Abstract
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the intestinal tract whose etiology has not yet been fully elucidated. Available medicines for treatment of IBD are not universally effective and result in marked deleterious effects. This challenge has thus heightened the need for research in order to adopt new therapeutic approaches for the treatment of IBD. 5-HT3 receptor antagonists have shown analgesic and anti-inflammatory properties in vitro and in vivo. Our aim was to investigate the effect of ondansetron, 5-HT3 receptor antagonist, in an immune-based animal model of IBD, trinitrobenzene sulfonic acid (TNBS)-induced rat colitis and probable involvement of 5-HT3 receptors. Two hours after induction of colitis (instillation of 50 mg/kg of TNBS dissolved in 0.25 ml of ethanol 50 % v/v) to male Wistar rats, ondansetron (2 mg/kg), dexamethasone (1 mg/kg), meta-chlorophenylbiguanide (mCPBG, 5 mg/kg), a 5-HT3 receptor agonist, or ondansetron + mCPBG were administrated intraperitoneally (ip) and continued daily for six days. The animals were sacrificed and distal colons were assessed macroscopically, histologically and biochemically [myeloperoxidase (MPO), tumor necrosis factor-alpha, interleukin-6 and interleukin-1 beta]. Ondansetron and dexamethasone resulted in a decrease in macroscopic and microscopic colonic damage significantly. In addition a dramatic reduction in MPO activity and colonic levels of inflammatory cytokines were seen. The protective effects of ondansetron were antagonized by concurrent administration of mCPBG. Our data suggests that the beneficial effects of ondansetron in TNBS-induced colitis could be mediated by 5-HT3 receptors.
Keywords: inflammatory bowel diseases, ondansetron, TNBS-induced colitis, 5-HT3 receptor
Introduction
The inflammatory bowel diseases (IBDs) include idiopathic, chronic, recurrent intestinal disorders. Their complex pathogenesis is mostly represented by Crohn's disease (CD) and ulcerative colitis (UC). Of the various forms of chronic inflammatory condition of the human bowels, IBD is the most common and the most serious (Podolsky, 1991[39]). In spite of continuous medical advances, IBDs are still-incurable diseases. Genetic and environmental factors, impaired immunity, and microbial factors are believed to give rise to this inflammatory condition (Baumaqart and Sandborn, 2007[3]; Kucharzik et al., 2006[29]). IBD is characterized by massive cellular infiltrates and pertains to immunological abnormalities showing increasing numbers of CD4+ T lymphocytes, mast cells, neutrophils, and eosinophils (Forbes et al., 2004[19]). Some medical therapies such as salicylates, glucocorticoids, and immunosuppressives have been administered for IBD (Sands, 2000[42]; Braus and Elliott, 2009[6]); however, its treatment remained challenging. To clarify, difficulties such as low efficacy and high incidence of side effects, alongside unreachable remission, are the major limitations in treatment of IBD (Dubinsky, 2004[14]). There is, therefore, a definite need for research to employ the new therapeutic approaches for IBD treatment. 5-Hydroxytryptamine (5-HT, serotonin) is a neurotransmitter and an important signaling molecule in the intestine (Gershon, 1999[21]). It is also found in the immune-inflammatory axis and affects the mammalian immune response (Kim et al., 2000[27]; Cloez-Tayarani et al., 2003[9]). Intestinal inflammation may arise from a change in 5-HT-producing enterochromaffin (EC) cells and an increase in 5-HT content associated with the pattern of IBD (Bishop et al., 1987[4]; Coates et al., 2004[10]). To date, multiple serotoninergic receptors have been identified. To illustrate, 5-HT3 receptor (5-HT3R) has been demonstrated to express in immune cells including T lymphocytes. It has also been found that 5-HT can modulate the T cells function through activation of 5-HT3R. T-cell activation and proliferation, for example, is potentiated by activation of 5-HT3Rs present on these cells (Fiebich et al., 2004[18]). Recently, there has been an increasing propensity for investigating cellular and molecular mechanisms predisposing to IBD using of different animal models (Hibi et al., 2002[24]). So much attention has recently been devoted to TNBS model of colitis since it can exhibit the inflammation related to cytokines secretion (Wirtz and Neurath, 2007[45]). It is assumed that UC is associated with the enhancement of serotonin secretion (Coates et al., 2004[10]). More to the point, an increase in 5-HT content and secretion resembling the pattern of UC was observed in TNBS model of colitis (Linden et al., 2003[30]). On the other hand, amongst the various models of induction of colitis, TNBS model is efficiently able to mimic both acute and chronic colitis resembling the human UC (Ajuebor et al., 2001[1]).
In order to prevent chemotherapy-induced emesis, 5-HT3 receptor antagonists have been commonly used (Kris, 1994[28]). Recent findings also demonstrated new clinical indications for this class of medicines (Liu et al., 2011[31]; McCleane et al., 2003[34]). 5-HT3 receptor antagonists, including tropisetron and ondansetron, were found to serve anti-inflammatory property (Fiebich et al., 2004[17]). Nonetheless, the complicated mechanisms for these impacts have not yet been completely identified. Fiebich et al. reported that lipopolysaccharide-stimulated secretion of tumor necrosis factor-α (TNF-α) was dose-dependently inhibited by ondansetron in human monocytes (Fiebich et al., 2004[17]). Moreover, preliminary data have shown that the intravenous injection of ondansetron has an analgesic affect in neuropathic pain (McCleane et al., 2003[34]). These findings produce new mechanistic insights into anti-inflammatory activity of ondansetron that might be at least partly mediated through its effect on 5-HT3 receptors pathways.
To date, no data are available on potential beneficial effect of ondansetron in IBD. Present study sets out to assess the anti-inflammatory property of ondansetron (through its effect on 5-HT3 receptors) upon colonic inflammation markers and to compare the effects of ondansetron with dexamethasone as a reference drug and investigate the probable involvement of 5-HT3Rs in protecting against colitis in TNBS-induced colitis in rats.
Materials and Methods
Animals
Male Wistar rats (250 ± 20 g, 12-week-old) bred in animal house of School of Pharmacy, Isfahan University of Medical Sciences were used. Animals were housed in groups of 6, under controlled temperature (23 ±1 C), relative humidity (55 ± 10 %), and lighting conditions (12/12 hours light/dark) and fed standard pelleted chow and water ad libitum. All experimental protocols were approved by the Animal Care Committee of the Isfahan University of Medical Sciences.
