Abstract
The evolution of altruistic behavior through group selection is generally viewed as possible in theory but unlikely in reality, because individual selection favoring selfish strategies should act more rapidly than group selection favoring cooperation. Here we demonstrate the evolution of altruism, in the form of conditional reproductive restraint based on an explicitly social mechanism, modulated by intrapopulation communication comprising signal and evolved response, in a spatially distributed predatory/parasitic/pathogenic model system. The predatory species consistently comes to exploit a signal implying overcrowding, individuals constraining their reproduction in response, with a corresponding increase in equilibrium reproduction rate in the absence of signal. This signaled restraint arises in a robust way for a range of model spatial systems; it outcompetes non-signal-based restraint and is not vulnerable to subversion by noncooperating variants. In these systems, communication is used to evaluate population density and regulate reproduction accordingly, consistent with central ideas of Wynne-Edwards [Wynne-Edwards, V. C. (1962) Animal Dispersion in Relation to Social Behavior (Hafner, New York)], whose claims about the evolutionary importance of group selection helped ignite decades of controversy. This quantitative simulation model shows how the key evolutionary transition from solitary living to sociality can occur. The process described here of cooperation evolving through communication may also help to explain other major evolutionary transitions such as intercellular communication leading to multicellular organisms.
Researchers from Darwin on have speculated about the evolutionary origins of cooperative behavior. In recent decades, evolutionary explanations have been rooted in individual- and gene-level selection, with selection above these levels considered too weak to play any significant role. Altruistic behavior is explained using inclusive fitness theory, through fundamentally selfish mechanisms such as kin selection, in which individual reproductive success is augmented by success of relatives with shared genes (1–4). However, experimental and theoretical metapopulation studies, with explicit partitioning into subpopulations, have shown that a lower reproduction rate can confer a long-term selective advantage with regard to population persistence (4–7). More recent studies of the evolution of reproductive restraint in spatially extended models (8–13) have demonstrated populations evolving such that individuals have lower reproduction ratios than they might. This restraint results in lower reproductive success for the individual, but over many generations, spatially and genetically correlated lineages avoid extinction that would otherwise result from exhaustion of all available resources. This strain extinction mechanism thus operates as a form of selection above the level of individuals. Whereas metapopulation studies display limited reproduction under restricted conditions, the spatially extended models demonstrate such behavior over a very wide range of model parameters (see figure 2 of ref. 13).
Although these studies indicate a kind of altruism, they do not reveal much about interactive social behavior and its effects on the outcomes of selection. Communication is ubiquitous in nature, with organisms using every available medium (auditory/vibrational, visual, olfactory/chemical, electrical, and even tactile signals) for social integration (14, 15), and it is a necessary aspect of true social community. Moreover, social interactions very commonly affect reproductive processes, among other behaviors (16–20). Pheromones influence breeding, development, and intraspecific interactions for many animals (19, 20); mammals, birds, fish, and arthropods all exhibit territorial behavior, which concerns reproduction no less than foraging (14–16, 19); bacteria use chemical signals to coordinate behaviors including cell division, spatial patterning, swarming motility, collective attack and defense, and dormancy (17); and the evolutionary step from unicellular to multicellular organisms required an unprecedented degree of communication and coordination between cells during all stages of the life cycle (21). In the work described here, we investigated the evolutionary role of reproduction-linked communication, using simulations with a generalized model system.
Our studies support the hypothesis that cooperation based on communication is an evolutionarily successful strategy under a wide range of conditions. In particular, for systems evolving in spatially extended environments:
Signal-based reproductive restraint is favored: individuals reliably come to restrict their reproduction in response to social signals associated with crowding.
Communicating individuals supplant noncommunicating ones: a single individual with a heritable capacity for communication (even if initially unused), introduced to a noncommunicating population, is much more likely than others to become the common ancestor of the entire population.
Cooperation is not vulnerable to invasion by noncooperators: selfish mutants do not succeed in invading a population that uses communication-based cooperation.
