |
| Age | 1.004 | 0.932-1.081 | 0.922 |
| Aetiology of HCC | |||
| Alcoholic liver disease | 0.775 | 0.155-3.887 | 0.757 |
| Hepatitis C | 3.272 | 0.798-13.417 | 0.100 |
| Haemochromatosis | 1.210 | 0.243-6.024 | 0.816 |
| Other | 0.027 | 0.000-16.500 | 0.271 |
| Pre-operative α-fetoprotein > 100 ng/mL | 6.668 | 1.661-26.768 | 0.007 |
| Compliant with Milan criteria | 1.354 | 0.271-6.761 | 0.712 |
| Compliant with UCSF criteria | 0.739 | 0.148-3.692 | 0.712 |
| Largest lesion | 1.326 | 0.976-1.801 | 0.071 |
| Cirrhosis | 23.025 | 0.000-327.873 | 0.604 |
| Steatosis | 0.045 | 0.000-546.731 | 0.664 |
| Multifocal lesions | 2.100 | 0.494-8.930 | 0.315 |
| Micro-vascular invasion | 1.560 | 0.376-6.463 | 0.540 |
| Tumour differentiation | |||
| Well | 0.249 | 0.046-1.340 | 0.105 |
| Moderate | 1.553 | 0.374-6.443 | 0.544 |
| Poor | 5.631 | 1.074-29.510 | 0.041 |
| Incidental lesions | 0.041 | 0.000-720.752 | 0.523 |
HCC: Hepatocellular carcinoma; UCSF: University of California San Francisco.
Figure 2.
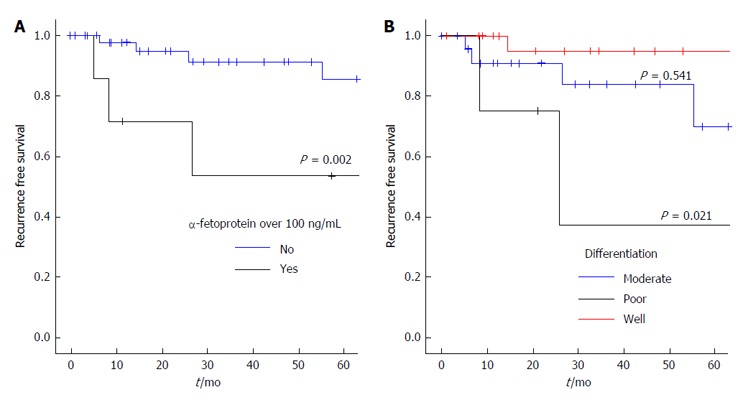
Kaplan-Meier estimates of recurrence free survival in relation to pre-operative α-fetoprotein (A) and tumour differentiation (B).
Patients exceeding Milan and UCSF criteria
Eight patients exceeded both Milan and UCSF criteria. Five were alive at 5 years and one patient with recurrence was alive after 3 years follow-up. Recurrence only occurred in 2 cases. One patient died from recurrence after 14 mo and one died from a separate malignancy at 2 years. Micro-vascular invasion was present in 4 cases. AFP exceeded 100 ng/mL in the patient who died from recurrence.
DISCUSSION
The current study confirms that OLT for HCC is an effective treatment modality and that survival rates are comparable to those undergoing OLT for non-malignant disease. Patients exceeding the Milan or UCSF criteria were not at increased risk of reduced overall survival or increased recurrence. Pre-operative serum AFP is an independent risk factor for recurrence.
The landmark Milan publication in 1996 established listing criteria based on a single HCC of less than 5 cm or up to 3 tumours, each less than 3 cm[4]. This was validated in other single-centre studies and together demonstrated a 5-year survival of 70% and recurrence rates of less than 15% which became the gold standard outcome in OLT for HCC[9-11]. These criteria continue to be used in Ireland and many centres worldwide. Our institution is a medium volume centre performing approximately 60-70 OLT per year. The outcomes of our patient cohort compare favourably to recently published series’ from higher volume centres[12,13]. Our patient demographic is different to most centres as in over 50% of patients the underlying liver pathology was alcoholic liver disease or haemochromatosis. Worldwide, the main causes of HCC are hepatitis B and C virus but in this study they only accounted for 33% of HCC[14]. However aetiology of HCC did not significantly impact on overall or disease free survival.
The majority of patients with HCC present with disease beyond the Milan criteria[15]. Acceptable 5-year survival and recurrence rates observed in a subgroup of patients with larger tumours led to the publication of the UCSF criteria which proposes listing patients with a single tumour up to 6.5 cm or up to 3 lesions, none larger than 4.5 cm and total tumour burden not exceeding 8 cm[16]. This has been reproduced in single-centre studies with short follow up but never in multi-centre or nationwide population studies but in recent years several units have called for an extension of the criteria. Patients beyond the Milan criteria did not experience inferior survival in our centre but our numbers are too small to support calls for extension of the criteria based simply on size and number of tumours. The limitations of pre-operative imaging for staging in the setting of cirrhosis also impede raising the threshold. One large study showed pre-operative imaging to under stage over 40% of patients[17]. In the current study almost 30% were not compliant with Milan criteria on explant pathology. Interval tumour growth is a possible explanation for patients who meet criteria on imaging and then exceed them on pathology. However our cohort experienced a short waiting period of 3 mo and relatively small tumours (median 3 cm) which makes tumour doubling unlikely. Even with advances such as double-contrast MRI, extending the criteria based solely on size risks transplanting patients with tumours too large to benefit.
