Abstract
Multiple myeloma (MM) is an incurable cancer of plasma cells localized preferentially in the bone marrow (BM). Resistance to chemotherapy represents one of the main challenges in MM management. BM microenvironment is known to play a critical role in protection of MM cells from chemotherapeutics; however, mechanisms responsible for this effect are largely unknown. Development of MM is associated with accumulation of myeloid-derived suppressor cells (MDSCs) mostly represented by pathologically activated relatively immature polymorphonuclear neutrophils (PMN-MDSCs). Here, we investigated whether PMN-MDSCs are responsible for BM microenvironment-mediated MM chemoresistance. Using in vivo mouse models allowing manipulation of myeloid cell number, we demonstrated a critical role for myeloid cells in MM growth and chemoresistance. PMN-MDSCs isolated from MM-bearing host are immunosuppressive and thus, functionally distinct from their counterpart in tumor-free host neutrophils. We found, however, that both PMN-MDSCs and neutrophils equally promote MM survival from doxorubicin and melphalan and that this effect is mediated by soluble factors rather than direct cell-cell contact. Our data indicate that targeting PMN-MDSCs would enhance chemotherapy efficacy in MM.
Keywords: multiple myeloma, myeloid-derived suppressor cells, neutrophils, chemoresistance
1. Introduction
Multiple myeloma (MM) is an incurable hematological malignancy characterized by a clonal proliferation of plasma cells that accumulate preferentially in the bone marrow (BM). One of the main challenges in this disease is chemotherapy resistance. The tumor microenvironment is now recognized as one of the leading factors that promotes chemoresistance; however, mechanisms responsible for this effect are still to be identified.
We and others have previously demonstrated that development of MM is associated with accumulation in BM of myeloid-derived suppressor cells (MDSCs) [3, 9, 12, 19, 24]. These cells are morphologically and phenotypically similar to immature neutrophils or monocytes but distinct in functional and biochemical characteristics and in their ability to suppress immune responses [11]. In mice, MDSCs are defined by co-expression of CD11b and Gr1 and lack of expression of markers of mature macrophages (MΦ) and dendritic cells (DC). Two major subsets of MDSCs have been identified: granulocytic or polymorphonuclear (PMN-MDSC) characterized by a CD11b+Ly6G+Ly6Clow phenotype and monocytic (M-MDSC) with a phenotype of CD11b+Ly6G−Ly6Chigh. Counterparts of PMN-MDSCs and M-MDSCs in control tumor-free mice are neutrophils and monocytes, respectively, that have similar phenotypes.
In cancer patients, MDSCs are defined as immature myeloid cells that are co-purified with the mononuclear cell (MNC) fraction and have a phenotype of CD33+CD14−CD11b+, with PMN-MDSCs distinguished from M-MDSCs by expression of CD15 or CD66b. In healthy donors, very few MNCs have the CD33+CD14−CD11b+ phenotype; these cells are called immature myeloid cells (IMC), since they are not immune suppressive. In MM patients, the vast majority (~95%) of MDSCs in the BM is represented by PMN-MDSCs. Human mature neutrophils are not co-purified with the MNC fraction but are found in the pellet formed following Ficoll-Paque gradient centrifugation. MDSCs together with mature neutrophils comprise the largest cellular population in the BM.
While some cellular populations in the human BM microenvironment, including BM stroma (BMS), macrophages (MΦ), plasmacytoid DCs (pDCs), conventional DCs (cDCs), and osteoclasts have been previously implicated in MM chemoresistance in vitro [1, 4, 17, 18, 29], the numbers of these cells in BM is small. At the same time, the contribution of BM PMN-MDSCs and mature neutrophils in MM chemoresistance has yet to be determined.
Our study for the first time demonstrates that cells of neutrophilic granulocyte lineage isolated from both MM-bearing and tumor-free hosts have similar chemoprotective effects on MM cells. This effect does not require direct contact with tumor cells and is mediated through soluble factors produced by these myeloid cells. Taken together, our data indicate that targeting PMN-MDSCs and neutrophils in patients with MM can improve the efficacy of chemotherapy and thus, outcome of this disease.
2. Materials and methods
2.1. Cell lines
Human MM U266, MM1.S, NCI-H929 and RPMI-8226 cell lines were obtained from ATCC. Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% Antibiotic-Antimycotic (Invitrogen, Grand Island, NY). Mouse MM DP42 and 38ATLN (ATLN) cell lines were established and kindly provided by Dr. Brian Van Ness (University of Minnesota, Minneapolis, MN) and were cultured in RPMI-1640 medium supplemented with 10% FBS, 5 mM glutamine, 50 μM 2-Mercaptoethanol, 1% Antibiotic-Antimycotic, and 0.5 ng/mL recombinant mouse IL-6 (R&D Systems). Cells were expanded upon arrival, and a new vial has been thawed every 2 months for experiments.
2.2. Reagents
Doxorubicin and melphalan were purchased from Sigma-Aldrich (St. Louis, MO). Fluorochrome-conjugated antibodies against mouse antigens CD138, siglec-H, GR-1, CD11b, CD11c, F4/80, B220, CD3, and those against human antigens CD138, CD123, HLA-DR, CD33, CD14, CD11b, CD19, CD56, CD3, CD11c used for flow cytometry were purchased from BD biosciences (San Jose, CA).
