Abstract
Food quality shapes life history traits either directly or through response of individuals to additional environmental factors, such as chemical cues. Plant extracts used as food additives modulate key life history traits; however little is known regarding such effects for olfactory chemical cues. Exploiting an interesting experimental system that involves the olive fly (Bactrocera oleae) and the plant metabolite α-pinene we asked whether exposure of adults to this compound modulates adult longevity and female reproduction in similar manner in a stressful – dietary (protein) restricted (DR) and in a relaxed- full diet (FD) feeding environment. Accordingly, we exposed males and females to the aroma of α-pinene and measured lifespan and age-specific fecundity in the above two dietary contexts. Our results demonstrate that exposure to α-pinene increased longevity in males and fecundity in females only under dietary restricted conditions. In relaxed food conditions, females exposed to α-pinene shifted high egg-laying towards younger ages compared to non-exposed ones. This is the first report demonstrating that a plant compound affects key life history traits of adult olive flies through olfaction. These effects are sex-specific and more pronounced in dietary restricted adults. Possible underlying mechanisms and the ecological significance are discussed.
Among the various challenges organisms face towards surviving and reproducing, quantitative and qualitative limitation of food stands out. Adaptive and/or plastic competence of living creatures to cope with adverse feeding conditions, such as scarce availability and low nutritional value of food, determines thriving and existence. Perhaps one of the most dramatic cases of environmental variance is living in high versus low nutritious environments (relaxed vs stressful respectively). Nutrient acquisition can generate variation in the amount of resources available for the individuals and may moderate the severity of trade-offs between survival and reproduction1,2. Caloric and/or dietary restriction may modulate the response of an organism to environmental stimuli3 eliciting hormetic responses in a variety of animal taxa4. Food quality, therefore, is expected to shape (positively, moderately or negatively) life history traits5 through successful response of individuals to additional environmental factors, such as chemical cues.
Plant extracts evoke various responses in several animal taxa (humans, insects, worms, etc.) modulating key life-history traits, prolonging lifespan and enhancing stress tolerance6, increasing fecundity7 and affecting behavior and physiology of organisms (for reviews see8,9). Plant compounds that are incorporated in food as diet supplements prolong longevity in model organisms by interacting either with nutrient-, energy- and stress-sensing pathways9 (and references therein). Among the many compounds tested, resveratrol constitutes probably the best studied phytochemical that prolongs longevity10,11 (and references therein). Likewise, blueberry extract extends the lifespan and increases thermotolerance in the nematode Caenorhabditis elegans12. Nectarine extracts have been shown to extend lifespan and simultaneously increase fecundity in Drosophila melanogaster7. Mixture of oregano and cranberry has been demonstrated to increase longevity without effects on the fecundity of Mexican fruit flies (Anastrepha ludens)13. Extracts of the acai berry improve survival of flies feeding on a high fat diet14. Despite being used as food additives the underlying mechanisms of extending longevity and modulating life history traits differ a great deal among the above compounds ranging from anti-oxidant, anti-flammatory, to immune-stimulatory properties. There is a great variation in the outcome of the above studies demonstrating positive effects of plant extracts used as food additives, which depend on the gender of tested individuals, diet conditions and other environmental factors.
Olfactory chemical cues -including phytochemicals- emitted from host plants of phytophagous insects may elicit a wide range of behavioral and physiological responses15, providing important signals for locating food sources16, oviposition sites and/or mediating oviposition behavior17,18. Phytochemicals may generate similar reactions in biochemical and physiological pathways towards increasing longevity as those reported earlier for plant extracts used as food additives, though through neurosensory regulation. Sensory cues (food and pheromone odors as well as additional environmental cues such as temperature) have been found to modulate longevity and reproduction in model organisms19,20,21,22, (reviewed in23). Food and carbon dioxide volatiles have been shown to expand longevity in Drosophila melanogaster20,24. Perception of olfactory, gustatory and thermosensory stimuli can modulate lifespan in C. elegans as well22,25,26. While there is a plethora of studies depicting beneficiaries of phytochemicals when added in food, very few refer to positive effects through olfactory perception.
The olive fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae) is a rather monophagous species feeding on cultivated and wild growing olives. Similar to other true fruit flies (tephritids) females lay eggs in olive fruit and larvae feed in olive fruit flesh before pupating27. Alpha-pinene, a common plant compound that is present in both olive fruit and leafs, consists also one of the four major compounds of the sex pheromone of female olive flies28 that has been found to evoke physiological responses and modulate behavioral ones in both male and female olive flies. A-pinene exhibits synergistic action with olean (the main compound of the female pheromone blend) in attracting males29 and in stimulating oviposition30 and most probably is acquired by olive leaves and/or olive fruit. More recent studies demonstrate that exposure to the aroma of α-pinene evokes strong electrophysiological responses in both male and female antennae31, and increases the mating performance of both sexes32. Thus, there is an intimate link between olive fruit flies and α-pinene since both adults and immatures (developing in α-pinene rich olive fruit) are frequently exposed to this compound. Similar to other fruit flies protein limitation in the adult food of the olive fly is expected to establish a nutritionally stressful environment33,34,35. Working towards exploring how chemical cues- closely related to the ecology of a species- can influence longevity and reproductive life history traits of olive flies and whether these effects are modulated by the dietary conditions that adults experience, we asked the following questions: Does exposure to α-pinene modulate: (a) longevity in olive flies, (b) reproductive traits of females and (c) life-history traits in similar manner in a dietary restricted (DR) and in a relaxed- full diet (FD) feeding environment. Accordingly by manipulating the availability of yeast hydrolyzate in adult food and exposure to α-pinene we tested the hypothesis that exposure to α-pinene influences longevity and reproduction regardless of dietary restriction, and the gender of individuals.
