Abstract
The aim of this review is to give a comprehensive and concise overview of coronary embryology and normal coronary anatomy, describe common variants of normal and summarize typical patterns of anomalous coronary artery anatomy. Extensive iconography supports the text, with particular attention to images obtained in vivo using non-invasive imaging. We have divided this article into three groups, according to their frequency in the general population: Normal, normal variant and anomaly. Although congenital coronary artery anomalies are relatively uncommon, they are the second most common cause of sudden cardiac death among young athletes and therefore warrant detailed review. Based on the functional relevance of each abnormality, coronary artery anomalies can be classified as anomalies with obligatory ischemia, without ischemia or with exceptional ischemia. The clinical symptoms may include chest pain, dyspnea, palpitations, syncope, cardiomyopathy, arrhythmia, myocardial infarction and sudden cardiac death. Moreover, it is important to also identify variants and anomalies without clinical relevance in their own right as complications during surgery or angioplasty can occur.
Keywords: Coronary arteries, Anomalies, Variants, Anatomy, Heart
Core tip: Congenital coronary artery anomalies are not common, but it is crucial to identify them as in some cases they can cause severe reduction of blood flow to the myocardium (ischaemia) and lead to chest pain, arrhythmias and sudden cardiac death, and that in themselves can increase the risk of routine procedures. The aim of this review is to give a comprehensive and concise overview of coronary embryology and normal coronary anatomy, describe common variants of normal and summarize typical patterns of anomalous coronary artery anatomy with the aid of non-invasive medical images.
CORONARY ARTERY ANOMALIES: THE NORMAL AND THE ABNORMAL
The aim of this review is to give a comprehensive and concise overview of normal coronary anatomy, describe common variants of normal and summarize typical patterns of anomalous coronary artery anatomy.
Where previously these anomalies could only be described from autopsy, due to huge developments in imaging modalities in recent years, anomalies can now be detected non-invasively.
The use of computed tomography (CT) angiography and cardiac magnetic resonance (CMR) to define these anomalies gives scope for clinicians to perform long term follow-up and the possibility to test anomalies functionally to judge hemodynamic significance.
This review will be heavily supported with imaging taken from invasive coronary angiography, CT and CMR whelps to illustrate the nature of the anomaly and alongside this, where possible, we will describe case reports detailing clinical events of affected patients.
CORONARY ARTERIES EMBRYOLOGY
To better understand coronary artery anomalies (CAAs) it is firstly useful to step back to briefly revise embryological development of the coronary arteries.
There are three basic elements for development of the coronary vasculature: (1) the sinusoids; (2) the in situ vascular endothelial network; and (3) the coronary buds on the aortic sinuses (Figure 1).
Figure 1.
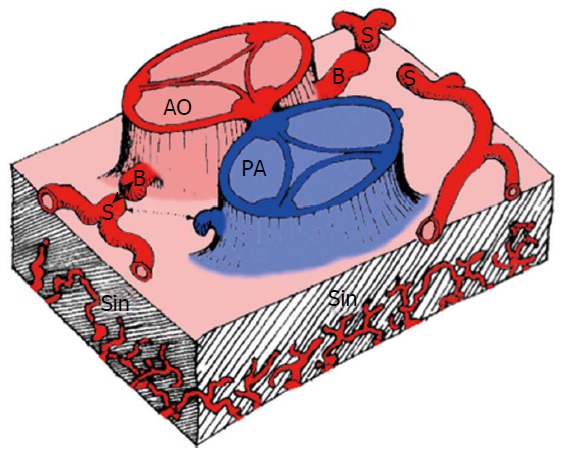
Schematic representation of the components involved in the embryogenesis of coronary arteries[3]. AO: Aorta; PA: Pulmonary artery; B: Coronary buds; S: Stems of right, circumflex and left anterior descending coronary artery; Sin: Sinusoids.
The sinusoids are an extension of the trabeculae of the spongiosa into the developing myocardium, formed in the subepicardial space in the first weeks of fetal development. They are the primitive sites of metabolic exchange between the blood in the cardiac cavities and the cardiac mesenchyma.
At this stage, the bulbus cordis has yet to separate into the aorta and the pulmonary artery (PA) and the two coronary arteries arise from this undivided proximal part: The points of origin of the coronary buds are located so that, with the subsequent division of this portion, they will be situated on the right and left sinuses of Valsalva (RSV and LSV). After the completion of aorto-pulmonary disjunction, the coronary buds and the in situ vascular networks fuse and the coronary circulation will begin to provide blood supply to the myocardium.
It is now understood that coronary arteries develop within the epicardial atrioventricular and interventricular grooves and that their proximal parts grow into the aortic valve sinuses[1]. The left coronary artery arises from the left posterior quadrant of the proximal bulbus cordis, while the right coronary artery arises from the right side.
The major coronary arteries reach their aortic connections subsequently to the aorto-pulmonary rotation, so the location of the aortic root relative to the cardiac base is responsible for the matching between the coronary arteries and their sinuses of origin.
There is a strict relationship between the distribution and size of the major epicardial coronary arteries and the extent of their dependent myocardium. A lack of coronary blood flow during cardiac development would induce hypoplasia of the dependent myocardial mass; conversely a reduction in the myocardial mass would produce relative hypoplasia of its coronary branch (an extreme example of this phenomenon is the case of a single common ventricle in which the left anterior descending artery is absent). Whilst the coronary ostia are formed soon after truncal separation, the distal coronary arteries remain as a loose network until myocardial mass develops during later developmental stages. The sinusoidal network gradually regresses and though trabeculae are present in the adult heart, their connections with the coronary arteries are small and physiologically insignificant and their flow is directed toward the cardiac cavities.
Abnormalities in the development of the coronary buds or the endothelial network are responsible for CAAs. Persistence of the sinusoids results in coronary fistulae[2-7].
DEFINITIONS
Defining what anatomy of the coronary arteries is normal can be challenging. Some normal features can be defined in numerical terms (for example, the number of coronary ostia), while in some other cases a more qualitative description is required. Angelini and coworkers proposed to classify “normal” as every feature with > 1% of frequency in an unselected general population. According to this approach, a CAA can be defined as a coronary pattern or feature that is encountered in less than 1% of the general population. In summary, we can divide the coronary feature in two groups: (1) Normal coronary anatomy, defined as any morphological characteristics seen in > 1% of unselected sample. This group also includes normal anatomical variants, defined as alternative and relatively unusual morphological feature observed in > 1% of the population; and (2) Anomalous coronary anatomy, defined as morphological features found in < 1% of the population[8-10].
NORMAL CORONARY ANATOMY
The coronary arteries arise from the aortic sinuses, converging towards the apex of the heart. Normally, there are three main coronary arteries, the right coronary artery (RCA), left circumflex artery (LCX) and left anterior descending (LAD), with the LCX and LAD arteries arising from a common stem, the left main coronary artery (LMCA). Table 1 shows the main characteristics of each of the three main coronary arteries and further detail on the typical course will be given for each artery in turn in the following section.
Table 1.
Main characteristic of the 3 main coronary arteries
| Dependent territory | Course | Branches | |
| RCA | RV free wall | Right AV sulcus | At least the acute marginal branch |
| LAD | Anterior IV septum | Anterior IV sulcus | Septal penetrating branches |
| LCX | LV free wall | Left AV sulcus | At least 1 obtuse marginal branch |
RCA: Right coronary artery; LAD: Left anterior descending; LCX: Left circumflex artery; RV: Right ventricle; IV: Interventricular; LV: Left ventricle; AV: Atrioventricular.
Figure 2 shows the typical origin of each coronary artery. If the aorta is uni or bicuspid rather than possessing the usual three cusps, the coronary ostia usually arise from the same radial position normally observed in subjects with tricuspid aortic valve. Table 2 shows the normal features of the coronary arteries to better understand how to differentiate normal features from anomalies[10].
Figure 2.
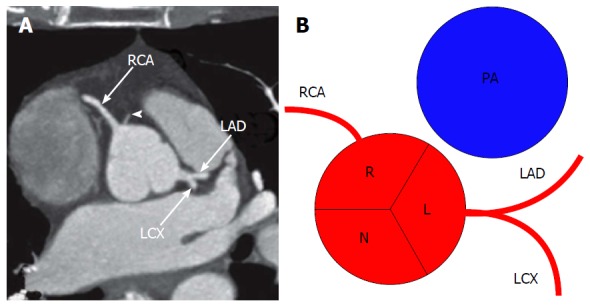
Conventional origin of coronary arteries. A: Origin of coronary arteries on computed tomography angiography maximum intensity projection image, modified from[2]. Arrowhead shows a small conus branch arising from right sinus of Valsalva; B: Schematic representation of the origin of coronary arteries. RCA: Right coronary artery; LAD: Left anterior descending; LCX: Left circumflex artery; R: Right sinus of Valsalva; L: Left sinus of Valsalva; N: Non-coronary sinus; PA: Pulmonary artery.
Table 2.
Normal features of the coronary anatomy in humans, modified from[10]
| Feature | Range |
| Number of ostia | 2 to 4 |
| Location | Right and left anterior sinuses (upper midsection) |
| Proximal orientation | 45° to 90° off the aortic wall |
| Proximal common stem or trunk | Only left (LAD and LCX) |
| Proximal course | Direct, from ostium to destination |
| Mid-course | Extramural (subepicardial) |
| Branches | Adequate for the dependent myocardium |
| Essential territories | RCA (RV free wall) |
| LAD (anteroseptal) | |
| OM (LV free wall) | |
| Termination | Capillary bed |
LAD: Left anterior descending artery; LCX: Left circumflex artery; RCA: Right coronary artery; RV: Right ventricle; OM: Obtuse marginal artery; LV: Left ventricle.
It is important to establish schemes for coronary anatomy to ensure standardization and reproducibility when results of pathology from imaging tests are reported. The coronary arteries are named with regards to their dependent territory rather than their origin. Indeed there are schemes describing myocardial segments typically served by each coronary artery (Figure 3)[2,10,11].
Figure 3.