Chemicals
Dexamethasone was purchased from Iran Hormone Pharmaceutical Co. (Tehran, Iran). Ondansetron hydrochloride was donated by Amin Pharmaceutical Co. (Tehran, Iran); metachlorophenylbiguanide (mPBG) and TNBS were purchased from Sigma (St. Louis, MO, USA). Hexadecyltrimethyl-ammonium bromide (HTAB), aprotinin A, bovine serum albumin, phenylmethylsulfonyl fluoride, benzethonium chloride, ethylene diamine tetra acetic acid (EDTA), and Tween 20 were all purchased from Sigma Chemical Company (St. Louis, MO, USA). TNF-α (ALPCO, USA), IL-1β (ALPCO, USA) and IL-6 (ALPCO, USA) kits were used for measurement of biochemical variables.
Grouping
Animals were randomly divided into the following groups of 6 rats in each: (I) TNBS-control group: 2 hours subsequent to induction of colitis, normal saline was administered intraperitoneally (ip); (II) normal group: rectal cannulation proceeded without induction of colitis, receiving normal saline (0.25 ml/rat) in lieu of TNBS; (III) Dexamethasone group: 2 hours subsequent to induction of colitis, dexamethasone (1 mg/kg) was administered ip (Minaiyan et al., 2012[36]); (IV) Ondansetron group: 2 hours subsequent to induction of colitis, ondansetron (2 mg/kg) was given ip (Saeki et al., 2001[41]); (V) Meta chlorophenylbiguanide group: 2 hours after induction of colitis, meta chlorophenylbiguanide (mCPBG; 5 mg/kg), a 5-HT3 receptor agonist, was given ip (Callahan and Cunningham, 1994[7]); (VI) Ondansetron + mCPBG group: 2 hours after induction of colitis, ondansetron and mCPBG were given simultaneously, ip.
Induction of colitis
Rats were fasted for 36 hours with free access to water before induction of colitis. Following assessment of their health, colitis was induced by means of method of Morris et al. (1989[37]). Having performed light ether anesthesia, we positioned the rats on their right. TNBS (50 mg/kg; dissolved in 50 % ethanol), was then administered intracolonically in a volume of 0.25 ml, via a polyethylene catheter inserted 8 cm proximal to the anus. Rats were positioned head-down for 2-3 minutes to preclude immediate anal leakage of the instillate and thereafter returned to their cages with access to food and water ad libitum. Normal group was given an enema of 0.25 ml of normal saline.
Measurment of body weight changes and diarrheal status
Body weight was recorded for each animal during the experimental period (prior to the administration of TNBS and subsequently daily for 6 days). Percent of body weight loss was thereafter measured. In addition, the fecal output was scored using arbitrary criteria as follows: 1. Formed stools, 2. Loosed stools and 3. Diarrhea, which were assessed daily for 6 days.
Macroscopic assessment
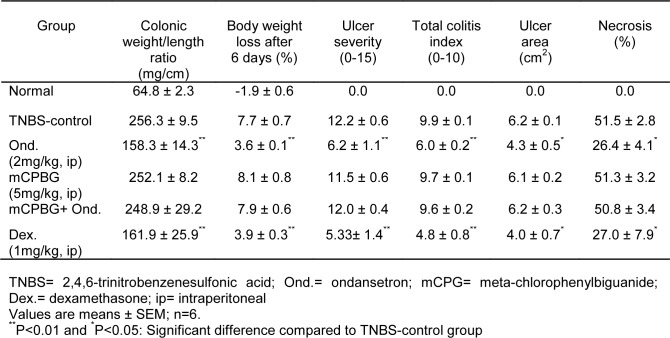
Animals were sacrificed by means of ether inhalation on day 6. Their abdomen was opened and the appearance of colon was then examined. Distal colon was removed, opened longitudinally and gently cleaned of fecal content using normal saline and processed for assessment by macroscopic, histological scores and biochemical markers. For each specimen, distal colon wet weight (mg) (8 cm from the anus) and weight/ length ratio (mg/cm) were measured. Using scoring system depicted in Table 1(Tab. 1) according to Ballester et al. (2007[2]), with some modifications, the severity of macroscopically visible colonic damage was scored. The intestinal segments were then divided longitudinally in 3-4 pieces and immediately frozen in liquid nitrogen for evaluation of biochemical variables.
Table 1. Scoring criteria for assessment of macroscopic colonic injuries.

Ulcer area and percent of necrosis measurement
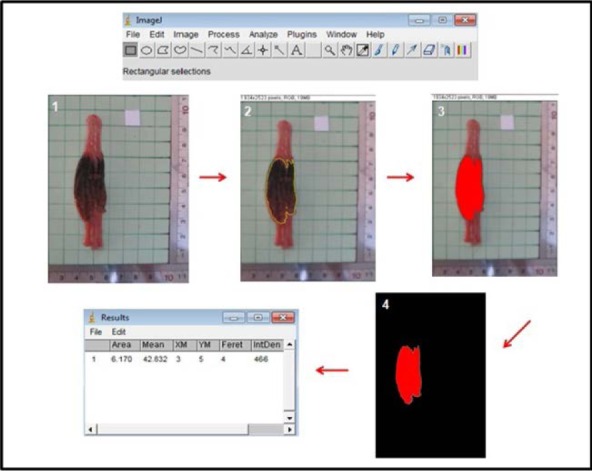
Using a digital camera (Canon IXUS 130.Tokyo, Japan), The RGB (red, green, blue) photos (24-bits) of the rats' colon was taken. The camera was positioned 20 cm above of the samples. Vertical and horizontal rulers were fixed on the table. The horizontal and vertical resolution of the images was 1934 and 2523 pixels, respectively. To provide a constant illumination, the images were taken at a shutter speed of 1/250 s using the external light source, by same investigator. All images were captured in a fixed setting of WB (Wight Balance) and brightness.
