Abstract
The remarkable versatility of the mammalian brain is made possible by a huge diversity of cellular plasticity mechanisms. These include long-term potentiation and depression at both excitatory and inhibitory synapses, as well as a variety of intrinsic and homeostatic plasticity mechanisms. A fundamental challenge for the field is to assemble our detailed knowledge of these specific mechanisms into a coherent picture of how plasticity within cortical circuits works to tune network properties.
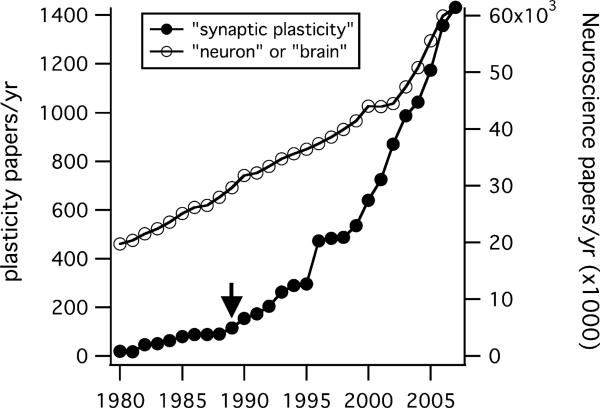
None of the articles in Neuron's inaugural issue, published nearly 20 years ago, concerned synaptic plasticity. Since that time, however, the synaptic plasticity field has undergone explosive growth, even relative to the growth of neuroscience as a whole (Figure 1). A substantial fraction of this communal effort (roughly a third of the “synaptic plasticity” publications in the last three years) has been devoted to understanding long-term potentiation (LTP) in the rodent hippocampus.
Figure 1. The Synaptic Plasticity Literature Explosion.
Filled symbols plot the number of papers published per year (left axis) estimated from a Medline search for the terms “synaptic” and “plasticity.” For much of this period (1980–2007) growth outstripped the broader neuroscientific literature estimated from searches for the terms “neuron” or “brain” (right axis). Neural plasticity papers accounted for 1/300 of this larger literature during the 1980s, but account for 1/42 papers in 2007. Arrow marks the year Neuron was founded.
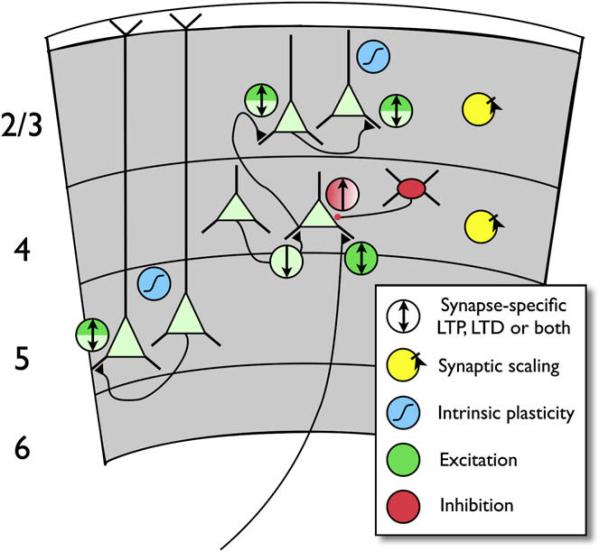
Over the last 20 years, what began as a search for the cellular basis of learning and memory zoomed downward, focusing on the molecular mechanisms underlying LTP at the Schaffer collateral synapse in (depending on your perspective) exquisite or excruciating molecular detail. Tremendous progress has been made in uncovering basic features of synaptic transmission and the regulation of excitatory synaptic strength (Malenka and Nicoll, 1999; Malinow et al., 2000). For example, we now understand the activation of kinase cascades in the postsynaptic density (Kennedy et al., 2005) and the trafficking of AMPA receptors (Nicoll et al., 2006; Shepherd and Huganir, 2007) with a level of detail that permits the functional role of these processes to be dissected. Nevertheless, success at linking these plasticity mechanisms directly to learning and sensory plasticity has been harder to come by. Perhaps part of the reason for this is that any behavioral manipulation—plucking a whisker, closing an eye, learning to navigate a maze, or for that matter even a seemingly simple manipulation like tetanizing a set of axons—sets into motion multiple plasticity mechanisms operating though distributed biochemical cascades at multiple sites within a large functionally interconnected circuit. In Figure 2, for example, we illustrate a sampling of the various forms of plasticity that have been documented at different synaptic connections within sensory cortex, many of which we will refer to in our discussion below.