2.3. Isolation and culture of primary human cells
BM samples from patients with MM and healthy donors were collected after receiving written informed consent according to the protocols approved by the University of South Florida and the University of Pennsylvania Institutional Review Boards. Mononuclear cells were isolated from BM samples from patients with MM and healthy donors by Ficoll-Paque density gradient centrifugation, labeled with anti-human CD33-PE-Cy7, CD14-APC-Cy7, CD11b-APC, and CD15-PerCP and stained with 4′,6-Diamidine-2′-phenylindole dihydrochloride (DAPI). CD33+CD14−CD11b+ MDSCs or IMCs and CD33+CD14−CD11b+CD15+ PMN-MDSCs were isolated from DAPI-negative BM mononuclear fraction by flow sorting using a FACS Aria instrument (BD) as previously described [19]. Neutrophils were isolated from the cell fraction remaining after removal of BM mononuclear cells. Briefly, Cells were resuspended in PBS and loaded on a step density gradient (Percoll 63% on top of Percoll 72%) to separate neutrophils in a monolayer between the 2 Percoll phases [6].
Primary MM cells were isolated from BM mononuclear fraction by positive selection of CD138+ cells using CD138 MicroBeads (Miltenyi Biotec). Human BMS was established as described previously [18]. MΦ and cDCs were generated in vitro from BM MNCs. Briefly, BM MNCs (4x106/mL) were adhered for 2h; non-adherent cells were then washed away and the remaining cells were cultured in the presence of 20 ng/mL GM-CSF and 10 ng/mL IL-4 for cDC cultures and 50 ng/mL human M-CSF (Peprotech) for MΦ. MΦ and cDCs were used after 7 days in culture. pDCs were isolated from healthy donor peripheral blood by positive selection using the CD304 Microbead kit (Miltenyi Biotech) as per manufacturer’s instructions.
2.4. Animal models
All experimental procedures involving animals in this study were reviewed and approved by the IACUC of the University of South Florida and the IACUC of the Wistar Institute. C57BL/6 and FVB/N mice were purchased from the National Cancer Institute (Frederick, MD), CD11b-diphtheria toxin receptor (DTR) mice on C57BL/6 and FVB/N background were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were crossed to obtain F1 progeny of mixed C57BL/6×FVB/N background. Mice were kept in pathogen-free conditions and handled in accordance with the requirements of the Guideline for Animal Experiments at the Moffitt Cancer Center and the Wistar Institute. Six to eight week old mice were used for experiments. MM tumors were established by i.v. inoculation of DP42 cells (1x104) into the tail vein. MDSCs were isolated from BM cells obtained from mice euthanized on day 12–14 after tumor inoculation.
For generation of CD11b-DTR BM chimeras, BM cells were obtained from the F1 progeny of C57BL/6 CD11b-DTR x FVB/N CD11b-DTR mice and injected i.v. into irradiated F1 progeny of C57BL/6 x FVB/N recipient mice (1–2 x 106 BM cells per mouse). For depletion of CD11b+ cells, mice were injected i.p. with 25 ng/g of body weight diphtheria toxin (DT) or control [GLU52]-DT (both from Sigma-Aldrich) every 4 days beginning 5–6 weeks after BM reconstitution. MM tumors were established one day after the first DT injection. Mice were treated with vehicle control (PBS) or doxorubicin (Pfizer, 4 mg/kg, i.v.). Treatment started on day 10 after tumor cell injection and was given every 4 days (total 6 times). Survival of mice was evaluated.
2.5. Hydrodynamic gene transfer
In vivo overexpression of GM-CSF was achieved by hydrodynamic gene transfer performed as previously described [15]. Briefly, 80 μg of endo-free purified plasmid encoding mouse GM-CSF [23] was resuspended in 1.6 ml saline and was injected i.v. into the tail vein within 5 sec. DP42 tumors were established 3 days after GM-CSF gene transfer. Treatment with doxorubicin (Pfizer, 4 mg/kg, i.v., every 4 days) or vehicle control (PBS) started on day 10 after tumor cell injection. Survival of mice was evaluated.
2.6. Mouse cell isolation and culture
CD11b+Gr1+ myeloid cells and MDSCs were isolated from BM cells obtained from femurs and tibias of control tumor-free or MM-bearing mice, respectively, using MACS technique. For isolation of these cell populations, BM cells were labeled with biotin-anti-Gr1 antibody (BD) followed by Streptavidin-conjugated MicroBeads (Miltenyi Biotec) and positive selection using LS columns. For MDSC isolation, BM cells were initially depleted of CD138+ MM cells followed by positive selection of Gr1+ cells. Flow cytometry analysis demonstrated that all Gr1+ cells also expressed the CD11b marker. CD11b+Ly6G+ BM neutrophil and PMN-MDSCs were isolated by MACS technique using biotin-Ly6G antibody and Streptavidin-conjugated MicroBeads.
