Abstract
AIM: To expand the living donor liver transplantation (LT) pool of eligible patients with hepatocellular carcinoma (HCC) using new morphological and biological criteria.
METHODS: Patients with HCC who underwent living donor LT (LDLT) from March 2005 to May 2013 at the National Cancer Center Korea (NCCK) were enrolled. We performed the 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) before LDLT. Overall and disease-free survival analysis was done in patients to evaluate the usefulness of new NCCK criteria using PET/CT and total tumor size (10 cm).
RESULTS: We enrolled a total of 280 patients who pathologically confirmed to have HCC and performed the PET/CT before transplantation. Among them, 164 (58.6%) patients fulfilled the NCCK criteria and 132 patients (47.1%) met the Milan criteria. Five-year overall and disease-free survival rates for patients who fulfilled the NCCK criteria showed 85.2% and 84.0%, respectively, and were significantly higher than those beyond the NCCK criteria (60.2% and 44.4%, respectively; P < 0.001). The correlation analysis between preoperative imaging tests and pathologic reports using Cohen’s Kappa demonstrated the better results in the NCCK criteria than those in the Milan criteria (0.850 vs 0.583). The comparison of disease-free analysis among the NCCK, Milan, and University of California, San Francisco (UCSF) criteria using the receiver operating characteristics curves revealed the similar area under the curve value criteria (NCCK vs Milan, P = 0.484; NCCK vs UCSF, P = 0.189 at 5-years).
CONCLUSION: The NCCK criteria using hybrid concept of both morphological and biological parameters showed an excellent agreement between preoperative imaging and pathological results, and favorable survival outcomes. These new criteria might select the optimal patients with HCC waiting LDLT and expand the selection pool.
Keywords: Hepatocellular carcinoma, Living donor, Liver transplantation, Selection criteria
Core tip: National Cancer Center Korea criteria using positron-emission tomography/computed tomography positivity and total tumor size (cutoff 10 cm) expanded the pool of living donor liver transplantation for patients with hepatocellular carcinoma. Patient identification on the bases of the criteria showed an excellent agreement between preoperative imaging and pathological results and favorable survival outcomes.
INTRODUCTION
The application of selection criteria for liver transplantation (LT) in patients with hepatocellular carcinoma (HCC) has changed the HCC treatment algorithm over the past 20 years. The Milan criteria proposed by Mazzaferro et al[1]. helped to increase the number of LTs in patients with HCC and demonstrated remarkably good survival outcomes for these patients. In particular, the Milan criteria, which use both tumor size and number are very useful and have been adopted as selection criteria. Based on these criteria, the patients for whom HCC was identified early had the best chance of being cured of cancer following LT. In Asian countries such as South Korea and Japan, the number of deceased donors is limited and living donor LT (LDLT) has become an important option for treatment in patients with HCC[2,3]. As the amount of experience and evidence on LDLT for HCC has increased in recent years, the selection criteria for LT have gradually been expanded in large-volume centers. Various expanded criteria based on tumor number and size, such as the University of California, San Francisco (UCSF) criteria, have been proposed[4-9]. Some Japanese centers have demonstrated that preoperative tumor markers such as the des-gamma-carboxy prothrombin (DCP) level and tumor size were associated with higher recurrence rates[10,11]. These expanded criteria revealed that selected patients who did not fulfill the Milan criteria showed good overall survival (OS) and disease-free survival (DFS) rates compared with those who fulfilled the Milan criteria. Although the Milan criteria always guarantee the best survival rates in patients with HCC, they are too restrictive and use modalities.
In HCC patients, tumor characteristics, including differentiation grade and microvascular invasion, are well-known independent prognostic factors for OS and DFS following LT[12]. However, these factors cannot be evaluated by preoperative imaging studies, which reveal the morphological characteristics such as number and size. Recently, several studies using 18F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) demonstrated that 18F-FDG PET/CT findings were a powerful prognostic marker in patients with HCC after LT and showed good correlation with pathological tumor characteristics, such as microvascular invasion and differentiation[13-15].
In the present study, we performed a retrospective analysis to identify prognostic factors in patients with HCC who underwent 18F-FDG PET/CT before LDLT. Based on this result, we developed new and simple expanded criteria [the National Cancer Center, Korea (NCCK) criteria], incorporating a hybrid concept of biological and morphological characteristics on PET/CT images, including total tumor size, and compared these criteria with the Milan criteria, which are based on only morphological evaluation.
MATERIALS AND METHODS
Patients
Patients who underwent LDLT due to HCC at NCCK between March 2005 and May 2013 were collected using prospectively collected database. All patients were diagnosed as HCC by pathologic reports, and underwent 18F-FDG PET/CT to check biologic status of the primary tumor and the presence of metastasis within 1 mo before LDLT. Routine preoperative imaging tools for clinical staging in patients with HCC before LDLT were ultrasonography, multi-detector CT (MDCT), and/or dual contrast-enhanced magnetic resonance imaging (MRI) including PET/CT without protocol tumor biopsy. We reviewed the medical records for clinicopathological data, including age, sex, serum α-fetoprotein (AFP), viral markers, C-reactive protein, Model for End-Stage Liver Disease (MELD) score, PET/CT reports, tumor maximum standardized uptake value (SUVmax), pre-transplant therapies, and pathologic data such as Edmondson and Steiner grade; vessel, serosa, and duct invasion; capsule formation; cirrhosis; intrahepatic metastasis; and dysplastic nodules. Prognostic factors using clinicopathological data were analyzed for their effect on OS and DFS. This study was approved by the institutional review board of NCCK.