Figure 2. Diverse Forms of Plasticity in Sensory Cortical Circuits.
A subset of known synaptic connections between excitatory (pale green) and inhibitory (red) neurons (black triangles are excitatory connections; red dot, inhibitory). Circles with arrows indicate LTP (up arrow), LTD (down arrow), or coexisting LTP and LTD (bidirectional arrow) of excitation (green) or inhibition (red). Light green indicates LTP/D believed to be primarily or exclusively presynaptic, dark green indicates postsynaptic. Both colors indicate evidence for mixed or coexisting pre- and postsynaptic mechanisms. Locus of LTP of inhibition is not known (red gradient). Axon entering layer 4 from below layer 6 indicates thalamocortical input to L4 spiny stellate cell. Excitatory and inhibitory synapses in layers 2/3 and 4 also exhibit bidirectional synaptic scaling (yellow symbols). Intrinsic plasticity (blue symbols) has been demonstrated in L2/3 and L5 pyramidal neurons. Some forms of plasticity shown are developmentally regulated.
As studies of synaptic plasticity have become more cell biological, it is natural to focus on mechanisms that may be common to all synapses. But one look at the range of cellular morphologies present in a single cortical column or a single hippocampal field drives home the importance of balancing the “depth” that comes with focusing on a single chain of events at a single synapse, with a broader appreciation of synaptic diversity. Synapses, like the pre- and postsynaptic neurons that comprise them, clearly come in different “types” that reflect very different morphological and molecular environments, and so it is perhaps not surprising that their plasticity mechanisms can differ widely. Here, we review some of these diverse forms of plasticity, with an emphasis on neocortical and hippocampal mechanisms. A fundamental challenge for the field is to assemble our detailed knowledge of these synapse-type specific mechanisms into a coherent picture of how plasticity within functional circuits works to tune network properties.
From Tetanus to Timing
Early studies emphasized the idea that the level of synaptic activity and subsequent postsynaptic depolarization controlled the polarity of synaptic plasticity: a rapid tetanus and/or rapid, strong depolarization produced LTP, while low frequency, milder depolarization produced LTD (Artola and Singer, 1993; Kirkwood and Bear, 1994). Critics argued that tetanic stimulation is non-physiologic since cortical and hippocampal neurons rarely fire at these rates and that spontaneous activity should produce constant depression in vivo (Holscher, 1997). While tetani and low-frequency stimulation (LFS) remain convenient experimental protocols, thinking about how plasticity is induced in vivo shifted radically with the discovery that both LTP and LTD could be induced at low frequency depending on the precise timing relationships between pre- and postsynaptic firing (for reviews see Abbott and Nelson, 2000; Dan and Poo, 2004; Linden, 1999). Early studies of spike-timing-dependent plasticity (STDP) showed that both the potentiation and depression were mediated by NMDARs. Subsequently, it has become clear that the LTP and LTD components of STDP are mechanistically distinct and may depend on different populations of NMDARs linked to distinct signaling pathways (Bender et al., 2006; Rodriguez-Moreno and Paulsen, 2008; Sjöström et al., 2007; Sjöström et al., 2003). Functionally, STDP may allow neurons to detect and enhance causal associations between their inputs. The time scales over which inputs must interact with postsynaptic activity varies with synaptic type and location and may themselves be altered by activity or neuromodulation.