In order to analyze the images, image processing software (ImageJ) was employed. ImageJ is public domain Java image processing and analysis software inspired by NIH Image for the Macintosh (Ghosh et al., 2004[22]). It is able to display, edit, analyze, process, save and print 8-bit, 16-bit and 32-bit photos. It can also calculate area and pixel value statistics of user-defined selections, measure distances and angles, make histograms and support standard image processing involving contrast manipulation, sharpening, smoothing, edge detection and median filtering. Our pilot studies indicated that this image analysis program was much more accurate and convenient than previously-made manual processing (Minaiyan and Ghafghazi, 1999[35]). Prior to taking photos, camera parameters and position had to be adjusted. The colon of the rat was gently pinched manually, so that the ulcer site was positioned at the intersection of the vertical and horizontal grid lines on the graphics table. After taking photos, it was followed by the image processing. This includes 3 steps in the following order: 1. Preprocess, 2. Noise reduction and 3. Analysis. Each image was devided in 3 segments: background, colon (raw wound, undamaged intact colon), rulers (vertical - horizontal). In the preprocess step, the colon segmented from the background of graphic table by applying a color filter using the prior knowledge that the table used was green in color. Rulers and grid lines were thereafter utilized. The two regions containing the horizontal (10 mm) and vertical (10 mm) rulers were firstly segmented and set on Image by the ability of ImageJ (Set Scale) to identify the horizontal and vertical gradation marks on the rulers to allow determination of the horizontal and vertical resolutions of the images in pixels per millimeter. The quality of image was enhanced by the Median (size = 5) and Gaussian (size = 5) filters in the noise reduction stage. Image was then ready for color threshold. Analysis stage incorporated color threshold and finally calculating the percent of necrosis [necrosis area is divided to total area based on 8cm of colon (from the anus)] and ulcer area of colon sample (Figure 1(Fig. 1)).
Figure 1. Measurement of ulcer area by ImageJ program. Horizontal and vertical charting rulers as horizontal and vertical scale were recognized and interpreted by the ImageJ. With regard to ulcer color differentiation, some template photographs with a series of red colors were applied in order to enable the program for recognition of ulcers and calculate the area automatically.
Microscopic assessment
For microscopic assessment, colon samples were fixed in 10 % formalin, dehydrated, paraffin embedded processed, sliced into 4 µm-thick sections and stained with H&E (haematoxylin and eosin). By means of a modified validated scoring system presented by Cooper et al. (1993[11]) and Dieleman et al. (1998[12]) total colitis index was then derived by summing 3 sub-scores (inflammation severity, inflammation extent, crypt damage) on H&E-stained and coded sections. Pathologist blinded to the study evaluated the microscopic and histological scoring study. It was implemented by means of a Zeiss® microscope equipped with a Sony® color video camera for digital imaging.
Determination of MPO activity
Using the modified method of Bradley et al. (1982[5]), MPO activity, which is a biochemical indicator of neutrophil infiltration into gastrointestinal tissue, was measured. Each segment was weighed and chopped in 1 ml of 50 mM potassium phosphate buffer involving 0.5 % HTAB. Having chopped, we placed tissue in a homogenizing tube. The container was then rinsed with 2×1 ml HTAB in buffer solution. Afterwards, we add more buffer in order to have a concentration which was equivalent to 5 ml per 0.1 g of colon tissue and homogenized (15,000 rpm) for 4×45 s at 1 min intervals. The homogenate was placed in a sample tube, sonicated in an ice bath for 10 s, subjected to 3 cycles of freezing and thawing, and sonicated again for 10 s. The suspensions were centrifuged (15,000 rpm for 15 min in 4 °C). The supernatant thereafter decanted for assessment. The MPO activity was analyzed spectrophotometrically as follows: 0.1 ml of the supernatant was added to 2.9 ml of 50 mM K3PO4 buffer (pH = 6.0) involving O-dianisidinedihydrochloride (0.167 mg/ml) and 0.005 % hydrogen peroxide. The absorbance of the reaction mixture was recorded at a wave length of 450 nm by means of a UV-Vis spectrophotometer. The data were reported as the change in absorbance/min/mg colonic wet weight.
Measurement of the IL-1β, IL-6 and TNF-α levels in the rat colon
Using enzyme-linked immunosorbent assay (ELISA) kit (ALPCO, USA), TNF-α, IL-1β and IL-6 levels in the inflamed colon tissues were determined as described earlier (Nacife et al., 2004[38]). The colon tissue segments were weighed and processed in order to determine IL-1β, IL-6 and TNF-α content. Thereafter colon samples were homogenized in phosphate buffered saline (PBS; pH = 7.4) containing 0.4 M NaCl, 0.05 % Tween-20, 0.5 % bovine serum albumin, 0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM benzethonium chloride, aprotinin A 20 KI, and 10 mM EDTA. They were then centrifuged at 12,000×g for 30 min at 4 °C, and ELISA was performed in order to assess the levels of IL-1β and TNF-α in the supernatants.
Statistical analysis
Data analysis was performed using the SPSS statistical package (Version 17.0). Comparison between groups was made using one-way analysis of variance (ANOVA) with TUKEY as post hoc test. Non-parametric data were analyzed by Mann-Whitney U test. Results were reported as mean ± standard error of mean (SEM). A P-value < 0.05 was considered significant.
Results
Animals' body weight and diarrheal status
TNBS-treated rats showed loss of body weight after 6 days (P<0.001). Animals in ondansetron and dexamethasone-treated groups also experienced a significant loss of body weight in comparison with normal group; however, percent of body weight loss in these groups was significantly lower than TNBS-control group after 6 days (P<0.01) (Table 2(Tab. 2)).
Table 2. Macroscopic and histologic parameters of colitis induced by TNBS (50mg/kg) in rats. All treatments were made daily for six days after induction of colitis.
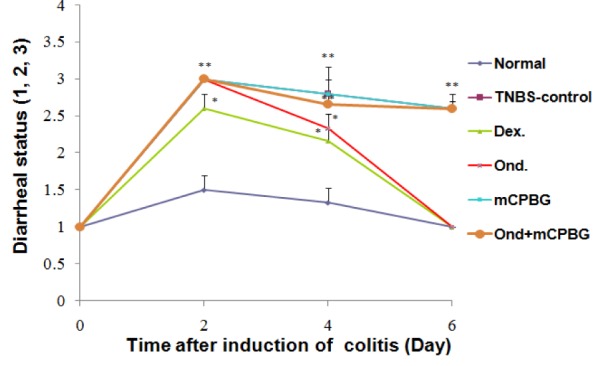
Figure 2(Fig. 2) illustrates that in all 6 days, diarrheal status of TNBS-control group was significantly higher than that of normal groups (P<0.01). In comparison with normal group, dexamethasone and ondansetron-treated rats showed a significant increase in the diarrhea index during the first 4 days after induction of colitis (P values are shown in Figure 2(Fig. 2)). However, diarrhea index diminished slowly from day 2 to 6 in these groups, and after day 4, no significant difference was observed in diarrheal status of aforementioned groups compared to that of normal group. Furthermore, there was no significant difference in the daily diarrheal status and percent of body weight loss between ondansetron-treated with dexamethasone-treated groups during the experiment. mCPBG as a serotonin agonist could not alter the diarrheal status of TNBS-treated rat; however, it nullified the therapeutic effects of ondansetron, suggesting a significant role for 5-HT3 receptors in diarrheal status of colitic rats.