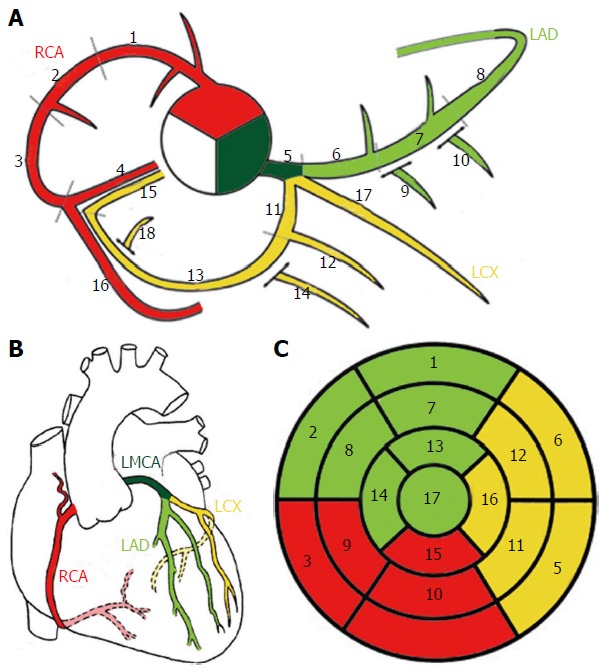
Schematic representation of normal coronary anatomy[11]. A: Coronary segments scheme proposed in SCCT guidelines for the interpretation and reporting of coronary computed tomography and used by Society of Cardiovascular Computer Tomography; B: Schematic representation of the coronary arteries. C: Circumferential polar plot of the 17 myocardial segments[56] with corresponding coronary artery territories. A-1, 2 and 3: Proximal, mid and distal RCA; 4: Right posterior descending artery; 5: Left main coronary artery; 6, 7 and 8: Proximal, mid and distal LAD; 9 and 10: First and second diagonal branches; 11: Proximal LCX; 12: First obtuse marginal branch; 13: Mid and distal LCX; 14: Second marginal branch; 15: Left posterior descending artery; 16: Right posterior-lateral branch; 17: Ramus intermedius; 18: Left posterior-lateral branch. RCA: Right coronary artery; LMCA: Left main coronary artery; LAD: Left anterior descending; LCX: Left circumflex artery.
RCA
The RCA typically arises from the right sinus of Valsalva (RSV) of the ascending aorta (Figure 2), passes anteriorly and to the right between the right auricle and the pulmonary artery and then descends vertically in the right atrioventricular sulcus. When the RCA reaches the acute margin of the heart, it turns to continue posteriorly in the sulcus onto the diaphragmatic surface and base of the heart. Its normal length varies from 12 cm to 14 cm.
The RCA is divided into a proximal segment (from the origin halfway to the acute margin), a middle segment (from this halfway point to the acute margin) and a distal segment (from the acute margin to the base of the heart at the junction of the atrial and ventricular septa).
During its course, several branches arise from the RCA: The conus branch arises as first branch in 50%-60% of the cases and supply the right ventricle (RV) outflow tract; the atrial branch passes in the groove between the right auricle and the ascending aorta; the sinus node artery originates from the right (66% of the cases) or left coronary artery (34%) and passes posteriorly around the superior vena cava to supply the sinus node[12]; the right marginal branch arises as the RCA approaches the acute margin of the heart and continues along this border toward the cardiac apex and it supplies the RV free wall; the atrioventricular (AV) nodal branch is a small branch to the atrioventricular node; the posterior interventricular branch is the final major branch, which lies in the posterior interventricular sulcus.
The RCA can be broadly considered the artery that supplies the right side of the heart. In detail, the RCA supplies the right atrium and right ventricle, the sino-atrial and atrioventricular nodes, the interatrial septum, a portion of the left atrium, the postero-inferior one-third of the interventricular septum and a portion of the posterior part of the left ventricle (LV)[2,13].
LMCA
The LMCA typically originates from the LSV, passes between the main pulmonary artery and the left auricle before entering the coronary sulcus and typically does not have significant branches of its own, but quickly bifurcates into the LAD and the LCX arteries. Its normal length varies from 2 mm to 4 cm[2,3].
LAD
The LAD arises from the bifurcation of LMCA, continues around the left side of the PA and descends in the epicardial fat obliquely toward the apex of the heart in the anterior interventricular sulcus. The LAD can be divided into a proximal segment (from the origin to the origin of the first septal perforator), a middle segment (from the first septal perforator origin halfway to the left ventricular apex) and a distal segment (from this halfway point to the apex itself). Its normal length is from 10 cm to 13 cm.
The LAD gives rise to diagonal branches, one or two large branches that may arise and descend diagonally across the anterior surface of the LV and to the septal perforator branches, which supply the anterior 2/3 of the basal interventricular septum and the entire septum at the mid and apical levels[2].
LCX
The LCX arises from the LMCA bifurcation, courses toward the left, in the coronary sulcus and onto the diaphragmatic cardiac surface and usually ends before reaching the posterior interventricular sulcus. Unlike the RCA and LAD artery, the LCX artery has only two segments, which are the proximal segment and the distal segment, divided by the origin of the first obtuse marginal branch. Its normal length is from 5 cm to 8 cm.
The LCX may give rise to the left marginal artery, a large branch, which continues across the rounded obtuse margin of the heart, the posterolateral branch, in which in some patients it arises from the LCX and the obtuse marginal branch, which supplies a portion of the inferior wall[2,13].
NORMAL VARIANTS
Coronary dominance
The posterior descending artery (PDA) runs in the posterior interventricular groove and supplies the inferior wall and inferior third of the interventricular septum. The artery that supplies the PDA and a posterolateral branch determines the coronary dominance, so there can be three situations: Right-dominance (approximately 70% of the cases) (supply from the RCA), left-dominance (10%) (supply from the LCX), and codominance (20%), the situation in which PDA and posterolateral branches arise from both right and left systems[14].
Inferior wall supply
Beside the variability in the origin of the PDA, there is a spectrum of normal variants regarding the vascular supply to the inferior wall. There could be multiple branches, in which case the PDA is very small and multiple branches from the distal RCA, LCX and obtuse marginal branches supply the inferior wall. When the PDA has a premature takeoff and then courses toward the cardiac apex along the diaphragmatic surface of the RV, the variant is called early takeoff of the PDA. Finally, the LAD may wrap around the cardiac apex and supply a part of the apical inferior wall, known as a “wraparound LAD”[15].
Atrioventricular nodal supply
Small branches from the dominant artery typically supply the atrioventricular node. These branches arise in most of the cases from the distal RCA, as right dominance is the most common[14].
Sinoatrial nodal supply
Typically a single sinoatrial nodal branch supplies the sinoatrial node and it can arise from the proximal RCA (60% of the cases), from the proximal LCX or from the distal RCA or LCX artery (unusual)[16].
Ramus intermedius
In this normal variant, the LMCA can trifurcate into a LAD, a LCX and a ramus intermedius. The ramus intermedius typically supplies the lateral and inferior walls, acting as a diagonal or obtuse marginal branch, while the arteries that usually supply this territory are small or absent[17].
Separate origin of the conus branch
In some patients the conus branch may arise directly from the aorta instead of arising from the proximal RCA[18].
Myocardial bridging
Normally the coronary arteries are surrounded by the epicardial fat, but in some cases a coronary artery may travel intramyocardially for a variable length. A muscular or myocardial bridge is defined as an atypical course of a coronary artery in which it dips intramyocardially with resulting compression of the vessel during systole. The prevalence of this anomaly has a wide range from 0.15%-25% angiographically, to between 5% and 86% at autopsy. However, many reports from angiography may underestimate the prevalence of this anomaly, as recent studies with computed tomography (CT)[19] have shown that myocardial bridging can be found in up to 25% of patients. In most of the cases the coronary involved was the proximal LAD, but it can occur in any segment. The myocardial bridging usually has a length that ranges from 10 mm to 50 mm. Most patients with myocardial bridging are asymptomatic and no abnormalities are observed during functional stress testing. An 11-year follow-up study[2] underlined the benign prognosis of this normal variant as none of the patients developed myocardial ischaemia and it was demonstrated that the prognosis is independent of the severity of systolic narrowing of the lumen. However, this has since been debated and it has been shown that in rare instances myocardial bridging may be related to atypical angina, particularly if the segment is long or deep. Indeed, a study from Morales et al[20] underlined that there is clinical evidence supporting the notion that, under certain conditions, systolic compression of an intramural LAD may impair coronary flow and precipitate myocardial ischaemia. The frequency of asymptomatic patients may seem surprising given that angiographic data show up to 50% systolic narrowing of the bridging artery, but it can be explained by the fact that most of the coronary blood flow occurs during diastole. Several studies showed that delayed diastolic reopening of the artery compressed during systole was at the basis for impaired coronary flow during diastole, decreased blood flow to the endocardium and a significant decrease in the vasodilator reserve and myocardial oxygen consumption. Ferreira et al[21] studied 50 cases of myocardial bridges and underlined the important distinction between superficial and deep muscle bridges in producing ischaemia. It is interesting to notice that frequently the coronary affected is spared of atherosclerotic disease.
Acute take-off of LCX
Angelini et al[22] defined this coronary anomaly in patients with an angle ≤ 45º between LMCA and LCX, in the left anterior oblique/caudal and/or right anterior oblique/caudal angiography X-ray projections. The origin and length of LMCA may be a relevant factor in this variant, as the acute take-off of the LCX may be caused by a usual distal point of origin so the LCX assumes a backward proximal course in order to join the normal distal LCX. This anatomic variant has an incidence around 2% and may be relevant in relation to the technical difficulties that can complicate angiographic procedures on the LCX[22].
High take-off
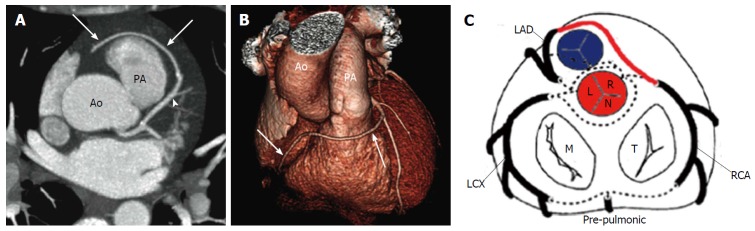
High take-off of a coronary ostium is defined as position of the ostium 5 mm or more above the aortic sinotubular junction. When the coronary ostium is quite far from the sinotubular junction there may be a funnel shape and the first part of the anomalous coronary artery courses vertically and intramurally within the aortic tunica media to reach its normal site. The coronary most frequently involved is the RCA and is reported with increased frequency in patients with a bicuspid aortic valve. This anomaly is benign most of the time, but may complicate coronary catheterization and its identification is important before an aortotomy. Cross clamping of the aorta below a high-origin coronary artery may result in unsuccessful induction of cardioplegia. Moreover, the higher the position of a coronary ostium, the higher the risk of coronary hypoperfusion as the sinuses of Valsalva facilitate maximal coronary diastolic perfusion[13,14,23,24] (Figure 4).