Presynaptic LTP/LTD and Retrograde Transmission
Despite evidence for a presynaptic form or component of LTP at CA3-CA1 synapses under some circumstances (Bayazitov et al., 2007; Emptage et al., 2003 ; Zakharenko et al., 2003), the weight of opinion favors the idea that the major form of potentiation at this synapse is the now canonical pathway beginning with postsynaptic calcium influx through NMDAR, activation of CaMKII, and ending with enhanced insertion of AMPAR subunits (for a more detailed analysis of the presynaptic/postsynaptic debate, see article by Südhof and Malenka [2008], this issue of Neuron). Some initial studies suggested that mechanistic insights into LTP and LTD at Shaffer collateral synapses would apply equally to neocortical synapses (Kirkwood et al., 1993). Subsequently it has become clear that different classes of synapses can exhibit different forms of plasticity. For example, synapses in L4 and L2/3 of visual cortex both exhibit LTD, but this LTD depends on cannabinoid receptors and presynaptic NMDA receptors in L2/3 (Bender et al., 2006; Rodriguez-Moreno and Paulsen, 2008), but not in L4 (Brasier and Feldman, 2008; Crozier et al., 2007). In some cases, plasticity rules for a single class of synapse depend on the dendritic location (Froemke et al., 2005; Letzkus et al., 2006; Sjöström and Hausser, 2006). Even for a single synaptic connection, multiple, mechanistically distinct forms of plasticity can coexist. For example, at Schaffer collaterals in CA1, two distinct forms of LTD can be induced, one depending on NMDAR activation and one depending on mGluR activation (Oliet et al., 1997). Neocortical LTP is expressed both pre- and postsynaptically at L4 to L2/3 synapses (Hardingham and Fox, 2006) and at synapses between L5 pyramidal neurons (Sjöström et al., 2007). Both forms require postsynaptic depolarization, calcium influx through NMDAR and activation of CaMKII. The postsynaptic form, like that in CA1, is absent in GluR1 knockout mice (Hardingham and Fox, 2006), while the presynaptic form depends on the retrograde messenger nitric oxide (NO); (Hardingham and Fox, 2006; Sjöström et al., 2007).
The role of retrograde transmission in synaptic plasticity, though initially highly controversial, has been a persistent theme in multiple systems. For example, in addition to contributing to presynaptic forms of neocortical LTP, NMDAR-dependent NO production is necessary for a heterosynaptic LTP of inhibition in the ventral tegmentum (Nugent et al., 2007), a form of plasticity that is blocked by opiates and may contribute to early phases of opiate addiction. NO-dependent LTP is also believed to underlie inflammatory hyperalgesia at pain pathways in the spinal cord (Ikeda et al., 2006).
Endocannaboids appear to be an even more ubiquitous retrograde messenger contributing to presynaptically expressed plasticity. Although first found to act as short-term modulators of synapses in several systems (for review see Kreitzer and Regehr, 2002; Wilson and Nicoll, 2002), it is now clear that LTD in the amygdala (Marsicano et al., 2002), neocortex (Sjöström et al., 2003), striatum and accumbens (Gerdeman et al., 2002; Robbe et al., 2002), and cerebellum (Safo and Regehr, 2005) require activation of CB1 receptors by endogenous cannabinoids. As mentioned above, at many of these synapses presynaptically expressed forms of LTD can coexist with postsynaptic forms of LTD mediated by internalization of AMPA receptors. Remaining questions, at least for many of these systems, include the precise identity of the endogenous ligand and the mode of its release.
Beyond LTP and LTD
Recent work has begun to challenge the view that synapse-specific forms of LTP and LTD at excitatory synapses can fully explain learning and experience-dependent plasticity. In particular, three additional forms of plasticity, each with its own interesting functional implications, have now been extensively documented in cortical and hippocampal networks and in a few cases have been tied to learning and/or sensory plasticity. These are (1) plasticity of intrinsic neuronal excitability, (2) plasticity of inhibitory synapses, and (3) stabilizing, homeostatic forms of intrinsic and synaptic plasticity. We briefly describe each in turn below, and discuss their possible functions within neuronal circuits.