Results
Effect of α-pinene on adult longevity
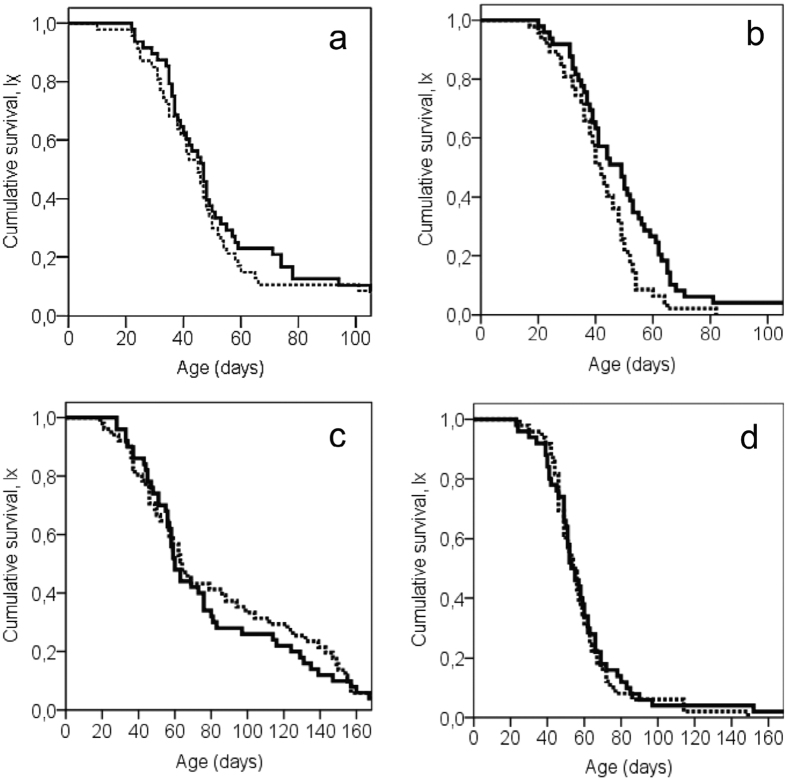
Table 1 gives the longevity parameters of both male and female cohorts and Fig. 1 the respective cumulative survivorship curves. Exposure to the aroma of α-pinene significantly increased the lifespan of DR males only (log-rank test; x2 = 6.027; df = 1; P = 0.014). There was a positive response to α-pinene in terms of longevity extension of males’ experienced FD conditions but the difference between exposed and non-exposed individuals was not significant (log-rank test; x2 = 0.417; df = 1; P = 0.518). Exposure to α-pinene did not prolong the longevity of females regardless of the food regime (Table 1; Fig. 1c,d, log-rank test; x2 = 0.403 and x2 = 0.197; df = 1; P = 0.525 and P = 0.657).
Table 1. Longevity parameters of adult olive flies that were exposed and non-exposed to the aroma of α-pinene and held in diet restriction (DR) and full diet (FD) food conditions.
| Adult cohort (Number of individuals) | Longevity parameters in days ± SE | |||
|---|---|---|---|---|
| Average | Quartiles | |||
| 25 | 50 | 75 | ||
| Males | ||||
| FD | ||||
| Exposed (n = 48) | 56.37 ± 5.01a | 58 ± 9.00 | 47 ± 2.15 | 36 ± 1.12 |
| Non-exposed (n = 47) | 51.42 ± 4.74a | 54 ± 3.73 | 45 ± 3.42 | 33 ± 1.99 |
| DR | ||||
| Exposed (n = 49) | 49.63 ± 2.76a | 61 ± 3.51 | 49 ± 4.49 | 37 ± 2.63 |
| Non-exposed (n = 47) | 41.95 ± 1.87b | 50 ± 1.60 | 42 ± 2.28 | 33 ± 2.98 |
| Females | ||||
| FD | ||||
| Exposed (n = 44) | 78.92 ± 6.03a | 114 ± 25.97 | 60 ± 2.94 | 48 ± 3.61 |
| Non-exposed (n = 53) | 83.96 ± 6.75a | 132 ± 19.69 | 64 ± 5.09 | 46 ± 5.96 |
| DR | ||||
| Exposed (n = 50) | 59.68 ± 3.70a | 66 ± 3.41 | 53 ± 3.03 | 46 ± 3.54 |
| Non-exposed (n = 51) | 58.87 ± 3.09a | 64 ± 3.39 | 56 ± 2.28 | 46 ± 1.56 |
Within sex, numbers followed by different letters are significantly different (pairwise comparisons log-rank test, P < 0.05).
Figure 1.