A Model for Density-Based Signaling
We considered a spatially distributed system of an interacting pair of organism types, whose interactions may be classified as predator–prey, pathogen–host, herbivore–plant, or any other association in which one population relies on the second population for survival to the latter's detriment. No general term for all such systems exists; in this report, we refer to the former as “consumers” and the latter as “hosts.” Consumers in our model sent out a local signal when crowded; their base reproduction rate and an adjustment thereto in response to the presence of a signal were allowed to evolve as independent traits. The host population was not subject to evolution.
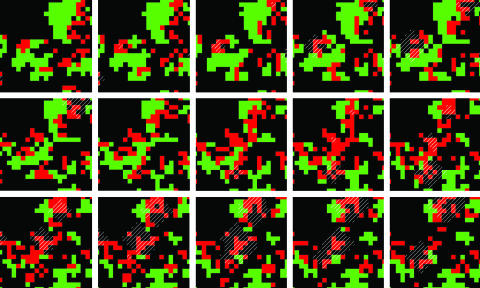
The model used a stochastic cellular automaton, based on those in refs. 12 and 13. Cells in a two-dimensional lattice represented areas populated by hosts alone, by both hosts and consumers, or by neither; a consumer could not occupy a cell in the absence of a host. At each time step, hosts alone reproduced into neighboring empty cells, with probability g for each empty cell; consumers spread to neighboring cells with hosts alone, with probability τ for each host-occupied cell; and consumers eliminated the hosts in their own cell (thereby causing their own demise), leaving empty space, with probability v. Host “growth rate” g and consumer “transmissibility” τ each reflect multiple factors, including reproduction rate and propensity to spread, combined into a single variable. Signaling was based on the fact that the behavior of many organisms depends on their local population density (19); bacteria, for instance, evaluate density through the concentration of diffusible “quorum-sensing” molecules (17). Here, consumers sent out a signal when surrounded on all four sides by other consumers, indicating crowding; this signal diffused a distance of one cell per time step up to a fixed maximum distance, after which it vanished. Consumers in cells in which signal was present adjusted their transmissibility for that time step by an amount δ. Host growth rate g and consumer “virulence” v were fixed at the beginning of a run; τ and δ were subject to mutation. Consumer transmissibility and virulence were modeled as independent variables, although the two are expected to be correlated in many natural systems. In summary, transmissibility across the consumer population could change over time and space either genetically by mutation, or as a temporary response to neighbor crowding, with the degree of that response also changing by mutation. Fig. 1 shows several successive snapshots in the time evolution of a small subsection of the lattice, illustrating the spread and death of hosts and consumers and the initiation and transmission of signal.
Fig. 1.
Snapshots of a small area (20 × 20 cells) of the lattice (250 × 250) at successive time steps (left to right and then top to bottom), showing the spread and death of hosts and consumers (g = 0.1 and v = 0.2) and the initiation and diffusion of signal. Black cells are empty, green cells are hosts alone, red cells are hosts in the presence of consumers, and signal is shown as a striped overlay.
Experiment I: Signal-Based Reproductive Restraint Is Favored
In our first set of simulation experiments, we used the model outlined above to track the distributions of transmissibility τ and response to signal δ in the consumer population over time for various choices of host growth rate g and consumer virulence v.
Methods. The lattice was initialized with 40% hosts alone, 5% hosts along with consumers with τ = 0.75v and δ = 0, and the remainder of grid cells empty. δ was initialized to 0 throughout the consumer population, so that consumers started out with no response to the signal. At each time step, every cell was assigned a new state based on the processes of host growth, consumer spread, and death of infected hosts. The neighbors of a cell were defined to be the four with which it shared an edge, and the lattice was updated synchronously. τ and δ were independently subject to mutation: with probability 1 – μτ (1 – μδ), a consumer offspring had τ (δ) identical to that of its parent; with probability μτ (μδ), it differed by ±ετ (±εδ). Because values of δ lower than –τ would have no meaning (adjusted transmissibility can be no lower than 0%), consumers were restricted to having δ ≥ –τ. Runs were conducted on a 250 × 250 lattice with periodic boundary conditions for 100,000 time steps per run; parameter values were μτ = 0.255, μδ = 0.0582, and ετ = εδ = 0.005; a signal diffused for four time steps before vanishing. These values were chosen arbitrarily and are not crucial to bring about the qualitative results reported.