Figure 4.
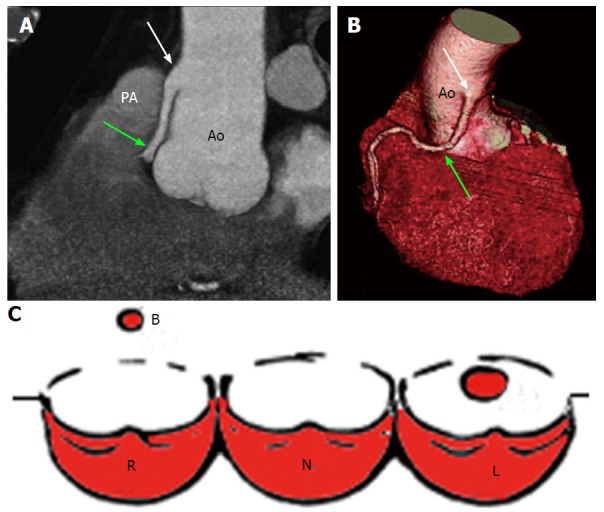
High take-off of right coronary artery in a 67-year-old man with equivocal exercise stress test[57]. Maximum intensity projection coronary CT angiogram (A) and three-dimensional volume-rendered CT reconstruction (B). Aortic step (white arrow) is seen to be the origin of RCA; RCA runs closely parallel to the proximal aorta (Ao) behind the pulmonary artery (PA). A: Regular position of left ostium; B: High position of right ostium; C: Schematic representation of high take-off. R: Right sinus of Valsalva; L: Left sinus of Valsalva; N: Non-coronary sinus; RCA: Right coronary artery; CT: Computed tomography.
Shepherd’s crook RCA
In this variant, the RCA has a normal origin, but takes a tortuous and high course, usually immediately after it originates from the aorta. The prevalence of this variant is estimated of approximately 5%. The shepherd’s crook is not clinically significant, but its presence may complicate percutaneous interventions in the RCA[14] (Figure 5).
Figure 5.
Shepherd’s crook right coronary artery. A: From[14]; B: Schematic representation.
CORONARY ARTERY ANOMALIES
According to the previous definition, CAAs are defined as a coronary pattern that is very rare among the general population. About 26% of coronary anomalies involve some kind of aortic root abnormality (such as bicuspid aortic valve), at least asymmetry of the aortic sinuses[2,8-10]. However, the incidence of CAAs varies widely in the literature. This is likely a reflection of referral bias and the variability in definitions of “anomalous” and “normal variant”. According to the literature, CAAs affect around 1% of the general population, ranging from 0.3%-5.6% in studies on patients undergoing coronary angiography, and in approximately 1% of routine autopsy. Neither of these figures may not be truly representative, the incidence of coronary anomalies in necropsy patients may be biased by cause of death and angiography is usually performed because of the suspicion of ischaemia.
The commonest CAA is a separate origin of the LAD and LCX, with an incidence of 0.41%, followed by LCX arising from the RCA, with an incidence of 0.37%[2,9,24-26].
Although congenital coronary artery anomalies are relatively uncommon, they are the second most common cause of sudden cardiac death (SCD) among young athletes. The risk of SCD in middle age or elderly individual with an incidentally discovered coronary anomaly is unclear, but is probably negligible. The anomaly most frequently associated with SCD is the anomalous origin of a coronary artery (AOCA), in particular with a course between the aorta and the PA.
Based on the functional relevance of each abnormality, CAAs can be classified as: (1) anomalies with obligatory ischaemia, as seen in anomalous origin of the LMCA from the pulmonary artery, or in coronary ostial atresia or severe stenosis; (2) anomalies without ischaemia; this group comprises the majority of CAAs and are not associated with clinical events; and (3) anomalies with exceptional ischemia: This is a group of CAAs that only occasionally cause critically severe clinical events, but are otherwise compatible with leading a normal life, including athletic training.
The clinical symptoms of a patient with a coronary anomaly will vary according to which group the anomaly falls into. In addition to ischemia, other clinical consequences can occur in correlation with CAAs. Fistulas can cause volume overload. Aortic-root distortion can be found in patients with very large coronary fistulas or aneurysms. Complications during aortic valve surgery or coronary angioplasty are described in different CAAs[2,8,9,12,25,27-33].
The risk correlated with a CAA usually depends on the location and course of the AOCA. A coronary artery that arises from the contralateral sinus of Valsalva has five potential paths it may take to its perfusion territory (Figure 6): (1) pre-pulmonic: Anterior to the right ventricular outflow tract. In this case usually there are no hemodynamic consequences, although in a small proportion of cases there may be an association with angina. This anomaly usually involves the LMCA and it is particularly common in tetralogy of Fallot (Figure 7); (2) retro-aortic: Posterior (Figure 8) to the aortic root. It takes a course posteriorly and passes into the space between the posterior sinus of Valsalva (PSV) and the interatrial septum, where normally no vascular structures are found. This variant does not seem haemodynamically significant, but may complicate valve surgery. This anomaly usually involves an artery arising from RCA or right sinus of Valsalva that supplies the distribution of LMCA or LCX artery; (3) inter-arterial: Between (Figure 9) the aorta and pulmonary artery. This course is associated with more severe prognosis and increased risk of SCD for reasons, which remain unclear. One hypothesis is based on the fact that exercise leads to expansion of the aortic root and pulmonary trunk and this may increase the existing angulation of the coronary artery, decreasing the luminal diameter. Another hypothesis is based on the fact that the vessel has an aberrant course within the aortic wall and is often hypoplastic and exposed to a lateral compression over the entire proximal intramural tract (intussusception into the aortic wall). However, in these patients, resting electrocardiograms are usually normal and stress tests are not always positive for inducible ischaemia; (4) trans-septal: This refers to a coronary artery taking a subpulmonic course. The artery traverses anteriorly and inferiorly through the interventricular septum and takes an intramyocardial course, giving off septal branches and finally emerging at its normal epicardial position. The arteries most commonly involved are the LAD or LMCA. Sometimes it can be difficult to differentiate a trans-septal coronary artery from an inter-arterial one: A trans-septal artery has a lower position, is usually surrounded by septal myocardium, should not have an oblong or slit like orifice and a downward dip of the trans-septal artery can be seen (known as the hammock sign) (Table 3) (Figure 10); and (5) retro-cardiac: In this case the path is behind mitral and tricuspid valves, in the posterior AV groove. The clinical relevance of this coronary anomaly may be due to underlying coronary atherosclerosis: It has been proposed that the abnormal origin and course of anomalous coronary arteries could make them more prone to atherosclerosis. In nearly 15% of patients with CAAs, myocardial ischemia can develop in the absence of atherosclerosis. In addition, some potential mechanisms have been proposed to explain ischaemia and SCD: Spasm of the anomalous coronary artery, possibly as a result of endothelial injury or ischemia caused by its long distance; the acute angle of take-off of the anomalous vessel, which may become kinked and occluded during exercise, and the related slit-like orifice[2,8,10,12,14,23,25,30,34-38].
Figure 6.
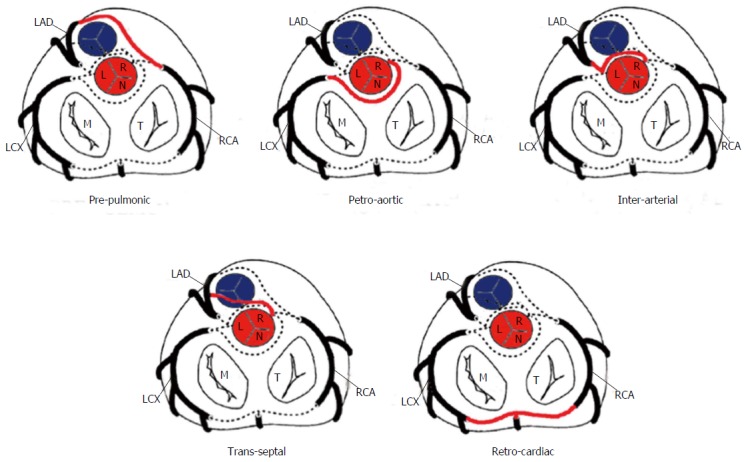
Schematic representation of the possible paths by which the coronary arteries can connect with the opposite coronary cusps[10]. LAD: Left anterior descending; LCX: Left circumflex; RCA: Right coronary artery; M: Mitral valve; T: Tricuspid valve; L: Left sinus of Valsalva; R: Right sinus of Valsalva; N: Non-coronary sinus.
Figure 7.
Prepulmonic coronary artery in a 41-year-old man with chest pain[14]. CT image (A) and volume-rendered image (B) show a prepulmonic RCA (arrows) that courses in front of and just caudal to the pulmonary artery (PA) after arising from the LAD artery (arrowhead in A). On the right: Schematic representation of prepulmonic course. Ao: Aorta; LAD: Left anterior descending; LCX: Left circumflex; RCA: Right coronary artery; M: Mitral valve; T: Tricuspid valve; L: Left sinus of Valsalva; R: Right sinus of Valsalva; N: Non-coronary sinus.
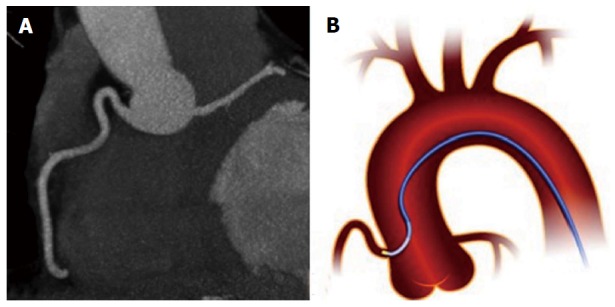
Figure 8.