Age-specific survival patterns of adult olive flies that were either exposed (solid line) or non-exposed (dashed line) to α-pinene: (a) males in full diet (FD) conditions, (b) males in diet restriction (DR), (c) females in full diet (FD) conditions, and (d) females in diet restriction (DR) conditions. (Log-rank pairwise tests reveal significant differences between exposed and non-exposed males fed in sugar, P < 0.05).
Effect of α-pinene on female reproductive traits
Figures 2 and 3 give the average lifetime and age-specific fecundity rates of females’ experienced FD (a) and DR (b) nutritious conditions respectively. Within cohort variability in age specific egg-laying patterns is given in Supplementary Figure 1. Exposure to α-pinene increased the number of eggs oviposited by females experiencing DR conditions (squared Wald test, x2 = 81.22; df = 1; P < 0.001, Fig. 2b), but not that of females having access to FD environment (P = 0.426). Interestingly, exposure to α-pinene shifts intensity of egg-laying to younger ages for females feeding in FD but not for those feeding in DR food (Fig. 3). ROC curve analysis (Supplementary Figure 3) highlights differences in the age-specific distribution of the egg laying output between non-exposed and exposed to α-pinene females that experienced FD environment (Supplementary Figure 3a, P = 0.002). The distribution of the egg laying output was similar between exposed and non-exposed to α–pinene females in the DR environment (Supplementary Figure 3b, P = 0.62). Exposure to α-pinene substantially (from 32.7 to 48.0%) and slightly (82.4 to 88.0%) increased the proportion of ovipositing females in cohorts maintained in DR (x2 = 2.42; df = 1; P = 0.119) and FD environments respectively (x2 = 0.402; df = 1; P = 0.525). Exposure to α-pinene had rather minor effect on both the oviposition (x2 = 0.019 and x2 = 0.505; df = 1; P = 0.889 and P = 0.477) and post-oviposition (x2 = 2.274 and x2 = 0.834; df = 1; P = 0.132 and P = 0.361) period of females experiencing FD and DR conditions respectively (Supplementary Figure 2).
Figure 2.
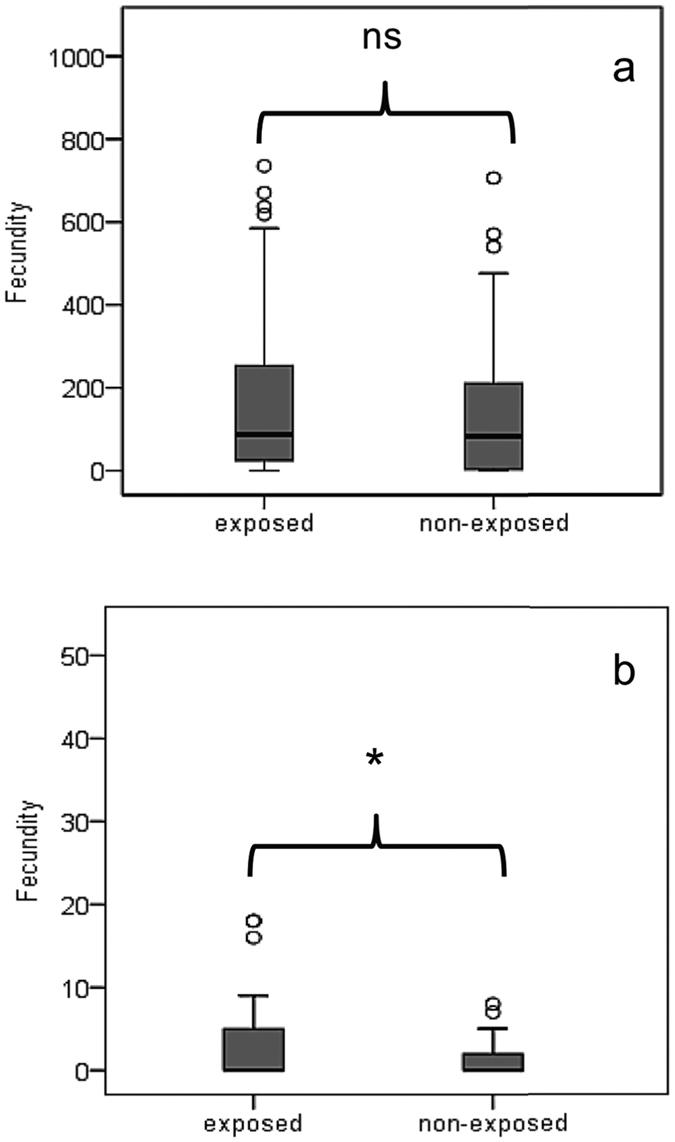
Box plots depicting life time fecundity rates (eggs per female) of females experiencing FD (a) and DR (b) food conditions and had either exposed or remained unexposed to α-pinene. Asterisk indicates significant differences between female cohorts (squared Wald test x2 = 81.219; df = 1; P < 0.05).
Figure 3.