Figure 2. Changes in diarrheal status before (day 0) and during 6 days of treatment after induction of colitis (TNBS, 50 mg/kg) in rats. Values are means ± SEM (n=6). **P<0.01 and *P<0.05 compared with normal group. Dex= Dexamethasone; TNBS-control= TNBS-control group; Ond= ondansetron group; mCPBG= meta chlorophenylbiguanide group; Ond+mCPBG= ondansetron+metachlorophenylbiguanide group. TNBS= 2,4,6-trinitrobenzenesulfonic acid. The rats were checked daily for stool consistency (1. Formed stools, 2. Loosed stools and 3. Diarrhea).
Assessment of macroscopic features
Severe inflammation, hemorrhage, ulcer, necrosis and thickened colon wall were found 6 days following induction of colitis in TNBS-control group, whereas macroscopic features of colon was quite intact in normal group (Table 2(Tab. 2)). Ondansetron or dexamethasone significantly decreased ulcer severity and weight/length ratio (P<0.01) as compared to TNBS-control group. No significant difference in aforementioned parameters was found between ondansetron and dexamethasone-treated groups. In addition, ulcer severity score (P<0.01) and weight length ratio (P<0.05) in ondansetron + mCPBG-treated rats were significantly high as compared to ondansetron group. We found that ulcer area and percent of necrosis were significantly lower in dexamethasone and ondansetron group, than in TNBS-control group. These macroscopic features were deteriorated significantly in ondansetron plus mCPBG-treated rats in comparison to ondansetron-treated rats (P<0.05). mCPBG exerted no effect itself upon macroscopic features of colitis.
Assessment of histopathological features
Histopathological assessment of colon tissued, exhibited a normal architecture with intact epithelium in colonic mucosa in normal group (Figure 3(Fig. 3)). We found however, a severe and intense transmural inflammation and/or diffuse necrosis, inflammatory granulomas and submucosal neutrophils infiltration in 50 mg/kg-TNBS-control rats.
Figure 3. Microscopic presentation of TNBS-induced colitis in rats (hematoxylin and eosin staining; original magnification 10×). (A) Normal group: Colon tissue with normal architecture; (B) TNBS-control group: epithelial distortion, architectural destruction of the crypts and inflammatory cell infiltrates; (C & D) Dexamethasone and ondansetron groups respectively: mild to moderate mucosal and submucosal inflammation and mucosal inflammatory cell infiltrates; (E & F): mCPBG and mCPBG + ondansetron, respectively: destruction of mucosal architecture and infiltration of neutrophils. TNBS= 2,4,6-trinitrobenzenesulfonic acid; mCPG= meta-chlorophenylbiguanide.
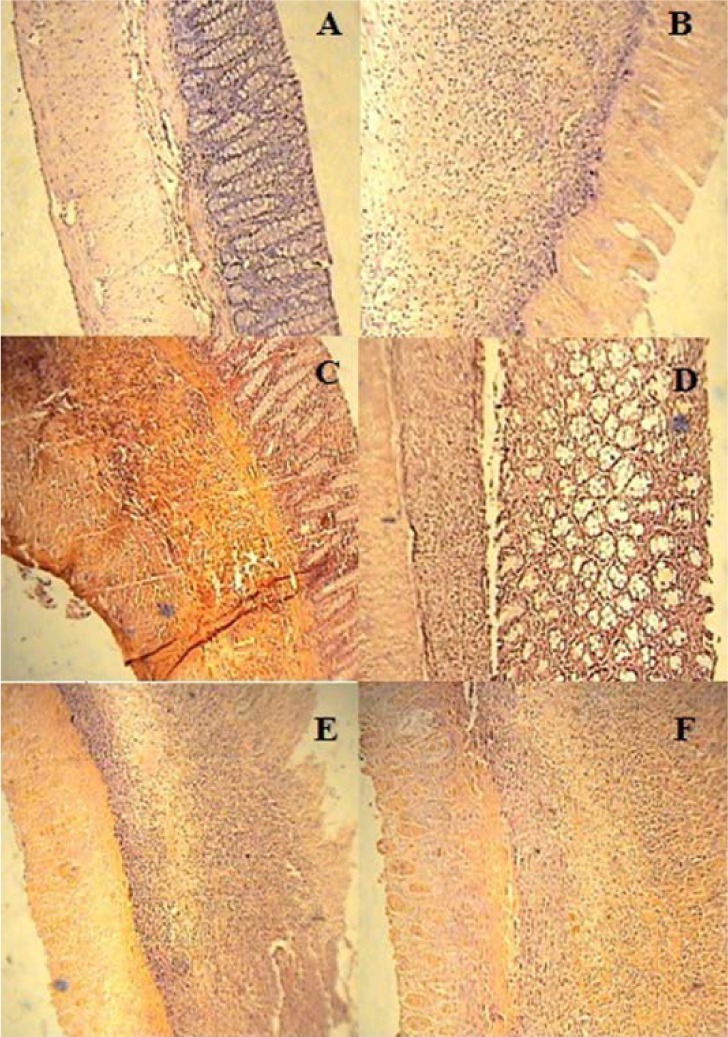
Ondansetron and dexamethasone-treated groups underwent significant decreased histopathological alterations. These treatments brought about a decline in total colitis index in injurious colons (Table 2(Tab. 2)). Re-epithelization of the mucosal layer and reduced inflammatory cell infiltration in lamina propria were observed in these groups. In this regard, no significant difference was seen between ondansetron and dexamethasone groups. Concomitant administration of mCPBG and ondansetron, significantly exacerbated histopathological aspects, compared to ondansetron administration alone (p<0.01). No significant difference was found in microscopic features between mCPBG-treated animals and TNBS-control group.
Assessment of MPO activity
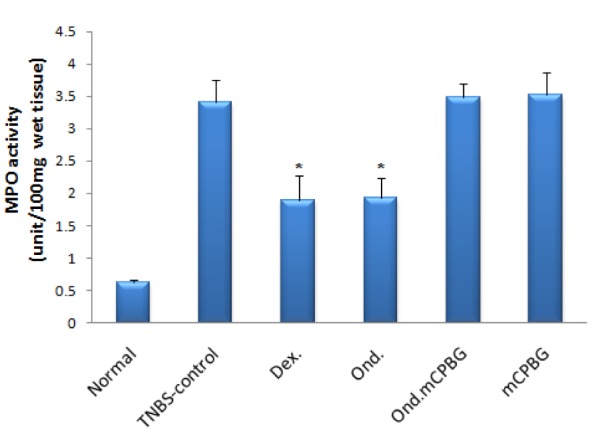
As can be noted in Figure 4(Fig. 4), we observed that MPO activity, which is a marker for leukocyte infiltration into the inflamed tissue, was conspicuously enhanced in the inflamed colon following the intrarectal TNBS administration. This result confirmed the histological assessment indicating increased leukocyte infiltration. In addition, we found that ondansetron or dexamethasone treatment significantly diminished the MPO activity level. A treatment with mCPBG as a 5-HT3 receptor agonist and ondansetron in tandem significantly brought about a high MPO activity, as opposed to ondansetron treatment alone (P<0.05). Data from mCPBG group was not different from TNBS-control group (P values are shown in Figure 4(Fig. 4)).