Mouse MΦ, BMS, cDC and pDC were generated in vitro from BM cells. MΦ were differentiated from BM cells in the presence of 50 ng/mL M-CSF (Peprotech). BMS were established in alpha-MEM media supplemented with 10% FBS, 1% Antibiotic-Antimycotic, 1% MEM Non-Essential Amino Acids Solution, 1% MEM Vitamin Solution, and 2-Mercaptoethanol. cDC were generated in the presence of 10 ng/mL GM-CSF and 10 ng/mL IL-4 and purified by MACS technique using biotin-anti-CD11c antibody (BD) and Streptavidin-MicroBeads (Miltenyi Biotec). For the generation of pDC, BM cells were cultured with 100 ng/mL Flt-3L for 9 days, collected, and pDC were positively selected using MACS technique by labeling with anti-mouse siglecH-PE antibody followed by incubation with anti-PE MicroBeads. The purity of all MACS isolated populations was more than 95%.
Supernatants of CD11b+Ly6G+Ly6Clow PMN-MDSCs, neutrophils, or BMS were collected after culturing these cells for 24h in RPMI-1640 medium supplemented with 25 mM Hepes and 10% FBS.
2.7. Flow cytometry and flow sorting
Apoptosis of MM cells was evaluated using Annexin V binding assay. Briefly, cells were washed twice with ice cold PBS and once with binding buffer followed by staining with Annexin V-APC or FITC (BD, San Jose, CA) and DAPI (Invitrogen). At least 10,000 events were acquired using a LSR II flow cytometer (BD).
MM cell proliferation was evaluated using APC-BRDU flow kit (BD) according to the manufacturer’s instructions.
For surface staining, cells were labeled with specific antibodies for 30 min on ice, washed with ice cold PBS, and resuspended in PBS containing DAPI. At least 10,000 events were acquired using a LSR II flow cytometer (BD). Data were analyzed using FlowJo software (TreeStar). Proportions of the following mouse cell populations were determined: CD11b+Gr1+ myeloid cells and MDSCs, CD11b+Ly6G+ neutrophils and PMN-MDSCs, CD11b+Ly6G−Ly6C+ monocytes and M-MDSCs, CD3+ T cells, B220+ B cells, CD11c+Gr1−CD11b+ cDC, Siglec H+B220+CD11c+CD11b−Gr1+ pDC, CD11b+Gr1−CD11c−F4/80+ MΦ, and CD138+ MM cells. The following human BM populations were evaluated: CD11b+CD14−CD33+ IMCs or MDSCs, CD138+ MM cells, Lin−CD11c−CD123+ pDCs and Lin−CD11c+HLA-DR+ cDCs.
CD11b+CD14−CD33+ MDSCs or IMCs, CD11b+CD14−CD33+CD15+ PMN-MDSCs, and CD3+ T cells were isolated by flow sorting using a FASCAria instrument (BD).
2.8. In vitro treatment
Different BM cells were labeled with CMFDA dye (Invitrogen) for 2 min at room temperature in the dark and washed 3 times with 10 mL of PBS supplemented with 0.5% FBS to remove unbound dye. CMFDA-labeled BM cell populations were cultured overnight in direct contact with MM cells followed by 24h treatment with chemotherapeutics or vehicle control. Mouse cells were treated with 0.25–1 μM doxorubicin or 7.5μM melphalan; human cells were treated with 0.5–2 μM doxorubicin or 5–10μM melphalan. Cells were then collected and apoptosis was evaluated by flow cytometry in the gated population of MM CMFDA-negative cells. Specific chemotherapy-induced apoptosis was calculated by subtracting background values (apoptosis of cells treated with vehicle control) from values of apoptosis in cells undergoing chemotherapy treatment. Values of specific chemotherapy-induced apoptosis are presented in figures.
2.9. Statistics
Statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software, Inc, La Jolla, CA). Differences between groups were calculated using a two-tailed Student t test and considered significant at p<0.05. A log-rank test was used to evaluate the statistical significance in mouse survival experiments.
3. Results
3.1. Proportion of different myeloid cell populations in MM-bearing mice
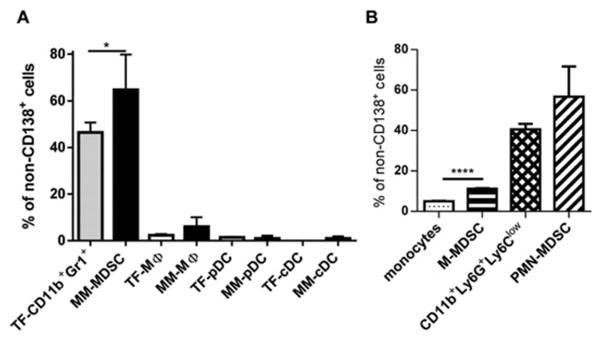
We used a DP42 mouse model of MM in which disease is localized to the BM and closely resembles the clinical characteristics of MM [5, 19]. Initially, we determined the proportion of different myeloid cell populations in the BM of tumor-free and MM-bearing mice. In the BM of tumor-free mice, CD11b+Gr1+ cells comprising CD11b+Ly6G+Ly6Clow neutrophils (89%) and CD11b+Ly6G−Ly6Chigh monocytes (11%) represented the largest population of myeloid cells (Fig. 1A). Their counterpart in MM-bearing mice, which we previously showed to be immune suppressive MDSCs [19], represented 65±15% of non-MM cells, whereas other populations of myeloid cells combined represented less than 10% (Fig. 1A). About 85% of the total MDSC population was comprised of PMN-MDSCs (Fig. 1B). While MDSCs were significantly increased in the BM of MM-bearing mice (p<0.02), the proportion of other BM myeloid cells was not substantially different from similar cell types in the BM of tumor-free mice.
