Abstract
Lyme Disease is caused by the bacterial pathogen Borrelia burgdorferi, and is transmitted by the tick-vector Ixodes scapularis. It is the most prevalent arthropod-borne disease in the United States. To determine the seroprevalence of B. burgdorferi antibodies in white-tailed deer (Odocoileus virginianus) from Texas, we analyzed serum samples (n = 1493) collected during the 2001–2015 hunting seasons, using indirect ELISA. Samples with higher sero-reactivity (0.803 and above) than the negative control group (0.662) were further tested using a more specific standardized western immunoblot assay to rule out false positives. Using ELISA, 4.7% of the samples were sero-reactive against B. burgdorferi, and these originated in two eco-regions in Texas (Edwards Plateau and South Texas Plains). However, only 0.5% of the total samples were sero-reactive by standardized western immunoblot assay. Additionally, both ELISA and standardized western immunoblot assay results correlated with an increased incidence in human Lyme Disease cases reported in Texas. This is the first longitudinal study to demonstrate fluctuation in sero-reactivity of white-tailed deer to B. burgdorferi sensu stricto antigens in southern United States. Future ecological and geographical studies are needed to assess the environmental factors governing the prevalence of Lyme Disease in non-endemic areas of the southern United States.
Keywords: Borrelia burgdorferi, Ixodes scapularis, Lyme disease, Sero-reactivity, Texas, White-tailed deer
Graphical abstract
Highlights
-
•
White-tailed deer serum samples were analyzed for anti Borrelia burgdorferi IgG.
-
•
This is the first 15-year longitudinal study reported in Texas, and provides data previously unavailable within the study of Lyme disease ecology.
-
•
White-tailed deer population density might be critical to sero-prevalence.
-
•
Further pathogenic landscape studies on Lyme disease in Texas are recommended.
-
•
Databased Lyme disease ecology models in Texas can be developed.
1. Introduction
Lyme Disease (LD) is a multisystem infectious bacterial disease caused by Borrelia burgdorferi and is transmitted by the tick vector Ixodes scapularis. This disease is considered the most prevalent arthropod-borne disease in the United States (US). In recent years, there has been an increase in the number of human LD cases confirmed by the Centers for Disease Control and Prevention (CDC, 2015) across its geographic distribution. In addition, recent studies project that LD is more prevalent than previously expected with over 300,000 infected individuals annually, and therefore significantly under-reported (Kuehn, 2013).
To date, most studies investigating LD prevalence in the US have focused on the endemic northeastern and midwestern states (Lane and Burgdorfer, 1986, Gill et al., 1994, Ostfeld and Keesing, 2000, Pepin et al., 2012) with few studies carried out in nonendemic southern US. Nevertheless, in recent years several studies on LD in the Texas-Mexico transboundary southern US region have emerged (Illoldi-Rangel et al., 2012, Clark et al., 2014, Feria-Arroyo et al., 2014, Rudenko et al., 2014, Szonyi et al., 2015; Mitchell et al., 2016). In addition, a number of reports about the isolation of B. burgdorferi spirochetes from humans, as well as from I. scapularis ticks removed from animals in Texas were published during the 1980’a and 1990’s (Burgdorfer and Keirans, 1983, Rawlings, 1987, Rawlings et al., 1987, Piesman and Sinsky, 1988, Teltow et al., 1991, Rawlings and Teltow, 1994).
In North America, B. burgdorferi sensu stricto causes LD while B. afzelii and B. garinii are considered the cause of most European cases. Other Borrelia genospecies possibly associated with human clinical cases are B. valaisiana, B. bissettii, B. americana, and the recently discovered B. mayonii (genospecies number 19) (Ryffel et al., 1999, Stanek and Reiter, 2011, Dolan et al., 2016). In the US, the ticks responsible for the transmission of this pathogen are I. scapularis and I. pacificus. Other Ixodes species known to participate in the enzootic cycle of this bacterial pathogen in the US are I. dentatus, I. affinis and I. uriae (Olsen et al., 1995, Brownstein et al., 2003).
Ixodes are three-host ticks with a lifecycle spanning two to four years during which they undergo four developmental stages including egg, larva, nymph and adult. The larval and nymphal stages of these ticks feed on a wide host range including small mammals such as the white-footed mouse (Peromyscus leucopus; a natural reservoir of B. burgdorferi), chipmunks and squirrels, birds, reptiles, and also larger animals such as white-tailed deer (WTD) (Frank et al., 1998, Ostfeld et al., 2006). Adult stages of Ixodes prefer to feed on large mammals, especially WTD, Odocoileus virginianus.
The literature has emphasized the importance of WTD as hosts for Ixodes. WTD not only facilitate mating by the adult stages of Ixodes, but also serve as a source of blood meal for female Ixodes egg production (Main et al., 1981, Wilson et al., 1985). Nevertheless, WTD is not considered a reservoir for B. burgdorferi (Ostfeld et al., 2006). It is also worthy to note that WTD do not show symptoms of diseases when infected female Ixodes feed on them. On the other hand, due to their role in maintaining tick populations, studies in the endemic areas of northeastern and midwestern US have shown that WTD densities and Ixodes abundance correlated positively with human cases of LD. A lower density of WTD and number of ticks in these areas is correlated with lower numbers of reported cases of LD in humans (Wilson et al., 1985, Kilpatrick et al., 2014). However, some studies showed that elimination of WTD does not remove the risk of LD in an area (Ostfeld and Keesing, 2000) due to the complexity of the ecology of this disease. In addition, data regarding the ecology of LD in nonendemic southern US is very limited.