The limitation of established criteria is that they are based on tumour dimensions. While results from single centre studies have justified its use for organ allocation, in a North American population study, a subgroup of patients with larger tumours within the Milan criteria had significantly poorer survival outcomes than those without HCC[18]. It is imperative that any selection criteria be accurate in predicting prognosis to justify the large proportion of transplants undertaken for HCC in the setting of a shortage of organs. For example 25% of all United States OLT have been for HCC since the introduction of priority MELD scores for HCC in 2002[18] and 11% of OLT in Ireland are for patients with HCC.
There is growing evidence that the biological behaviour of the tumour rather than size dictates recurrence. Patients with larger tumours beyond the Milan criteria but without micro-vascular invasion can have excellent survival, such as outlined in the “up-to-seven-criteria”, but this cannot be diagnosed pre-operatively[19]. The impact of micro-vascular invasion was not found to be statistically significant in our cohort but in large studies it has been shown to double the risk of death[7]. Pre-operative AFP may be the best available surrogate marker for micro-vascular invasion and the biological aggressiveness of the tumour. Several studies have identified a high pre-operative AFP as a risk factor for recurrence and reduced survival[18,20-22]. We have shown AFP predicted reduced disease free survival, independent of both tumour size and micro-vascular invasion. Furthermore, patients exceeding Milan or even UCSF criteria experienced excellent overall and recurrence free survival with a pre-operative AFP < 100 ng/mL. This supports the finding of another group where an AFP level < 30 ng/mL predicted disease free survival in patients beyond Milan criteria[23]. Both studies are limited by the small number of patients exceeding Milan criteria. Recent large studies have not examined the impact of AFP in the context of tumours beyond the Milan criteria. The largest study reporting survival in patients with tumours exceeding the Milan criteria (1112 patients) unfortunately did not examine the impact of AFP level[7]. Analysis from the United Network for Organ Sharing on 2253 patients demonstrated a significant survival advantage in patients with low pre-transplant AFP (< 20 ng/mL) but this effect wasn’t explored in patients with tumours outside Milan criteria[24]. It would therefore be intriguing if AFP could be examined in a large population database or multicentre study to determine if patients with large tumours but low pre-operative AFP had higher survival rates. Only then can AFP be used to augment existing eligibility criteria to safely expand the pool of patients suitable for OLT.
In conclusion, in appropriately selected patients with HCC undergoing OLT, survival was comparable to non-HCC patients. A subgroup of patients with larger tumours and low AFP may benefit from OLT but this association should be examined in larger, multicentre studies.
ACKNOWLEDGMENTS
Anne Cooney, database manager, the Liver Unit, for assistance in database retrieval.
COMMENTS
Background
Orthotopic liver transplantation (OLT) is the most effective treatment for hepatocellular carcinoma (HCC) in the setting of cirrhosis. Survival in well selected patients with a small burden of tumour is similar to patients undergoing OLT for non-cancer related indications.
Research frontiers
Existing selection criteria are based on the size and number of the tumour. Several datasets have demonstrated good survival outcomes in patients exceeding these criteria. The biological characteristics, for example micro-vascular invasion may just as important as the tumour dimensions. However these cannot be reliably detected pre-operatively.
Innovations and breakthroughs
This study also demonstrates that patients with larger tumours can still have good survival outcomes. Pre-operative alpha-fetoprotein (AFP) predicted tumour recurrence. AFP may be a useful surrogate marker for less favourable biological characteristics of the tumour.
Applications
The prognostic value of AFP could be evaluated in large, multi-centre datasets to determine its potential as an adjunct to existing selection criteria.
Terminology
OLT: Orthotropic liver transplant involves fully explanting the diseased liver immediately prior to the transplant; HCC: Hepatocellular carcinoma is the most common primary liver tumour. Because most cases occur in the setting of cirrhosis, it is often not amenable to resection; AFP: Alpha-fetoprotein has no known function in adults but it has clinical significance as a tumour marker in the diagnosis of HCC.
Peer-review
This is an interesting attempt to evaluate the results of liver transplantation for HCC with regard to potential relation with pre-operative values of AFP. The paper is well written and results are clarified.
Footnotes
Institutional review board statement: The study was approved by the St Vincent’s University Hospital ethics review board.