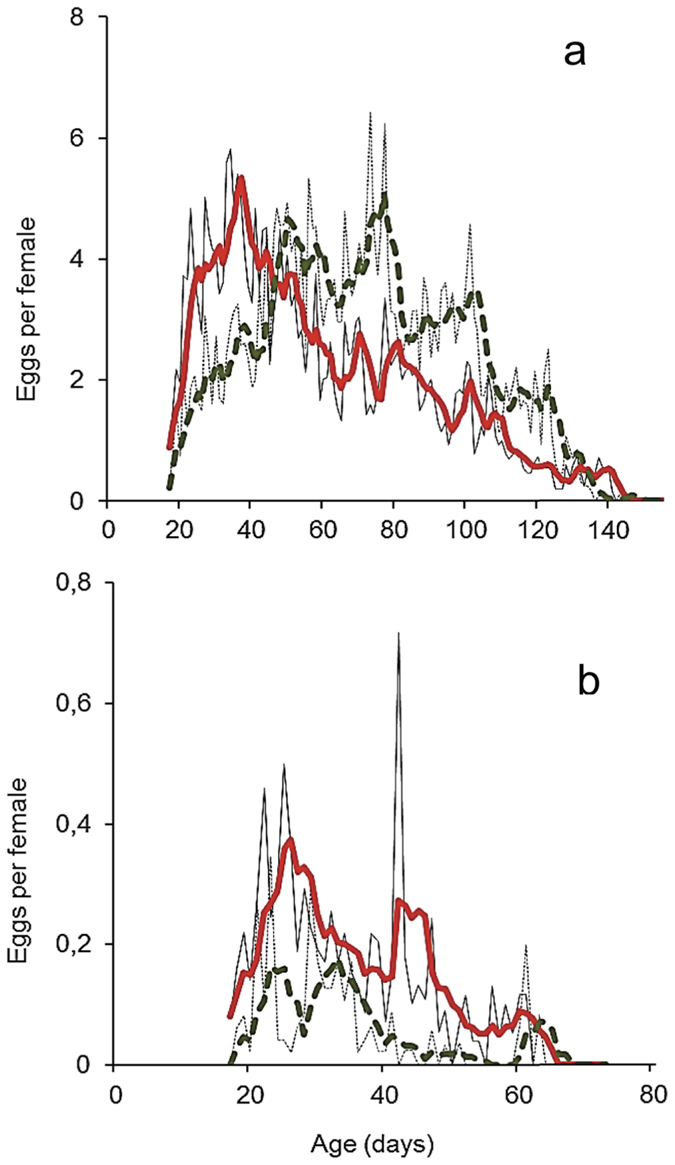
Age-specific fecundity of exposed (solid black line) and non-exposed (dashed black line) females in FD (a) and in DR food conditions (b). Red solid (exposed) and green dashed (non-exposed) lines represent data of female fecundity after smoothing of 5-days period, in the respective food regimes.
Discussion
Our results demonstrate that (a) exposure to the aroma of the plant metabolite α-pinene affects major life history traits such as lifespan and reproduction in adult olive flies, (b) the effects of α-pinene are modulated by both the gender of adults and the dietary context. Exposure to α-pinene (c) increases longevity only on dietary restricted males but has no apparent effect on female longevity. Interestingly, the aroma of α-pinene (d) increases fecundity of dietary restricted females only and modulates age–specific patterns of egg laying on females fed in full diet conditions. Therefore, our hypothesis that exposure to α-pinene increases longevity and reproduction of adult olive flies regardless of gender and dietary context should be rejected.
A-pinene modulates life-history traits and chemosensory stimulation
To our knowledge this is the first report demonstrating that a plant compound affects key life history traits of adult olive flies, such as longevity, lifetime and age-specific reproduction, through olfaction. Our experimental manipulations were designed to minimize any direct contact of α-pinene with the gustatory system of the exposed individuals. Although, we cannot exclude that in FD treatments, food odors could have an effect and overlay with effects of the odor of α-pinene, yet comparisons were performed within the same nutritional environments depicting a clear effect of α-pinene in extending longevity of males and increasing fecundity of DR females. The clear and more pronounced effect of the induced phenomenon in adults fed only sugar (free of any odors) further strengthens our conclusions. Therefore, we strongly suggest that the observed effects result from the stimulation of B. oleae chemosensory system that is triggered by the detection and olfaction of α-pinene aroma. Apparently future studies should concentrate on exploiting the underlying physiological and/or molecular mechanisms of the above phenomenon.
As it is stated earlier, the aroma of α-pinene is known to elicit behavioral changes in both male and female olive flies. Perception of α-pinene molecules by the olfactory system of B. oleae males could modulate the activity of neurotransmitters and of different peptide or steroid hormones21,22,36, which in turn may affect different homeostatic mechanisms that regulate longevity, as shown in other model organisms37,38. Compelling evidence that chemosensory systems (mainly olfactory but also gustatory) can modulate longevity has been reported in both C. elegans and D. melanogaster20,24,26,39,40.
For females exposure to the aroma of α-pinene modulates lifetime fecundity of DR females and generates differences in age-specific egg-laying patterns of FD ones. Interestingly our study demonstrates differential results of females held in rich and poor food conditions. Although exposure of females to α-pinene ended on day 16th of life, effects on patterns of egg-laying were evident weeks or months later. Previous studies report stimulatory effects of α-pinene on initiation of oviposition30 and induction of electrophysiological responses on females31. The odor of various plant volatiles can act either as an ovipositional stimuli and attractant or as a repellent18, (reviewed in41). Although evidences regarding the underlying regulative mechanisms do not exist, it is plausible to argue that the aroma of α-pinene may serve as an olfactory input, which activates transcription factors (e.g., DAF-16/FOXO, insulin, steroid signaling) and triggers release of secondary messengers (e.g., hormones) that regulate physiological changes in female olive flies, some of which modulate longevity and reproductive functions23,42. Direct contact (feeding or application) of plant compounds (e.g plant adaptogens, phytochemicals) can have profound effects on life prolonging and shaping life-history traits (larval development, fecundity) by activating common signaling pathways9 with those activated through chemosensory stimulation (for reviews see)23,36,38. Further studies on the causal mechanisms underlying exposure to the aroma of α-pinene could provide useful information towards this direction.