Serological tests have revealed the presence of antibodies to B. burgdorferi in various animal species. These include WTD as well as other wild mammals (white-footed mouse, raccoon), and domestic animals (dog, cat, horse, cattle) (Main et al., 1981, Magnarelli et al., 1984, Brownstein et al., 2003). The application of serologic surveillance in WTD has been used to establish geographic locations where B. burgdorferi circulates (Magnarelli et al., 1984, Magnarelli et al., 1986, Lane and Burgdorfer, 1986, Gill et al., 1994, Martinez et al., 1999).
With little being known about LD ecology in the southern US (Esteve-Gassent et al., 2015, Szonyi et al., 2015), the detection of I. scapularis ticks infected with B. burgdorferi in Texas (Feria-Arroyo et al., 2014), and the growing population of WTD nationwide (Rawinski and Square, 2008, McShea, 2012, Raizman et al., 2013), the objective of the current study was to determine the sero-reactivity of Texas WTD to B. burgdorferi during a 15-year longitudinal study (2001–2015).
2. Materials and methods
2.1. White-tailed deer serum sample collection
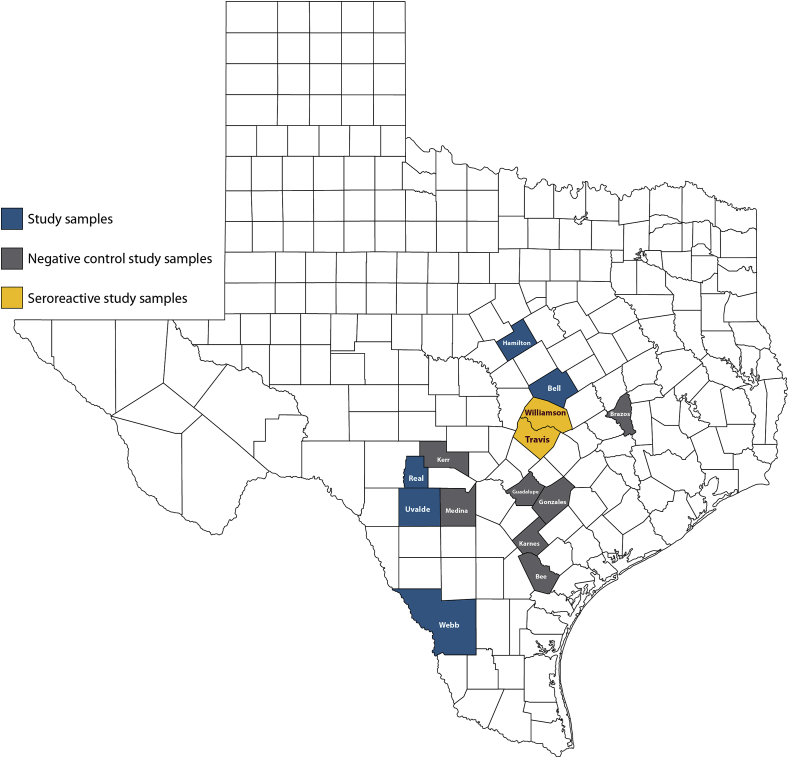
From October 2001 to February 2015, a total of 1493 male and female WTD ranging from 0.5 to 6.5 years of age were sampled during the Texas hunting season from 14 counties of the state. About 56.9% of the sampled WTD were adults (two years and older), 23% were yearlings (one to two years old), and 20.1% fawns (less than one year of age). The counties from which samples were collected included Bee, Bell, Brazos, Gonzales, Guadalupe, Hamilton, Karnes, Kerr, Medina, Real, Travis, Uvalde, Webb, and Williamson (Fig. 1).
Fig. 1.
Texas map showing 14 counties in which white-tailed deer (WTD) were sampled for Borrelia burgdorferi antibodies from 2001 to 2015. Blue counties: samples negative by ELISA and standardized western immunoblot; Gray counties: negative control samples; Yellow counties: samples sero-reactive by standardized western immunoblot assay (Travis and Williamson counties). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
All blood samples were collected, centrifuged, sera separated and stored in a −20 °C freezer until used. Dr. J. Morrill from the University of Texas Medical Branch (UTMB) (Galveston, Texas), and the Orion Research and Management Services, Inc. Belton, Texas provided samples. Additional WTD serum samples, which were provided by Dr. Alice Blue-McLendon at the Texas A&M University Winnie Carter Wildlife Center, served as negative controls. These were collected from 2003 to 2013 from pen-raised WTD with no known exposure to ticks or B. burgdorferi. These animals received ivermectin injections (for its acaricidal properties) triple the recommended dose annually in the fall, and repeated every 10–14 days. In addition, a second group of negative controls were obtained in 2015 from WTD on deer ranches that implemented tick control measures, and where Ixodes is less prevalent.
2.2. Serological detection of the pathogen
Indirect ELISA was used to detect antibodies to B. burgdorferi in the sera of WTD following previously described protocols (Small et al., 2014), and modified for WTD. This modification used B. burgdorferi B31 strain A3 grown in (Barbour-Stoenner-Kelly II) BSK-II medium (pH 7.6), and supplemented with 1% inactivated rabbit serum, at 32 °C and 1% CO2. ELISA plates were blocked with 3% bovine serum albumin (BSA) to reduce nonspecific reactivity. The primary antibody dilution used was 1:200 (WTD serum samples) and 1:2000 was used for the secondary antibody dilution (Horseradish peroxidase-conjugated Rabbit anti-deer Immunoglobin G, Rockland Immunochemicals, Inc., Limerick, PA, USA). Both primary and secondary dilutions were carried out in 0.1 M phosphate-buffered saline (pH 7.4) with 0.1% Tween 20. The substrate used for the enzyme included both o-phenylene diamine dihydrochloride (OPD) (Thermo Fisher Scientific, Life Technologies, Carlsbad, CA, USA) and hydrogen peroxide. Optical density values were read at 450 nm. Samples were considered sero-reactive when the optical density 450 nm (OD) values were three standard deviations (SD) above the mean for the negative controls (OD = 0.662). All samples were tested in triplicates.