Intrinsic Plasticity
Until recently, the activity-dependent refinement of cortical circuits has largely been ascribed to synaptic plasticity mechanisms. However, changes in intrinsic excitability that alter the input-output function of a neuron can also strongly affect network behavior, and there is mounting evidence for activity-dependent modulation of intrinsic excitability in a variety of neurons (Marder and Goaillard, 2006; Zhang and Linden, 2003). Interestingly, some of the first reports of hippocampal LTP found that, along with synaptic changes, tetanic stimulation also induced “ES potentiation”—or an increase in the probability that a synaptic input of a given size will elicit a spike—through a mechanism that was suggested to be due, in part, to changes in the voltage-dependent conductances that shape neuronal input-output properties (Andersen et al., 1980). More recent studies have found that the classic stimuli used to induce hippocampal LTP and LTD induce synaptic and intrinsic changes in parallel (Fan et al., 2005; Frick et al., 2004). These changes can take a variety of forms: for example, LTP-inducing stimuli can enhance local dendritic excitability and thus act synergistically with LTP or conversely can lower the probability of somatic spike generation and thus serve a stabilizing function (see section on Homeostatic Plasticity below; Johnston and Narayanan, 2008; Kim and Linden, 2007). Thus, just as synaptic plasticity comes in a variety of flavors and can be induced through a variety of signaling cascades, intrinsic plasticity also exhibits a great diversity which we are just beginning to appreciate.
While the phenomenon of intrinsic plasticity has been widely documented, little is currently known about the underlying induction and expression mechanisms. A number of classic signaling pathways can regulate ion channel function through phosphorylation and other posttranslational modifications. It is also clear that the membrane ion channels that underlie neuronal firing and dendritic integration are nonuniformly distributed in neurons, and the cell biological processes that regulate the abundance and localization of neurotransmitter receptors are likely to apply as well to voltage-gated ion channels. The functions of intrinsic plasticity are also likely to be diverse. For example, activity-dependent regulation of neuronal input/output curves serves as a gain control mechanism underlying adaptive plasticity of the vesibulo-ocular reflex (Gittis and du Lac, 2006) and is correlated with some forms of learning (Matthews et al., 2008; Saar and Barkai, 2003). In general, the contribution of a neuron to circuit function can be boosted or reduced by modifying the somatic input-output function, in a way that is independent of changes in synaptic input. This provides an additional higher level of control over circuit function to that provided by synapse-specific plasticity mechanisms. In addition, local control over ion channel function in dendritic regions could act to boost or attenuate the function of particular groups of synaptic inputs onto that dendrite, thus enhancing cooperativity between inputs. Finally, some forms of intrinsic plasticity could serve a “metaplastic” function, because changing dendritic or somatic excitability can make it easier or harder to generate forms of synaptic plasticity that rely on dendritic depolarization and calcium influx, or on postsynaptic spiking. Understanding how activity shapes network function will require integrating these mechanisms into our view of circuit plasticity.
Inhibitory Plasticity
Approximately 20% of cortical and 10% of hippocampal neurons are GABAergic (Lawrence and McBain, 2003), and inhibition plays a number of critical roles in information processing. Given the importance of cortical and hippocampal inhibition, it seems likely that inhibitory synapses are an important locus of change during learning and experience-dependent plasticity. However, reports of rapidly-inducible plasticity at GABAergic synapses are relatively rare compared to reports of excitatory LTP/LTD. A number of factors have probably contributed to this paucity of reports of inhibitory plasticity. First, theoretical work has focused on changes at excitatory synapses. A second reason is the technical difficulty of studying identified inhibitory synapses in cortical or hippocampal networks. Because interneurons are extremely heterogeneous, extracellular stimulation typically activates a mixed set of inhibitory inputs which may have heterogeneous plasticity rules—and so to be interpretable many such experiments require technically challenging approaches that allow activation of indentified neurons. With the advent of mouse lines in which particular classes of GABAergic neurons are fluorescently labeled (Monyer and Markram, 2004), this has become a tractable approach. Finally, the patterns of activation required to induce plasticity at inhibitory synapses are sometimes very different from those at excitatory synapses, so finding the right combination of presynaptic and postsynaptic activation to generate inhibitory plasticity can be challenging. Recent work suggesting that inhibitory plasticity plays important roles in processes as diverse as balancing excitation and inhibition during development (Akerman and Cline, 2006; Lien et al., 2006), opiate addiction in the ventral tegmental area (Nugent et al., 2007), and the deprivation-induced loss of visual responsiveness in the rodent visual system (Maffei et al., 2006), is likely to fuel growing interest in the mechanisms and function of inhibitory plasticity.