Figure 1. Myeloid cell populations in mouse BM.
(A) Proportion of indicated populations of myeloid cells in BM of MM DP42-bearing mice 12 days after tumor cell inoculation (n=6, MM) and tumor-free (TF) was determined by flow cytometry. (B) Proportion of neutrophils (CD11b+Ly6G+Ly6Clow cells) and monocytes was determined by flow cytometry in BM of tumor-free mice (n=3) and proportion of PMN-MDSCs and M-MDSCs in BM of MM-bearing mice (n=3).
3.2. In vivo effect of myeloid cells on MM
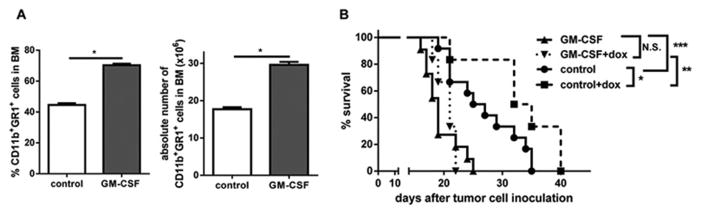
To determine the role of total population of myeloid cells in regulating MM progression in vivo we used CD11b-DTR chimera mice (lethally irradiated wild type (WT) mice reconstituted with BM cells isolated from CD11b-DTR mice). The presence of CD11b+Gr1+ cells in the BM of mice was significantly reduced in vivo by injections of DT (Fig. 2A). Populations of BM MΦ and cDCs represented a small proportion of cells and were also decreased (Fig. 2B). There were no significant changes in the proportion of BM B-cells and T-cells (Fig. 2C). DP42 MM tumors were established in CD11b-DTR chimera mice after the depletion of CD11b+ myeloid cells and treated either with doxorubicin or vehicle control. Depletion of myeloid cells resulted in a significant (p< 0.0005) improvement in mouse survival and in the anti-MM effect of doxorubicin (p<0.005) (Fig. 2D). To confirm the role of myeloid cells in the regulation of MM response to chemotherapy, we stimulated myelopoiesis with GM-CSF by hydrodynamic transfer of a plasmid containing gm-csf. This resulted in a significant increase of CD11b+Gr1+ cells in the BM (Fig. 3A). Increase in the population of CD11b+Gr1+ cells was associated with significant decrease in mouse survival (p<0.001) and abrogation of the anti-MM effect of chemotherapy (p<0.01) (Fig. 3B). These results demonstrated a critical role for myeloid cells in the regulation of MM growth and chemosensitivity.
Figure 2. In vivo effect of myeloid cell depletion on MM growth and chemosensitivity.
(A–C) Proportion and absolute number of BM CD11b+Gr1+ cells (A), indicated populations of myeloid cells (B), or lymphocytes (C) in CD11b-DTR-chimeras 48h after DT injection (n=3). (D) DP42 tumors were established in CD11b-DTR chimeras. Treatment with doxorubicin (dox, Pfizer, i.v., 4 mg/kg every 4 days) started 10 days after tumor cell inoculation (n=11–14 per group). Survival of mice was determined. ** - p<0.005; and *** - p< 0.001.
Figure 3. In vivo effect of myeloid cell expansion on MM growth and chemosensitivity.
(A) Proportion and absolute number of BM CD11b+Gr1+ cells 7 days after hydrodynamic gm-csf gene transfer. (B) DP42 tumors were established following hydrodynamic gm-csf gene transfer. Treatment with doxorubicin (dox, Pfizer, i.v., 4 mg/kg every 4 days) started 10 days after tumor cell inoculation (6–12 per group). Mice survival was evaluated. * - p<0.05; ** - p<0.005; and *** - p< 0.001.