Commercially developed standardized western immunoblot assays for the analyses of WTD sera are not available. Therefore, the samples with a high sero-reactivity (high optical density above the cut off value) when compared to the negative controls, were tested further with a standardized western immunoblot assay. This assay was used to determine the specificity of the immune reaction to B. burgdorferi specific antigens, and to rule out false positives. The standardized western immunoblot assay used in this study was modified using previous studies (Gill et al., 1994). B. burgdorferi B31 strain A3 was the test antigen used. This modification used B. burgdorferi pure cell lysates, which were separated in 12% SDS-PAGE gels following standardized electrophoresis protocols (Maruskova et al., 2008). After Borrelia proteins were separated, gels were transferred to nitrocellulose membranes (GE HealthCare) using the RTA transfer blot kit (Bio-Rad Laboratories, Inc. Hercules, CA, USA) following manufacturer’s recommendations. The membranes were blocked using 1% nonfat skimmed milk in Tris Buffer Saline (TBS) containing 0.2% Tween 20. Primary antibody (WTD serum samples) was utilized at 1:1500 dilution and incubated overnight at 4 °C, while secondary antibody (Peroxidase conjugated Rabbit anti-deer IgG, Rockland Immunochemicals, Inc., Limerick, PA, USA) dilution at 1:5000 was incubated for one hour at room temperature. All blots were visualized using Chemiluminescence (Bio-Rad Chemiluminescence and Colorimetric detection kit, Bio-Rad Laboratories, Inc., Hercules, CA, USA) and imaged using a ChemiDoc ™ Touch (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Samples with five or more bands (excluding cross-reactive bands that were also present on the immunoblots of the negative control samples) were considered sero-reactive, in line with the LD diagnostic standards established by the CDC, while samples with four or less bands were considered negative.
The sero-reactive samples were further analyzed using the B. burgdorferi (IgG) Marblot Strip System (Trinity Biotech Plc., Bray, Ireland) following manufacturer’s recommendations with modifications to adapt the protocol to WTD. Unbound sera were washed from the strip, and bound B. burgdorferi specific antibodies were reacted with alkaline phosphatase conjugated anti-deer IgG. The strips were then washed to remove the unbound IgG, and the strips were eventually reacted with a precipitating color developing solution, which deposited a purple precipitate on antibody-reacted bands. Bands were visualized, and scored for intensity relative to the 41 kDa band of the weakly reactive control and recorded.
2.3. Statistical analysis
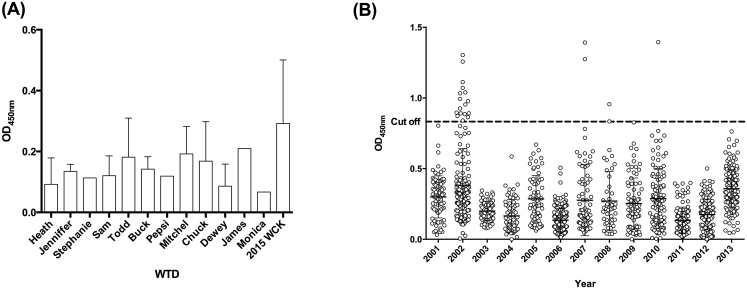
The statistical analysis used to determine the cut-off value for the indirect ELISA was STATA 12.0 statistical software (STATA, StataCorp LP, College Station, Texas 77845 USA). The cut-off value (Fig. 2) was calculated as three standard deviations plus the average of negative controls.
Fig. 2.
Optical density values of the white-tailed deer (WTD) serum samples analyzed with indirect ELISA for Borrelia burgdorferi. (A.) ELISA data from 109 WTD serum samples used as negative controls, collected from 2003 to 2015. (B.) ELISA data from 1384 WTD serum samples collected from 2001 to 2013. The dashed line denotes the cut off value used in this study. The samples above this line were analyzed with standardized western immunoblot assay.
3. Results
Serum samples of 1493 WTD were collected and evaluated for sero-reactivity against B. burgdorferi. Of these samples, 1384 were categorized as the study samples while 109 were the negative controls. The indirect ELISA results were read at the optical density of 450 nm (OD) and ranged from 0 to 1.395 for the test samples, and 0–0.662 for the negative control samples. The cut off value (0.803) used to detect the highly sero-reactive samples was calculated by adding the average of the negative controls to three times their standard deviation. Therefore, test samples above 0.803 were considered sero-reactive and thereafter further analyzed with standardized western immunoblot assay (Fig. 2).