Homeostatic Plasticity
Learning-related and experience-dependent adaptations in circuit function require that neurons detect correlations in the environment and store these as changes in synaptic or intrinsic properties. This means that, in living organisms, neuronal circuit properties are constantly being perturbed by all the forms of plasticity we have described above. This raises the question of how neurons and circuits maintain stability of function when virtually every aspect of neuronal physiology is subject to ongoing activity-dependent modifications.
An answer that has emerged over the past 15 years or so is that neuronal circuits possess an array of “homeostatic” plasticity mechanisms that serve to stabilize neuron and circuit function. A homeostatic form of plasticity can be thought of as one that acts to stabilize neuronal activity in the face of perturbations, such as changes in synapse number or strength, that alter excitability. A large number of plasticity phenomena have now been identified in a wide range of organisms and brain regions that appear to serve such a stabilizing function (Davis and Bezprozvanny, 2001; Marder and Prinz, 2003; Turrigiano, 2007; Turrigiano and Nelson, 2004). For example, neocortical neurons can detect changes in their average firing rates and scale excitatory synaptic strengths up or down to keep firing relatively constant. Interestingly, this “synaptic scaling” is thought to adjust all of a neuron's synapses up or down in strength proportionally, so that while average synaptic strength is regulated in a homeostatic manner, the relative strengths of individual synapses remain constant. This has the nice property of allowing neurons to preserve the synapse-specific differences in synaptic weights that presumably encode information, while allowing neurons to keep their activity within a functional range.
This work on homeostatic plasticity has shown that neuronal activity, like other critical physiological variables, is subject to classic homeostatic negative feedback control. How do neurons accomplish, for example, global negative feedback control of synaptic strength? The best current evidence suggests that during synaptic scaling neurons detect changes in their own firing rates through a set of calcium-dependent sensors that then regulate receptor trafficking to increase or decrease the accumulation of glutamate receptors at synaptic sites (Turrigiano, 2008). Additional mechanisms may allow synapse-specific homeo-static changes in synaptic function or network-wide changes in activity to be sensed through parallel pathways, generating a nested set of homeostatic mechanisms that operate over different temporal and spatial scales to stabilize network function.
Recent work suggests that homeostatic plasticity plays a number of important roles in the experience-dependent development of sensory systems. For example, in the visual system there is evidence that synaptic scaling mediates an activity-dependent trade-off between synapse number and strength, which serves to keep activation relatively constant despite developmental changes in connectivity (Chandrasekaran et al., 2007; Desai et al., 2002). There is mounting evidence that synaptic scaling or other forms of homeostatic plasticity underlie the potentiation of open-eye responses that follow closure of one eye during a classic form of visual system plasticity, ocular dominance plasticity (Kaneko et al., 2008; Mrsic-Flogel et al., 2007). Currently direct evidence that homeostatic plasticity helps to stabilize hippocampal function during LTP or LTD is lacking, although hippocampal circuits clearly display several forms of homeo-static plasticity.
Connecting Plasticity to Behavior
Ultimately the challenge for neurobiologists is not just to understand the cellular and molecular mechanisms of synaptic and intrinsic plasticity, but to identify how these processes contribute to behaviors such as spatial learning, fear conditioning, or sensory plasticity. Despite many successes, it has been very difficult to convincingly link any specific synaptic plasticity mechanism directly to behavioral plasticity or learning. For example, in some cases, manipulations that block hippocampal LTP do not block spatial learning (Zamanillo et al., 1999). Similar dissociations between manipulations that affect visual cortical LTD and monocular deprivation have been observed (Hensch, 2005), although the degree to which these linkages remain valid is quite contentious in both systems. There are many reasons why causal links between molecular mechanisms of plasticity and behavior have been elusive, but an important one is that individual synapses are embedded in complex circuits and many forms of plasticity operate in these circuits.