3.3. Mouse BM MDSCs and neutrophils equally promote MM chemoresistance
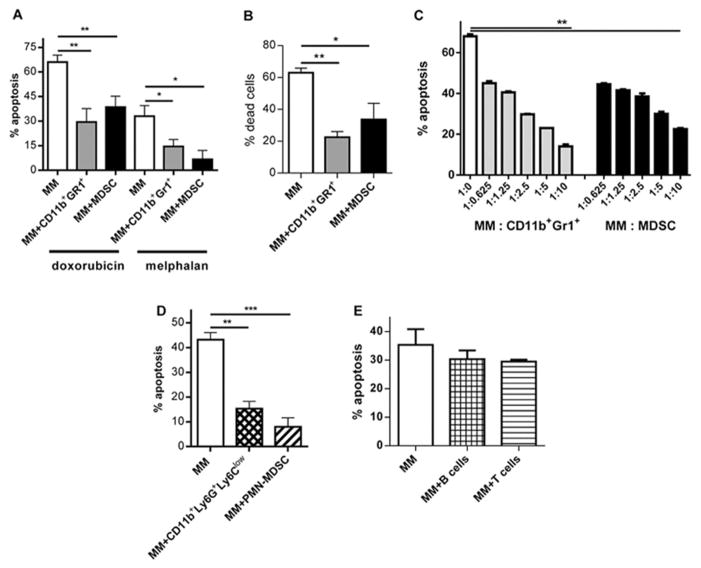
Previous studies have implicated BM stroma (BMS) and several relatively rare BM cells (MΦ, pDCs, and osteoclasts) in MM cell chemoresistance [1, 4, 17, 18, 29]. Since CD11b+Gr1+ cells represented the largest population of myeloid cells in MM-bearing mice, we investigated the possible role of these cells in MM chemosensitivity. MDSCs isolated from the BM of MM-bearing mice significantly reduced the cytotoxic effect of doxorubicin and melphalan in two mouse MM cell lines studied: DP42 and ATLN (Fig. 4A,B). The same protective effect was observed with CD11b+Gr1+ cells isolated from the BM of tumor-free mice (Fig. 4A,B). Titration of the number of CD11b+Gr1+ cells co-cultured with MM showed no difference in the anti-apoptotic effects of CD11b+Gr1+ cells isolated from either tumor-free or tumor-bearing mice (Fig. 4C), indicating that this effect was not limited to MDSC.
Figure 4. Regulation of MM by mouse neutrophils.
(A–C) CD11b+Gr1+ MDSC were isolated from BM of MM DP42-bearing mice 12 days after tumor cell inoculation; CD11b+Gr1+ cells were isolated from BM of tumor-free mice. (A,B) Mouse DP42 (A) or ATLN (B) cells were cultured overnight with or without CD11b+Gr1+ cells or MDSCs (ratio 1:5) followed by 24h treatment with doxorubicin or melphalan and detection of apoptosis. (C) MM DP42 cells were cultured with or without myeloid cells in indicated ratios overnight followed by 24h treatment with doxorubicin and detection of apoptosis. (D) CD11b+Ly6G+Ly6Clow neutrophils were isolated from BM of tumor-free mice and CD11b+Ly6G+Ly6Clow PMN-MDSCs were isolated from BM of MM DP42-bearing mice. These myeloid cells were cultured with DP42 cells overnight. Cells were then treated with doxorubicin followed by detection of apoptosis. Each experiment was performed independently at least 3 times and the combined results are shown. (E) DP42 cells were cultured with or without lymphocytes overnight followed by 24h treatment with doxorubicin and detection of apoptosis.
Since more than 85% of CD11b+Gr1+ cells were CD11b+Ly6G+Ly6Clow neutrophils in tumor-free mice and PMN-MDSCs in MM-bearing mice, we investigated whether these cells were chemoprotective in MM. Neutrophils and PMN-MDSCs were isolated using a Ly6G specific antibody and demonstrated potent protection of MM cells from doxorubicin (p<0.001 and p<0.0005, respectively) (Fig. 4D). To verify that this effect was not the result of higher cell density in the co-culture system, B- or T-cells were co-cultured with MM at the same concentrations. The presence of lymphocytes did not alter the cytotoxic effect of doxorubicin on MM (Fig. 4E).
3.4. BM MDSCs and neutrophils protect human MM cell from chemotherapy
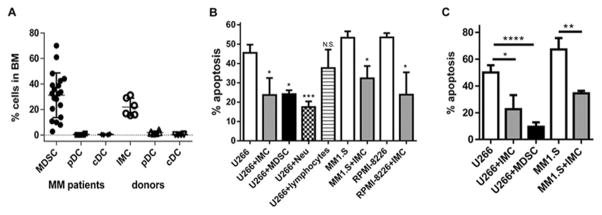
To confirm the clinical relevance of our data we used human cells. Separation of human mature neutrophils from PMN-MDSCs in the same patient is possible since PMN-MDSCs are localized in the low density mononuclear fraction of BM, whereas mature neutrophils are in the high density fraction and can be separated using a Percoll gradient. Analysis of the cellular composition of BM obtained from MM patients revealed that MDSCs comprised the most abundant myeloid population in the BM low density mononuclear cell fraction (31.2±17.6%) (Fig. 5A). In MM, more than 95% of BM MDSCs were represented by CD33+CD14−CD11b+CD15+ PMN-MDSCs [19]. PMN-MDSCs and mature neutrophils, but not lymphocytes, from the BM of MM patients significantly reduced cytotoxicity of doxorubicin (Fig. 5B) and melphalan (Fig. 5C) against different human MM cell lines in vitro. IMC from the BM of healthy donors had a similar effect (Fig. 5B,C).
Figure 5. Effect of human neutrophils on MM.
(A) Proportion of different myeloid cell populations in BM of healthy donors and patients with MM was determined by flow cytometry and presented as a frequency of DAPI-negative cells. (B,C) Human MM cell lines were cultured with or without IMCs from BM of healthy donors (n=4–5), or MDSCs (n=4–5), neutrophils (Neu) (n=5), or T cells (n=6) isolated from BM of patients with MM. Cells were then treated with doxorubicin (B) or melphalan (C) followed by detection of apoptosis. * - p<0.05; ** - p<0.01; *** - p<0.0005; **** - p<0.0001.
