Abstract
The role of P2X2/3, P2X3, P2X4 or P2X7 and P2Y2, P2Y6, and P2Y12 receptors in neuropathic pain has been widely studied. In contrast, the role of P2Y1 receptors is scarcely studied. In this study we assessed the role of P2Y1 receptors in several neuropathic pain models in the rat. Furthermore, we analyzed the expression of P2Y1 receptors in the ipsilateral dorsal root ganglia (DRG) and dorsal part of the spinal cord during the development and maintenance of neuropathic pain. We also determined the effect of the P2Y1 receptor antagonist on the expression of P2Y1 receptors. Spinal nerve ligation (SNL), chronic constriction injury (CCI) or spared nerve injury (SNI) produced tactile allodynia from 1 to 14 days after nerve injury. SNL, CCI and SNI enhanced expression of P2Y1 receptors in DRG but not in the dorsal part of the spinal cord at 1-3 days after injury. Intrathecal injection of the selective P2Y1 receptor antagonist MRS2500, but not vehicle, reduced tactile allodynia in rats 1-3 days after SNL, CCI or SNI. Moreover, intrathecal injection of MRS2500 (at day 1 or 3) reduced neuropathy-induced up-regulation of P2Y1 receptors expression. Intrathecal injection of MRS2500 lost most of the antiallodynic effect when injected 14 days after injury. Our results suggest that P2Y1 receptors are localized in DRG, are up-regulated by nerve injury and play a pronociceptive role in development and, to a lesser extent, maintenance of neuropathic pain.
Keywords: Neuropathic pain, P2Y1 receptors, Tactile allodynia, Spinal cord
1. Introduction
ATP (adenosine 5′-triphosphate) is released from primary afferents following nerve damage (Chizh and Illes, 2001). After it is released, ATP can bind to P2X (P2X1–7) or P2Y (P2Y1/2/4/6/11/12/13/14) receptors (Lazarowski et al., 2003). P2X receptors are ion channels whereas P2Y receptors are coupled to Gq/11 (P2Y1, 2, 4, 6, 11) or Gi/o (P2Y12, 13, 14) protein families (Burnstock et al., 2011). The latter receptors show affinity for different endogenous nucleotides including adenosine-5′-diphosphate (ADP) (P2Y1/2/13), ATP (P2Y1/11), uridine-5′-diphosphate (UDP) (P2Y6), uridine-5′-triphosphate (UTP) (P2Y4), UDP-glucose (P2Y14) or both ATP and UTP nucleotides (P2Y2) (Jacobson et al., 2009).
The participation of P2X2/3, P2X3 (Jarvis et al., 2002; Honore et al., 2002; Sharp et al., 2006), P2X4 (Tsuda et al., 2003) and P2X7 (Chessell et al., 2005; Honore et al., 2006) receptors in neuropathic pain has been widely studied. These studies have revealed a pronociceptive role for these channels. Likewise, P2Y2 (Li et al., 2014; Magni et al., 2015), P2Y6 (Kobayashi et al., 2012; Barragán-Iglesias et al., 2014), P2Y11 (Barragán-Iglesias et al., 2014) and P2Y12/13/14 (Tozaki-Saitoh et al., 2008; Kobayashi et al., 2008, 2012; Horváth et al., 2014) receptors have been related to maintenance of neuropathic pain. The role of P2Y1 receptors in neuropathic pain is scarcely studied.
Previous studies have reported a pronociceptive role for the P2Y1 receptors in inflammatory pain. For instance, local peripheral administration of selective P2Y1 receptor antagonists into the rat hind paw reduces formalin-induced nociception and ATP-, carrageenan-, acidic saline/ischemia- or complete Freund's adjuvant (CFA)-induced hyperalgesia (Andó et al., 2010; Seo et al., 2011; Jankowski et al., 2012; Kwon et al., 2014a, 2014b; Barragán-Iglesias et al., 2015). Also, intrathecal injection of a P2Y1 receptor antagonist diminishes bone cancer pain in rats (Chen et al., 2012). Conversely, the role of P2Y1 receptors in neuropathic pain is unclear. Intraperitoneal administration of a selective P2Y1 receptor agonist reduces tactile allodynia in rats with neuropathic pain (Andó et al., 2010) suggesting that P2Y1 receptors play an antinociceptive role in neuropathic pain. These authors also found that activation of P2Y1 receptors reduces acute and inflammatory pain. Interestingly, P2Y1 receptor mRNA is up-regulated in rat dorsal root ganglia (DRG) neurons after chronic constriction injury (CCI) or peripheral axotomy of the sciatic nerve (Xiao et al., 2002; Tsuchihara et al., 2009). In addition, it has been suggested that P2Y1 receptors are expressed in astrocytes and these cells contribute to neuropathic pain (Morán-Jiménez and Matute, 2000; Kobayashi et al., 2013). Thus, these studies have suggested that P2Y1 receptors may have a pronociceptive rather than an antinociceptive role in neuropathic pain.
In this study, we assessed the role of P2Y1 receptors, by using a selective P2Y1 receptor antagonist, in neuropathic pain models in the rat to shed light on the role of spinal P2Y1 receptors in neuropathic pain. Furthermore, we analyzed the expression of P2Y1 receptors in DRG and spinal cord during the development and maintenance of neuropathic pain. We also determined the effect of the P2Y1 receptor antagonist on the expression of P2Y1 receptors.