To illustrate this point, consider what we know about the changes induced within the rodent visual cortex by a relatively simple manipulation—closing one eye for a few days. Over 50 years ago, Hubel and Wiesel showed that closing one eye during a critical period of mammalian development could dramatically alter functional circuits in the visual cortex. The cellular mechanisms were initially attributed to homosynaptic LTP and LTD like those operating in CA1, but we now know that multiple forms of plasticity are engaged. Monocular deprivation has been shown to, among other things, potentiate excitatory synapses onto inhibitory interneurons and generate LTP at inhibitory synapses within layer 4 (Maffei et al., 2006), depress excitatory synapses from layer 4 to layer 2/3 (Crozier et al., 2007; Maffei and Turrigiano, 2008), and increase the intrinsic excitability of layer 2/3 pyramidal neurons (Maffei and Turrigiano, 2008). To further complicate matters, the constellation of changes produced at these sites and others depend critically on the exact age at which the manipulation is performed (Desai et al., 2002; Maffei et al., 2006; Maffei et al., 2004), as well as how vision is deprived—lid suture and blockade of retinal activity with TTX, for example, produce dramatically different effects within layer 2/3 (Maffei and Turrigiano, 2008), presumably because these different sensory manipulations have different effects on cortical activity, and thus engage a different subset of the available plasticity mechanisms. We are currently quite far from being able to assemble these observations into a unified view of how visual experience shapes microcircuitry within visual cortex. A similar “embarrassment of riches” appears to hold for activity-dependent reorganization in somatosensory cortex (Feldman and Brecht, 2005). Nonetheless, it seems clear that this diversity of cellular mechanisms is tightly orchestrated so that each form of plasticity occurs in the right place at the right time to allow the developmentally appropriate tuning of sensory circuits.
Moving Forward in the Face of Complexity
In the face of all of this diversity of cellular and molecular plasticity mechanisms, it is tempting to throw up one's hands in despair and, for example, decry the entire enterprise of trying to identify the molecular pathways that mediate changes in synaptic strength (Lisman et al., 2003). But recognizing that mechanisms of plasticity are diverse and often imperfectly isolated from one another is not at all the same as saying “anything goes.” Instead, it seems likely that there is a finite set of plasticity “modules” that subserve particular functions—such as synaptic normalization or encoding short time-scale correlations—that will be recruited as appropriate. Similarly, there is likely to be a set of molecular modules that sense patterned neural activity and transduce it under appropriate conditions into changes in transmitter release, transmitter sensitivity and neuronal excitability. Core components, like the NMDAR-Calmodulin-CaMKII components of the calcium sensing module are likely linked to dozens of additional, more specialized, molecular participants that fine tune module operation in a cell-type- or synapse-specific manner. Other modules include those that mediate trafficking and anchoring of AMPAR subunits, the retrograde signaling pathways mentioned above and core signaling pathways like that linking GPCRs to cAMP production, A-kinase activation, and CREB activation. The number of these molecular modules is probably on the order of many dozens rather than a few. It is likely these molecular modules can be mixed, interconnected, and turned on and off developmentally to fine-tune the functional plasticity modules they underlie. Viewed this way, our brains are strong and flexible by virtue of the very diversity that has been driving neuroscientists to distraction over the past 20 years.
A major experimental difficulty with sorting out which signaling modules are central to a particular form of plasticity, rather than permissive or modulatory, arises because the exquisite spatial and temporal segregation of signaling complexes is often disrupted or circumvented by the experiments we devise to study them. Examples include genetic manipulations that permit developmental compensation or that overexpress signaling elements in a non-spatially-restricted manner, biochemical measurements that homogenize distinct cell types, and physiology experiments that coactivate disparate circuit elements in non-physiological ways. But methods of circumventing these difficulties are improving. LTP and LTD can be readily induced at individual synaptic connections using more naturalistic patterns of activity. Activation of neurons can be achieved optically. Single release sites can be imaged directly (Oertner et al., 2002; Ryan et al., 1997). Biochemical analyses can be performed on single cells or cell types (Nelson et al., 2006; Tietjen et al., 2003; Trimarchi et al., 2007). And increasingly sophisticated genetic manipulations can be used for gain or loss of function with cell type and developmental specificity, both in genetic model organisms and in other organisms using viral vectors (Dymecki and Kim, 2007; Luo et al., 2008). Taken together, these feats of technical progress offer hope that over the next 20 years of plasticity research the toolbox will finally be up to the task of tackling the complexity which has stymied the linkage of particular molecular and cellular mechanisms to changes in perception and behavior.