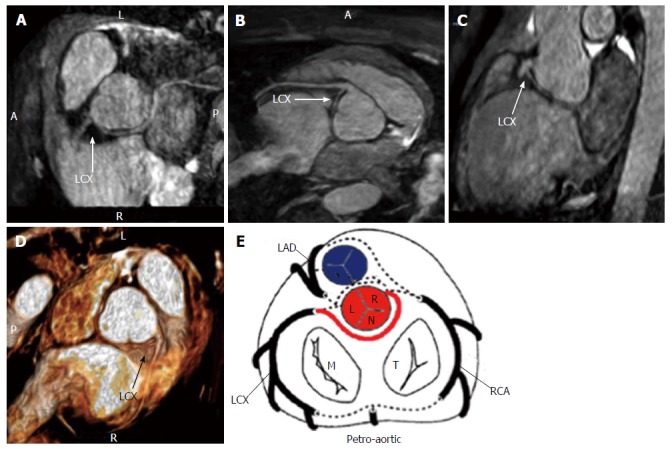
Anomalous origin of left circumflex from right coronary artery with retro-aortic course seen with three-dimensional cardiac magnetic resonance maximum intensity projection, multiplanar reformation and virtual reality imaging[19] (A-E). E: Schematic representation of retro-aortic course. LAD: Left anterior descending; LCX: Left circumflex; RCA: Right coronary artery; M: Mitral valve; T: Tricuspid valve; L: Left sinus of Valsalva; R: Right sinus of Valsalva; N: Non-coronary sinus.
Figure 9.
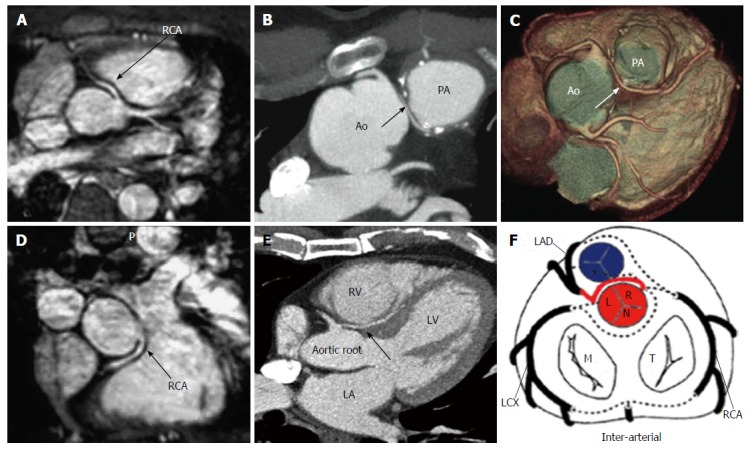
Example of interarterial course. A and D: Anomalous origin of the right coronary artery from the left sinus of Valsalva with an interarterial course between the aorta and the pulmonary artery (arrow)[19]; B and C: Interarterial coronary artery in a 56-year-old man with chest pain. CT image (B) and volume-rendered image (C) show an interarterial LAD artery (arrow) arising from the RCA and coursing between the aorta (Ao) and pulmonary artery (PA). Note the short-segment narrowing of the proximal LAD artery, a finding likely related to the patient’s anomalous anatomy[14,15]; E: CT image, venous phase, three chamber view shows interarterial course of the left coronary artery between the aortic root and the right ventricular outflow tract (black arrow). RV: Right ventricle; LA: Left atrium; LV: Left ventricle; F: Schematic representation of interarterial course; LAD: Left anterior descending; LCX: Left circumflex; RCA: Right coronary artery; M: Mitral valve; T: Tricuspid valve; L: Left sinus of Valsalva; R: Right sinus of Valsalva; N: Non-coronary sinus.
Table 3.
Comparison of trans-septal and interarterial coronary arteries[14]
| Trans-septal | Interarterial | |
| Artery surrounding | Septal myocardium | Epicardial fat |
| Course downward | Yes (hammock sign) | No |
| Oblong orifice | No | Possible |
Figure 10.
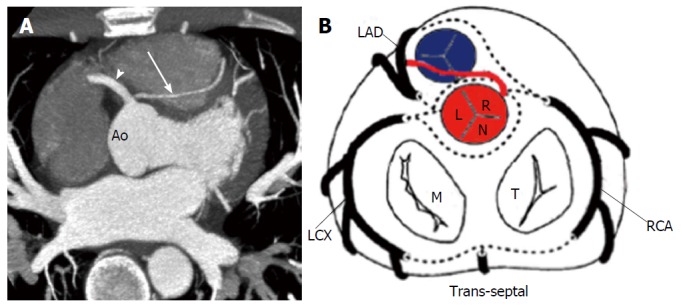
Trans-septal coronary artery in a 39-year-old man who underwent cardiac computed tomography after a stress test with equivocal results. A: Axial computed tomography image shows a trans-septal LAD artery (arrow) that arises from the RCA (arrowhead)[14]; B: Schematic representation of trans-septal course. Ao: Aorta; RCA: Right coronary artery; LAD: Left anterior descending; LCX: Left circumflex; RCA: Right coronary artery; M: Mitral valve; T: Tricuspid valve; L: Left sinus of Valsalva; R: Right sinus of Valsalva; N: Non-coronary sinus.
CLASSIFICATION
Other authors prefer to categorize CAAs on the basis of their anatomical appearance regardless of the functional importance of the lesion. Many classification schemes have been developed, although no single classification scheme is widely employed[10,14,35,39]. In this review we suggest a scheme based on the anatomical features (Table 4).
Table 4.
Classification of coronary artery anomalies based on anatomical features
| Coronary artery anomalies | |||
| Of ostium | Ostial atresia | ||
| valve-like ridge | |||
| Of origin | From PA | RCA from PA | |
| LMCA from PA | |||
| LAD from PA | |||
| All from PA | |||
| Accessory cor. from PA | |||
| From aorta | SCA | SCA from LSV | |
| SCA from RSV | |||
| RCA | RCA ectopic from RSV | ||
| RCA from LSV | |||
| RCA from PSV | |||
| LMCA | LMCA from PSV | ||
| LAD | LAD from RCA | ||
| LAD from RSV | |||
| LCX | LCX from RSV | ||
| LCX from RCA | |||
| Of anatomy | Duplication | Duplication of RCA | |
| Duplication of LAD | |||
| Duplication of LCX | |||
| Congenital absence | Congenital absence of LMCA | ||
| Atresia LMCA | |||
| Congenital absence of LCX | |||
| Hypoplasia | Hypoplasia of RCA and LCX | ||
| Of termination | Fistulae | ||
| Systemic termination |
PA: Pulmonary artery; RCA: Right coronary artery; LMCA: Left main coronary artery; LAD: Left anterior descending; SCA: Single coronary artery; LSV: Left sinus of Valsalva; RSV: Right sinus of valsalva; PSV: Posterior sinus of Valsalva; LCX: Left circumflex artery.
ANOMALIES OF OSTIUM
Atresia of coronary ostium
It can happen that coronary arteries become obstructed or totally occluded in fetal or neonatal life due to a coronary ostial hypoplasia or atresia (Figure 11). In this case, the distal coronary bed is supplied by collateral circulation from the opposite side. Congenital atresia must be differentiated from single coronary artery, a situation that involves the ectopic origin of a coronary artery from the opposite coronary system (in the latter situation all of the normal coronary arteries are present). Coronary ostial atresia is usually associated with ischaemic manifestations. Karadag et al[16] reported a case of a 34-year-old man with a 10-year history of typical angina. In this case, the only abnormal test was the myocardial perfusion scintigraphy, showing a perfusion defect of the inferior walls. Coronary angiogram showed the absence of RCA ostium and the RCA filling through collateral vessels from left system. CT showed also hypoplasia of the proximal segment of the RCA, a characteristic feature of congenital ostial atresia[3,16,40].
Figure 11.
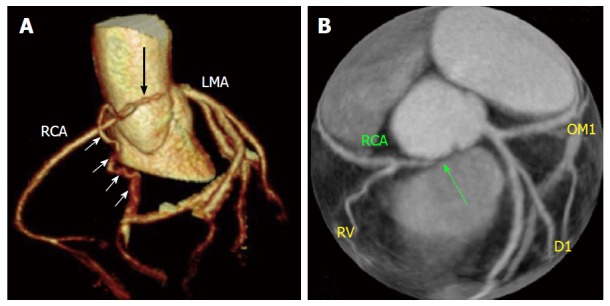
Cases of congenital atresia[16] (A and B). Computed tomography revealed congenital atresia of RCA ostium, a hypoplastic proximal RCA that ends blindly (black arrow) and collaterals from left coronary system (white arrow). RCA: Right coronary artery; LMA: Left main coronary artery; RCA: Right coronary artery; D1: First diagonal branch, OM1: Obtuse marginal branch.
Valve-like ridge
Frescura et al[23] observed cases with a regular coronary artery origin, but a stenosis of the coronary ostium attributable to a valve-like ridge as a consequence of a fold in the elastic tunica media of the aorta. Ostial valve-ridge is created by aortic and coronary artery walls as they meet and form the ostial orifice. The ostial ridge has been recognized only at post-mortem. This anomaly is considered a possible cause of SCD if the surface area of the ridge > 50% of the coronary ostial luminal area. It is hypothesized that an ostial valve-ridge may act as occlusive valve during aortic root dilation, predisposing to myocardial ischemia.
ANOMALIES OF ORIGIN FROM PULMONARY ARTERY
RCA from PA
The first report of this anomaly was in 1885. In this anomaly blood flows from the LCA to the RCA via collaterals, then retrograde to the PA. This anomaly is extremely rare (0.002%), far less common than the origin of the LMCA from PA. Patients with this anomaly are usually asymptomatic and there is no evidence of myocardial ischaemia. However, SCD, syncope and congestive heart failure have been reported[24,41] (Figure 12).
Figure 12.