Our policy for selecting recipients with HCC for LDLT was basically based on the Milan criteria by preoperative imaging tools such as MDCT, MRI, or PET/CT. However, considering the specificity of living related donation, we performed LDLT on patients without major vascular invasion and extrahepatic metastasis on preoperative imaging tools even though they do not satisfy the Milan criteria. We do not recommend the downstaging or bridging therapy before LDLT even though the patient had advanced HCC. The operative techniques, immunosuppression, and management for hepatitis virus of donor and recipient have been described in detail in previous our reports[16,17]. Patients were followed up periodically with interval 3 or 6 mo using imaging studies such as ultrasonography, abdomen, and chest MDCT with AFP and DCP level. As the tumor recurrence was suspected by imaging tools and serologic tests, additional PET/CT was performed to evaluate the recurrent tumor and distant metastasis. For one or two nodules in the liver, lung, bone, or brain, we performed the resections. However, in case of multiple metastases, we treated tumors with a multimodality approach such as radiofrequency ablation, transarterial chemoembolization (TACE), radiation therapy, or chemotherapy.
18F-FDG PET/CT
Our protocol of 18F-FDG PET/CT was described in detail previously[14]. In brief, 18F-FDG PET/CT was performed using a PET/CT scanner (Biograph LSO; Siemens Medical Systems and Discovery LS; GE Healthcare, New Jersey, United States). The mean period between PET/CT and LDLT was 14.8 d. All PET/CT images were analyzed by experienced nuclear medicine physicians. SUV was calculated as (decay-corrected activity kBq/mL of tissue volume)/(injected FDG activity kBq/body mass gram). SUVs of the lesions were checked by placing a region of interest (ROI) at the site of the maximum FDG uptake in the PET images. The ROI was drawn to encircle the highest activity of each tumor, by the results of the CT scans that were acquired from PET/CT or MRI scans. PET/CT positivity was defined by experienced nuclear medicine physicians by checking whether the SUVmax of the tumor by CT or MRI scans was higher than that in the surrounding noncancerous hepatic tissue. Mean SUVmax of tumors for PET/CT positivity and negativity in this study was 4.46 and 3.08, respectively (P < 0.001).
NCCK criteria
In a multivariable analysis of our data, we identified two significant prognostic factors by evaluating pathological examination results (Table 1). These were positive findings on PET/CT (HR = 2.652, 95%CI: 1.384-50.085, P = 0.003 for OS; HR = 2.517, 95%CI: 1.481-4.279, P = 0.001 for DFS) and total tumor size of > 10 cm (HR = 2.909, 95%CI: 1.230-6.880, P = 0.015 for OS; HR = 3.003, 95%CI: 1.536-5.870, P = 0.001 for DFS). Although microvascular invasion was a significant factor only for DFS (HR = 2.148, 95%CI: 1.064-4.336, P = 0.033), it was not included because these data are typically not available before transplantation. We analyzed our data in comparison with the Milan and UCSF criteria using the NCCK criteria (negative findings on PET/CT and total tumor size < 10 cm vs others). The NCCK criteria were assessed both preoperatively and postoperatively.
Table 1.
Multivariable analysis of prognostic factors for overall and disease-free survival
|
Overall survival |
Disease-free survival |
||||||
| Multivariable analysis | HR | 95%CI | P | HR | 95% CI | P | |
| Variables | |||||||
| AFP | > 400 ng/mL | 1.145 | 0.543-2.418 | 0.722 | 1.003 | 0.556-1.811 | 0.991 |
| PET/CT | Positive | 2.652 | 1.384-5.085 | 0.003 | 2.517 | 1.481-4.279 | 0.001 |
| Tumor number | > 3 | 0.647 | 0.294-1.425 | 0.280 | 0.814 | 0.425-1.557 | 0.534 |
| Maximum tumor size | > 5 cm | 0.696 | 0.307-1.580 | 0.386 | 1.551 | 0.836-2.877 | 0.164 |
| Total tumor size | > 10 cm | 2.909 | 1.230-6.880 | 0.015 | 3.003 | 1.536-5.870 | 0.001 |
| Differentiation1 | III-IV | 1.206 | 0.616-2.358 | 0.585 | 1.010 | 0.594-1.717 | 0.972 |
| Microvascular invasion | Present | 1.269 | 0.522-3.084 | 0.599 | 2.148 | 1.064-4.336 | 0.033 |
| Capsule formation | Present | 0.439 | 0.166-1.162 | 0.097 | 0.737 | 0.353- 1.542 | 0.418 |
| Major vessel invasion | Present | 2.017 | 0.829-4.905 | 0.122 | 1.712 | 0.850-3.449 | 0.132 |
| Ductal invasion | Present | 0.907 | 0.265-3.100 | 0.876 | 1.409 | 0.534-3.720 | 0.489 |
| Serosal invasion | Present | 1.463 | 0.670-3.195 | 0.339 | 1.047 | 0.553-1.984 | 0.887 |
| Intrahepatic metastasis | Present | 1.471 | 0.595-3.640 | 0.404 | 1.519 | 0.752-3.070 | 0.244 |
| Dysplastic nodule | Present | 0.744 | 0.365-1.514 | 0.414 | 0.840 | 0.478-1.479 | 0.546 |
Edmondson-Steiner Grade. CT: Computed tomography; PET: Positronemission tomography; AFP: α-fetoprotein.
Statistical analysis
Survival rates were estimated using Kaplan-Meier method, and survival curves were compared with log-rank test. Multivariable Cox proportional hazard regressions were fitted to identify factors that affected post-transplant survival. T-test and χ2 test analyses were also used in comparing the differences between groups for continuous and categorical variables, respectively. Cohen’s Kappa was used to assess classification consistency of each criteria. The prediction model of DFS using each criteria (the NCCK, Milan, and UCSF) adjusted for significant prognostic factors was developed using multivariable Cox proportional hazard regression. The receiver operating characteristic (ROC) curves and the associated area under the curves (AUC) of these models predicting 1, 3 and 5 years DFS rates were evaluated to compare the discrimination ability of different criteria. Differences in AUCs were tested using Delong’s method[18]. All statistical analyses were performed using SAS software (9.2 version). P-value less than 0.05 was used to evaluate statistical significance.