Figure 4. Changes in MPO activity of rat colon with TNBS-induced colitis. All treatments were made daily for 6 days. Ond.= ondansetron (2 mg/kg); Dex.= dexamethasone (1 mg/kg); mCPBG= meta chlorophenylbiguanide (5 mg/ kg); TNBS= 2,4,6-trinitrobenzenesulfonic acid (50 mg/kg); MPO= myeloperoxidase. Values are presented as mean ± SEM, n=6. *P<0.05 vs. TNBS-control group.
Assessment of cytokine profiles (TNF-α, IL-6, IL-1β)
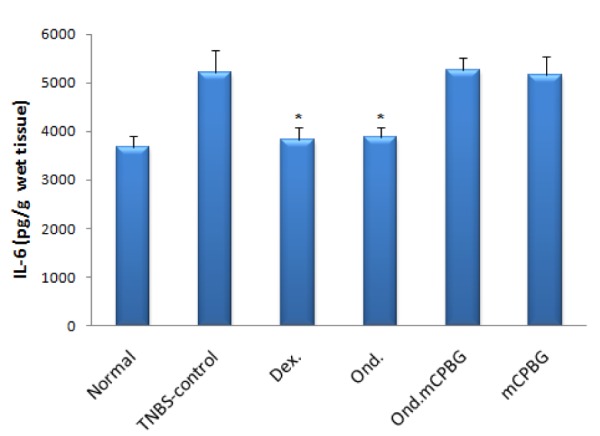
Colonic levels of TNF-α, IL-1β and IL-6 in TNBS-treated rats were assessed in different groups and compared with TNBS-control group. As it is shown in Figures 5-7(Fig. 5)(Fig. 6)(Fig. 7) the levels of cytokines were significantly lower in rats treated with either ondansetron or dexamethasone. In the group treated with the co-administration of ondansetron and mCPBG, the levels of these values were significantly higher than the group taken exclusively ondansetron (P<0.05 for TNF-α and IL-6, P<0.01 for IL-1β). No significant difference was found in TNF-α, IL-1β and IL-6 levels between ondansetron and dexamethasone-treated animals. mCPBG-treated group did not show any significant changes in the content of these inflammatory cytokines, as compared to TNBS-control group.
Figure 5. Colonic TNF-α level of rats with TNBS-induced colitis quantified by ELISA. Ond.= ondansetron (2 mg/kg); Dex.= dexamethasone (1 mg/kg); mCPBG= metachlorophenylbiguanide (5 mg/kg); TNBS= 2,4,6-trinitrobenzenesulfonic acid (50 mg/kg); TNF-α= tumor necrosis factor-alpha. Values are presented as mean ± SEM, n=6. *P<0.05 vs. TNBS-control.
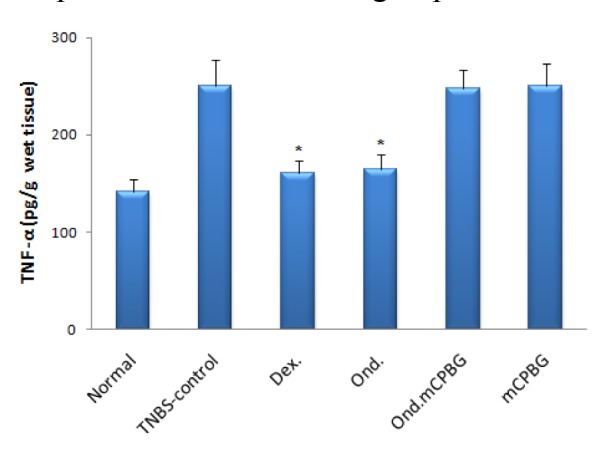
Figure 6. Colonic IL-1β level of rats with TNBS-induced colitis quantified by ELISA. Ond.= ondansetron (2 mg/kg); Dex.= dexamethasone (1 mg/kg); mCPBG= metachlorophenylbiguanide (5 mg/kg); TNBS= 2,4,6-trinitrobenzenesulfonic acid (50 mg/kg); IL-1β= interleukin-1 beta. Values are presented as mean ± SEM, n=6. *P<0.01 vs. Normal group; #P<0.01 vs. TNBS- control group.
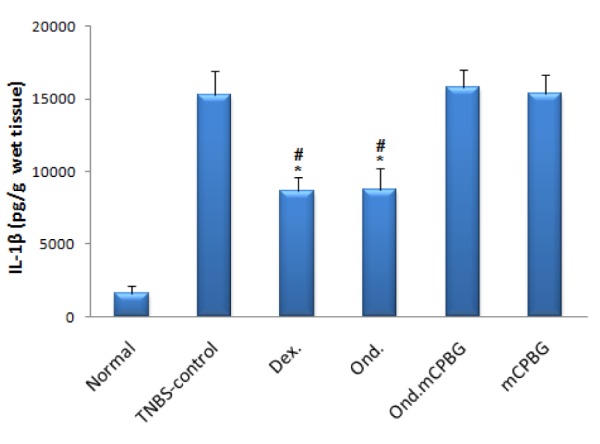
Figure 7. Colonic IL-6 level of rats with TNBS-induced colitis quantified by ELISA. Ond.= ondansetron (2 mg/kg); Dex.= dexamethasone (1 mg/kg); mCPBG= metachlorophenylbiguanide (5 mg/kg); TNBS= 2,4,6-trinitrobenzenesulfonic acid (50 mg/kg); IL-6= interleukin-6 .Values are presented as mean ± SEM, n=6. *P<0.05 vs. TNBS-control.
Discussion
It is interesting to note that the present study is the first one indicating alleviation of immune-based animal model of IBD; TNBS-induced colitis in rats by ondansetron. Ondansetron healed colonic macroscopic and histological damages, reduced levels of proinflammatory cytokines and diminished elevated tissue MPO. On the grounds that concurrent administration of mCPBG negates these effects, it can be inferred that 5-HT3 receptor contributes to the protection produced by ondansetron.