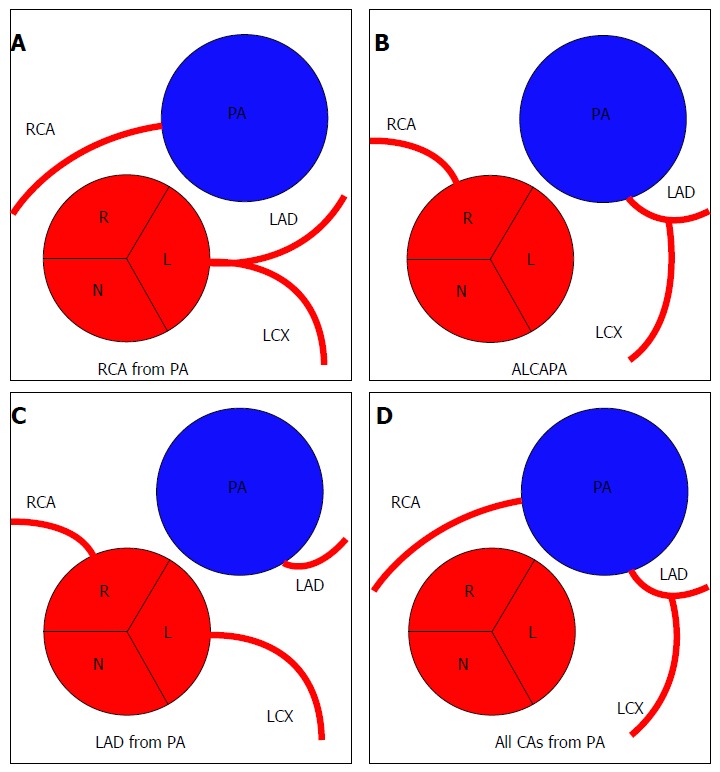
Anomalies of origin from pulmonary artery (A-D). R: Right sinus of Valsalva; L: Left sinus of Valsalva; N: Non-coronary sinus; PA: Pulmonary artery; RCA: Right coronary artery; LAD: Left anterior descending; LCX: Left circumflex; ALCAPA: Anomalous left coronary artery from pulmonary artery; CAs: Coronary arteries.
LMCA from PA (anomalous origin of LCA from PA)
Anomalous origin of LCA from PA (ALCAPA) is also known as Bland-White-Garland syndrome from the eponym of the authors who described it for the first time in 1956. ALCAPA is a very rare congenital CAA (0.008%). In this anomaly the function of the LMCA territory often requires extensive collateral formation from the RCA, which is often dilated. In the first month of life, physiologic pulmonary hypertension tends to preserve anterograde flow within the left coronary artery, and infants are usually asymptomatic. As pulmonary pressure drops, left-to-right shunting from the higher pressure left coronary arterial system to the lower pressure pulmonary arterial system occurs, and patients become symptomatic. Imaging findings may include markedly dilated collateral arteries and coronary veins. In a study by Alexander et al[26] all the patients with ALCAPA had the additional finding of aortic coarctation with a patent ductus arteriosus. Ninety percent of patients with this anomaly die during the first year of life, and very few survive into adulthood. According to some authors, this could be explained by inadequate development of inter-coronary collaterals. The preferred method for treatment is surgical reimplantation of LMCA onto the aorta[2,14,23,24,26,41,42].
LAD from PA
Pulmonary origin of the LAD is a very rare CAA with a frequency of 0.0008%. This anomaly leads to myocardial ischaemia and sudden cardiac death[24,43].
All coronary arteries from PA
In this anomaly all the coronary arteries arise from PA so the entire coronary circulation is supplied by the pulmonary artery. The prognosis of this CAA is poor and these patients usually die during the first month of life. This CAA is also associated with patent ductus arteriosus (failure of the fetal connection from aorta to pulmonary artery to close after birth) and with other major anomalies of the heart or great arteries[26,41].
Accessory coronary artery from PA
In most of the cases the accessory coronary artery involved is a conus artery. This anomaly is of no functional significance[41].
ANOMALIES OF ORIGIN FROM AORTA
Single coronary artery
This anomaly was described for the first time in 1903 and several cases of single coronary artery (SCA) have been reported. It is well recognized that SCA is commonly associated with other congenital cardiovascular anomalies such as transposition of the great vessels, coronary fistulas, bicuspid aortic valve and tetralogy of Fallot[25,41].
SCA arising from LSV: In most of the cases published the RCA originates from the proximal or mid segment of the LAD and usually courses anterior to the PA to reach the right AV groove or in between the great vessels. This is an extremely rare congenital anomaly appearing in approximately 0.024%-0.066% of the general population undergoing coronary angiography[25,34]. This anomaly is less frequently associated with other congenital cardiovascular defects in comparison to the other SCA anomalies.
SCA arising from RSV: In this CAA the entire left system arises from RSV, separately or sharing a common ostium with the RCA. Anomalous origin of LCA from RSV is very rare, with a reported prevalence of 0.02%-0.05% on angiographic studies[12,24]. Although this condition is rare, it is an important associated finding in patients with SCD before age 20, usually after vigorous exertion; with the majority of the patients being asymptomatic before the SCD episode. The prognosis changes with regards to the anatomical subtypes: The anterior variant is usually benign, while the interarterial course is the most potentially serious variant. Venturini et al[35] described a case of an acute myocardial infarction in a 74-year-old woman with a single coronary artery originating from RSV with a single ostium bifurcating into LMCA and a dominant RCA. In this case the coronary arteries were normal, without any stenosis or extrinsic compression. This case is interesting because of the advanced age of the patient and the combination of a benign course of ectopic coronary artery with acute myocardial infarction without coexisting coronary atherosclerotic lesions. Baltaxe et al[43] reported a case of a SCA arising from RSV in a patient with tetralogy of Fallot, where only a tiny muscular branch arose from an orifice in the LSV. Flessas et al[12] reported the case of a patient with stable angina who was found to have an anomalous origin of LCA from the RSV with a common ostium with RCA. LCA followed a cranial and leftward direction, before its bifurcation, and the LMCA was longer than usual (2 cm in length) following an interarterial course between aortic root and conus arteriosus, bifurcating immediately after its exit. In this case the LCA anomaly was associated with an aneurysm of the descending aorta and an anomalous origin of the left common carotid artery from the proximal part of the innominate artery; the coronary arteriogram showed no coronary obstructive lesions[12,24,27,35,43,44] (Figure 13).
Figure 13.
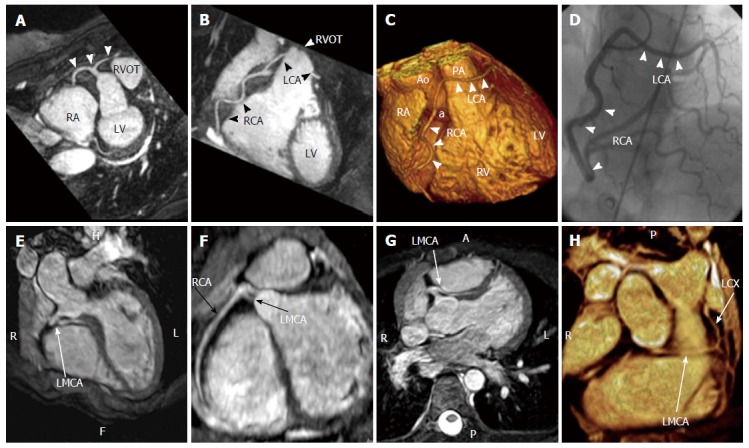
Cases of single coronary artery. A-D: Coronary cardiac magnetic resonance, T2-prepared navigator-gated whole-heart technique shows the anomalous origin of a single coronary artery from the right sinus of Valsalva in a patient complaining of angina. A: Maximum intensity projection (MIP) image demonstrating the anomalous origin of the single coronary artery from the right sinus of Valsalva (white arrows); B: MIP image showing the path of the right coronary artery and of the left coronary artery, crossing in front of the right ventricle outflow tract; C: Volume rendering reconstruction (VRT) of the heart, showing the anomalous origin of the single coronary artery (a) from the aorta and the course of the RCA and the LCA; D: Coronary angiography in right anterior oblique projection, showing the anomalous coronary tree[17,18]; E-H: Case of single coronary artery arising from the right sinus of Valsalva with interarterial course of the left main coronary artery and distal origin of left circumflex (arrows) seen on three-dimensional cardiac magnetic resonance multiplanar reconstruction, MIP and volume rendering images[19]. Ao: Aorta; LCA: Left coronary artery; PA: Pulmonary artery; RVOT: Right ventricle outflow tract; RA: Right atrium; LMCA: Left main coronary artery; LCX: Left circumflex artery; RCA: Right coronary artery; LV: Left ventricle; RV: Right ventricle.
Anomalies of origin of RCA
The RCA can originate anomalously from an ectopic position from RSV, LSV, PSV, but also from the ascending aorta and from the left ventricle.
Ectopic RCA from RSV: This coronary anomaly has a frequency of 1.13% and therefore by some classifications can be considered a variant.
Ectopic RCA from LSV: This anomaly has a wide frequency range from 0.03%-0.92% of patients undergoing cardiac catheterization, with most authors reporting an incidence of about 0.1%. Ectopic RCA from LSV is associated with SCD. Angina pectoris, syncope, myocardial infarction and ventricular tachycardia have been reported in patients with this CAA in the absence of coronary atherosclerosis. Sloan et al[34] presented a case of an inferior myocardial infarction following exercise in a 50-year-old patient with no obstructive disease, but an anomalous RCA originating from LSV and an interarterial course. This case is interesting because the patient was older than usual at the time of presentation and he suffered a myocardial infarction without previous cardiac symptoms[24,34,44].
RCA from PSV: This is an extremely rare anomaly (0.003%) in hearts without other congenital anomalies. The anomaly is usually not associated with symptoms or complications so should be considered benign[24,41].
Anomalies of origin of LMCA
LMCA from PSV: The origin of the LMCA from posterior sinus of Valsalva is usually seen in patients with other anomalies of heart and great vessels.
This situation is extremely rare (0.0008%) and even more rarely associated with sudden death. No clinical manifestations or perfusion abnormalities have been associated with these anomalies. This anomaly should be considered benign[23,24,41].
Anomalies of origin of LAD
LAD from RCA: The origin of LAD is influenced by the development of the pulmonary conus: In tetralogy of Fallot, for example, the hypoplasia of the pulmonary infundibulus seems to be associated with a high probability of abnormal origin of LAD from the RCA. Baitaxe et al[43] reported a case of LAD arising from RCA in 18-year-old man who had undergone repair of a ventricular septal defect two years earlier. This patient had typical exertional angina[3,43] (Figure 14).
Figure 14.
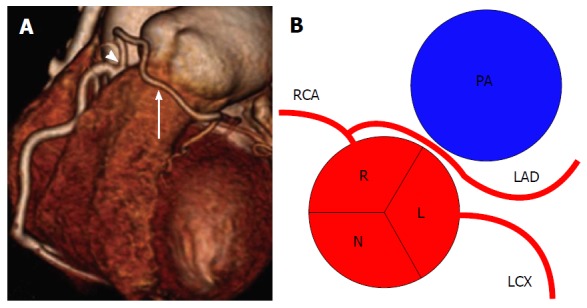
Left anterior desceding from right coronary artery[2]. A: Volume-rendered computed tomography image of LAD (arrow) artery arising from proximal RCA (arrowhead); B: Schematic representation. RCA: Right coronary artery; LCX: Left circumflex; LAD: Left anterior descending; R: Right sinus of Valsalva; L: Left sinus of Valsalva; N: Non-coronary sinus; PA: Pulmonary artery.