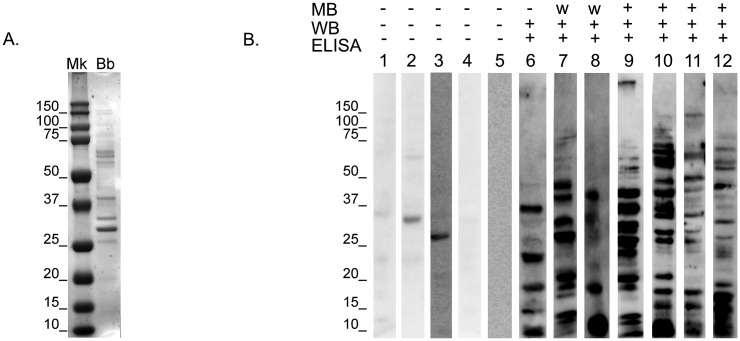
A total of 65/1384 (4.7%) WTD had a high sero-reactivity to B. burgdorferi by indirect ELISA and 7/1384 (0.5%) by standardized western immunoblot assay (Table 1). Control samples (109) were utilized to evaluate cross-reactivity of WTD negative serum to B. burgdorferi antigens. In this experiment, we observed that a number of bands (28 kDa, 32 kDa, 35 kDa, 70 kDa) appeared cross-reactive because they were also found in the negative control samples (Fig. 2). Consequently, those four bands were removed from analyses of the test samples, and not considered when marking the highly sero-reactive WTD samples (Table 2). The samples marked as highly sero-reactive were those that had five or more reactive bands by the standardized western immunoblot assay (Fig. 3B). Consequently, from the sero-reactive group, 10.8% (7 out of 65) of the highly sero-reactive samples by ELISA were also sero-reactive by standardized western immunoblot assay, which represented approximately 0.5% (7 out of 1384) of the total samples studied. The gender distribution of the sero-reactive samples by standardized western immunoblot assay was as follows; 42.9% (3/7) were males (two fawns and a yearling) while 57.1% (4/7) were adult females. There is no apparent age or sex trend observed with respect to the sero-reactive samples. In addition, the sero-reactive samples by standardized western immunoblot assay were further tested using the commercially available Marblot western blot assay (Fig. 3B). This assay is designed for qualitative in vitro detection of human immunoglobulin G (IgG) antibody to individual proteins of B. burgdorferi (B31) in human serum (http://www.trinitybiotech.com) to confirm diagnostics of Lyme disease (CDC, 1995). As shown in Fig. 3, four of the seven sero-reactive WTD samples also provided a sero-reactive result using the commercially available Marblot Strip System. The presence of bands on these Marblot strips indicated that specific antibodies to individual B. burgdorferi proteins were present in the WTD sera. In particular, the reactive bands correspond with Borrelia antigens at 66 kDa, 60 kDa, 58 kDa, 34 kDa. The remaining two samples provided a very weak immunoreactivity to the different Borrelia antigens presented in this test, and therefore we consider those two samples negative for the test.
Table 1.
White-tailed deer serum samples positive for Borrelia burgdorferi by ELISA and standardized western immunoblot assay, with their respective years and location.
| Year | Texas County | Number of samples positive by ELISA | Number of samples positive by standardized western immunoblot assay |
|---|---|---|---|
| 2001 | Travis | 3 | 0 |
| 2002 | Travis | 29 | 6 |
| 2005 | Travis | 3 | 0 |
| 2007 | Travis, Uvalde | 7 | 0 |
| 2008 | Travis | 3 | 0 |
| 2009 | Travis, Williamson | 4 | 1 |
| 2010 | Travis | 10 | 0 |
| 2013 | Travis, Williamson | 6 | 0 |
| Total | 65 | 7 |
Only seven of the samples highly sero-reactive by ELISA were positive by standardized western immunoblot assay, indicating a potential cross-reactivity with proteins similar to Borrelia burgdorferi.
Table 2.
The seven white-tailed deer serum samples positive by standardized western immunoblot assay, and the respective Borrelia burgdorferi antigens they reacted against upon testing with the diagnostic standardized Marblot protocol for human Lyme Disease.
| No of WTD samples | Molecular weights in kDa of B. burgdorferi antigens |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 93 | 70 | 66 | 60 | 58 | 45 | 41 | 39 | 34 | 31 | 30 | 28 | 23 | 18 | |
| Negative | 5 | 4 | 2 | 3 | 3 | 5 | 2 | 5 | 6 | 7 | 7 | 6 | 3 | 6 |
| Weak | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – |
| Positive | 2 | 3 | 5 | 4 | 4 | 2 | 5 | 2 | 1 | 0 | 0 | 1 | 3 | 1 |
This table summarizes the number of WTD serum samples, which were negative, weak or positive for each of the B. burgdorferi antigens on the standardized marblot protocol for human Lyme Disease.
Fig. 3.
Assays used to demonstrate reactivity to Borrelia burgdorferi antigens in white-tailed deer serum samples. (A.) The molecular weight marker (Mk) showing estimated molecular weights of Borrelia antigens (Bb). (B.) From left to right, negative control samples (1–5) and samples highly sero-reactive (6–12). The three immunoassays used were ELISA, standardized western immunoblot (WB) and Marblot (MB) assays.
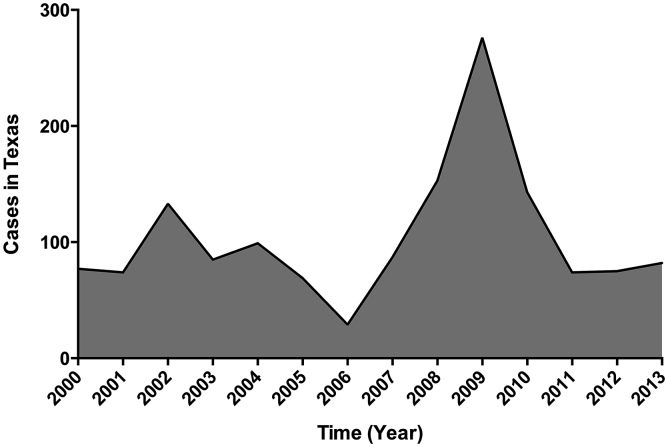
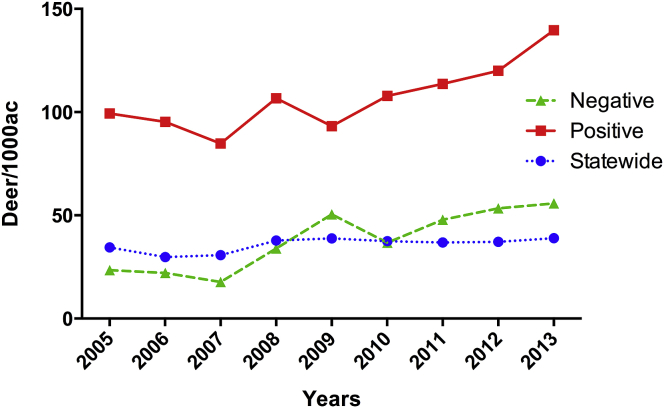
Overall, the counties with the highest prevalence of sero-reactive samples were Travis and Williamson, located in central region of Texas (Fig. 1). Interestingly, those sero-reactive WTD samples were detected in years 2002 (6 samples) and 2009 (1 sample), which correlate with the years in which Texas reported to CDC their highest numbers of human LD (Fig. 4). In addition, when looking at the density of WTD in the counties with the sero-reactive samples, we observed that they are located in the eco-regions with the highest WTD densities in the state of Texas. Furthermore, those densities were above state average, with a continuous increase since 2007 (Fig. 5).