In spite of the fact that ondansetron, a potent antiemetic, has commonly been administered in order to ameliorate vomiting caused by surgery or chemo/radiation therapies, new potential applications have been suggested for this drug (Kris, 1994[28]; Liu et al., 2011[31]; McCleane et al., 2003[34]). It was also suggested that ondansetron, which is a selective 5-HT3 receptor antagonist, exerts antiphlogistic and analgesic activities (Faerber et al., 2007[16]).
TNBS method is one of the most common models of induction of colitis. This model provides a simple process, reproducible colonic damage and long-lasting damage along with inflammatory cell infiltration and ulcers. It is efficiently able to mimic the pattern of inflammation with human ulcerative colitis (Zheng et al., 2000[46]). Considering the fact that the serotonin content increase during TNBS-induced colitis, it is likely to be beneficial and suitable for analyzing the drugs affecting serotonin pathway in intestinal inflammation (Linden et al., 2003[30]). Taking into account of the immune system involvement, which is a considerable similarity of TNBS-induced colitis with human ulcerative colitis, serves a pivotal role in the pathogenesis of IBD (Wirtz and Neurath, 2007[45]). TNBS model could activate both Th1 and Th2 responses (Dohi and Fujihashi, 2006[13]) and mimic both acute and chronic phases of colitis based on experiment period (Ajuebor et al., 2001[1]). Present study was designed on a six-days-experiment in order to investigate the effect of daily administration of ondansetron upon TNBS-induced colitis. The time period of experiment adopted in present study is similar to the previous studies conducted on the model of TNBS-induced chronic inflammation in the rat (Morris et al., 1989[37]).
It has been demonstrated that an increase in the number of enterochromaffin (EC) cells and in 5-HT content is associated with intestinal mucosal inflammation such as ulcerative colitis (Coates et al., 2004[10]). It is also worth noting that serotoninergic receptors were found in immune cells such as macrophages, a principle source of proinflammatory cytokines IL-1, IL-6, and TNF (Fiebich et al., 2004[18]). Owing to the strategic location of EC, it is possible to infer that 5-HT serves a key role in infiltration and activation of macrophages in intestinal inflammation.
Linden et al. (2003[30]) observed that an increase in the bioavailability of 5-HT from mucosal epithelial cells affected TNBS-induced colitis. The authors also reported that EC cell hyperplasia occured during inflammation and SERT expression was downregulated in TNBS-induced colitis. These findings are consistent with those of study conducted on rectal biopsy specimens of patients with ulcerative colitis (Coates et al., 2004[10]). In addition Magro et al. (2006[32]) reported the increase in 5-HT levels in the inflamed colon in TNBS-induced colitis may result from mast cell infiltration.
Releasing cytokines by intestinal immunocytes can activate adjacent EC cells for 5-HT secretion. This increased 5-HT secretion via a positive feedback mechanism, consequently, results in activation of more 5-HT3 receptors on EC cells (Gebauer et al., 1993[20]). More to the point, serotonin also is secreted by immune cells. As a consequence, this exogenously added serotonin augments T-cell proliferation (Fiebich et al., 2004[18]). It is evident that T cells serve a crucial role in pathogenesis of IBD. Not only does an activation of Th1 cell bring about a secretion of the proinflammatory cytokines, but also it stimulates tissue macrophages to liberate additional proinflammatory cytokines (e.g. TNF-α, IL-1β, IL-6, IL-8, and IL-12), nitric oxide and reactive oxygen species (Cloez-Tayarani and Changeux, 2007[8]). These, in turn, cause tissue damage in IBD. Due to the fact that ondansetron inhibits T-cell proliferation (Vega et al., 2005[44]), it can be inferred that ondansetron can reduce T-cell induced tissue damage in IBD. Furthermore, ondansetron, which is an antagonist of 5-HT3 receptor, modulates 5-HT secretion from EC cells. In addition, a decrease in the 5-HT content gives rise to lower number of activated macrophages, the main proinflammatory cytokines source through their serotoninergic receptors (Cloez-Tayarani and Changeux, 2007[8]).
Amongst the intestinal immunomodulatory factors, cytokines are believed to serve a fundamental role. To clarify, an imbalance between proinflammatory and anti-inflammatory cytokines contributes to the pathogenesis of IBD. Proinflammatory cytokines (e.g., TNF-α, IL-1β and IL-6) released from macrophages, neutrophils and endothelial cells are overproduced in TNBS-induced colitis (Hosseini-Tabatabaei et al., 2009[25]) and in human IBD (Rahimi et al., 2007[40]). What should also be considered is that they can result in secretion of other cytokines, arachidonic acid metabolites and lytic enzymes and in turn, develop edema, fibrosis and necrosis (Maunder, 2000[33]). In present study, we observed that the content of these proinflammatory cytokines augments as a result of following TNBS instillation. Our findings indicated that ondansetron alleviated the colonic profile of these mediators. This result is perhaps due to inhibition of their synthesis or release. In vivo and in vitro studies confirmed that 5-HT3 receptor antagonists possess anti-inflammatory properties. Seidel et al. (2008[43]) reported that these antagonists inhibited serotonin-induced release of PGE2 from synovial cells. Lipopolysaccharide-induced TNF-α release, in human monocytes was dose-dependently inhibited by ondansetron (Fiebich et al., 2004[17]). Additionally, Hrycaj et al. (2004[26]) argued that patients with chronic inflammatory joint disease took advantage from 5-HT3 receptor antagonists.
On the other hand, ondansetron, which is a 5-HT3 receptor antagonist, is likely to reduce the proinflammatory cytokine release by blocking the serotoninergic receptors of intestinal macrophages. Vega et al. (2005[44]) reported that the molecular target for the immunosuppressive activity of 5-HT3 receptor antagonists may be independent of its binding to 5-HT3 receptor. They said different T-cell suppression profiles of these antagonists may imply that their effects are mediated via pathways other than 5-HT3. However, based on our finding, the beneficial effects of ondansetron upon colonic profile of cytokines in TNBS-induced colitis (an immune-based animal model of IBD with enhancement of serotonin content), are at least partly mediated through its effects in a receptor-dependent fashion.
MPO refers to an enzyme which is distributed mostly in neutrophils and to a lesser extent in monocytes and macrophages. Its activity has been a marker used for assessment of neutrophil infiltration to the site of inflammation in both human and experimental models of IBD (Esmaily et al., 2009[15]). In the present study, elevated MPO activity in TNBS-treated rats was considerably attenuated by ondansetron. The plausible explanation is that diminution of MPO level by ondansetron is due to its ability to decline neutrophil infiltration to the inflamed tissue. Therefore, it may preclude the release of components deteriorating inflammatory conditions.