REFERENCES
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat. Neurosci. 2000;3:1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J. Neurosci. 2006;26:5117–5130. doi: 10.1523/JNEUROSCI.0319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Sundberg SH, Sveen O, Swann JW, Wigstrom H. Possible mechanisms for long-lasting potentiation of synaptic transmission in hippocampal slices from guinea-pigs. J. Physiol. 1980;302:463–482. doi: 10.1113/jphysiol.1980.sp013256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. doi: 10.1016/0166-2236(93)90081-v. [DOI] [PubMed] [Google Scholar]
- Bayazitov IT, Richardson RJ, Fricke RG, Zakharenko SS. Slow presynaptic and fast postsynaptic components of compound long-term potentiation. J. Neurosci. 2007;27:11510–11521. doi: 10.1523/JNEUROSCI.3077-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J. Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier DJ, Feldman DE. Synapse-specific expression of functional presynaptic NMDA receptors in rat somatosensory cortex. J. Neurosci. 2008;28:2199–2211. doi: 10.1523/JNEUROSCI.3915-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran AR, Shah RD, Crair MC. Developmental homeostasis of mouse retinocollicular synapses. J. Neurosci. 2007;27:1746–1755. doi: 10.1523/JNEUROSCI.4383-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier RA, Wang Y, Liu CH, Bear MF. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc. Natl. Acad. Sci. USA. 2007;104:1383–1388. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Davis GW, Bezprozvanny I. Maintaining the stability of neural function: a homeostatic hypothesis. Annu. Rev. Physiol. 2001;63:847–869. doi: 10.1146/annurev.physiol.63.1.847. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat. Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Dymecki SM, Kim JC. Molecular neuroanatomy's “Three Gs”: a primer. Neuron. 2007;54:17–34. doi: 10.1016/j.neuron.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A, Bliss TV. Optical quantal analysis reveals a presynaptic component of LTP at hippocampal Schaffer-associational synapses. Neuron. 2003;38:797–804. doi: 10.1016/s0896-6273(03)00325-8. [DOI] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in I(h). Nat. Neurosci. 2005;8:1542–1551. doi: 10.1038/nn1568. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat. Neurosci. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Poo MM, Dan Y. Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature. 2005;434:221–225. doi: 10.1038/nature03366. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gittis AH, du Lac S. Intrinsic and synaptic plasticity in the vestibular system. Curr. Opin. Neurobiol. 2006;16:385–390. doi: 10.1016/j.conb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Hardingham N, Fox K. The role of nitric oxide and GluR1 in presynaptic and postsynaptic components of neocortical potentiation. J. Neurosci. 2006;26:7395–7404. doi: 10.1523/JNEUROSCI.0652-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Holscher C. Long-term potentiation: a good model for learning and memory? Prog. Neuropsychopharmacol. Biol. Psychiatry. 1997;21:47–68. doi: 10.1016/s0278-5846(96)00159-5. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T, Sandkuhler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- Johnston D, Narayanan R. Active dendrites: colorful wings of the mysterious butterflies. Trends Neurosci. 2008;31:309–316. doi: 10.1016/j.tins.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB, Beale HC, Carlisle HJ, Washburn LR. Integration of biochemical signalling in spines. Nat. Rev. Neurosci. 2005;6:423–434. doi: 10.1038/nrn1685. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Linden DJ. Ubiquitous plasticity and memory storage. Neuron. 2007;56:582–592. doi: 10.1016/j.neuron.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J. Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Dudek SM, Gold JT, Aizenman CD, Bear MF. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science. 1993;260:1518–1521. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde signaling by endocannabinoids. Curr. Opin. Neurobiol. 2002;12:324–330. doi: 10.1016/s0959-4388(02)00328-8. [DOI] [PubMed] [Google Scholar]
- Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation–feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26:631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Kampa BM, Stuart GJ. Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. J. Neurosci. 2006;26:10420–10429. doi: 10.1523/JNEUROSCI.2650-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien CC, Mu Y, Vargas-Caballero M, Poo MM. Visual stimuli-induced LTD of GABAergic synapses mediated by presynaptic NMDA receptors. Nat. Neurosci. 2006;9:372–380. doi: 10.1038/nn1649. [DOI] [PubMed] [Google Scholar]
- Linden DJ. The return of the spike: postsynaptic action potentials and the induction of LTP and LTD. Neuron. 1999;22:661–666. doi: 10.1016/s0896-6273(00)80726-6. [DOI] [PubMed] [Google Scholar]
- Lisman J, Lichtman JW, Sanes JR. LTP: perils and progress. Nat. Rev. Neurosci. 2003;4:926–929. doi: 10.1038/nrn1259. [DOI] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J. Neurosci. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat. Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malinow R, Mainen ZF, Hayashi Y. LTP mechanisms: from silence to four-lane traffic. Curr. Opin. Neurobiol. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- Marder E, Prinz AA. Current compensation in neuronal homeostasis. Neuron. 2003;37:2–4. doi: 10.1016/s0896-6273(02)01173-x. [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat. Rev. Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Weible AP, Shah S, Disterhoft JF. The BK-mediated fAHP is modulated by learning a hippocampus-dependent task. Proc. Natl. Acad. Sci. USA. 2008;105:15154–15159. doi: 10.1073/pnas.0805855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Markram H. Interneuron Diversity series: Molecular and genetic tools to study GABAergic interneuron diversity and function. Trends Neurosci. 2004;27:90–97. doi: 10.1016/j.tins.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Hempel C, Sugino K. Probing the transcriptome of neuronal cell types. Curr. Opin. Neurobiol. 2006;16:571–576. doi: 10.1016/j.conb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Oertner TG, Sabatini BL, Nimchinsky EA, Svoboda K. Facilitation at single synapses probed with optical quantal analysis. Nat. Neurosci. 2002;5:657–664. doi: 10.1038/nn867. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc. Natl. Acad. Sci. USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Paulsen O. Spike timing-dependent long-term depression requires presynaptic NMDA receptors. Nat. Neurosci. 2008;11:744–745. doi: 10.1038/nn.2125. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Reuter H, Smith SJ. Optical detection of a quantal presynaptic membrane turnover. Nature. 1997;388:478–482. doi: 10.1038/41335. [DOI] [PubMed] [Google Scholar]
- Saar D, Barkai E. Long-term modifications in intrinsic neuronal properties and rule learning in rats. Eur. J. Neurosci. 2003;17:2727–2734. doi: 10.1046/j.1460-9568.2003.02699.x. [DOI] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Hausser M. A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron. 2006;51:227–238. doi: 10.1016/j.neuron.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Multiple forms of long-term plasticity at unitary neocortical layer 5 synapses. Neuropharmacology. 2007;52:176–184. doi: 10.1016/j.neuropharm.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Malenka RC. Understanding synapses: Past, present, and future. Neuron. 2008;60:469–476. doi: 10.1016/j.neuron.2008.10.011. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietjen I, Rihel JM, Cao Y, Koentges G, Zakhary L, Dulac C. Single-cell transcriptional analysis of neuronal progenitors. Neuron. 2003;38:161–175. doi: 10.1016/s0896-6273(03)00229-0. [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Stadler MB, Roska B, Billings N, Sun B, Bartch B, Cepko CL. Molecular heterogeneity of developing retinal ganglion and amacrine cells revealed through single cell gene expression profiling. J. Comp. Neurol. 2007;502:1047–1065. doi: 10.1002/cne.21368. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic signaling: the positive side of negative feedback. Curr. Opin. Neurobiol. 2007;17:318–324. doi: 10.1016/j.conb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. The self-tuning neuron: homeostatic synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1–CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat. Rev. Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]