Fig. 4.
Confirmed human Lyme Disease cases in Texas from 2000 to 2013 reported to the CDC (www.cdc.com).
Fig. 5.
White-tailed deer (WTD) population density in Texas eco-regions from 2005 to 2013. This graph is a representation of WTD population density statewide in Texas; in two counties (Travis and Williamson) where sero-reactive samples, and negative control samples for Borrelia burgdorferi antibodies were found.
4. Discussion
The current study was designed to evaluate the sero-reactivity of WTD to LD pathogen (B. burgdorferi) in central and south Texas, and we showed a sero-reactivity of only 4.7% (65/1384) by indirect ELISA and 0.5% (7/1384) by standardized western immunoblot assay. In addition, the sero-reactive samples are distributed across different eco-regions of Texas, in particular, the central region of the state (Texas Parks and Wildlife Department, 2015; https://tpwd.texas.gov/education/hunter-education/online-course/wildlife-conservation/texas-ecoregions). Additionally, the years of high WTD sero-reactivity correlate with the years during which there was a peak of reported LD cases in Texas (Fig. 4). The observed discrepancy between the ELISA and western blot results presented in this study may be due to potential cross-reactivity of WTD IgG with antigens from different Borrelia species. The animals tested were in sylvatic environments and may have had multiple bites by different tick species that might have exposed them to a number of bacterial species with antigenic structures similar to those present in the B. burgdorferi cultures used for the ELISA assay. For instance, the presence of Relapsing Fever Borrelia such as B. turicatae and B. lonestari has been documented in Texas (Barbour et al., 1996; (Whitney et al., 2007). These bacterial species have been shown to have cross-reactive antigens with B. burgdorferi (Bunikis and Barbour, 2002). Taking together, this could explain the higher number of sero-reactive animals by ELISA.
The negative control samples were obtained from pen-raised WTD at the Texas A&M University Winnie Carter Wildlife Center, with no known exposure to ticks or B. burgdorferi; deer ranches where ectoparasites and acarid control measures were implemented; and areas where there was a lower possibility of Ixodes survival. Due to the fact that WTD are free roaming animals and do not get spirochetemia when exposed to B. burgdorferi infected ticks, the objective of our study was to obtain a large pool of negative samples, including those with known lack of tick exposure, as well as those that could have been exposed to ticks but not to the bacterial pathogen, to account for a more realistic background reactivity of WTD serum samples in the different serological tests performed.
Upon further analysis of the 65 samples that were sero-reactive by ELISA with standardized western immunoblot assay, samples with five or more bands were considered highly sero-reactive, while samples with four or less bands were marked negative. There is a lack of standardized tests for WTD and any information on their reactivity to specific B. burgdorferi antigens. Therefore, the decision of high sero-reactivity was based on the LD diagnostic standards of the Center for Disease Control and Prevention (CDC) and the immunoblot results obtained in this study (Fig. 3). Some of the reactive bands seen with the samples sero-reactive by standardized western immunoblot assay were consistent with the bands seen on western immunoblots of samples from humans with LD (Craft et al., 1986, Grodzicki and Steere, 1988, Gill et al., 1993). In the current study, the 28 kDa, 32 kDa, 35 kDa and 70 kDa Borrelia antigens may be nonspecific as these were found in most of the WTD serum samples negative by ELISA, as well as the negative control samples (Fig. 3). Furthermore, only one of the seven samples sero-reactive by western immunoblot assay showed a response to one of the two major outer surface proteins, OspB (34 kDa) (Table 2). However, animals immunized with killed LD spirochetes have been shown to respond to the outer surface proteins OspA and OspB (Gill et al., 1993). Previous studies have also reported a lack of response to the outer surface proteins of B. burgdorferi in humans infected in the early stage of LD (Craft et al., 1986, Grodzicki and Steere, 1988, Guy, 1993). Therefore, we could hypothesize that the sero-reactive animals had a recent exposure to B. burgdorferi, because our sampling period (fall and winter months in Texas) correlates with the season in which I. scapularis adult ticks shows its highest activity.
Three of the seven sero-reactive WTD samples did not give a positive result when the Marblot Strip System was used, but these samples showed a number of reactive bands in the developed in-house immunoblot test, and high ELISA readings. This discrepancy could be due to the fact that other Borrelia species such as B. lonestari, the causative agent of Southern Tick Associated Rash Illness (STARI), is also present in the state of Texas. In this respect, B. lonestari is known to be transmitted by Amblyomma americanum (Lone Star tick), which also feeds on WTD, and co-infections could be present. Unfortunately, the sero-reactivity of our WTD samples against B. lonestari antigens was not possible to evaluate due to the lack of any available isolate.