Control experiments were performed both without any temporary modulation of reproduction rate (δ fixed at 0) and with modulation not linked to any signal. In the latter case, consumers had traits τ and δ subject to mutation as before, but here there was no communication to trigger the modulation. Instead, consumers modified or failed to modify their transmissibility by δ randomly at each time step, with the same probability of modification as consumers in the standard communication-based experiment (i.e., the fraction of the time that the latter were in the presence of a signal; see Fig. 3C).
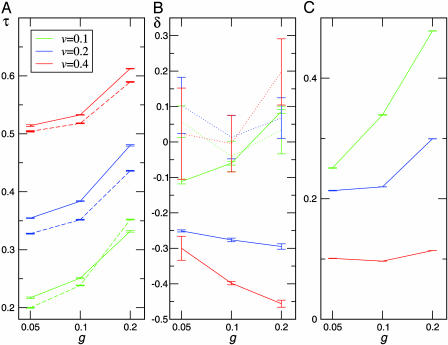
Fig. 3.
Shown are mean transmissibility τ (A), response to signal δ (B), and fraction of consumers in the presence of signal (C) for various values of host growth rate (g) and consumer virulence (v). The dashed lines mark runs without response (δ fixed at 0), and dotted lines mark runs where consumers do not signal but modify their transmissibility by δ at random with the probability given in C. In most cases with communication-based modulation of transmissibility, δ evolves to be significantly negative (more so in runs where signal is more infrequently present), and mean τ is increased accordingly compared with runs without response (A, dashed lines). When modulation occurs at random rather than in response to signal, mean δ is not significantly different from 0 (B, dotted lines). Means were taken over the consumer population, over time between steps 100,000 and 150,000 (or 50,000 and 100,000 for modulation-free runs, which converge very rapidly), and over 10 independent runs; error bars give the expected error in the final average over runs. Fluctuations over time of the population average within individual runs with communication-based modulation had standard deviations in both τ and δ 1/2 to 1 order of magnitude larger than the error bars shown.
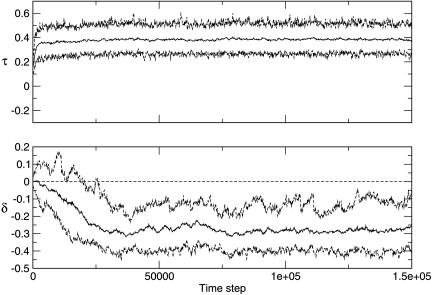
Results and Discussion. Fig. 2 shows a typical example of the evolution of τ and δ during a single run. As in previous studies using similar models without communication (8, 10–13), average transmissibility τ in the consumer population evolves to fluctuate about a moderate equilibrium value rather than increasing to the τ = 1 limit as individual-level selection would predict. The maximum τ trace shows the repeated emergence of higher-τ variants, evidence of individual-level selection winning out at a spatially and temporally local level; however, local extinctions eliminate these strains over the long term (10, 12, 13). The same result occurred for all tested values of g and v (Fig. 3A).
Fig. 2.
Transmissibility τ (Upper) and response to signal δ (Lower) during a typical single run with g = 0.1 and v = 0.2. The solid line shows the mean value across the population, and the dashed lines show maximum and minimum values.
The restraint corresponding to this limited mean τ arises directly from overexploitation of local resources, as follows. In spatial models, rapidly reproducing individuals have numerous offspring, so that their direct descendants populate an increasingly resource-poor environment; eventually, resources are exhausted and the strain goes extinct. Slower-reproducing strains remain viable in the long term, spreading no more quickly than new hosts become available, as hosts reproduce and spreading patches of hosts merge. The patchy structure itself results from prior consumption and extinction events. In a self-regulating feedback process, local extinctions generate the patchy spatial structure, separating regions populated by different strains, which in turn spread through the hosts at their own characteristic rates and leave the patchy structure in their wake. This feedback process, occurring both with and without communication, gives rise to the observed robustness of the qualitative system behavior for a wide range of parameter settings. The role of multiple time scales and the transition between short- and long-term behavior, and the composition of the population that results from continued appearance and extinction of rapidly reproducing strains, has been described (12).