RESULTS
Clinicopathological characteristics
During the study period, a total of 280 patients underwent LDLT for HCC. Among them, 116 (41.4%) patients did not fulfil the NCCK criteria. The comparisons of clinicopathological characteristics between patients who did and did not fulfill the NCCK criteria are presented in Table 2. C-reactive protein level, tumor SUVmax, total tumor size (> 10 cm), AFP (> 400 ng/mL), positive findings on PET/CT, differentiation (grade III-IV), microvascular invasion, intrahepatic metastasis, and serosal invaion were significantly greater in patients who did not fulfill the NCCK criteria compared with those who did. The mean C-reactive protein levels in two groups were 0.58 mg/dL and 1.37 mg/dL, and tumor SUVmax were 3.08 and 4.13, in patients who did and did not fulfill the NCCK criteria, respectively. On the other hand, patients who did not fulfill the NCCK criteria had significantly lower MELD scores compared to those within the NCCK criteria (12.5 vs 14.4, respectively, P = 0.029). Pre-transplant therapy type, viral hepatitis type, ductal invasion, capsule formation, dysplastic nodules, and cirrhosis were not significantly different between the two groups.
Table 2.
Clinicopathologic characteristics of patients according to National Cancer Center Korea criteria
| Variables | Within NCCK (n = 164) | Beyond NCCK (n = 116) | P value | |
| Sex, n (%) | Male | 138 (84.1) | 97 (83.6) | 1 |
| Female | 26 (15.9) | 19 (16.4) | ||
| Age (yr), mean (SD) | 54.2 (7) | 54.7 (7.7) | 0.561 | |
| MELD score, mean (SD) | 14.4 (7.9) | 12.5 (6.1) | 0.029 | |
| C-reactive protein (mg/dL), mean (SD) | 0.58 (1.11) | 1.37 (2.67) | 0.004 | |
| Tumor maximum SUV, mean (SD) | 3.08 (0.64) | 4.13 (1.79) | < 0.001 | |
| Tumor total size, n (%) | ≤ 10 cm | 164 (100) | 56 (48.3) | < 0.001 |
| > 10 cm | 0 (0) | 60 (51.7) | ||
| AFP, n (%) | ≤ 400 ng/mL | 151 (92.1) | 88 (75.9) | < 0.001 |
| > 400 ng/mL | 13 (7.9) | 28 (24.1) | ||
| PET/CT, n (%) | Negative | 164 (100) | 26 (22.4) | < 0.001 |
| Positive | 0 (0) | 90 (77.6) | ||
| Pretransplant therapy, n (%) | No therapy | 39 (23.8) | 29 (25) | 0.77 |
| Surgery only | 8 (4.9) | 4 (3.4) | ||
| TACE only | 71 (43.3) | 52 (44.8) | ||
| RFA only | 7 (4.3) | 2 (1.7) | ||
| Combination | 39 (23.8) | 29 (25) | ||
| Viral hepatitis, n (%) | HBV | 142 (86.6) | 103 (88.8) | 0.442 |
| HCV | 9 (5.5) | 8 (6.9) | ||
| NBNC | 11 (6.7) | 3 (2.6) | ||
| HBV + HCV | 2 (1.2) | 2 (1.7) | ||
| Differentiation1, n (%) | I-II | 102 (62.2) | 55 (47.4) | 0.02 |
| III-IV | 62 (37.8) | 61 (52.6) | ||
| Microvascular invasion, n (%) | Absent | 127 (77.4) | 47 (40.5) | < 0.001 |
| Present | 37 (22.6) | 69 (59.5) | ||
| Capsule formation, n (%) | No complete | 134 (81.7) | 94 (81) | 1 |
| Complete | 30 (18.3) | 22 (19) | ||
| Ductal invasion, n (%) | Absent | 161 (98.2) | 109 (94) | 0.123 |
| Present | 3 (1.8) | 7 (6) | ||
| Serosal invasion, n (%) | Absent | 146 (89) | 72 (62.1) | < 0.001 |
| Present | 18 (11) | 44 (37.9) | ||
| Intrahepatic metastasis, n (%) | Absent | 129 (78.7) | 55 (47.4) | < 0.001 |
| Present | 35 (21.3) | 61 (52.6) | ||
| Cirrhosis, n (%) | Absent | 10 (6.1) | 11 (9.5) | 0.407 |
| Present | 154 (93.9) | 105 (90.5) | ||
| Dysplastic nodule, n (%) | Absent | 120 (73.2) | 81 (69.8) | 0.633 |
| Present | 44 (26.8) | 35 (30.2) | ||
Edmondson-Steiner Grade. HBV: Hepatitis B virus; HCV: Hepatitis C virus; NBNC: Non-hepatitis B and non-hepatitis C virus; B + C: Hepatitis B and C virus; NCCK: National Cancer Center Korea; TACE: Transarterial chemoembolization; RFA: Radiofrequency ablation; PET/CT: Positron emission tomography/computed tomography; AFP: α-fetoprotein; MELD: Model for End-Stage Liver Disease; SUV: Standardized uptake value.
NCCK criteria: Survival rates and comparison between preoperative imaging and explant pathological reports
OS and DFS according to the NCCK criteria are presented in Figure 1. Patients fulfilling the NCCK criteria according to preoperative imaging findings revealed significantly higher OS and DFS than those who did not fulfill the NCCK criteria (five-year OS: 83.6% vs 59.8%, P < 0.001; five-year DFS: 80.7% vs 45.1%, P < 0.001). In patients who fulfilled the NCCK criteria according to explant pathological reports, five-year OS and DFS were 85.2% and 84.0%, respectively; these values were significantly higher than those among patients who did not fulfill the NCCK criteria (60.2% and 44.7%, respectively, P < 0.001).