One of the limitations of our study was the lack of a positive control. This is due to the fact that currently, there is no isolate of B. burgdorferi from Texas that may be used to immunize or infect WTD in order to generate a positive control group. Moreover, the conditions that will make WTD seroconvert have not yet been clearly established. In addition, this is the first study in Texas, so there is no prior data collection to compare our new WTD data. Therefore, we evaluated the high sero-reactivity based on a comparison with our negative controls. Taken together, it is therefore likely that, the generally low sero-reactivity recorded in this serological analysis of WTD in Texas, may be an indication of a low incidence of LD in this non-endemic southern region of the US.
In reviewing the literature, no data was found on the correlation of a high population density of WTD with LD incidence in Texas, but there have been reports of a positive correlation between WTD population and LD cases in endemic regions of the US (Wilson et al., 1985, Kilpatrick et al., 2014). Nonetheless, there has been an increase in population density of WTD from 14,000 to 3.8 million across Texas over the past decade (Alan, 2013). As shown in Fig. 5, the highly sero-reactive WTD were reported in one of the Texas eco-regions in which WTD population density has significantly increased (Alan, 2013) over the past decade. Therefore, we hypothesize that the increased populations of WTD may be a contributing factor to the increased LD cases in these two counties.
Moreover, this study is unique by virtue of being the first of its kind with such large number of samples obtained over an extended 15-year longitudinal period (2001–2015). The results from this study serve as the underlying basis for further exploration of the important factors that contribute to the pathogenic landscape of LD in Texas. In addition, through the evaluation of WTD sera across multiple continuous years, we have provided experimental data that will facilitate the development of accurate LD ecology models in non-endemic regions of the country, rather than using extrapolations and/or assumptions from models generated in endemic areas. From our data, the overall seroprevalence for B. burgdorferi in WTD in Texas is low, but two counties in the central part of the state in which we recorded higher levels of antibodies correlate with the years during which a peak of human LD cases occurred. Additionally, we have demonstrated, for the first time, the distribution of B. burgdorferi antibodies in WTD in Texas. Future research is aimed at Geographical Information System (GIS) mapping methods and spatio-temporal analyses to evaluate land use changes over the period of time during which these serum samples were obtained. This study will be relevant to understand the link between WTD sero-reactivity to B. burgdorferi, land use changes in Texas, the observed increase in WTD populations in the central region of the state, and their impact on LD risk in non-endemic areas.
Conflict of interest
All authors declare no conflict of interest.
Acknowledgements
We thank the following for their financial support: Texas A&M AgriLife Research: “Molecular ecology of vector-borne zoonosis in the Gulf of Mexico: A One Health approach”, “Improving diagnostic methods for Lyme Disease, and epidemiology of human and animal infections in TX”, project TEXV6579 (I-9524); Department of Veterinary Pathobiology, College of Veterinary Medicine & Biomedical Sciences, Texas A&M University for graduate degree stipends; the TAMU Lyme Lab team; and Orion Research and Management Inc. We also thank Dr. Michael Criscitiello for critical reading of the manuscript.
Contributor Information
Shakirat A. Adetunji, Email: saadetunji@cvm.tamu.edu.
Rosina C. Krecek, Email: tkrecek@cvm.tamu.edu.
Gabrielle Castellanos, Email: gabs.castellanos@gmail.com.
John C. Morrill, Email: jcmorril@utmb.edu.
Alice Blue-McLendon, Email: ABlue@cvm.tamu.edu.
Walt E. Cook, Email: Wcook@cvm.tamu.edu.
Maria D. Esteve-Gassent, Email: mesteve-gassent@cvm.tamu.edu.
References
- Alan C. 2013. Performance Report Federal Aid Project No. W-127-r-20. Big Game Research and Surveys.https://tpwd.texas.gov/huntwild/wild/research/highlights/taxa/publications/Gray_2013_PronghornDiseases.pdf White-Tailed Deer Harvest Recommendations. [Google Scholar]
- Barbour A.G., Maupin G.O., Teltow G.J., Carter C.J., Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J. Infect. Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- Brownstein J.S., Holford T.R., Fish D. A climate-based model predicts the spatial distribution of the Lyme disease vector Ixodes scapularis in the United States. Env. Health Persp. 2003;111:1152–1157. doi: 10.1289/ehp.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis J., Barbour A.G. Laboratory testing for suspected Lyme disease. Med. Clin. N. Am. 2002;86(2):311–340. doi: 10.1016/s0025-7125(03)00089-0. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Keirans J.E. Ticks and lyme disease in the United States. Ann. Intern. Med. 1983;99 doi: 10.7326/0003-4819-99-1-121. 121–121. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, CDC . 2015. This CDC and the Following Are Presented Here in Different Formats.http://www.cdc.gov/lyme/ Available at. (accessed 16.12.15.) [Google Scholar]