The spatial component of these models is crucial to their behavior. In a mean-field version of such a model, in which consumers may spread not just to neighboring grid cells but to any available host with equal probability, higher transmissibility is always favored, and the mean transmissibility of the consumer population continues to increase until extinction or τ = 1 (8). Spatially patchy systems, however, permit very different dynamics in general, allowing, e.g., the coexistence of competing species through self-generated spatial segregation (22, 23). The size of grid cells in a spatial model is characteristic of the length scale over which consumers are able to migrate; spatial effects appear when the size of the full grid is much larger than that of a single patch. The patchy structure on the scale of many grid cells and the inability of consumers to move distances greater than a single cell at a time allow the segregation and coexistence of multiple competing strains.
The above discussion concerns individual reproductive restraint; we now turn to the results on communication.
The average δ evolves to fluctuate about an equilibrium value significantly less than 0. This result corresponds to conditional restraint elicited by the presence of signal. A consumer with τ and δ equal to these population averages, when it detects a signal or becomes surrounded itself, temporarily reduces its transmissibility by ≈75%. The model behavior as a function of g and v is shown in Fig. 3. Over most of this part of the (g, v) parameter space, the average δ over the population reliably reaches a consistent value significantly less than 0; it stays close to that value and far from 0 for the full course of the run. Also shown in Fig. 3 are the results of control experiments, both with no modulation δ at all and with δ not linked to any signal. In the latter case, the mean δ wanders randomly through the course of a run, reaching no consistent value significantly different from 0 across runs (Fig. 3B). Thus, in the absence of social context, δ has no utility, and consumers evolve to exhibit neither additional restraint nor exploitation. Control experiments without δ (where purely individual restraint occurs) show that the equilibrium value of τ is higher in the presence of signaling than in its absence (Fig. 3A): reducing transmissibility when resources are close to exhausted allows it to remain higher the rest of the time. Thus, a population of such consumers has the ability to adapt rapidly, on an ecological rather than evolutionary time scale, to rapid environmental changes; it can spread faster than can a noncommunicative population through a region of abundant hosts, quickly respond by restricting transmissibility when resources near exhaustion, and quickly return to higher transmissibility to take advantage of a sudden increase in local resource abundance, without relying on the slower course of evolutionary change to accomplish these shifts. By contrast, a population without this social signaling mechanism must compromise, with a single value of τ not optimal for either situation alone. The evolution of such an adaptive capacity has been modeled before strictly as a “flexible” individual strategy for response to fluctuations in resource abundance (24, 25). Here, it evolves because of the social context; it is an added benefit to the individual of evolving in response to social signaling.
These results are not sensitive to the form of the signal. We performed experiments in which the signal is relayed only to neighboring consumers and does not diffuse through empty space; in which the signal instantaneously blankets an area rather than spreading slowly; in which δ modifies τ multiplicatively, rather than additively; in which consumers signal at random with some fixed probability in addition to doing so when surrounded; in which consumers signal not only when surrounded but also when in the presence of signal [as is the case, e.g., with the well studied slime molds Dictyostelium (18)]; and for different values of the maximum distance for signal diffusion. All give rise to the same qualitative model behavior.
Experiment II: Communicating Individuals Supplant Noncommunicating Ones
We next considered the origins of a capacity for signaling and cooperative social behavior. Consumers in the first experiments started out with no response to signaling (δ initially 0); however, they had the capability to evolve a response from the beginning. How might that capability have originated, and if initially unused, would such a capability ultimately confer an evolutionary advantage? In this section we explore the transition from a truly noncommunicating state to full-fledged communication.