Figure 1.

Overall and disease-free survival rates according to the National Cancer Center Korea criteria. A: By preoperative imaging; B: By explant pathology; C: OS and DFS rates for all patients. OS: Overall survival; DFS: Disease-free survival; NCCK: National Cancer Center Korea.
The number of patients who fulfilled the NCCK criteria according to preoperative imaging and explant pathology reports were 178 (63.6%) and 164 (58.6%). According to the Milan criteria, these were 167 (59.6%) and 132 (47.1%) patients (Table 3). The NCCK criteria exhibited 95.0% accuracy of preoperative imaging and explant pathological reports; in contrast, the Milan criteria demonstrated only 78.9% accuracy. Compared with the Milan criteria, the NCCK criteria exhibited almost perfect agreement between preoperative imaging and explant pathological reports (Cohen’s Kappa 0.850 vs 0.583).
Table 3.
Comparison between preoperative imaging and explant pathology by the Milan and National Cancer Center Korea criteria
| Milan criteria | NCCK criteria |
Preoperative imaging |
|||
| Within | Beyond | ||||
| Explant | Within | 120 (42.86) | 12 (4.29) | ||
| Pathology | Beyond | 47 (16.79) | 101 (36.07) | ||
| Explant | Within | 161 (57.50) | 3 (1.07) | ||
| Pathology | Beyond | 17 (6.07) | 99 (35.36) | ||
Cohen’s Kappa = 0.850. NCCK: National Cancer Center Korea.
Comparative survival analysis among the NCCK, Milan, and UCSF criteria
In a survival analysis including all patients, five-year OS and DFS were 75.2% and 67.7% (Figure 1). The patients who fulfilled the Milan criteria according to preoperative imaging and explant pathological reports showed good five-year OS and DFS (83.4% and 82.0% according to preoperative imaging; 85.5% and 84.4% by explant pathological reports, Figure 2). These survival results are very similar to those of patients fulfilling the NCCK criteria, particularly with regard to explant pathological reports. There were 34 (12.14%) patients who did not fulfill the NCCK criteria but fulfilled the Milan criteria according to preoperative imaging findings, and 22 (7.9%) according to explant pathological reports. This group showed a trend toward low five-year OS and DFS according to both preoperative imaging and explant pathological reports, compared with those who fulfilled the NCCK criteria; however, the differences between the two groups were not statistically significant (P = 0.148 in OS and P = 0.212 in DFS according to preoperative imaging findings; P = 0.658 in OS and P = 0.376 in DFS according to explant pathological reports, Figure 3).
Figure 2.
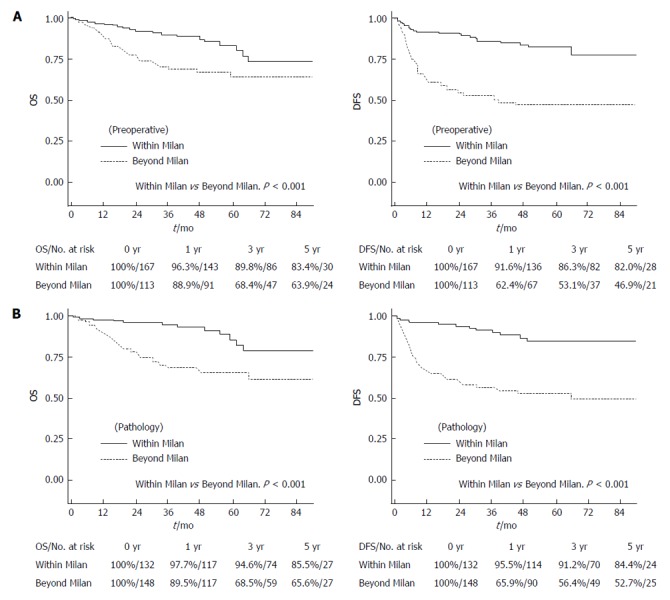
Overall and disease-free survival rates according to the Milan criteria. A: By preoperative imaging; B: By explant pathology. OS: Overall survival; DFS: Disease-free survival.
Figure 3.
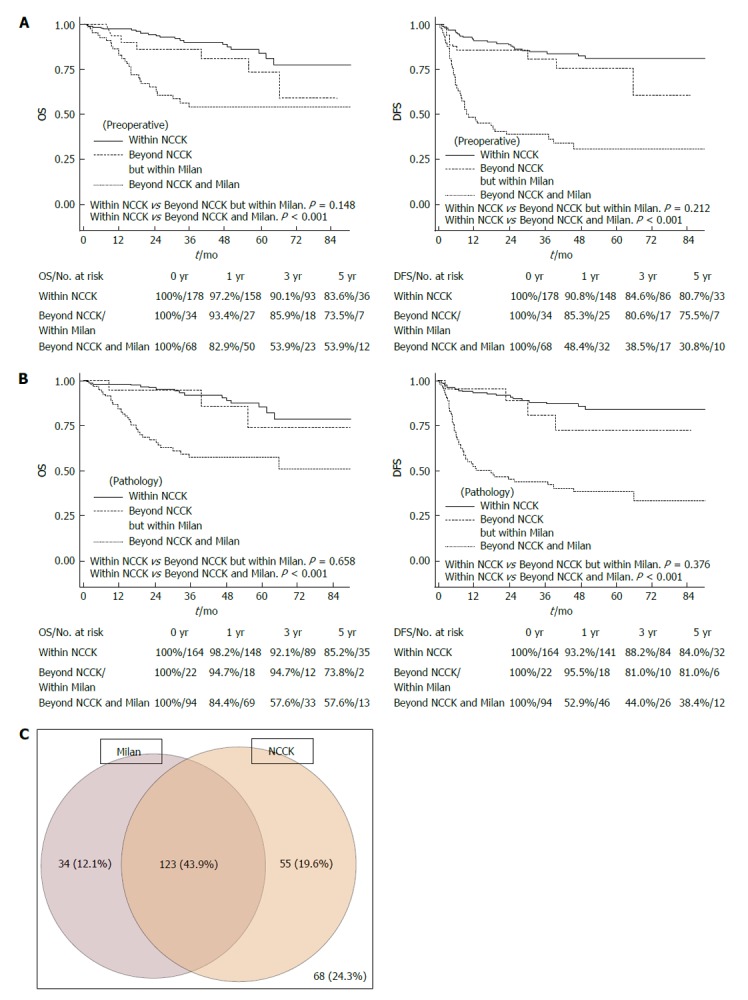
Overall and disease-free survival rates according to three groups (within the National Cancer Center Korea criteria, Beyond the National Cancer Center Korea but within the Milan criteria, Beyond both the National Cancer Center Korea and Milan criteria). A: By preoperative imaging; B: By explant pathology; C: The diagram of the portion of patients in Milan and NCCK criteria by preoperative imaging. OS: Overall survival; DFS: Disease-free survival; NCCK: National Cancer Center Korea.