- Centers for Disease Control and Prevention, CDC Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of lyme disease. MMWR. 1995;44:590. [PubMed] [Google Scholar]
- Clark K.L., Leydet B.F., Threlkeld C. Geographical and genospecies distribution of Borrelia burgdorferi sensu lato DNA detected in humans in the USA. J. Med. Micr. 2014;63:674–684. doi: 10.1099/jmm.0.073122-0. [DOI] [PubMed] [Google Scholar]
- Craft J.E., Fischer D.K., Shimamoto G.T., Steere A.C. Antigens of Borrelia burgdorferi recognized during lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J. Clin. Invest. 1986;78:934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M.C., Hojgaard A., Hoxmeier J.C., Replogle A.J., Respicio-Kingry L.B., Sexton C., Williams M.A., Pritt B.S., Schriefer M.E., Eisen L. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete. Candidatus Borrelia Mayonii. Ticks Tick. Borne Dis. 2016 doi: 10.1016/j.ttbdis.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Esteve-Gassent M.D., Grover A., Feria-Arroyo T.P., Castro-Arellano I., Medina R.F., Gordillo-Pérez G., de León A.A.P. Prevalence of Borrelia burgdorferi-infected ticks from wildlife hosts, a response to Norris et al. Parasit. Vectors. 2015;8:129–136. doi: 10.1186/s13071-015-0739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feria-Arroyo T.P., Castro-Arellano I., Gordillo-Perez G., Cavazos A.L., Vargas-Sandoval M., Grover A., Esteve-Gassent M.D. Implications of climate change on the distribution of the tick vector Ixodes scapularis and risk for Lyme disease in the Texas-Mexico transboundary region. Parasit. Vectors. 2014;7:199–215. doi: 10.1186/1756-3305-7-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D.H., Fish D., Moy F.H. Landscape features associated with lyme disease risk in a suburban residential environment. Landsc. Eco. 1998;13:27–36. [Google Scholar]
- Gill J.S., McLean R.G., Neitzel D.F., Johnson R.C. Serologic analysis of white-tailed deer sera for antibodies to Borrelia burgdorferi by enzyme-linked immunosorbent assay and western immunoblotting. J. Clin. Micro. 1993;31:318–322. doi: 10.1128/jcm.31.2.318-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J.S., McLean R.G., Shriner R.B., Johnson R.C. Serologic surveillance for the Lyme disease spirochete, Borrelia burgdorferi, in Minnesota by using white-tailed deer as sentinel animals. J. Clin. Micr. 1994;32:444–451. doi: 10.1128/jcm.32.2.444-451.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicki R.L., Steere A.C. Comparison of immunoblotting and indirect enzyme-linked immunosorbent assay using different antigen preparations for diagnosing early Lyme disease. J. Infect. Dis. 1988;157:790–797. doi: 10.1093/infdis/157.4.790. [DOI] [PubMed] [Google Scholar]
- Guy E.C. The laboratory diagnosis of Lyme borreliosis. Rev. Med. Micro. 1993;4:89–96. [Google Scholar]
- Illoldi-Rangel P., Rivaldi C.L., Sissel B., Trout Fryxell R., Gordillo-Pérez G., Rodríguez-Moreno A., Williamson P., Montiel-Parra G., Sánchez-Cordero V., Sarkar S. Species distribution models and ecological suitability analysis for potential tick vectors of Lyme disease in Mexico. J. Trop. Med. 2012;2012:959101. doi: 10.1155/2012/959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick H.J., Labonte A.M., Stafford K.C. The relationship between deer density, tick abundance, and human cases of lyme disease in a residential community. J. Med. Ent. 2014;51:777–784. doi: 10.1603/me13232. [DOI] [PubMed] [Google Scholar]
- Kuehn B.M. CDC Estimates 300 000 US cases of lyme disease annually. J. Am. Med. Assoc. 2013;310:1110. doi: 10.1001/jama.2013.278331. [DOI] [PubMed] [Google Scholar]
- Lane R.S., Burgdorfer W. Potential role of native and exotic deer and their associated ticks (Acari: Ixodidae) in the ecology of Lyme disease in California, USA. Zentralblatt Für Bakteriologie, Mikrobiologie Und Hygiene.Series A. Med. Micro. Infect. Dis. Virol. Par. 1986;263:55–64. doi: 10.1016/s0176-6724(86)80103-1. [DOI] [PubMed] [Google Scholar]
- Magnarelli L.A., Anderson J.F., Apperson C.S., Fish D., Johnson R.C., Chappell W.A. Spirochetes in ticks and antibodies to Borrelia burgdorferi in white-tailed deer from Connecticut, New York State, and North Carolina. J. Wildl. Dis. 1986;22:178–188. doi: 10.7589/0090-3558-22.2.178. [DOI] [PubMed] [Google Scholar]
- Magnarelli L.A., Anderson J.F., Chappell W.A. Antibodies to spirochetes in white-tailed deer and prevalence of infected ticks from foci of Lyme disease in Connecticut. J. Wildl. Dis. 1984;20:21–26. doi: 10.7589/0090-3558-20.1.21. [DOI] [PubMed] [Google Scholar]
- Main A.J., Sprance H.E., Kloter K.O., Brown S.E. Ixodes dammini (Acari: Ixodidae) on white-tailed deer (Odocoileus virginianus) in Connecticut. J. Med. Entomol. 1981;18:487–492. [Google Scholar]
- Martinez A., Salinas A., Martinez F., Cantu A., Miller D.K. Serosurvey for selected disease agents in white-tailed deer from Mexico. J. Wildl. Dis. 1999;35:799–803. doi: 10.7589/0090-3558-35.4.799. [DOI] [PubMed] [Google Scholar]
- Maruskova M., Esteve-Gassent M.D., Sexton V.L., Seshu J. Role of the BBA64 locus of Borrelia burgdorferi in early stages of infectivity in a murine model of Lyme disease. Infect. Immun. 2008;76:391–402. doi: 10.1128/IAI.01118-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShea W.J. Ecology and management of white-tailed deer in a changing world. Ann. NY. Acad. Sci. 2012;1249:45–56. doi: 10.1111/j.1749-6632.2011.06376.x. [DOI] [PubMed] [Google Scholar]