Methods. In a set of “invasion” experiments, there were two primary variants of consumers: those, as in the first set of experiments described above, capable of evolving a response to signals (“responsive”), and those unable to evolve any reproduction-modulating response, i.e., δ was fixed at 0 for all individuals of this type in all generations (“nonresponsive”). We assumed heritable mutations between the two forms to occur with very low frequency. These experiments consisted of taking a population of nonresponsive consumers that had been allowed to evolve to an equilibrium distribution of τ and converting one consumer to the responsive type, still with its δ at 0. Each consumer had offspring of its own type with respect to responsiveness: offspring of the former always had δ fixed at 0; those of the latter could have δ vary through mutation as described above. If the responsive type went extinct, a new single responsive mutant with δ = 0 was introduced. This process continued until the responsive invaders had taken over the entire consumer population or until 200,000 time steps had passed; we recorded the number of times the invading variant took over in 20 such independent runs.
Two features of these invasion experiments are worth emphasizing. First, the probability that a single mutant will overtake a population is small for almost any mutation, because of the role of chance in survival. Second, if we continue to introduce mutants, eventually invasion is inevitable, again simply by chance. The key to analysis of the results is characterizing the number of attempts until successful invasion as a measure of the likelihood of evolutionary success of the invader as compared with the originally existing type. In control experiments, both original and invading consumers were of the same type, the only difference between the two being whether they were marked as members of the invading or original population.
Results and Discussion. Invasion was successful in 18 of 20 runs; these runs totaled 37,618 introductions, giving a success rate of 4.8 × 10–4. By comparison, when the control experiment was performed with nonresponsive consumers as both invaders and original population, 7 of 72,217 introductions resulted in successful invasion, giving a rate of 9.7 × 10–5 (see also the converse experiments discussed in the next section). We thus find that the ability to evolve a transmissibility-modulating response to signal is favored, so that those consumers that have such a response outcompete those that lack it. If a single, rare mutation can toggle the presence or absence of that ability, then a simpler, noncommunicating population will tend to give way over time to a responsive one, bringing about full-fledged social communication in the population.
It is commonly speculated that molecules originally serving one function in cells became co-opted as the signal molecule for another, and indeed it is not uncommon for a given single morphogen to play multiple roles in the same organism (21). Such a mechanism has been proposed to have been responsible for the inception of multicellularity (21). Nonresponsive consumers in our model had no ability to respond, nor to evolve a response, to a signal carrier (which may not be accurate to describe as a “signal” in the absence of any response). However, they did give off this indicator when surrounded by other consumers, a property we assume to preexist for metabolic or incidental reasons unrelated to the signal-bearing potential of the carrier. The results of these experiments thus support this hypothesis for how communication-based cooperation between unicellular organisms may first have emerged. Similarly, our results suggest that the development of a response to an existing protosignal may apply to the emergence of sociality based on other sensory modalities and at various levels of organization including among macroscopic organisms.
Experiment III: Cooperation Is Not Vulnerable to Invasion by Noncooperators
A classic concern about the evolution of cooperative social behaviors is the possibility that they could easily be undermined and evolutionarily overtaken by noncooperating selfish individuals. We therefore performed a second set of invasion experiments to address this issue. In a population of consumers that reduce their transmissibility in response to a signal, mutants that signal when surrounded but do not modify their own transmissibility in response can be considered as “cheaters” for continuing to reproduce with a higher rate, counter to community convention but in favor of their own short-term self-interest, and as “manipulators” for inducing cooperating neighbors to restrain their reproduction without doing so themselves, to the neighbors' immediate competitive disadvantage. These experiments tested whether cheaters and manipulators could successfully invade a population of cooperators.
Methods. Here we performed invasion experiments similar to those described for the second experiment above. In this case, nonresponsive mutants were introduced into a population of responsive consumers (altruists) with an equilibrium distribution of τ and δ.