3.5. Mechanism of BM neutrophil pro-survival effect on MM cell
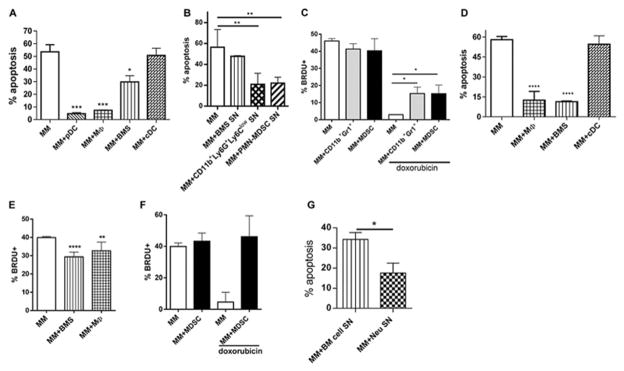
Previous studies have demonstrated that the chemoprotective effect of BMS and other BM cells depends on direct cell-cell contact and is mediated primarily via adhesion molecules [7, 18, 29, 30]. Consistent with these observations, mouse BMS, MΦ, and pDCs provided chemoprotection when co-cultured with mouse MM cells (p< 0.05, p<0.001, p<0.01, respectively) (Fig. 6A). Supernatants from BMS cultures did not protect MM cells (Fig. 6B). In contrast, supernatants from CD11b+Ly6G+Ly6Clow PMN-MDSCs or neutrophils significantly reduced the anti-MM effect of doxorubicin (p<0.005) (Fig. 6B). Similar data was obtained when cells were treated with melphalan (data not shown). This effect was not associated with growth arrest as neutrophils and MDSCs did not affect MM proliferation (Fig. 6C). In addition, significantly higher proportions of MM cells retained their proliferative capacity after treatment with doxorubicin in the presence of these myeloid cells (Fig. 6C). Taken together, these results suggest a distinct protective mechanism for neutrophils.
Figure 6. Neutrophils mediate chemoprotective effect on MM through production of soluble factors.
(A) Indicated mouse BM cells were cultured with mouse DP42 cells overnight followed by 24h doxorubicin treatment and detection of apoptosis. (B) DP42 cells were cultured in the presence of supernatants (SN) from BMS, CD11b+Ly6G+Ly6Clow neutrophils or PMN-MDSCs. Cells were then treated with doxorubicin followed by detection of apoptosis. Each experiment was performed independently at least 3 times and the combined results are shown. (C) Proliferation of mouse MM DP42 cells cultured in the presence or absence of CD11b+Gr1+ cells or MDSC for 48h with or without addition of doxorubicin for the last 24h of culture (n=3). (D) Doxorubicin-induced apoptosis of human MM U266 cells cultured in the presence or absence of indicated BM cell populations. (E) Proliferation of U266 cells cultured for 48h with or without indicated cell populations. (F) U266 were cultured with or without MDSCs isolated from BM of 4 different patients with MM and treated with doxorubicin for the last 24h of culture where indicated. Proliferation of MM cells was determined by BrdU incorporation after 48h of co-culture. (G) U266 cells cultured with BM Neu SN or with SN from BM cells depleted of myeloid and MM cells were treated with doxorubicin followed by detection of apoptosis. Combined results from 6 independent experiments performed with SN collected from BM cells obtained from 6 different MM patients are shown. * - p<0.05; ** - p<0.01; *** - p<0.001; **** - p<0.0001.
We used human cells to confirm the relevance of our results in the mouse system. In agreement with previously published data human BMS and MΦ protected human MM cells from doxorubicin-induced apoptosis (p<0.0001) (Fig. 6D). This effect was associated with significantly decreased proliferation of MM cells (Fig. 6E). Despite a similar chemoprotective effect of MDSCs, these cells did not affect MM cell ability to incorporate BrDU (Fig. 6F). Moreover, MM cells retained their proliferation in the presence of doxorubicin (Fig. 6F). Similar to the effect observed in mice, the presence of supernatants from human neutrophils isolated from patient BM protected MM cells from chemotherapy (p<0.05) (Fig. 2G).
4. Discussion
Tumor microenvironment (TME) is known to play a critical role in regulation of tumor cell chemosensitivity. However, cellular mechanisms responsible for this effect and contributions of various TME cell populations in this process are not entirely understood. The majority of the cellular compartment in the BM where MM cells reside is constituted of cells of myeloid lineage. Previous in vitro studies have implicated some types of myeloid cells including MΦ, cDC and pDC in MM chemoprotection [4, 17, 29]. These cells, however, are present in the BM at very low numbers. The contribution of the major myeloid population represented by neutrophils as well as overall significance of the myeloid lineage in MM response to chemotherapy has not been determined. Using a MM mouse model where a number of myeloid cells could be manipulated we, for the first time, demonstrated that elimination of CD11b+ cells in the BM significantly reduced tumor growth and improved response to chemotherapy while their expansion resulted in reduced mice survival and decreased chemosensitivity.