Informed consent statement: Informed consent was not required for this study.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Data sharing statement: No additional data.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 15, 2015
First decision: January 4, 2016
Article in press: May 9, 2016
P- Reviewer: Boucek CD, Balaban YH, Panda CK, Silva R, Smyrniotis V S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
References
- 1.Botha JF, Langnas AN. Liver transplantation for hepatocellular carcinoma: an update. J Natl Compr Canc Netw. 2006;4:762–767. doi: 10.6004/jnccn.2006.0066. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 3.Jarnagin W, Chapman WC, Curley S, D’Angelica M, Rosen C, Dixon E, Nagorney D. Surgical treatment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford) 2010;12:302–310. doi: 10.1111/j.1477-2574.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Selby R, Kadry Z, Carr B, Tzakis A, Madariaga JR, Iwatsuki S. Liver transplantation for hepatocellular carcinoma. World J Surg. 1995;19:53–58. doi: 10.1007/BF00316980. [DOI] [PubMed] [Google Scholar]
- 6.Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, Ko GY, Park KM, Ha TY, Song GW. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14:935–945. doi: 10.1002/lt.21445. [DOI] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 8.Zaman MB, Hoti E, Qasim A, Maguire D, McCormick PA, Hegarty JE, Geoghegan JG, Traynor O. MELD score as a prognostic model for listing acute liver failure patients for liver transplantation. Transplant Proc. 2006;38:2097–2098. doi: 10.1016/j.transproceed.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999;19:311–322. doi: 10.1055/s-2007-1007120. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 11.Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 12.Cescon M, Ravaioli M, Grazi GL, Ercolani G, Cucchetti A, Bertuzzo V, Vetrone G, Del Gaudio M, Vivarelli M, D’Errico-Grigioni A, et al. Prognostic factors for tumor recurrence after a 12-year, single-center experience of liver transplantations in patients with hepatocellular carcinoma. J Transplant. 2010;2010:pii: 904152. doi: 10.1155/2010/904152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levi DM, Tzakis AG, Martin P, Nishida S, Island E, Moon J, Selvaggi G, Tekin A, Madrazo BL, Narayanan G, et al. Liver transplantation for hepatocellular carcinoma in the model for end-stage liver disease era. J Am Coll Surg. 2010;210:727–734, 735-736. doi: 10.1016/j.jamcollsurg.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Hwang S, Lee SG, Belghiti J. Liver transplantation for HCC: its role: Eastern and Western perspectives. J Hepatobiliary Pancreat Sci. 2010;17:443–448. doi: 10.1007/s00534-009-0241-0. [DOI] [PubMed] [Google Scholar]
- 15.Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer. 1996;77:2217–2222. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2217::AID-CNCR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 17.Ioannou GN, Perkins JD, Carithers RL. Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Freeman RB, Mithoefer A, Ruthazer R, Nguyen K, Schore A, Harper A, Edwards E. Optimizing staging for hepatocellular carcinoma before liver transplantation: A retrospective analysis of the UNOS/OPTN database. Liver Transpl. 2006;12:1504–1511. doi: 10.1002/lt.20847. [DOI] [PubMed] [Google Scholar]
- 19.D’Amico F, Schwartz M, Vitale A, Tabrizian P, Roayaie S, Thung S, Guido M, del Rio Martin J, Schiano T, Cillo U. Predicting recurrence after liver transplantation in patients with hepatocellular carcinoma exceeding the up-to-seven criteria. Liver Transpl. 2009;15:1278–1287. doi: 10.1002/lt.21842. [DOI] [PubMed] [Google Scholar]
- 20.Kondili LA, Lala A, Gunson B, Hubscher S, Olliff S, Elias E, Bramhall S, Mutimer D. Primary hepatocellular cancer in the explanted liver: outcome of transplantation and risk factors for HCC recurrence. Eur J Surg Oncol. 2007;33:868–873. doi: 10.1016/j.ejso.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 21.McHugh PP, Gilbert J, Vera S, Koch A, Ranjan D, Gedaly R. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB (Oxford) 2010;12:56–61. doi: 10.1111/j.1477-2574.2009.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grąt M, Krasnodębski M, Patkowski W, Wronka KM, Masior Ł, Stypułkowski J, Grąt K, Krawczyk M. Relevance of Pre-Transplant α-fetoprotein Dynamics in Liver Transplantation for Hepatocellular Cancer. Ann Transplant. 2016;21:115–124. doi: 10.12659/aot.894644. [DOI] [PubMed] [Google Scholar]
- 23.Sotiropoulos GC, Malagó M, Bockhorn M, Schmitz KJ, Radtke A, Molmenti EP, Schaffer R, Beckebaum S, Cicinnati VR, Fouzas I, et al. Liver transplantation for hepatocellular carcinoma and cirrhosis in candidates with undetectable or very low alpha-fetoprotein levels: is an expansion of the listing criteria justified? Hepatogastroenterology. 2008;55:1671–1677. [PubMed] [Google Scholar]
- 24.Mailey B, Artinyan A, Khalili J, Denitz J, Sanchez-Luege N, Sun CL, Bhatia S, Nissen N, Colquhoun SD, Kim J. Evaluation of absolute serum α-fetoprotein levels in liver transplant for hepatocellular cancer. Arch Surg. 2011;146:26–33. doi: 10.1001/archsurg.2010.295. [DOI] [PubMed] [Google Scholar]