Results and Discussion. When nonresponsive consumers were introduced as invaders into an equilibrium population of responsive ones, not one introduction in 141,369 attempts was successful, and the large number of attempts made in the fixed number of constant-length runs reflects the relatively shorter period for which invaders were able to persist before being driven to extinction. By contrast, in the control experiment in which responsive consumers were both invader and invaded, 5 invasions of 71,232 introductions were successful, giving a success rate of 7.0 × 10–5. Thus, this framework of communication-based restraint is not susceptible to undermining by noncooperative variants of this type. These results should also be compared with the converse (origin) experiment and the baseline invasion results reported above; when transitions between the two consumer variants can take place, the cooperative/restraining variant is the stable one. Note that in this system there is no explicit evaluation by consumers of cooperation on the part of others, nor is there punishment of those that do not cooperate.
Conclusions
The consumers in our model system evolve to show conditional reproductive restraint, sacrificing short-term individual reproductive success for long-term benefit to the lineage, which responds to fluctuations in resource availability by a mechanism of social communication. Consumers with this communication-based cooperative behavior have a competitive advantage in that they can spread faster in times of plenty (increased τ) and still avoid extinction during those bottlenecks in which resources run short (negative δ). This exploitation of social interaction returns to one of the central ideas of Wynne-Edwards (14), whose reliance on the group selection hypothesis helped ignite the controversy over that topic; less remembered is his emphasis on the importance of communicative social mechanisms in directing the behavior and evolution of nearly all animal species. He proposed that social mechanisms are used to evaluate local population density and regulate reproduction accordingly. His conclusions may have been based on insufficient evidence, but the idea has gained some support (5, 16, 19), and in the system described here, we can observe the evolution of just such a process. Wynne-Edwards believed that in order for any social group to avoid overexploiting its resources, it must substitute some convention (e.g., territory or social status) for food as the proximate goal for competition (14, 26); however, our model requires no such feature.
Wynne-Edwards relied on group selection to explain how the behaviors for which he argued could have evolved (14, 26). In our simulations, local extinctions of consumer subpopulations do occur, and this process is crucial to the results. However, individuals are not confined to well defined groups (4, 5, 27): the boundaries between subpopulations are highly fluid, with fission and fusion both common, and descendants of an individual consumer do not necessarily share a common fate. It is likely that our results will apply equally well to discrete groups of animals or to the cells in a multicellular organism, but the present framework is one of a less rigidly structured population of agents. For such systems, another description, although harder to quantify, is that provided by clade selection (28, 29), where an important component of selection operates at the level of transient collections of spatially and genetically correlated organisms whose fates are coupled over an extended interval of time. The amorphousness of these collections inhibits a quantitative formalization in the case of the simulations discussed here, but the conceptual framework may be a useful one. A time-dependent notion of fitness could be used to quantify the time scale over which selection operates (12, 13).
The model may be applied to systems at many biological levels, with a single lattice cell corresponding to a single-celled host infected by a viral consumer, or equally well to a patch of land populated by macroscopic plants and the herbivores that feed on them. When a spatial cell represents a subpopulation of more than one individual, the details of reproduction (whether sexual or asexual, for example) are unimportant. The essence of the model is in how traits originate and propagate spatially.
The extent to which social communication is used to modulate reproductive activity and other behaviors in a wide range of biological systems suggests the importance of such mechanisms to evolutionary success. Pheromones and other intraspecific chemical cues mediate breeding, moderate relationships in mammal societies, and coordinate development in insects based on resource availability (19, 20). In some cases, signaling is known to be explicitly used in situations of deprivation to coordinate group response: when faced with hostile circumstances, many bacteria sporulate or enter dormant forms, processes regulated by intercellular signaling (17). Similarly, when food is scarce, the cellular slime molds stop foraging and use a chemical signal to coordinate a cooperative stage in which a substantial proportion of the cells are guaranteed to perish without reproducing (18). Our results provide a possible evolutionary explanation for these connections between signaling, collective behavior, reproductive restraint, and other forms of altruism.
Acknowledgments
We thank E. Rauch for useful discussions and C. Goodnight, S. Pimm, M. Wade, G. Williams, D. S. Wilson, and an anonymous referee forhelpful comments on the manuscript. This work was supported by a U.S. Department of Defense National Defense Science and Engineering Graduate Fellowship (to J.W.), a Packard Foundation Fellowship (to H. S. Seung), National Institutes of Health Training Grant GM07484 (to the Massachusetts Institute of Technology), and National Science Foundation Grant 0083885 (to Y.B.).