Although Gr1+CD11b+ myeloid cells isolated from control tumor-free and MM-bearing mice are phenotypically similar they have distinct biochemical properties and an ability to affect T cell responses. Thus, only Gr1+CD11b+ MDSCs from tumor-bearing animals but not their counterpart from control mice induced immune suppression [11, 19, 24]. We hypothesized that BM MDSCs are also different from Gr1+CD11b+ cells isolated from tumor-free animals in an ability to promote MM cell survival from chemotherapy. However, our results demonstrated that the source of Gr1+CD11b+ has no effect on their pro-survival properties.
Previous studies have demonstrated a dependence of MM cell chemoresistance on direct contact with some components of BM TME leading to growth arrest of these tumor cells [7, 18]. However, we found that the chemoprotective effect of neutrophils is mechanistically different from the one caused by extracellular matrices, BM stroma, MΦ, pDC, or osteoclasts, which is mediated by direct contact with MM cells and involve signaling from integrins, CD28, ICAM-1, or PSGL-1 [7, 18, 29, 30]. In contrast, the presence of neutrophils did not inhibit MM growth. Moreover, the protective effect of neutrophils did not require cell-cell contact as it could be reproduced by the addition of supernatant from these cells to MM. Several soluble factors have been previously implicated in the modulation of tumor cell chemosensitivity. Chemoresistance of cancer cells to doxorubicin, cisplatin or paclitaxel has been associated with the overproduction of several cytokines including interleukin 6 (IL6), IL8, IL10, vascular endothelial growth factor, epidermal growth factor, and tumor necrosis factor [2, 8, 10, 25–27]. IL6 and insulin-like growth factor-1 have been found to mediate MM cell survival from dexamethasone [13, 28]. Increased levels of basis fibroblast growth factor observed in patients with chronic lymphocytic leukemia correlated with their resistance to fludarabine [16]. Many of these soluble factors are produced by neutrophils and potentially could contribute to neutrophil-mediated MM chemoresistance. Identification of the nature of neutrophil-derived soluble factors responsible for the chemoprotective effect of these cells on MM is of high significance and therefore, warrants further studies.
It is well accepted that targeting MDSCs in cancer improves the immune response and increases the efficacy of immunotherapy [14]. Our study, for the first time, demonstrated that targeting this cell population may have an additional significant benefit – improvement of tumor cell response to chemotherapy. This is especially important in light of recent data suggesting the benefit of combined chemo- and immunotherapy treatment protocols [20–22].
Highlights.
Myeloid cells play a critical role in regulation of myeloma growth
PMN-MDSCs and neutrophils equally protect myeloma cells from chemotherapy
PMNMDSCs and neutrophils mediate chemoprotective effect through soluble factors
Acknowledgments
Support for this work was provided by the Multiple Myeloma Research Foundation (Y.N.) and NIH grant T32CA009171 (S.E.H.). Support for Shared Resources utilized in this study was provided by Cancer Center Support Grant CA010815 to The Wistar Institute and P30-CA76292 to the H. Lee Moffitt Cancer Center and Research Institute.
The authors would like to thank Dr. Alexandra Pisklakova, Eileen R. Grigson and Tess Chase for technical assistance and Ashley Durand (H. Lee Moffitt Cancer Center) and Rebecca Kotcher (University of Pennsylvania) for assistance with collection of patient samples.
Abbreviations
- MM
multiple myeloma
- BM
bone marrow
- MDSC
myeloid-derived suppressor cells
- PMN-MDSC
polymorphonuclear MDSC
- M-MDSC
monocytic MDSC
- IMC
immature myeloid cells
- MΦ
macrophages
- DC
dendritic cells
- cDC
conventional DC
- pDC
plasmacytoid DC
- MNC
mononuclear cell
- BMS
bone marrow stroma
- TME
tumor microenvironment
Footnotes
Conflict of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T, Kido S, Oshima T, Shibata H, Ozaki S, Inoue D, Matsumoto T. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104:2484–2491. doi: 10.1182/blood-2003-11-3839. [DOI] [PubMed] [Google Scholar]
- 2.Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay J. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. 2003;88:1721–1726. doi: 10.1038/sj.bjc.6600956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brimnes M, Vangsted A, Knudsen L, Gimsing P, Gang A, Johnsen H, Svane I. Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA-DR−/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol. 2010;72:540–547. doi: 10.1111/j.1365-3083.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 4.Chauhan D, Singh A, Brahmandam M, Carrasco R, Bandi M, Hideshima T, Bianchi G, Podar K, Tai Y, Mitsiades C, Raje N, Jaye D, Kumar S, Richardson P, Munshi N, Anderson K. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16:309–323. doi: 10.1016/j.ccr.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung W, Kim J, Linden M, Peng L, Van Ness B, Polakiewicz R, Janz S. Novel targeted deregulation of c-Myc cooperates with Bcl-X(L) to cause plasma cell neoplasms in mice. J Clin Invest. 2004;113:1763–1773. doi: 10.1172/JCI20369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Condamine T, Kumar V, Ramachandran I, Youn J, Celis E, Finnberg N, El-Deiry W, Winograd R, Vonderheide R, English N, Knight S, Yagita H, McCaffrey J, Antonia S, Hockstein N, Witt R, Masters G, Bauer T, Gabrilovich D. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J Clin Invest. 2014;124:2626–2639. doi: 10.1172/JCI74056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 8.Dedoussis G, Mouzaki A, Theodoropoulou M, Menounos P, Kyrtsonis M, Karameris A, Maniatis A. Endogenous interleukin 6 conveys resistance to cis-diamminedichloroplatinum-mediated apoptosis of the K562 human leukemic cell line. Exp Cell Res. 1999;249:269–278. doi: 10.1006/excr.1999.4442. [DOI] [PubMed] [Google Scholar]