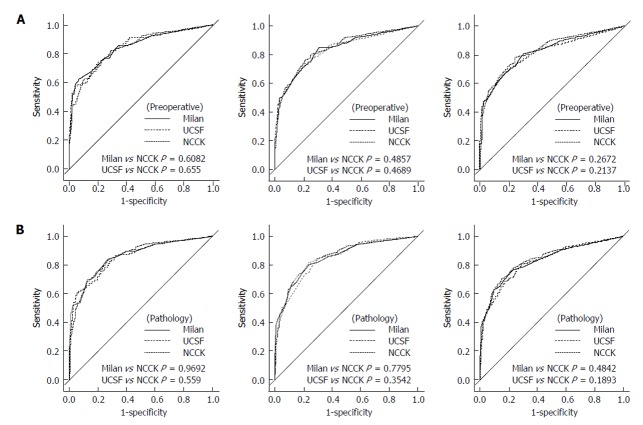
ROC curve and AUC of the Milan, UCSF and NCCK criteria for the prediction of one, three, and five years DFS are presented in Figure 4 and Table 4. The value of AUC by three criteria was similar in both preoperative imaging and explant pathological reports, and there were no significant differences in the area under the ROC curve at one, three, and five years by three groups (five-year DFS, Delong’s P = 0.267 for Milan vs NCCK, P = 0.213 for UCSF vs NCCK in preoperative imaging; P = 0.484 for Milan vs NCCK, P = 0.189 for UCSF vs NCCK in explant pathological reports).
Figure 4.
Receiver operating characteristic curves of three criteria (the National Cancer Center Korea, Milan and University of California, San Francisco) at 1, 3, and 5 years. A: By preoperative imaging; B: By explant pathology. UCSF: University of California, San Francisco; NCCK: National Cancer Center Korea.
Table 4.
Area under the curves and 95%CI for the Milan, University of California, San Francisco, and National Cancer Center Korea criteria for the prediction of 1, 3, and 5 years disease-free survival
| Diagnostic approach | Criteria |
AUC (95%CI) |
||
| 1 yr | 3 yr | 5 yr | ||
| Preoperative | Milan1 | 0.814 | 0.804 | 0.799 |
| imaging | (0.754, 0.873) | (0.750, 0.858) | (0.747, 0.851) | |
| UCSF2 | 0.812 | 0.800 | 0.793 | |
| (0.754, 0.871) | (0.747, 0.853) | (0.741, 0.844) | ||
| NCCK3 | 0.810 | 0.806 | 0.802 | |
| (0.753, 0.867) | (0.755, 0.857) | (0.753, 0.852) | ||
| Explant pathology | Milan4 | 0.824 | 0.815 | 0.807 |
| (0.767, 0.880) | (0.764, 0.866) | (0.757, 0.856) | ||
| UCSF5 | 0.819 | 0.811 | 0.803 | |
| (0.761, 0.877) | (0.759, 0.863) | (0.752, 0.853) | ||
| NCCK6 | 0.823 | 0.817 | 0.810 | |
| (0.769, 0.878) | (0.767, 0.866) | (0.762, 0.857) | ||
Adjusted by PET, X, Y and Z;
By PET, X and Y;
By maximum tumor size, X, Y, and Z;
By PET, total tumor size, X and Y;
By PET, X, Y, and Z;
By total tumor size, X, Y, and Z. X: Microvascular invasion; Y: Major vessel invasion; Z: Intrahepatic metastasis; AUC: Area under the curves; UCSF: University of California, San Francisco; PET: Positron emission tomography; NCCK: National Cancer Center Korea; 95%CI and P value were calculated by Cox PH regression analyses adjusted by the following covariates for each criteria.
DISCUSSION
In the present study, the NCCK criteria were associated with favorable survival outcomes and expanded the selection pool for LDLT among patients with HCC. Over the past 10 years, the Milan criteria have been regarded as a well-established tool for assessing the prognosis of HCC for LT. However, limited selection and inaccurate assessment using preoperative imaging modalities, such as CT, have been constantly recognized as a limitation of the criteria. Tumor biological characteristics, such as microvascular invasion and differentiation, are strong predictive factors for HCC recurrence. 18F-FDG PET/CT findings are a useful marker to predict these factors before LT, as well as to detect extrahepatic metastases. Furthermore, total tumor size itself can be simple and relatively accurate measure rather than using both tumor number and size which are used in the Milan and UCSF criteria. The proposed NCCK criteria, therefore, presented with better correlation with preoperative imaging and explant pathological reports than the Milan criteria.