LAD from RSV: This anomaly has a frequency of 0.03%[24].
Anomalies of origin of LCX
LCX from RSV: The anomalous origin of the LCX from the proximal RSV was described for the first time in 1933. LCX and RCA can arise from a common ostium or from separate ostia. Usually LCX courses posterior to the aorta and provides branches to the LV lateral wall. This anomaly is relatively common with an estimated frequency varying from 0.32%-0.67%. In the absence of coronary atherosclerosis, the anomaly may be considered benign[24,45].
LCX from RCA: The LCX may arise as a proximal branch of the RCA. The frequency of this coronary anomaly is around 0.37%[23,25,43,46] (Figure 15).
Figure 15.
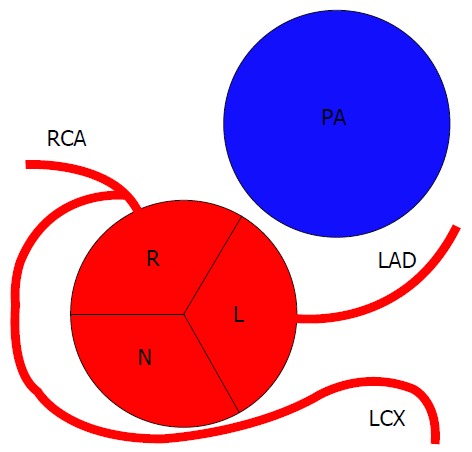
Schematic representation of left circumflex artery arising from right coronary artery. RCA: Right coronary artery; LAD: Left anterior descending; LCX: Left circumflex; R: Right sinus of Valsalva; L: Left sinus of Valsalva; N: Non-coronary sinus; PA: pulmonary artery.
DUPLICATION
Split RCA
A split RCA is defined as an RCA that features a split PDA with the anterior subdivision of the RCA leading to the distal portion of the PDA leading to the anterior free wall of the RV. The other (posterior) bifurcation of the RCA maintains a course in the atrioventricular groove and forms the uppermost portion of the posterior descending branch. The length of each of the two posterior descending branches varies from patient to patient. Split RCA is sometimes called “double RCA”, even if in reality there are not two RCAs, but split portions of the posterior descending branch of the RCA with separate proximal courses. Split RCA has been reported as the most common type of coronary anomaly (1.23%). Sawaya et al[47] reported the case of a patient with an occlusion of the anterior bifurcation of a split RCA with no distal filling through collateral vessels, which resulted in an infarct that involved both the inferoseptal LV wall and the anterior RV free wall. With this case they demonstrated the extent of the territory fed by the anterior bifurcation of the split RCA: Both inferior and anterior RV free walls. Gulel et al[48] reported the case of a patient with anterior myocardial infarction in which the coronary angiogram showed two separate RCAs originating from the RSV with the same ostium: One of them was normal, while the other one had atherosclerotic plaques. In this case the arteriosclerotic changes affected only the AV branch and this is what happens in most reported cases, likely related to the higher amount of fat in the AV sulcus[14,47-49].
Duplication of LAD
Duplication of the LAD has been reported in several reviews and case series. Often one of the duplicated LAD arteries may arise from the RCA and take a pre-pulmonic, septal or inter-arterial course. This anomaly is not intrinsically haemodynamically significant, but its presence may complicate surgical intervention when aorto-coronary bypass or other coronary artery surgery is performed[14] (Figure 16).
Figure 16.
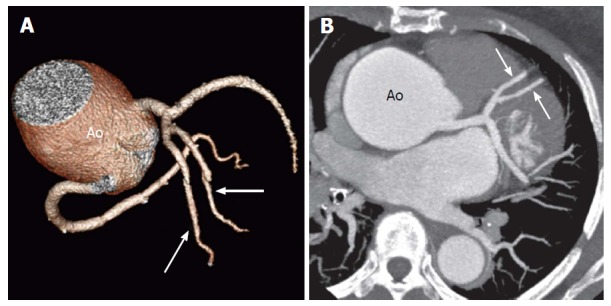
Duplication of the left anterior descending artery in a 52-year-old woman at preoperative evaluation[14]. Volume- rendered image (A) and oblique axial computed tomography image (B) show two equally sized LAD arteries (arrows). The Ao is noted to be aneurysmal. LAD: Left anterior descending; Ao: Aorta.
Duplication of LCX
Van der Velden et al[4] described a case of a patient with chest pain and history of myocardial infarction. Coronary angiography demonstrated three large coronary arteries without any significant stenotic lesion. An additional duplicated LCX was found originating from the RCA. A 90% stenosis was found at the ostium of this aberrant vessel. Moreover, several small coronary artero-venous fistulae were found to arise from a large diagonal branch (Figure 17).
Figure 17.
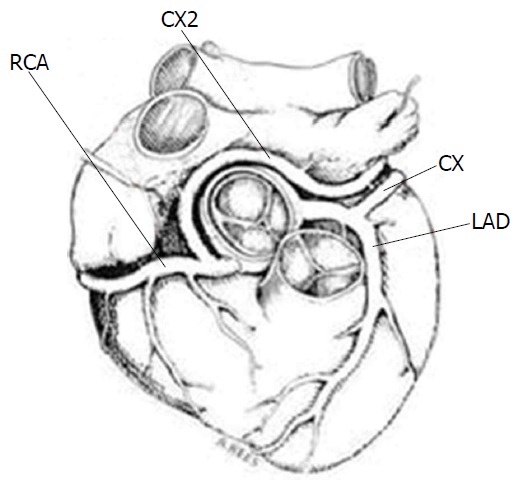
Schematic illustration of the double circumflex coronary system[4]. CX2 anomalous circumflex coronary artery arises from RCA. RCA: Right coronary artery; CX: Circumflex; LAD: Left anterior descending.
CONGENITAL ABSENCE
Absent LCMA (separate origin of LAD and LCX)
In this anomaly the LCMA is absent and LAD and LCX arise from separate, but adjacent ostia in the LSV; the vessels are otherwise normal in their distribution pattern. This CAA is fairly common, with a frequency of 0.41%-0.67%, and is found with increased incidence in aortic valve disease and left system dominance. This anomaly causes no hemodynamic impairment and should be considered benign, although could potentially have clinical consequences if unrecognized during surgery or coronary catheterization[24,25,41] (Figure 18).
Figure 18.
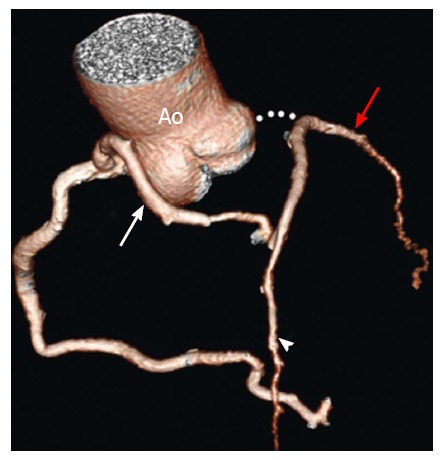
Atresia of the left main coronary artery in a 47-year-old woman with exertional chest pain and positive results on a stress test. The patient presented with LMCA atresia in adulthood. Volume-rendered image shows large conus artery (white arrow) collateral to the left anterior descending artery (arrowhead). The LAD artery and LCX (red arrow) are diminutive overall[14]. LMCA: Left main coronary artery; LAD: Left anterior descending; LCX: Left circumflex; Ao: Aorta.
LMCA atresia
When the LMCA is atretic a fibrous connection is often present between the LAD-LCX arterial junction and the LSV. In some cases a very thin, but abnormal, near-obliterated lumen has been reported, while in most cases, no lumen can be identified. Often, there are prominent right-to-left collateral vessels between the coronary arteries, although these vessels are usually inadequate to meet the oxygen requirements of LV. In most reported cases, presentation is early in the first year of life, although delayed presentation in adulthood has been reported. A large conus collateral branch supplying the LAD may mimic a pre-pulmonic vessel[14]. According to a systematic review of patient with LMCA atresia[40], though this anomaly is usually associated with the onset of symptoms in young patients, cases in elderly patients are also described.
Congenital absence of LCX
The congenital absence of the LCX results from the failure of LCX development in the left AV groove. According to some authors, this condition represents a normal variant, usually associated with anomalous origin of the LCX from the distal RCA. Indeed, in this CAA a large “superdominant” RCA crosses the crux of the heart where it ascends in the AV groove to perfuse the posterolateral and lateral wall, while the LAD arises from LSV and has normal distribution. Congenitally absent LCX is a very rare anomaly and not many cases have been reported. Its reported incidence is between 0.003%[50] and 0.067%[24]. This anomaly is considered a benign condition in the absence of coronary occlusive disease, however it can cause angina-like symptoms, particularly on exertion. It is hypothesized that “steal phenomenon” could explain symptoms, where increased metabolic demand in the LCX territory would cause ischaemia in the LAD or RCA territories. Hongsakul et al[50] reported a case of absent LCX in a 52-year-old man with acute chest pain and palpitations. Multiple enlarged diagonal branches of LAD were present to supply the lateral wall of LV in addition to a super-dominant RCA with its posterolateral branch continuing into the territory of the LCX[24,50].
HYPOPLASIA
Congenital hypoplasia of RCA and LCX
Hypoplastic RCA and LCX coronary arteries can be defined as small sized arteries with shorter courses so that neither reaches the crux. When the RCA is dominant, the LCX is usually quite small and therefore may be considered hypoplastic, and similarly when the LCX is dominant, the RCA is usually small. Hypoplasia of both RCA and LCX however is a rare occurrence. This anomaly has been reported in a 17-year-old girl who died suddenly after a 3-mile race[51] and in a 30-year-old man who died suddenly during a basketball game[13]: In the second case both RCA and LCX were hypoplastic and significantly reduced in length (4.5 cm and 2.5 cm respectively and the RCA had a high take-off position) (Figure 19).
Figure 19.