- 9.Favaloro J, Liyadipitiya T, Brown R, Yang S, Suen H, Woodland N, Nassif N, Hart D, Fromm P, Weatherburn C, Gibson J, Ho P, Joshua D. Myeloid derived suppressor cells are numerically, functionally and phenotypically different in patients with multiple myeloma. Leuk Lymphoma. 2014;12:1–8. doi: 10.3109/10428194.2014.904511. [DOI] [PubMed] [Google Scholar]
- 10.Frankel A, Mills G. Peptide and lipid growth factors decrease cis-diamminedichloroplatinum-induced cell death in human ovarian cancer cells. Clin Cancer Res. 1996;2:1307–1313. [PubMed] [Google Scholar]
- 11.Gabrilovich D, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorgun G, Whitehill G, Anderson J, Hideshima T, Maguire C, Laubach J, Raje N, Munshi N, Richardson P, Anderson K. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013;121:2975–2987. doi: 10.1182/blood-2012-08-448548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hideshima T, Nakamura N, Chauhan D, Anderson K. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 14.Iclozan C, Antonia S, Chiappori A, Chen D, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62:909–918. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;7:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 16.Menzel T, Rahman Z, Calleja E, White K, Wilson E, Wieder R, Gabrilove J. Elevated intracellular level of basic fibroblast growth factor correlates with stage of chronic lymphocytic leukemia and is associated with resistance to fludarabine. Blood. 1996;87:1056–1063. [PubMed] [Google Scholar]
- 17.Nair J, Carlson L, Koorella C, Rozanski C, Byrne G, Bergsagel P, Shaughnessy JJ, Boise L, Chanan-Khan A, Lee K. CD28 expressed on malignant plasma cells induces a prosurvival and immunosuppressive microenvironment. J Immunol. 2011;187:1243–1253. doi: 10.4049/jimmunol.1100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nefedova Y, Landowski T, Dalton W. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17:1175–1182. doi: 10.1038/sj.leu.2402924. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran I, Martner A, Pisklakova A, Condamine T, Chase T, Vogl T, Roth J, Gabrilovich D, Nefedova Y. Myeloid-derived suppressor cells regulate growth of multiple myeloma by inhibiting T cells in bone marrow. J Immunol. 2013;190:3815–3823. doi: 10.4049/jimmunol.1203373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramakrishnan R, Antonia S, Gabrilovich D. Combined modality immunotherapy and chemotherapy: a new perspective. Cancer Immunol Immunother. 2008;57:1523–1529. doi: 10.1007/s00262-008-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramakrishnan R, Gabrilovich D. Mechanism of synergistic effect of chemotherapy and immunotherapy of cancer. Cancer Immunol Immunother. 2011;60:419–423. doi: 10.1007/s00262-010-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramakrishnan R, Gabrilovich D. Novel mechanism of synergistic effects of conventional chemotherapy and immune therapy of cancer. Cancer Immunol Immunother. 2013;62:405–410. doi: 10.1007/s00262-012-1390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Valckenborgh E, Schouppe E, Movahedi K, De Bruyne E, Menu E, De Baetselier P, Vanderkerken K, Van Ginderachter J. Multiple myeloma induces the immunosuppressive capacity of distinct myeloid-derived suppressor cell subpopulations in the bone marrow. Leukemia. 2012;26:2424–2428. doi: 10.1038/leu.2012.113. [DOI] [PubMed] [Google Scholar]
- 25.Voorzanger-Rousselot N, Favrot M, Blay J. Resistance to cytotoxic chemotherapy induced by CD40 ligand in lymphoma cells. Blood. 1998;92:3381–3387. [PubMed] [Google Scholar]
- 26.Wang Y, Niu X, Qu Y, Wu J, Zhu Y, Sun W, Li L. Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett. 2010;295:110–123. doi: 10.1016/j.canlet.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Qu Y, Niu X, Sun W, Zhang X, Li L. Autocrine production of interleukin-8 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cytokine. 2011;56:365–375. doi: 10.1016/j.cyto.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Xu F, Gardner A, Tu Y, Michl P, Prager D, Lichtenstein A. Multiple myeloma cells are protected against dexamethasone-induced apoptosis by insulin-like growth factors. Br J Haematol. 1997;97:429–440. doi: 10.1046/j.1365-2141.1997.592708.x. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, Li H, Wang M, Yang J, Yi Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y, Yang J, Qian J, Qiu P, Hanabuchi S, Lu Y, Wang Z, Liu Z, Li H, He J, Lin P, Weber D, Davis R, Kwak L, Cai Z, Yi Q. PSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myeloma. Leukemia. 2013;27:702–710. doi: 10.1038/leu.2012.272. [DOI] [PMC free article] [PubMed] [Google Scholar]