Diet-specific effects
Our results demonstrate that the beneficial effects of exposure to α-pinene depend on the dietary context of adult olive flies. Interestingly, our work goes in the opposite direction of the seminal study of Libert and co-workers20 where DR individuals that could smell food lost lifespan extension. In this work yeast odor (which is accepted as an exclusively nutritional cue for many organisms including insects) has been used while in our work a species-specific odorant stimulus (α-pinene) is involved, which elicits various behavioral and physiological responses (see introduction) but is not connected with adult feeding. A-pinene may activate ‘private’ mechanisms (i.e. organism-specific) of lifespan extension and not ‘public’ ones, whose effects are still unknown for olive fly43. Private mechanisms influencing lifespan refer to unique links developed between specific chemical stimuli (usually closely related to ecological challenges each species faces) and receptors, while ‘public’ ones (evolutionary conserved across many taxa) refer to links that arise due to response of organisms to fundamental needs, such as the need for food and mates. Furthermore, in the study of Libert and co-workers DR individuals had access to low levels of yeast (protein), whereas in our study DR individuals were deprived of yeast. Even small amounts of yeast may evoke the expression of different genes, which could account for the opposite effects observed in our study. Diet composition has a significant impact on lifespan and reproduction and may interact with other factors. Similar to other studies on tephritids44 (and references therein) absence of yeast hydrolyzate in adult diet decreases lifespan by 18.41% in male olive flies (FD vs DR). Longevity extension was observed for males exposed to the aroma of α-pinene, though differences were significant only in DR males. Variation in longevity extension observed between DR and FD exposed males may be attributed to trade-offs between behavioral and physiological processes related to adult food.
For females direct prolongevity effects after exposure to α-pinene have not been depicted. Although, reproduction does not predict lifespan and these two can be uncoupled45,46 (and references therein) trade-offs between them may still exist to some extent47,48,49. In many studies artifactual lifespan enhancement has been observed as a result of reduced fecundity. In our study, we found an increase in lifetime fecundity of DR females, without longevity trade-off. This suggests that α-pinene could promote longevity.
On the other hand, yeast availability in female food diet could mask measurable/quantitative trade-offs between reproduction and longevity. In our study, FD olive fruit fly females laid significantly more eggs and lived longer than DR ones, despite the increased reproduction cost, demonstrating the high impact of full diet on female fitness. Yeast by itself is a strong mediator of lifespan and fecundity in female fruit flies50,51. Full diet conditions may drive fecundity and longevity extension to a plateau that exposure to the aroma of α-pinene could not enhance further. As a consequence beneficial effect of α-pinene aroma could be difficult to detect and could be represented as changes in other life history traits. A shift of age-specific fecundity pattern towards early reproduction of females exposed to α-pinene aroma may serve as a support to the above arguments. Age specific patterns of egg laying in olive fly and other fruit flies are quite plastic and subject to several external and internal factors. We have recently shown that female fecundity is greatly regulated by seminal fluid and the mating history of male patterns and that effect may be long lasting52.
Sex-specific effects
Another interesting outcome of our study was the differential response of the two sexes to the aroma of α-pinene. Life-prolonging effects have been shown for males but not for females where reproductive traits such as fecundity have been regulated. Since males were deprived of females, they were prevented of costly reproductive activities such as courting and mating. Therefore, beneficial stimulatory effects of exposure to α-pinene may have been directed to somatic maintenance and longevity extension. On the other hand, since females are capable of producing and laying eggs without the involvement of males, they had to balance the “allocation” of beneficial effects of the aroma of α-pinene between reproduction and lifespan, as discussed.
Differential responsiveness of males and females could also possibly have arisen because of activation of different mechanisms and/or signaling pathways. A-pinene aroma through the stimulation of the neurosensory system of B. oleae, could lead to secretion of same or different kinds of hormones in males and females, whose effects may regulate different physiological processes (homeostasis, reproduction, feeding etc.). Additionally, different activated hormonal pathways could act both separately or coordinately to influence both longevity and reproduction. For example juvenile hormone regulates multiple aspects of physiology (and behavior), representing remarkably variable functions between males and females of Drosophila melanogaster (for review see53). Other possible explanations on differential response between genders to olfaction cues sensing (e.g. CO2 sensing see24) include differences between the two sexes in the olfactory stimulation/sensitivity and/or in the procession of odor information.