Neurogenic inflammation can be associated with colitis (Hassani et al., 2005[23]). In addition, Faerber et al. (2007[16]) found that substance P-induced neurogenic inflammation was associated with 5-Hydroxytryptamine (5-HT) through acting at 5-HT3 receptors on capsaicin-sensitive fibers.
It can be also inferred that the effects of ondansetron in experimental rat colitis may be at least partly explained by its ability to block 5-HT-induced inflammatory neuropeptide liberation.
We found that mCPBG neutralized the protective effect of ondansetron on TNB-induced colitis which acts through 5-HT3 receptors. This, in turn, can antagonize the influence of serotonin highly produced in colon over the course of inflammation. Animal treated with mCPBG experienced a colitis which was comparable to that of TNBS- control group. This might result from the severity of colitis reached its peak at the day of evaluation of colon damage and thus no more severity could be caused by mCPBG, a selective 5-HT3 receptor agonist. In addition, it can be inferred that there is the maximum level of involvement of serotonin pathway in TNBS-induced colitis and the administration of mCPBG as a potent 5-HT3 agonist, cannot further aggravate colitis through activation of this pathway.
In conclusion, we found that ondansetron reduced colonic damage, neutrophil infiltration and colonic levels of pro-inflammatory cytokines, in the TNBS model of rat colitis, a common employed preclinical model of IBD, with an activity comparable to that of dexamethasone. In addition clinical efficacy of ondansetron, as an analgesic agent in patients with chronic inflammatory joint diseases has been documented. Therefore, it is possible that some anti-inflammatory effects of ondansetron upon colonic damage in TNBS-induced colitis are at least partly mediated through its effects on 5-HT3 receptor. Considering the low incidence of adverse effects of ondansetron, it is inferred that IBD patients could benefit from 5-HT3 antagonists therapy. However, additional randomized clinical trials are warranted to establish the therapeutic usefulness of this drug for treatment of IBD as an add-on therapy.
Acknowledgements
We would like to thank the School of Pharmacy, Isfahan University of Medical Sciences for financially supporting this project.
References
- 1.Ajuebor MN, Hogaboam CM, Kunkel SL, Proudfoot AEI, Wallace JL. The chemokine RANTES is a crucial mediator of the progression from acute to chronic colitis in the rat. J Immunol. 2001;166:552–558. doi: 10.4049/jimmunol.166.1.552. [DOI] [PubMed] [Google Scholar]
- 2.Ballester I, Daddaoua A, Posadas RL, Nieto A, Suárez MD, Zarzuelo A, et al. The bisphosphonate alendronate improves the damage associated with trinitrobenzenesulfonic acid-induced colitis in rats. Brit J Pharmacol. 2007;151:206–215. doi: 10.1038/sj.bjp.0707227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumaqart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 4.Bishop AE, Pietroletti R, Taat CW, Brummelkamp WH, Polak JM. Increased populations of endocrine cells in Crohn’s ileitis. Virchows Arch A Pathol Anat Histopathol. 1987;410:391–396. doi: 10.1007/BF00712758. [DOI] [PubMed] [Google Scholar]
- 5.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 6.Braus NA, Elliott DE. Advances in the pathogenesis and treatment of IBD. Clin Immunol. 2009;132:1–9. doi: 10.1016/j.clim.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callahan PM, Cunningham KA. Involvement of 5-HT2C receptors in mediating the discriminative stimulus properties of m-chlorophenylpiperazine (mCPP) Eur J Pharmacol. 1994;257:27–38. doi: 10.1016/0014-2999(94)90690-4. [DOI] [PubMed] [Google Scholar]
- 8.Cloez-Tayarani I, Changeux JP. Nicotine and serotonin in immune regulation and inflammatory processes: a perspective. J Leukoc Biol. 2007;81:599–606. doi: 10.1189/jlb.0906544. [DOI] [PubMed] [Google Scholar]
- 9.Cloez-Tayarani I, Petit-Bertron AF, Venters HD, Cavaillon JM. Differential effect of serotonin on cytokine production in lipopolysaccharide-stimulated human peripheral blood mononuclear cells: involvement of 5-hydroxytryptamine 2A receptors. Int Immunol. 2003;15:233–240. doi: 10.1093/intimm/dxg027. [DOI] [PubMed] [Google Scholar]
- 10.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Cooper H, Murthy S, Shah R, Sedergran D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 12.Dieleman L, Palmen M, Akol H, Bloemena E, Pena A, Meuwissen S. Chronic experimental colitis induced by dextran sulfate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dohi T, Fujihashi K. Type 1 and 2 T helper cell-mediated colitis. Curr Opin Gastroenterol. 2006;22:651–657. doi: 10.1097/01.mog.0000245545.80160.0f. [DOI] [PubMed] [Google Scholar]
- 14.Dubinsky MC. Targeting therapy in pediatric inflammatory bowel disease. Curr Treat Options Gastroenterol. 2004;7:391–405. doi: 10.1007/s11938-004-0052-y. [DOI] [PubMed] [Google Scholar]
- 15.Esmaily H, Hosseini-Tabatabaei A, Rahimian R, Khorasani R, Baeeri M, Barazesh-Morgani A, et al. On the benefits of silymarin in murine colitis by improving balance of destructive cytokines and reduction of toxic stress in the bowel cells. Centr Eur J Biol. 2009;4:204–213. [Google Scholar]
- 16.Faerber L, Drechsler S, Ladenburger S, Gschaidmeier H, Fischer W. The neuronal 5-HT3 receptor network after. 20 years of research – evolving concepts in management of pain and inflammation. Eur J Pharmacol. 2007;560:1–8. doi: 10.1016/j.ejphar.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Fiebich BL, Akundi RS, Lieb K, Candelario-Jalil E, Gmeiner D, Haus U, et al. Anti-inflammatory effects of 5-HT3 receptor antagonists in lipopolysaccharide-stimulated primary human monocytes. Scand J Rheumatol Suppl. 2004;119:28–32. [PubMed] [Google Scholar]
- 18.Fiebich BL, Akundi RS, Seidel M, Geyer V, Haus U, Müller W, et al. Expression of 5-HT3A receptors in cells of the immune system. Scand J Rheumatol Suppl. 2004;119:9–11. [PubMed] [Google Scholar]