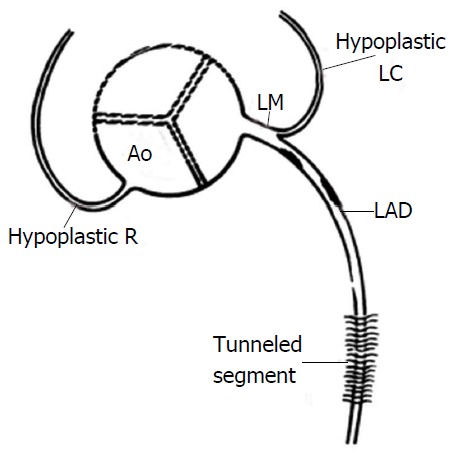
Congenital hypoplasia of right coronary artery and left circumflex artery[13]. LAD: Left anterior descending; Ao: Aorta; LM: Left main coronary artery; LC: Left circumflex; R: Right coronary artery.
ANOMALIES OF TERMINATION
Coronary fistulas and arteriovenous malformations
This CAA is the result of a communication between the termination of a coronary artery or its branches and a cardiac chamber, a great vessel or other vascular structure. Unfortunately, in the literature it is not clear the distinction between discrete coronary fistulas, which are quite common, and complex coronary arteriovenous malformations (AVMs). Most instances of fistulae are congenital. The incidence of coronary fistulae in patients who undergo coronary angiography is about 0.3%-0.87%. Coronary artery fistulae with large intra-cardiac shunts are rare in adults, since the majority are now detected and repaired during childhood. Fistulae terminating in the right heart chambers account for about 60% of the cases. The vast majority of patients are asymptomatic and coronary fistulae and AVMs usually have a benign clinical course. However, patients may become symptomatic, usually the 5th or 6th decade of life, and may present with SCD, myocardial ischemia, pulmonary hypertension, heart failure, arrhythmia, rupture or endocarditis. In one study of 51 patients with coronary steal phenomenon[52] the 5-year mortality was only 4%. Just a few cases of spontaneous closure have been reported. Termination into a cardiac chamber or vascular structure with lower pressure may lead to enlargement and tortuosity of the artery. Moreover, fistulae draining into right heart chambers function as left to right shunts and may result in RV volume overload. Coronary fistulae can cause ischemia by two possible mechanisms: First, there can be a steal of blood flow to the fistulous tract from the normal coronary branches, leading to ischemia and rarely to infarction; and, second, there can be stenosis of side branches secondary to thrombus associated with fistulous tracts, ulcerations and atherosclerosis. The steal can be persistent, caused by large fistulous tract, which also feed nutrient branches or receive collateral vessels that originate in the opposite coronary vessels, or episodic, caused by physiologic factors that increase shunting flow into the fistula at the expense of nutrient flow.
Symptomatic patients with large fistulae should undergo surgical closure of the fistulae at the drainage site. There is no consensus whether asymptomatic coronary fistulae should be treated or not[2,8,14,24,43].
Systemic termination
In this CAA the coronary artery terminates into a systemic artery. This CAA should be differentiated from fistulae, as in this case the coronary artery is not enlarged and tortuous as there is no significant pressure difference between it and the systemic artery. Systemic termination of a coronary artery is an uncommon finding, but some authors have suggested that the prevalence can be underestimated because the vessels involved have small caliber and may be difficult to identify. In the absence of a pressure gradient, such as that due to a stenosis, a coronary-systemic arterial communication is unlikely to be significant[14].
IMAGING
When a coronary artery anomaly is found, the exact origin, course and its relationship with other cardiac structures must be described in detail. Coronary angiography is still considered the reference standard for CAA evaluation. However, coronary angiography is invasive, therefore carrying a risk and more importantly provides only a two-dimensional representation of coronary course. Transthoracic echocardiogram (TTE) can visualize anomalous coronary arteries non-invasively. However, the reported incidence of AOCA on TTE was 0.17%, significantly lower compared with 1.07% incidence found at coronary angiography. Davies et al[36] found four coronary origin anomalies in a series of 2388 TTE, but importantly, in one case a negative echocardiographic finding was followed by SCD related to a coronary anomaly. These reports raise doubts about the sensitivity of TTE for the detection of AOCA. Moreover, TTE is limited in the identification of coronaries with intramural course. Trans-esophageal echocardiography may detect anomalies of origin of the coronary arteries. However, these are usually incidental findings.
Coronary CT is currently regarded as the diagnostic standard for the identification and visualization of CAAs. Coronary artery CT offers the best performance in terms of spatial resolution, acquisition time and image contrast. However, its widespread use is limited due the dose of ionizing radiation and the use of contrast agents, in particular taking into account that most patients are young. A recent study showed that the prevalence of CAAs is substantially higher with CT than coronary angiography, suggesting a possible underestimation of CAAs based on invasive angiography[53].
CMR is capable of visualizing the origin of the coronary arteries non-invasively, without use of ionizing radiation and contrast agents. Currently however, due to spatial resolution, the capability of CMR to visualize smaller coronary branches is still a limiting factor preventing full assessment of coronary arteries using this modality[2,9,10,12,14,17,19,23,25,44,46,54,55].
This aim of this review was to give a good overview of coronary artery anomalies and their clinical sequelae. What is covered is by no means exhaustive but covers a broad range of the common and less common anomalies and tries to give guidance based on previous case reports on whether a detected anomaly can be considered incidental or whether close follow-up or correction is advised. The developments and more widespread access to advanced cardiac imaging will undoubtedly lead to a deeper understanding of the natural progression of these.
ACKNOWLEDGMENTS
The authors acknowledge financial support from the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King's College London and King’s College Hospital NHS Foundation Trust. The Centre of Excellence in Medical Engineering funded by the Wellcome Trust and EPSRC under grant number WT 088641/Z/09/Z. King’s College London and UCL Comprehensive Cancer Imaging Centre. Funded by the CRUK and EPSRC in association with the MRC and DoH (England). Funded by the British Heart Foundation award RE/08/003.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 30, 2015
First decision: December 28, 2015
Article in press: March 16, 2016
P- Reviewer: Kettering K, Peteiro J, Raja SG, Salemi VMC, Said SAM, Ueda H, van Beek EJR S- Editor: Qiu S L- Editor: A E- Editor: Li D
References
- 1.Chiu IS, Anderson RH. Can we better understand the known variations in coronary arterial anatomy? Ann Thorac Surg. 2012;94:1751–1760. doi: 10.1016/j.athoracsur.2012.05.133. [DOI] [PubMed] [Google Scholar]
- 2.Young PM, Gerber TC, Williamson EE, Julsrud PR, Herfkens RJ. Cardiac imaging: Part 2, normal, variant, and anomalous configurations of the coronary vasculature. AJR Am J Roentgenol. 2011;197:816–826. doi: 10.2214/AJR.10.7249. [DOI] [PubMed] [Google Scholar]
- 3.Angelini P. Normal and anomalous coronary arteries: definitions and classification. Am Heart J. 1989;117:418–434. doi: 10.1016/0002-8703(89)90789-8. [DOI] [PubMed] [Google Scholar]
- 4.van der Velden LB, Bär FW, Meursing BT, Ophuis TJ. A rare combination of coronary anomalies. Neth Heart J. 2008;16:387–389. doi: 10.1007/BF03086184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldsmith JB, Butler HW. The development of the cardiac‐coronary circulatory system. American J Anato. 1937;60:185–201. [Google Scholar]
- 6.Clark EB. Cardiac embryology. Its relevance to congenital heart disease. Am J Dis Child. 1986;140:41–44. doi: 10.1001/archpedi.1986.02140150043030. [DOI] [PubMed] [Google Scholar]
- 7.Conte G, Pellegrini A. On the development of the coronary arteries in human embryos, stages 14-19. Anat Embryol (Berl) 1984;169:209–218. doi: 10.1007/BF00303151. [DOI] [PubMed] [Google Scholar]
- 8.Angelini P. Coronary artery anomalies--current clinical issues: definitions, classification, incidence, clinical relevance, and treatment guidelines. Tex Heart Inst J. 2002;29:271–278. [PMC free article] [PubMed] [Google Scholar]
- 9.Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002;105:2449–2454. doi: 10.1161/01.cir.0000016175.49835.57. [DOI] [PubMed] [Google Scholar]
- 10.Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation. 2007;115:1296–1305. doi: 10.1161/CIRCULATIONAHA.106.618082. [DOI] [PubMed] [Google Scholar]
- 11.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, Cheng V, DeFrance T, Hellinger JC, Karlsberg RP. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–136. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Flessas D, Mamarelis I, Maniatis V, Souretis G, Laschos N, Kotoulas C, Lazaridis K. An unusual pattern of three major components of the cardiovascular system: multimodality imaging and review of the literature. J Cardiothorac Surg. 2013;8:61. doi: 10.1186/1749-8090-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menke DM, Waller BF, Pless JE. Hypoplastic coronary arteries and high takeoff position of the right coronary ostium. A fatal combination of congenital coronary artery anomalies in an amateur athlete. Chest. 1985;88:299–301. doi: 10.1378/chest.88.2.299. [DOI] [PubMed] [Google Scholar]
- 14.Shriki JE, Shinbane JS, Rashid MA, Hindoyan A, Withey JG, DeFrance A, Cunningham M, Oliveira GR, Warren BH, Wilcox A. Identifying, characterizing, and classifying congenital anomalies of the coronary arteries. Radiographics. 2012;32:453–468. doi: 10.1148/rg.322115097. [DOI] [PubMed] [Google Scholar]