A-pinene may regulate ecological responses of adult olive flies
A-pinene emitted from olive fruit may represent a cue informing B. oleae females about fruit abundance and suitability and, therefore, favorable conditions for larvae survival. A-pinene aroma acting synergistically with female pheromone attracts males29 and increases the mating success on both male and female olive flies32. Once females mated, α-pinene stimulates and increases fecundity or modulates age-specific patterns of egg laying, boosting early reproduction and maximizing individual fitness. On the other hand, increased longevity observed in males, coupled with higher mating success32, may contribute to a higher number of copulations during lifetime; either for replenishment of the sperm reservoirs of already mated females or fertilization of virgin females. Differential effects of α-pinene aroma in the two dietary contexts, could reveal plastic responses generating alternate life history strategies, which ultimately enhance the chances of survival and reproduction under available resources37 or serve as sensory cue triggering mechanisms towards cell protection, allowing animals to react quickly in rapid changing environmental conditions.
Materials and Methods
The experiments were conducted during autumn 2013 in the laboratory of Entomology and Agricultural Zoology at the University of Thessaly, Greece at 25 ± 1 °C, 65 ± 5% R.H. and a photoperiod of L14:D10. Light was provided by daylight fluorescent tubes and by natural light from four windows with the intensity inside the test cages ranging from 1500 to 2000 Lux. All flies used were obtained from infested fruit (collected in the area of Volos, Greece) and were reared in the above standard laboratory conditions on olive fruit (female oviposition and immature development) for one generation (F1). Using an aspirator, adults were separated by sex within one-day post emergence that is several days before attaining sexual maturation32. Experimental flies experienced a simulated dawn and dusk during which the light intensity ramped up and down during 1 h period, respectively. We used unmated flies (males and females) to avoid potential effects of mating on longevity and egg production that could confound our results. Especially for female tephritids, male insemination is not necessary for egg production52,54,55 and virgin females are widely used35,44,56 to avoid possibly confounding components costs, such as potential mortality risk due to copulation, exposure to males and thus to chemical communication between them, that are associated with copulation in the olive fly and other female insects52,57,58.
Effect of α-pinene and protein availability on male longevity
Soon after sexing males were placed in groups of 40 in plexiglass cages (20 by 20 by 20 cm bearing two 225 cm2 windows covered with mesh cloth for ventilation) with water and adult food (ad libitum) consisting of either a mixture of yeast hydrolyzate (MP Biomedicals LLC, France), sugar and water at 1:4:5 (FD conditions) or a mixture of sugar and water (DR conditions). On day 14 of adult life, 10 adults of each food regime were transferred into small cubic plexiglass cages (15 by 15 by 15 cm bearing a round opening of 7.5 cm in diameter on one side that served as the entrance to the cage) and exposed to 20 μl (-)-α-pinene (98% purity, Alfa, Aesar, France) or water for control, for three successive days. Comprehensive details and exact procedure of exposure to α-pinene are given by Gerofotis32. Therefore, we established four male cohorts that experienced FD conditions and were either exposed (a) or non-exposed (control) to α-pinene (b) and DR conditions and were either exposed (c) or non-exposed (control) to α-pinene (d). On day 17 (one day after last exposure to α-pinene), males of each group were transferred and held individually till the end of adult life into transparent plastic cages (400-ml capacity plastic cups) bearing a 24 cm2 lateral opening covered with mesh cloth to allow adequate ventilation and offered the respective adult food they had experienced before. Male lifespan was recorded daily until the death of last individual in the cohort. Fifty individuals were considered for each treatment.
Effect of α-pinene and protein availability on female fitness
Following the procedures described above we established four female cohorts: females that experienced FD conditions and were either exposed (a) or non-exposed (control) to α-pinene (b) and DR conditions that were exposed (c) or non-exposed to α-pinene (d). On day 17 (one day after last exposure to α-pinene), females of each group were transferred and held individually into transparent plastic cages (similar to those described above) maintaining them with the respective adult food they had experienced till then. As oviposition substrates we used five 18 mm, yellow, hollow, ceresin hemispheres (domes) that were fitted into five holes of respective size perforated on the base of each female cage (see52). Egg production and female age at death were recorded until the death of last individual in the cohort. Fifty individuals were considered for each treatment.
Data analysis
Kaplan-Meier estimators followed by the log-rank test were used for modeling and comparing lifespans of both males and females, and oviposition and post oviposition periods of females. Pairwise log-rank tests were adjusted for multiple comparisons. Poisson regression was used for the assessment of the effect of α-pinene and protein availability on fecundity. Squared Wald test results for the model parameters are presented. The comparison of oviposition distributions in a pairwise fashion, between exposed and non-exposed individuals within each diet regime, was assessed through ROC (Receiver Operation Curve) analysis along the lines presented in Alonso59. Chi-square was used to compare the proportion of ovipositing females in a pairwise manner among the four female cohorts. For all analyses, p-values less than 0.05 were considered statistically significant. SPSS 21.0 (IBM Corp., Armonk, NY) and R 3.1.3 (R Core Team, Vienna, Austria) were used for data analysis.
Additional Information
How to cite this article: Gerofotis, C. D. et al. The odor of a plant metabolite affects life history traits in dietary restricted adult olive flies. Sci. Rep. 6, 28540; doi: 10.1038/srep28540 (2016).
Supplementary Material
Acknowledgments
We thank Georgia Papadogiorgou and Kyriakos Pavlidis (University of Thessaly) for technical support, and Daniel Hahn (University of Florida) for his valuable comments on an earlier draft of the manuscript. We also acknowledge the contribution of the two anonymous reviewers whose comments substantially improved the manuscript.