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Maynard Smith, J. (1964) Nature 201, 1145–1147. [Google Scholar]
- 2.Williams, G. (1966) Adaptation and Natural Selection: A Critique of Some Current Evolutionary Thought (Princeton Univ. Press, Princeton).
- 3.Dawkins, R. (1976) The Selfish Gene (Oxford Univ. Press, New York).
- 4.Sober, E. & Wilson, D. S. (1998) Unto Others: The Evolution and Psychology of Unselfish Behavior (Harvard Univ. Press, Cambridge, MA).
- 5.Gilpin, M. (1975) Group Selection in Predator-Prey Communities (Princeton Univ. Press, Princeton). [PubMed]
- 6.Nathanson, M. (1975) Bull. Entomol. Res. 65, 1–12. [Google Scholar]
- 7.Wade, M. (1980) Ecology 61, 1056–1064. [Google Scholar]
- 8.Rand, D., Keeling, M. & Wilson, H. B. (1995) Proc. R. Soc. London Ser. B 259, 55–63. [Google Scholar]
- 9.Savill, N. J. & Hogeweg, P. (1998) Proc. R. Soc. London Ser. B 265, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haraguchi, Y. & Sasaki, A. (2000) J. Theor. Biol. 203, 85–96. [DOI] [PubMed] [Google Scholar]
- 11.Boots, M. & Sasaki, A. (2000) Ecol. Lett. 3, 181–185. [Google Scholar]
- 12.Rauch, E., Sayama, H. & Bar-Yam, Y. (2002) Phys. Rev. Lett. 88, 228101. [DOI] [PubMed] [Google Scholar]
- 13.Rauch, E., Sayama, H. & Bar-Yam, Y. (2003) J. Theor. Biol. 221, 655–664. [DOI] [PubMed] [Google Scholar]
- 14.Wynne-Edwards, V. C. (1962) Animal Dispersion in Relation to Social Behavior (Hafner, New York).
- 15.Sebeok, T., ed. (1977) How Animals Communicate (Indiana Univ. Press, Bloomington).
- 16.McLaren, I., ed. (1971) Natural Regulation of Animal Populations (Atherton, New York).
- 17.Shapiro, J. A. (1998) Annu. Rev. Microbiol. 52, 81–104. [DOI] [PubMed] [Google Scholar]
- 18.Davey, J., ed. (1999) Semin. Cell Dev. Biol. 10, 565–619. [DOI] [PubMed] [Google Scholar]
- 19.Wallen, K. & Schneider, J., eds. (2000) Reproduction in Context (MIT Press, Cambridge, MA).
- 20.Agosta, W. (1992) Chemical Communication: The Language of Pheromones (Freeman, New York).
- 21.Bonner, J. T. (2000) First Signals: The Evolution of Multicellular Development (Princeton Univ. Press, Princeton).
- 22.Comins, H., Hassell, M. & May, R. (1992) J. Anim. Ecol. 61, 735–748. [Google Scholar]
- 23.Comins, H., Hassell, M. & May, R. (1994) Nature 370, 290–292. [Google Scholar]
- 24.Lack, D. (1967) The Natural Regulation of Animal Numbers (Oxford Univ. Press, London).
- 25.Hau, M., Wikelski, M. & Wingfield, J. C. (2000) J. Exp. Zool. 286, 494–504. [PubMed] [Google Scholar]
- 26.Wynne-Edwards, V. C. (1963) Nature 200, 623–626. [Google Scholar]
- 27.Wade, M. (1977) Evolution (Lawrence, Kans.) 31, 134–153. [DOI] [PubMed] [Google Scholar]
- 28.Stearns, S. C. (1986) in Patterns and Processes in the History of Life: Report of the Dahlem Workshop, eds. Jablonski, D. & Raup, D. M. (Springer, Berlin), pp. 23–44.
- 29.Williams, G. (1992) Natural Selection: Domains, Levels, and Challenges (Oxford Univ. Press, New York).