- 15.Apitzsch J, Kühl HP, Mühlenbruch G, Mahnken AH. Unusual malignant coronary artery anomaly: results of coronary angiography, MR imaging, and multislice CT. Cardiovasc Intervent Radiol. 2010;33:389–393. doi: 10.1007/s00270-009-9663-y. [DOI] [PubMed] [Google Scholar]
- 16.Karadag B, Ayan F, Ismailoglu Z, Goksedef D, Ataev Y, Vural VA. Extraordinary cause of ischemic chest pain in a young man: congenital ostial atresia of the right coronary artery. J Cardiol. 2009;54:335–338. doi: 10.1016/j.jjcc.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Chiribiri A, Ishida M, Nagel E, Botnar RM. Coronary imaging with cardiovascular magnetic resonance: current state of the art. Prog Cardiovasc Dis. 2011;54:240–252. doi: 10.1016/j.pcad.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Boffano C, Chiribiri A, Cesarani F. Native whole-heart coronary imaging for the identification of anomalous origin of the coronary arteries. Int J Cardiol. 2009;137:e27–e28. doi: 10.1016/j.ijcard.2008.05.075. [DOI] [PubMed] [Google Scholar]
- 19.Clemente A, Del Borrello M, Greco P, Mannella P, Di Gregorio F, Romano S, Morra A. Anomalous origin of the coronary arteries in children: diagnostic role of three-dimensional coronary MR angiography. Clin Imaging. 2010;34:337–343. doi: 10.1016/j.clinimag.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Morales AR, Romanelli R, Tate LG, Boucek RJ, de Marchena E. Intramural left anterior descending coronary artery: significance of the depth of the muscular tunnel. Hum Pathol. 1993;24:693–701. doi: 10.1016/0046-8177(93)90004-z. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira AG, Trotter SE, König B, Décourt LV, Fox K, Olsen EG. Myocardial bridges: morphological and functional aspects. Br Heart J. 1991;66:364–367. doi: 10.1136/hrt.66.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angelini P, Trujillo A, Sawaya F, Lee VV. “Acute takeoff” of the circumflex artery: a newly recognized coronary anatomic variant with potential clinical consequences. Tex Heart Inst J. 2008;35:28–31. [PMC free article] [PubMed] [Google Scholar]
- 23.Frescura C, Basso C, Thiene G, Corrado D, Pennelli T, Angelini A, Daliento L. Anomalous origin of coronary arteries and risk of sudden death: a study based on an autopsy population of congenital heart disease. Hum Pathol. 1998;29:689–695. doi: 10.1016/s0046-8177(98)90277-5. [DOI] [PubMed] [Google Scholar]
- 24.Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21:28–40. doi: 10.1002/ccd.1810210110. [DOI] [PubMed] [Google Scholar]
- 25.Yurtdaş M, Gülen O. Anomalous origin of the right coronary artery from the left anterior descending artery: review of the literature. Cardiol J. 2012;19:122–129. [PubMed] [Google Scholar]
- 26.Alexander RW, Griffith GC. Anomalies of the coronary arteries and their clinical significance. Circulation. 1956;14:800–805. doi: 10.1161/01.cir.14.5.800. [DOI] [PubMed] [Google Scholar]
- 27.Camarda J, Berger S. Coronary artery abnormalities and sudden cardiac death. Pediatr Cardiol. 2012;33:434–438. doi: 10.1007/s00246-012-0168-0. [DOI] [PubMed] [Google Scholar]
- 28.Burke AP, Farb A, Virmani R, Goodin J, Smialek JE. Sports-related and non-sports-related sudden cardiac death in young adults. Am Heart J. 1991;121:568–575. doi: 10.1016/0002-8703(91)90727-y. [DOI] [PubMed] [Google Scholar]
- 29.Drory Y, Turetz Y, Hiss Y, Lev B, Fisman EZ, Pines A, Kramer MR. Sudden unexpected death in persons less than 40 years of age. Am J Cardiol. 1991;68:1388–1392. doi: 10.1016/0002-9149(91)90251-f. [DOI] [PubMed] [Google Scholar]
- 30.Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–1501. doi: 10.1016/s0735-1097(00)00566-0. [DOI] [PubMed] [Google Scholar]
- 31.Maron BJ, Thompson PD, Puffer JC, McGrew CA, Strong WB, Douglas PS, Clark LT, Mitten MJ, Crawford MH, Atkins DL, et al. Cardiovascular preparticipation screening of competitive athletes. A statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation. 1996;94:850–856. doi: 10.1161/01.cir.94.4.850. [DOI] [PubMed] [Google Scholar]
- 32.Van Camp SP, Bloor CM, Mueller FO, Cantu RC, Olson HG. Nontraumatic sports death in high school and college athletes. Med Sci Sports Exerc. 1995;27:641–647. [PubMed] [Google Scholar]
- 33.Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol. 1992;20:640–647. doi: 10.1016/0735-1097(92)90019-j. [DOI] [PubMed] [Google Scholar]
- 34.Sloan K, Majdalany D, Connolly H, Schaff H. Inferior wall myocardial infarction caused by anomalous right coronary artery. Can J Cardiol. 2008;24:e102–e103. doi: 10.1016/s0828-282x(08)70704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venturini E, Magni L. Single coronary artery from the right sinus of Valsalva. Heart Int. 2011;6:e5. doi: 10.4081/hi.2011.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies JE, Burkhart HM, Dearani JA, Suri RM, Phillips SD, Warnes CA, Sundt TM, Schaff HV. Surgical management of anomalous aortic origin of a coronary artery. Ann Thorac Surg. 2009;88:844–847; discussion 847-848. doi: 10.1016/j.athoracsur.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Cacici G, Angelini P. Unusual case of single coronary artery: questions of methods and basic concepts. Ital Heart J. 2005;6:345–347. [PubMed] [Google Scholar]
- 38.Angelini P, Walmsley RP, Libreros A, Ott DA. Symptomatic anomalous origination of the left coronary artery from the opposite sinus of valsalva. Clinical presentations, diagnosis, and surgical repair. Tex Heart Inst J. 2006;33:171–179. [PMC free article] [PubMed] [Google Scholar]
- 39.Dodge-Khatami A, Mavroudis C, Backer CL. Congenital Heart Surgery Nomenclature and Database Project: anomalies of the coronary arteries. Ann Thorac Surg. 2000;69:S270–S297. doi: 10.1016/s0003-4975(99)01248-5. [DOI] [PubMed] [Google Scholar]
- 40.Musiani A, Cernigliaro C, Sansa M, Maselli D, De Gasperis C. Left main coronary artery atresia: literature review and therapeutical considerations. Eur J Cardiothorac Surg. 1997;11:505–514. doi: 10.1016/s1010-7940(96)01121-9. [DOI] [PubMed] [Google Scholar]
- 41.Roberts WC. Major anomalies of coronary arterial origin seen in adulthood. Am Heart J. 1986;111:941–963. doi: 10.1016/0002-8703(86)90646-0. [DOI] [PubMed] [Google Scholar]
- 42.Alexi-Meskishvili V, Nasseri BA, Nordmeyer S, Schmitt B, Weng YG, Böttcher W, Hübler M, Berger F, Hetzer R. Repair of anomalous origin of the left coronary artery from the pulmonary artery in infants and children. J Thorac Cardiovasc Surg. 2011;142:868–874. doi: 10.1016/j.jtcvs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Baltaxe HA, Wixson D. The incidence of congenital anomalies of the coronary arteries in the adult population. Radiology. 1977;122:47–52. doi: 10.1148/122.1.47. [DOI] [PubMed] [Google Scholar]
- 44.Frommelt PC, Frommelt MA, Tweddell JS, Jaquiss RD. Prospective echocardiographic diagnosis and surgical repair of anomalous origin of a coronary artery from the opposite sinus with an interarterial course. J Am Coll Cardiol. 2003;42:148–154. doi: 10.1016/s0735-1097(03)00503-5. [DOI] [PubMed] [Google Scholar]
- 45.Click RL, Holmes DR, Vlietstra RE, Kosinski AS, Kronmal RA. Anomalous coronary arteries: location, degree of atherosclerosis and effect on survival--a report from the Coronary Artery Surgery Study. J Am Coll Cardiol. 1989;13:531–537. doi: 10.1016/0735-1097(89)90588-3. [DOI] [PubMed] [Google Scholar]
- 46.Bunce NH, Lorenz CH, Keegan J, Lesser J, Reyes EM, Firmin DN, Pennell DJ. Coronary artery anomalies: assessment with free-breathing three-dimensional coronary MR angiography. Radiology. 2003;227:201–208. doi: 10.1148/radiol.2271020316. [DOI] [PubMed] [Google Scholar]
- 47.Sawaya FJ, Sawaya JI, Angelini P. Split right coronary artery: its definition and its territory. Tex Heart Inst J. 2008;35:477–479. [PMC free article] [PubMed] [Google Scholar]
- 48.Gulel O, Yazici M, Durna K, Demircan S. A rare coronary anomaly: double right coronary artery. Clin Cardiol. 2007;30:309. doi: 10.1002/clc.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erbagci H, Davutoglu V, Turkmen S, Kizilkan N, Gumusburun E. Double right coronary artery: review of literature. Int J Cardiovasc Imaging. 2006;22:9–11. doi: 10.1007/s10554-005-5139-6. [DOI] [PubMed] [Google Scholar]
- 50.Hongsakul K, Suwannanon R. Congenital absence of left circumflex artery detected by computed tomography coronary angiography: a case report. Case Rep Vasc Med. 2012;2012:204657. doi: 10.1155/2012/204657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts WC, Glick BN. Congenital hypoplasia of both right and left circumflex coronary arteries. Am J Cardiol. 1992;70:121–123. doi: 10.1016/0002-9149(92)91407-u. [DOI] [PubMed] [Google Scholar]
- 52.Said SA, Van der Werf T. Dutch survey of congenital coronary artery fistulas in adults. Neth Heart J. 2006;14:5–13. [PMC free article] [PubMed] [Google Scholar]
- 53.Ghadri JR, Kazakauskaite E, Braunschweig S, Burger IA, Frank M, Fiechter M, Gebhard C, Fuchs TA, Templin C, Gaemperli O, et al. Congenital coronary anomalies detected by coronary computed tomography compared to invasive coronary angiography. BMC Cardiovascular Disorders. 2014;14:1. doi: 10.1186/1471-2261-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kassaian SE, Mahmoodian M, Salarifar M, Alidoosti M, Abbasi SH, Rasekh A. Stent-graft exclusion of multiple symptomatic coronary artery fistulae. Tex Heart Inst J. 2007;34:199–202. [PMC free article] [PubMed] [Google Scholar]
- 55.Erol C, Seker M. Coronary Artery Anomalies: The Prevalence of Origination, Course, and Termination Anomalies of Coronary Arteries Detected by 64-Detector Computed Tomography Coronary Angiography. Journal of Computer Assisted Tomography. 2011;35:618–624. doi: 10.1097/RCT.0b013e31822aef59. [DOI] [PubMed] [Google Scholar]
- 56.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 57.Purvis J, Howe A, Morgan D. Aortic step: a clue to unusually high origin of right coronary artery. Heart. 2010;96:1334. doi: 10.1136/hrt.2010.198242. [DOI] [PubMed] [Google Scholar]