Footnotes
Author Contributions C.D.G., N.T.P. and C.S.I. conceived and designed the experiments; C.D.G. and C.S.I. performed the experiments; C.D.G., N.T.P. and C.T.N. analyzed the data, and C.D.G., N.T.P., C.S.I. and C.T.N. interpreted data, constructed table and graphics, and wrote the paper.
References
- Van Noordwijk A. J. & Dejong G. Acquisition and allocation of resources: Their influence on variation in life history tactics. Am. Nat. 128, 137–142 (1986). [Google Scholar]
- Tatar M. & Carey J. R. Nutrition mediates reproductive trade-offs with age-specific mortality in the beetle Callosobruchus maculatus. Ecology 76, 2066–2073 (1995). [Google Scholar]
- Costantini D., Metcalfe N. B. & Monaghan P. Ecological processes in a hormetic framework. Ecol. Lett. 13, 1435–1447 (2010). [DOI] [PubMed] [Google Scholar]
- Mattson M. P. Dietary factors, hormesis and health. Ageing Res. Rev. 7, 43–48 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns S. C. The Evolution Of Life Histories, Oxford University Press, New York (1992). [Google Scholar]
- Wiegant F. A. et al. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology 10, 27–42 (2009). [DOI] [PubMed] [Google Scholar]
- Boyd O. et al. Nectarine promotes longevity in Drosophila melanogaster. Free Radic. Biol. Med. 50, 1669–1678 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S. I. Hormesis in aging. Ageing Res. Rev. 7, 63–78 (2008). [DOI] [PubMed] [Google Scholar]
- Leonov A. et al. Longevity extension by phytochemicals. Molecules 20, 6544–6572 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur J. A. et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. et al. The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. Age 35, 69–81 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. A. et al. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 5, 59–68 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S. et al. Prolongevity effects of an oregano and cranberry extract are diet dependent in the Mexican fruit fly (Anastrepha ludens). J. Gerontol. A Biol. Sci. Med. Sci. 65A, 41–50 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. et al. Acai palm fruit (Euterpe oleracea Mart.) pulp improves survival of flies on a high fat diet. Exp. Gerontol. 45, 243–251(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonhoven L. M., Van Loon J. J. A. & Dicke M. Insect-Plant Biology. Oxford University Press, New York (2005). [Google Scholar]
- Stensmyr M., Larsson M., Bice S. & Hansson B. Detection of fruit- and flower-emitted volatiles by olfactory receptor neurons in the polyphagous fruit chafer Pachnoda marginata (Coleoptera: Cetoniinae). J. Comp. Physiol. A 187, 509–519 (2001). [DOI] [PubMed] [Google Scholar]
- Fletcher B. S. & Prokopy R. J. Host location and oviposition in tephritid fruit flies. Reproductive Behaviour of Insects. Individuals and populations. Chapman & Hall, New York (1991). [Google Scholar]
- Linz J. et al. Host plant-driven sensory specialization in Drosophila erecta. Proc. R. Soc. B 280, 1760 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T. et al. Lifespan extending activity of substances secreted by the nematode Caenorhabditis elegans that include the Dauer-inducing pheromone. Biosci. Biotechnol. Biochem. 69, 2479–2481 (2005). [DOI] [PubMed] [Google Scholar]
- Libert S. et al. Regulation of Drosophila life span by olfaction and food-derived odors. Science 315, 1133–1137 (2007). [DOI] [PubMed] [Google Scholar]
- Libert S. & Pletcher S. D. Modulation of longevity by environmental sensing. Cell 131, 1231–1234 (2007). [DOI] [PubMed] [Google Scholar]
- Lee S.-J. & Kenyon C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr. Biol. 19, 715–722 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong D. E., Artan M., Seo K. & Lee S. J. Regulation of lifespan by chemosensory and thermosensory systems: findings in invertebrates and their implications in mammalian aging. Front. Genet. 3, 218 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon P. C., Kuo T. H., Linford N. J., Roman G. & Pletcher S. D. Carbon dioxide sensing modulates lifespan and physiology in Drosophila. PLoS Biol. 8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld J. & Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 402, 804–809 (1999). [DOI] [PubMed] [Google Scholar]
- Alcedo J. & Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41, 45–55 (2004). [DOI] [PubMed] [Google Scholar]
- Tzanakakis M. E. Insects and Mites Feeding on Olive. Distribution, Importance, Habits, Seasonal Development & Dormancy, Brill Academic Publishes, Leiden, The Netherlands (2006). [Google Scholar]
- Mazomenos B. E. & Haniotakis G. E. A multicomponent female sex pheromone of Dacus oleae Gmelin: Isolation and bioassay. J. Chem. Ecol. 7, 437–444 (1981). [DOI] [PubMed] [Google Scholar]
- Mazomenos B. E. & Haniotakis G. E. Male olive fruit-fly attraction to synthetic sex-pheromone components in laboratory and field-tests. J. Chem. Ecol. 11, 397–405 (1985). [DOI] [PubMed] [Google Scholar]
- Scarpati M. L., Loscalzo R. & Vita G. Olea europaea volatiles attractive and repellent to the olive fruit-fly (Dacus oleae, Gmelin). J. Chem. Ecol. 19, 881–891 (1993). [DOI] [PubMed] [Google Scholar]