- 19.Forbes E, Murase T, Yang M, Matthaei KI, Lee JJ, Lee NA, et al. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol. 2004;5664:75. doi: 10.4049/jimmunol.172.9.5664. [DOI] [PubMed] [Google Scholar]
- 20.Gebauer A, Merger M, Kilbinger H. Modulation of 5-HT3 and 5-HT4 receptors of the release of 5-hydroxytryptamine from the guinea-pig small intestine. Naunyn-Schmiedebergs Arch Pharmacol. 1993;347:137–140. doi: 10.1007/BF00169258. [DOI] [PubMed] [Google Scholar]
- 21.Gershon MD. Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13:15–30. [PubMed] [Google Scholar]
- 22.Ghosh M, Song X, Mouneimne G, Sidani M, Lawrence DS, Condeelis JS. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304(5671):743–746. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]
- 23.Hassani H, Lucas G, Rozell B, Ernfors P. Attenuation of acute experimental colitis by preventing NPY Y1 receptor signaling. Am J Physiol Gastrointestinal Liver Physiol. 2005;288:G550–G556. doi: 10.1152/ajpgi.00182.2004. [DOI] [PubMed] [Google Scholar]
- 24.Hibi T, Ogata H, Sakuraba A. Animal models of inflammatory bowel disease. J Gastroenterol. 2002;37:409–417. doi: 10.1007/s005350200060. [DOI] [PubMed] [Google Scholar]
- 25.Hosseini-Tabatabaei A, Esmaily H, Rahimian R, Khorasani R, Baeeri M, Barazesh-Morgani A, et al. Benefit of nicorandil using an immunologic murine model of experimental colitis. Centr Eur J Biol. 2009;4:74–85. [Google Scholar]
- 26.Hrycaj P. Serotonin type 3 receptor antagonist tropisetron in the treatment of chronic inflammatory rheumatic conditions;preliminary clinical experience. Scand J Rheumatol Suppl. 2004;119:55–58. doi: 10.1080/03009740410007069. [DOI] [PubMed] [Google Scholar]
- 27.Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2668–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 28.Kris MG. Ondansetron: a specific serotonin antagonist for the prevention of chemotherapy-induced vomiting. Important Adv Oncol. 1994:165–177. [PubMed] [Google Scholar]
- 29.Kucharzik T, Maaser C, Lügering A, Kagnoff M, Mayer L, Targan S, et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006;12:1068–1083. doi: 10.1097/01.mib.0000235827.21778.d5. [DOI] [PubMed] [Google Scholar]
- 30.Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- 31.Liu FC, Liu FW, Yu HP. Ondansetron attenuates hepatic injury via p38 MAPK-dependent pathway in a rat haemorrhagic shock model. Resuscitation. 2011;82:335–340. doi: 10.1016/j.resuscitation.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Magro F, Fraga S, Azevedo I, Soares-Da-Silva P. Intestinal 5-hydroxytryptamine and mast cell infiltration in rat experimental colitis. Dig Dis Sci. 2006;51:495–501. doi: 10.1007/s10620-006-3161-8. [DOI] [PubMed] [Google Scholar]
- 33.Maunder R. Mediators of stress effects in inflammatory bowel disease: not the usual suspects. J Psychosomatic Res. 2000;48:569–577. doi: 10.1016/s0022-3999(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 34.McCleane GJ, Suzuki R, Dickenson AH. Does a single intravenous injection of the 5-HT3 receptor antagonist ondansetron have an analgesic effect in neuropathic pain? A double-blinded, placebo-controlled cross-over study. Anesth Analg. 2003;97:1474–1478. doi: 10.1213/01.ANE.0000085640.69855.51. [DOI] [PubMed] [Google Scholar]
- 35.Minaiyan M, Ghafghazi T. Effects of selective dopamine receptor agonists (D1/DA1) and (D2/DA2) on stress induced gastric lesions in rats. Physiol Pharmacol. 1999;1:1–10. [Google Scholar]
- 36.Minaiyan M, Ghannadi AR, Etemad, Mahzouni P. A study of the effects of Cydonia oblonga Miller (Quince) on TNBS-induced ulcerative colitis in rats. Res Pharm Sci. 2012;7:in press. [PMC free article] [PubMed] [Google Scholar]
- 37.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 38.Nacife VP, Soeiro MN, Gomes RN, D'Avila H, Castro-Faria Neto HC, Meirelles MN. Morphological and biochemical characterization of macrophages activated by carrageenan and lipopolysaccharide in vivo. Cell Struct Funct. 2004;29:27–34. doi: 10.1247/csf.29.27. [DOI] [PubMed] [Google Scholar]
- 39.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 1991;325:1008–1016. doi: 10.1056/NEJM199110033251406. [DOI] [PubMed] [Google Scholar]
- 40.Rahimi R, Nikfar S, Abdollahi M. Meta-analysis technique confirms the effectiveness of anti-TNF-alpha in the management of active ulcerative colitis when administered in combination with corticosteroids. Med Sci Monit. 2007;13:PI13–PI18. [PubMed] [Google Scholar]
- 41.Saeki M, Sakai M, Saito R, Kubota H, Ariumi H, Takano Y. Effects of HSP-117, a novel tachykinin NK1-receptor antagonist, on cisplatin-induced pica as a new evaluation of delayed emesis in rats. Jpn J Pharmacol. 2001;86:359–362. doi: 10.1254/jjp.86.359. [DOI] [PubMed] [Google Scholar]
- 42.Sands BE. Therapy of inflammatory bowel disease. Gastroenterology. 2000;118:S68–S82. doi: 10.1016/s0016-5085(00)70007-2. [DOI] [PubMed] [Google Scholar]
- 43.Seidel M, Fiebich BL, Merzenich GU, Candelario-Jalil E, Koch FW, Vetter H. Serotonin mediates PGE2 overexpression through 5-HT2A and 5-HT3 receptor subtypes in serum-free tissue culture of macrophage-like synovial cells. Rheumatol Int. 2008;28:1017–1022. doi: 10.1007/s00296-008-0564-1. [DOI] [PubMed] [Google Scholar]
- 44.Vega Lde L, Muñoz E, Calzado MA, Lieb K, Candelario-Jalil E, Gschaidmeir H, et al. The 5-HT3 receptor antagonist tropisetron inhibits T cell activation by targeting the calcineurin pathway. Biochem Pharmacol. 2005;70:369–380. doi: 10.1016/j.bcp.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 45.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliver Rev. 2007;59:1073–1083. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Zheng L, Gao ZQ, Wang SX. A chronic ulcerative colitis model in rats. World J Gastroenterol. 2000;6:150–152. doi: 10.3748/wjg.v6.i1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]