There were several expanded criteria for patients with HCC beyond the Milan criteria. The main factors that were present in these criteria were tumor size and number. The UCSF, Tokyo, and “up-to-seven” criteria are based on tumor morphological characteristics using preoperative imaging or explant pathological reports[4,8,19]. However, recent studies reported the expanded criteria using markers of tumor aggressiveness as well as tumor morphological characteristics. These included responses to TACE, the degree of differentiation, the gene-expression profile, the presence of microvascular invasion, and the levels of tumor markers, including AFP or DCP[11,20-24]. In particular, it is well known that microvascular invasion and the degree of differentiation are associated with decreased survival and an increased risk of recurrence following LT. However, these pathological examination results are not routinely available before LT because fine-needle biopsy before surgery has not shown significant correlations with explant pathological reports[25]. Some promising attempts to identify microvascular invasion before LT through 18F-FDG PET or PET/CT have been reported[13,14,26]. Moreover, positive findings on PET/CT in patients with HCC predicted the prognosis and tumor recurrence after LT[13-15]. In the present study, the patients beyond the NCCK criteria, including positive findings on PET/CT, showed more microvascular invasion (59.5% vs 22.6%, P < 0.001) and poor differentiation (52.6% vs 37.8%, P = 0.02). One concern regarding the use of PET/CT in patients with HCC is that the sensitivity is low for the primary detection of HCC compared with many other cancers, because glucose metabolism is high in liver tissue[27,28]. On the other hand, PET/CT has been shown to differentiate between well-differentiated and poorly-differentiated HCC, and is useful in the detection of extrahepatic metastases and recurrence of HCC after transplantation[29].
The concept of the NCCK criteria began from the observation that good survival rates without recurrence could occur in patients who did not fulfill the Milan criteria. In our data, patients beyond the Milan criteria who also had negative findings on PET/CT showed significantly better survival rates than those who had positive findings on PET/CT (five-year OS, 74.6% vs 51.4%, P < 0.001; five-year DFS, 73.3% vs 37.5%, P < 0.001). When another significant factor for survival in multivariable analysis (total tumor size < 10 cm) was considered, patients who did not fulfill the Milan criteria with negative findings on PET/CT and total tumor size < 10 cm showed similar OS and DFS compared with those who met the Milan criteria (OS: mean 90.7 mo vs 83.8 mo, P = 0.235; DFS: mean 94.4 mo vs 84.4 mo, P = 0.076). Furthermore, positive findings on PET/CT and total tumor size were significant prognostic factors of OS and DFS for all patients (Table 1). Therefore, we applied the NCCK criteria to all patients and analyzed their usefulness and associated survival rates as new expanded criteria that could be used instead of the traditional Milan criteria.
Numerous expanded criteria based on tumor number and size have been reported, but are not used widely due to limited clinical usefulness. The major reason for this is that the risk of underestimating tumor status is considerable regardless the recent developments of new technologies in radiological assessment of liver tumors[30]. Freeman et al[31] studied the results from the United Network for Organ Sharing database on 789 LT recipients to analyze the accuracy of imaging findings compared with the explant pathological reports. In that report, radiological imaging underestimated tumor staging in 26.6% of cases, and the risk of overestimation was almost 30%. The overall preoperative accuracy was approximately 50%, regardless of the radiological technique used. In our data, among 167 patients who fulfilled the Milan criteria according to preoperative imaging modalities, 47 patients (28.1%) were found as not fulfilling the Milan criteria in explant pathological reports. Therefore, some authors proposed that total tumor volume or size was more likely to result in accurate staging before LT[32-34]. We also used the total tumor size (cutoff 10 cm), which was a significant prognostic factor in multivariable analysis for the NCCK criteria. In our study, among a total of 243 patients with preoperative total tumor size < 10 cm measured with imaging modalities, only 27 patients (11.1%) were confirmed to have a total tumor size of > 10 cm according to pathological reports. Compared with the Milan criteria, the percentage of underestimation in the NCCK criteria using total tumor size (cutoff 10 cm) was lower (9.6%), and Cohen’s Kappa was high (0.850), explaining the near-perfect agreement between preoperative imaging and explant pathological reports (Table 3).
In particular, the survival rates of patients who fulfilled the NCCK criteria were quite good and showed similar outcomes compared with the Milan and UCSF criteria (five-year DFS; 80.7% according to preoperative imaging findings, 84.0% in explant pathological reports, Figure 2). Furthermore, the number of patients who fulfilled the NCCK criteria was higher than the Milan criteria [preoperative imaging findings, 178 (63.6%) vs 164 (58.6%) patients; explant pathological reports, 167 (59.6%) vs 132 (47.1%) patients]. The patients who did not fulfill the NCCK, but fulfilled the Milan criteria did not show statistically significant differences compared with those who fulfilled the NCCK criteria; however, a trend toward low five-year OS and DFS according to both preoperative imaging and explant pathological reports was observed (Figure 3). This result was likely because of the fact that the Milan criteria are too restrictive and limited. There was no significant difference observed when the values of AUC and ROC curves for predicting DFS at one, three, and five years were compared among the three criteria (NCCK, Milan, and UCSF) (Figure 4 and Table 4).
There are some limitations to the present study. First, we analyzed LDLT patients without including deceased donor LT patients; therefore, comparison with other studies that included deceased donor LT patients was not possible. However, we included a considerable proportion of patients who were beyond the Milan criteria; thus, the dilution effect on the analysis was less than that in other studies. Second, the present study was retrospective in nature, and selection bias could have influenced the survival analysis. However, we enrolled all consecutive cases and performed routine PET/CT before LDLT in patients with HCC. Therefore, exclusions during the study period were rare.