- Liscia A. et al. Characterization of olfactory sensilla of the olive fly: Behavioral and electrophysiological responses to volatile organic compounds from the host plant and bacterial filtrate. J. Insect Physiol. 59, 705–716 (2013). [DOI] [PubMed] [Google Scholar]
- Gerofotis C. D., Ioannou C. S. & Papadopoulos N. T. Aromatized to find mates: alpha-Pinene aroma boosts the mating success of adult olive fruit flies. PLoS ONE 8, 11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanson B. G., Petterson I. E. & Taylor P. W. Diet quality mediates activity patterns in adult Queensland fruit fly (Bactrocera tryoni). J. Insect Physiol. 59, 676–681 (2013). [DOI] [PubMed] [Google Scholar]
- Carey J. R., Liedo P., Muller H. G., Wang J. L. & Vaupel J. W. Dual modes of aging in Mediterranean fruit fly females. Science 281, 996–998 (1998). [DOI] [PubMed] [Google Scholar]
- Carey J. R. et al. Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell 1, 140–148 (2002). [DOI] [PubMed] [Google Scholar]
- Alcedo J., Flatt T. & Pasyukova E. G. Neuronal inputs and outputs of aging and longevity. Front. Genet. 4, 71 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielenbach N. & Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes & Development 22, 2149–2165 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J. The genetics of ageing. Nature 464, 504–512 (2010). [DOI] [PubMed] [Google Scholar]
- Waterson M. J. et al. Water sensor ppk28 modulates Drosophila lifespan and physiology through AKH signaling. Proc. Natl. Acad. Sci. USA 111, 8137–8142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostojic I. et al. Positive and negative gustatory inputs affect Drosophila lifespan partly in parallel to dFOXO signaling. Proc. Natl. Acad. Sci. USA 111, 8143–8148 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. & Liberles Stephen D. Aversion and attraction through olfaction. Curr. Biol. 25, 120–129 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M., Flatt T. & Aguilaniu H. Reproduction, fat metabolism, and life span: what is the connection? Cell Metab. 17, 10–19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L. & Gems D. Mechanisms of aging: public or private? Nat. Rev. Genet. 3, 165–175 (2002). [DOI] [PubMed] [Google Scholar]
- Fanson B. G., Weldon C. W., Pérez-Staples D., Simpson S. J. & Taylor P. W. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni). Aging Cell 8, 514–523 (2009). [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell 120, 449–460 (2005). [DOI] [PubMed] [Google Scholar]
- Flatt T. Survival costs of reproduction in Drosophila. Exp. Gerontol. 46, 369–375 (2011). [DOI] [PubMed] [Google Scholar]
- Williams G. C. Natural selection, the costs of reproduction, and a refinement of Lack’s Principle. Am. Nat. 100, 687–690 (1966). [Google Scholar]
- Reznick D., Nunney L. & Tessier A. Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol. Evol. 15, 421–425 (2000). [DOI] [PubMed] [Google Scholar]
- Partridge L., Gems D. & Withers D. J. Sex and death: what is the connection? Cell 120, 461–472 (2005). [DOI] [PubMed] [Google Scholar]
- Taylor P. W. et al. Post-teneral nutrition as an influence on reproductive development, sexual performance and longevity of Queensland fruit flies. J. Appl. Entomol. 137, 113–125 (2013). [Google Scholar]
- Lee K. P. et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 105, 2498–2503 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerofotis C. D., Yuval B., Ioannou C. S., Nakas C. T. & Papadopoulos N. T. Polygyny in the olive fly–effects on male and female fitness. Behav. Ecol. Sociobiol. 69, 1323–1332 (2015). [Google Scholar]
- Flatt T., Tu M. P. & Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays 27, 999–1010 (2005). [DOI] [PubMed] [Google Scholar]
- Chapman T., Miyatake T., Smith H. K. & Partridge L. Interactions of mating, egg production and death rates in females of the Mediterranean fruit fly, Ceratitis capitata. Proc. Biol. Sci. 265, 1879–1894 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S., Kattel R., Bhatia B., Petherwick A. & Chapman T. The effect of diet, sex and mating status on longevity in Mediterranean fruit flies (Ceratitis capitata), Diptera: Tephritidae. Exp. Gerontol. 40, 784–792 (2005). [DOI] [PubMed] [Google Scholar]
- Carey J. R. et al. Stochastic dietary restriction using a Markov-chain feeding protocol elicits complex, life history response in medflies. Aging Cell 4, 31–39 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi S. & Miyatake T. Costs of mating and egg production in female Callosobruchus chinensis. J. Insect Physiol. 49, 823–827 (2003). [DOI] [PubMed] [Google Scholar]
- Kuijper B., Stewart A. D. & Rice W. R. The cost of mating rises nonlinearly with copulation frequency in a laboratory population of Drosophila melanogaster. J. Evol. Biol. 19, 1795–1802 (2006). [DOI] [PubMed] [Google Scholar]
- Alonzo T. A., Nakas C. T., Papadopoulos N. T. & Papachristos D. P. A Receiver Operating Characteristic Analysis Approach for the assessment of the separation of female Mediterranean fruit fly (Diptera: Tephritidae) oviposition distributions. J. Econ. Entomol. 102, 1985–1991 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.