- Mitchell E.A., Williamson Phillip C., Billingsley Peggy M., Seals Janel P., Ferguson Erin E., Allen Michael S. Frequency and Distribution of Rickettsiae, Borreliae, and Ehrlichiae Detected in Human-parasitizing Ticks. Emerg. Infect. Dis. 2016;22:312–315. doi: 10.3201/eid2202.150469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B., Duffy D.C., Jaenson T.G., Gylfe A., Bonnedahl J., Bergström S. Transhemispheric exchange of Lyme disease spirochetes by seabirds. J. Clin. Microbiol. 1995;33:3270–3274. doi: 10.1128/jcm.33.12.3270-3274.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld R.S., Keesing F. Biodiversity and disease risk: the case of Lyme disease. Con. Biol. 2000;14:722–728. [Google Scholar]
- Ostfeld R.S., Keesing F., LoGiudice K. Community ecology meets epidemiology: the case of Lyme disease. In: Sharon K.C., Chris R., editors. Disease Ecology. Oxford University Press Inc.; New York: 2006. pp. 28–40. [Google Scholar]
- Pepin K.M., Rebecca J.E., Paul S.M., Joseph P., Durland F., Anne G.H., Alan G.B., Sarah H., Maria A., Diuk-Wasser M.A. Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am. J. Trop. Med. Hyg. 2012;86:1062–1071. doi: 10.4269/ajtmh.2012.11-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J., Sinsky R.J. Ability of Ixodes scapularis, Dermacentor variabilis, and Amblyomma americanum (Acari: Ixodidae) to acquire, maintain, and transmit Lyme disease spirochetes (Borrelia burgdorferi) J. Med. Entomol. 1988;23(5):336–339. doi: 10.1093/jmedent/25.5.336. [DOI] [PubMed] [Google Scholar]
- Raizman E.A., Holland J.D., Shukle J.T. White-Tailed Deer (Odocoileus virginianus) as a potential sentinel for human lyme disease in Indiana. Zoonoses Public Health. 2013;60:227–233. doi: 10.1111/j.1863-2378.2012.01518.x. [DOI] [PubMed] [Google Scholar]
- Rawinski T.J., Square N. USDA Forest Service; Newton Square, PA: 2008. Impacts of White-tailed Deer Overabundance in Forest Ecosystems: an Overview. (Available online at: http://www. na. fs. fed. us/fhp/special_interests/white_tailed_deer. pdf) [Google Scholar]
- Rawlings J.A. Lyme disease in Texas. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene. Ser. A Med. Micro. Infect. Dis. Virol. Parasit. 1987;263:483–487. doi: 10.1016/s0176-6724(87)80115-3. [DOI] [PubMed] [Google Scholar]
- Rawlings J.A., Fournier P.V., Teltow G.J. Isolation of Borrelia spirochetes from patients in Texas. J. Clin. Micro. 1987;25:1148–1150. doi: 10.1128/jcm.25.7.1148-1150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings J.A., Teltow G.J. Prevalence of Borrelia (Spirochaetaceae) spirochetes in Texas ticks. J. Med. Entomol. 1994;31:297–301. doi: 10.1093/jmedent/31.2.297. [DOI] [PubMed] [Google Scholar]
- Rudenko N., Golovchenko M., Belfiore N.M., Grubhoffer L., Oliver J.H., Jr. Divergence of Borrelia burgdorferi sensu lato spirochetes could be driven by the host: diversity of Borrelia strains isolated from ticks feeding on a single bird. Parasit. Vectors. 2014;7:3305–3307. doi: 10.1186/1756-3305-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryffel K., Péter O., Rutti B., Suard A., Dayer E. Scored antibody reactivity determined by immunoblotting shows an association between clinical manifestations and presence of Borrelia burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana in Humans. J. Clin. Microbiol. 1999;37:4086–4092. doi: 10.1128/jcm.37.12.4086-4092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small C.M., Ajithdoss D.K., Hoffmann A.R., Mwangi W., Esteve-Gassent M.D. Immunization with a Borrelia burgdorferi BB0172-Derived peptide protects mice against Lyme disease. PLoS One. 2014;9:e88245. doi: 10.1371/journal.pone.0088245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek G., Reiter M. The expanding Lyme Borrelia complex—clinical significance of genomic species? Clin. Microbiol. Infect. 2011;17:487–493. doi: 10.1111/j.1469-0691.2011.03492.x. [DOI] [PubMed] [Google Scholar]
- Szonyi B., Srinath I., Esteve-Gassent M., Lupiani B., Ivanek R. Exploratory spatial analysis of Lyme disease in Texas–what can we learn from the reported cases? BMC Pub. Health. 2015;15:924–932. doi: 10.1186/s12889-015-2286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teltow G.J., Fournier P.V., Rawlings J.A. Isolation of Borrelia burgdorferi from arthropods collected in Texas. Am. J. Trop. Med. Hyg. 1991;44:469–474. doi: 10.4269/ajtmh.1991.44.469. [DOI] [PubMed] [Google Scholar]
- TPWD . 2015. Texas Parks and Wildlife Department.https://tpwd.texas.gov/education/hunter-education/online-course/wildlife-conservation/texas-ecoregions Available at. (accessed 11.13.15.) [Google Scholar]
- Whitney M.S., Schwan T.G., Sultemeier K.B., McDonald P.S., Brillhart M.N. Spirochetemia caused by Borrelia turicatae infection in 3 dogs in Texas. Veterinary Clin. Pathol./Am. Soc. Veterinary Clin. Pathol. 2007;36:212–216. doi: 10.1111/j.1939-165x.2007.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Wilson M.L., Adler G.H., Spielman A. Correlation between abundance of deer and that of the deer tick, Ixodes dammini (Acari: Ixodidae) Ann. Ent. Soc. Am. 1985;78:172–176. [Google Scholar]