In conclusion, our data show that the NCCK criteria, utilizing total tumor size and PET/CT findings, successfully expanded the recipient pool and demonstrated better ability of tumor assessment before LT and similar survival rates compared with the well-known criteria, such as the Milan and UCSF. These criteria represent a new approach to selection for LT that incorporates both tumor biological and morphological characteristics. Therefore, the NCCK criteria are simple and useful expanded criteria for LDLT in HCC, showing excellent agreement between preoperative imaging and explant pathological reports and favorable survival outcomes.
COMMENTS
Background
Several expanded criteria based on morphological features have been proposed to identify appropriate candidates for liver transplantation (LT). However, the definitions are still complex, and the benefit of expanding the pool remains controversial. In this study, the authors evaluated the new criteria using positron-emission tomography/computed tomography (PET/CT) and total tumor size, called as National Cancer Center Korea criteria.
Research frontiers
The expanding criteria for living donor liver transplantation (LDLT) for hepatocellular carcinoma (HCC) is issued recently. The results of this study contribute to clarifying exact criteria using PET/CT and tumor morphologic characteristics.
Innovations and breakthroughs
In this study, they used the PET/CT for all patients underwent LDLT in their institute before transplantation. These results are so unique and included relatively large number of patients. PET/CT is very useful tool for selecting recipients with HCC in LDLT.
Applications
This study suggested that PET/CT is useful for selecting recipient and total tumor size is simple for marker in preoperative imaging tests. If a patient is diagnosed with HCC and waiting the LDLT, PET/CT can be chosen for diagnostic metastasis and prediction of prognosis.
Peer-review
The author this paper evaluated the usefulness of PET/CT and total tumor size for predicting the prognosis after LDLT for HCC, and showed the expanded criteria using these tools. Further trials using these criteria in large population of LDLT will be valuable.
Footnotes
Institutional review board statement: This study was reviewed and approved by the National Cancer Center Institutional Review Board.
Informed consent statement: This is the retrospective study and we analyzed data using only medical records. Therefore, waiver of informed consent for this study subjects might be justifiable. In our institute IRB, waiver of informed consent in this study was approved.
Conflict-of-interest statement: The authors declare no potential conflicts of interest and funding resources.
Data sharing statement: Technical appendix, statistical code, and dataset available from the first author at 1sd@ncc.re.kr. Participent's consent was not obtained but the presented data are anonymized and risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 23, 2015
First decision: January 15, 2016
Article in press: March 9, 2016
P- Reviewer: Chiu KW, Goral V, Kabir A, Mihaila RG, Mizuguchi T S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
References
- 1.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 2.Chen CL, Fan ST, Lee SG, Makuuchi M, Tanaka K. Living-donor liver transplantation: 12 years of experience in Asia. Transplantation. 2003;75:S6–11. doi: 10.1097/01.TP.0000046533.93621.C7. [DOI] [PubMed] [Google Scholar]
- 3.Lee SG. Asian contribution to living donor liver transplantation. J Gastroenterol Hepatol. 2006;21:572–574. doi: 10.1111/j.1440-1746.2006.04283.x. [DOI] [PubMed] [Google Scholar]
- 4.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 5.Herrero JI, Sangro B, Quiroga J, Pardo F, Herraiz M, Cienfuegos JA, Prieto J. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl. 2001;7:631–636. doi: 10.1053/jlts.2001.25458. [DOI] [PubMed] [Google Scholar]
- 6.Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, Miller CM, Schwartz ME. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533–539. doi: 10.1097/00000658-200204000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onaca N, Davis GL, Goldstein RM, Jennings LW, Klintmalm GB. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2007;13:391–399. doi: 10.1002/lt.21095. [DOI] [PubMed] [Google Scholar]
- 8.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Ahn C, Ha T, Moon D, Choi K, Song G, Chung D, Park G, Yu Y, Choi N, et al. Liver transplantation for hepatocellular carcinoma: Korean experience. J Hepatobiliary Pancreat Sci. 2010;17:539–547. doi: 10.1007/s00534-009-0167-6. [DOI] [PubMed] [Google Scholar]
- 10.Taketomi A, Sanefuji K, Soejima Y, Yoshizumi T, Uhciyama H, Ikegami T, Harada N, Yamashita Y, Sugimachi K, Kayashima H, et al. Impact of des-gamma-carboxy prothrombin and tumor size on the recurrence of hepatocellular carcinoma after living donor liver transplantation. Transplantation. 2009;87:531–537. doi: 10.1097/TP.0b013e3181943bee. [DOI] [PubMed] [Google Scholar]
- 11.Takada Y, Ito T, Ueda M, Sakamoto S, Haga H, Maetani Y, Ogawa K, Ogura Y, Oike F, Egawa H, et al. Living donor liver transplantation for patients with HCC exceeding the Milan criteria: a proposal of expanded criteria. Dig Dis. 2007;25:299–302. doi: 10.1159/000106908. [DOI] [PubMed] [Google Scholar]
- 12.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 13.Kornberg A, Freesmeyer M, Bärthel E, Jandt K, Katenkamp K, Steenbeck J, Sappler A, Habrecht O, Gottschild D, Settmacher U. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009;9:592–600. doi: 10.1111/j.1600-6143.2008.02516.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee SD, Kim SH, Kim YK, Kim C, Kim SK, Han SS, Park SJ. (18)F-FDG-PET/CT predicts early tumor recurrence in living donor liver transplantation for hepatocellular carcinoma. Transpl Int. 2013;26:50–60. doi: 10.1111/j.1432-2277.2012.01572.x. [DOI] [PubMed] [Google Scholar]
- 15.Kornberg A, Küpper B, Tannapfel A, Büchler P, Krause B, Witt U, Gottschild D, Friess H. Patients with non-[18 F]fludeoxyglucose-avid advanced hepatocellular carcinoma on clinical staging may achieve long-term recurrence-free survival after liver transplantation. Liver Transpl. 2012;18:53–61. doi: 10.1002/lt.22416. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Kim YK, Lee SD, Park SJ. Selection and outcomes of living donors with a remnant volume less than 30% after right hepatectomy. Liver Transpl. 2013;19:872–878. doi: 10.1002/lt.23677. [DOI] [PubMed] [Google Scholar]
- 17.Lee SD, Kim SH, Kim YK, Lee SA, Park SJ. Graft-to-recipient weight ratio lower to 0.7% is safe without portal pressure modulation in right-lobe living donor liver transplantation with favorable conditions. Hepatobiliary Pancreat Dis Int. 2014;13:18–24. doi: 10.1016/s1499-3872(14)60002-3. [DOI] [PubMed] [Google Scholar]
- 18.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 19.Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007;25:310–312. doi: 10.1159/000106910. [DOI] [PubMed] [Google Scholar]
- 20.Otto G, Herber S, Heise M, Lohse AW, Mönch C, Bittinger F, Hoppe-Lotichius M, Schuchmann M, Victor A, Pitton M. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260–1267. doi: 10.1002/lt.20837. [DOI] [PubMed] [Google Scholar]
- 21.Cillo U, Vitale A, Grigoletto F, Gringeri E, D’Amico F, Valmasoni M, Brolese A, Zanus G, Srsen N, Carraro A, et al. Intention-to-treat analysis of liver transplantation in selected, aggressively treated HCC patients exceeding the Milan criteria. Am J Transplant. 2007;7:972–981. doi: 10.1111/j.1600-6143.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz M, Dvorchik I, Roayaie S, Fiel MI, Finkelstein S, Marsh JW, Martignetti JA, Llovet JM. Liver transplantation for hepatocellular carcinoma: extension of indications based on molecular markers. J Hepatol. 2008;49:581–588. doi: 10.1016/j.jhep.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirabe K, Itoh S, Yoshizumi T, Soejima Y, Taketomi A, Aishima S, Maehara Y. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma-with special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol. 2007;95:235–240. doi: 10.1002/jso.20655. [DOI] [PubMed] [Google Scholar]
- 24.Sotiropoulos GC, Malagó M, Bockhorn M, Schmitz KJ, Radtke A, Molmenti EP, Schaffer R, Beckebaum S, Cicinnati VR, Fouzas I, et al. Liver transplantation for hepatocellular carcinoma and cirrhosis in candidates with undetectable or very low alpha-fetoprotein levels: is an expansion of the listing criteria justified? Hepatogastroenterology. 2008;55:1671–1677. [PubMed] [Google Scholar]
- 25.Pawlik TM, Gleisner AL, Anders RA, Assumpcao L, Maley W, Choti MA. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: implications for transplant eligibility. Ann Surg. 2007;245:435–442. doi: 10.1097/01.sla.0000250420.73854.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375–1382. doi: 10.1245/s10434-008-9846-9. [DOI] [PubMed] [Google Scholar]
- 27.Hatano E, Ikai I, Higashi T, Teramukai S, Torizuka T, Saga T, Fujii H, Shimahara Y. Preoperative positron emission tomography with fluorine-18-fluorodeoxyglucose is predictive of prognosis in patients with hepatocellular carcinoma after resection. World J Surg. 2006;30:1736–1741. doi: 10.1007/s00268-005-0791-5. [DOI] [PubMed] [Google Scholar]
- 28.Okazumi S, Isono K, Enomoto K, Kikuchi T, Ozaki M, Yamamoto H, Hayashi H, Asano T, Ryu M. Evaluation of liver tumors using fluorine-18-fluorodeoxyglucose PET: characterization of tumor and assessment of effect of treatment. J Nucl Med. 1992;33:333–339. [PubMed] [Google Scholar]
- 29.Kim YK, Lee KW, Cho SY, Han SS, Kim SH, Kim SK, Park SJ. Usefulness 18F-FDG positron emission tomography/computed tomography for detecting recurrence of hepatocellular carcinoma in posttransplant patients. Liver Transpl. 2010;16:767–772. doi: 10.1002/lt.22069. [DOI] [PubMed] [Google Scholar]
- 30.Sherman M. The radiological diagnosis of hepatocellular carcinoma. Am J Gastroenterol. 2010;105:610–612. doi: 10.1038/ajg.2009.663. [DOI] [PubMed] [Google Scholar]
- 31.Freeman RB, Mithoefer A, Ruthazer R, Nguyen K, Schore A, Harper A, Edwards E. Optimizing staging for hepatocellular carcinoma before liver transplantation: A retrospective analysis of the UNOS/OPTN database. Liver Transpl. 2006;12:1504–1511. doi: 10.1002/lt.20847. [DOI] [PubMed] [Google Scholar]
- 32.Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, Grant DR, Greig PD, Shapiro AM, Kneteman NM. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008;14:1107–1115. doi: 10.1002/lt.21484. [DOI] [PubMed] [Google Scholar]
- 33.Silva M, Moya A, Berenguer M, Sanjuan F, López-Andujar R, Pareja E, Torres-Quevedo R, Aguilera V, Montalva E, De Juan M, et al. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transpl. 2008;14:1449–1460. doi: 10.1002/lt.21576. [DOI] [PubMed] [Google Scholar]
- 34.Guiteau JJ, Cotton RT, Washburn WK, Harper A, O’Mahony CA, Sebastian A, Cheng S, Klintmalm G, Ghobrial M, Halff G, et al. An early regional experience with expansion of Milan Criteria for liver transplant recipients. Am J Transplant. 2010;10:2092–2098. doi: 10.1111/j.1600-6143.2010.03222.x. [DOI] [PubMed] [Google Scholar]