2. Results
2.1. Expression of P2Y1 receptors and effect of the P2Y1 receptor antagonist in rats with CCI, spared nerve injury (SNI) or spinal nerve ligation (SNL)
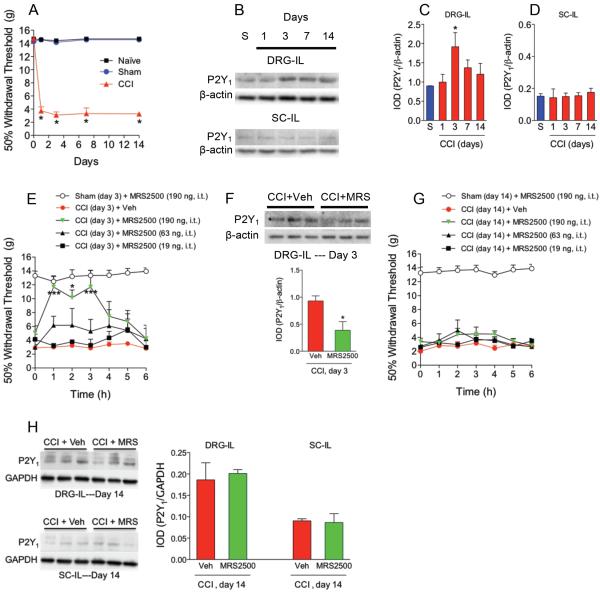
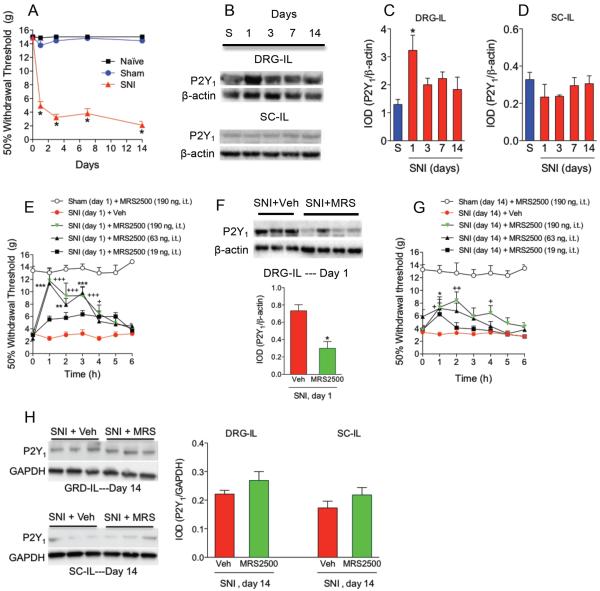
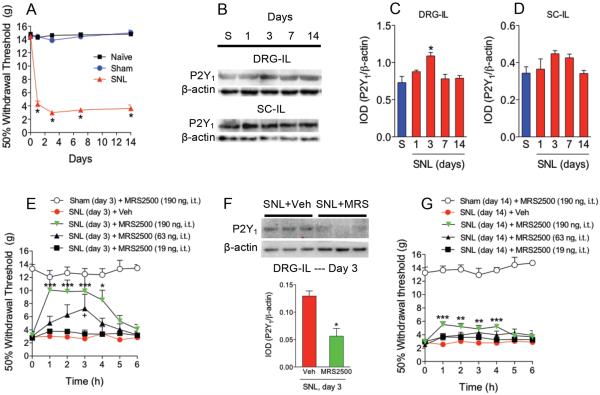
CCI, SNI or SNL dramatically decreased 50% paw withdrawal threshold response to values of about 4 g, as compared to the sham or naïve groups, which was interpreted as tactile allodynia (Figs. 1A, 2A and 3A). Tactile allodynia was observed from 1 to 14 days after nerve injury. Using Western blotting, a band of about 45 kDa corresponding to P2Y1 receptors was found in both ipsilateral DRG and dorsal part of the spinal cord (Figs. 1B, 2B and 3B). CCI (Figs. 1B/C/D) enhanced expression of P2Y1 receptors 3-days after injury whereas SNI (Figs. 2B/C/D) increased that expression at 1-day post- injury in the ipsilateral DRG but not in the dorsal part of the spinal cord. SNL (Figs. 3B/C/D) had a similar pattern than CCI. SNI produced the greatest enhancement of protein expression followed by CCI and SNL. Based on these results, we injected the selective P2Y1 (MRS2500) receptor antagonist in neuropathic rats at those times. Intrathecal injection of the drug (19-190 ng), but not vehicle, dose-dependently increased 50% paw withdrawal threshold response in rats after CCI (Fig. 1E), SNI (Fig. 2E) or SNL (Fig. 3E). In contrast, intrathecal MRS2500 did not modify pain threshold in naïve (data not shown) or sham-operated rats at 1, 3 or 14 days (Figs. 1E/G, 2E/G and 3E/G). Besides the antiallodynic effect, intrathecal injection of MRS2500 (at day 1 or 3) reduced neuropathy-induced up-regulation of P2Y1 receptors expression (Figs. 1F, 2F and 3F). In contrast with the antiallodynic effects observed at the first or third day after injury, intrathecal injection of MRS2500 (19-190 ng) only produced a modest enhancement of the 50% withdrawal threshold response when injected 14 days after spinal nerve ligation (Figs. 1G, 2G and 3G). Accordingly, MRS2500 did not modify P2Y1 receptors expression in DRG nor spinal cord at 14 days in rats subjected to CCI and SNI (Figs. 1H and 2H). Based on these negative results, we did not carry out the same experiment in the SNL model.
Figure 1.
Expression of P2Y1 receptors in DRG and spinal cord and effect of the selective P2Y1 receptor antagonist MRS2500 (MRS) in rats subjected to chronic constriction injury (CCI). Time course of the withdrawal threshold observed after CCI (panel A). Expression of P2Y1 receptors in the ipsilateral (IL) dorsal root ganglia (DRG) and dorsal spinal cord (SC) in sham (S) and neuropathic rats at 1, 3, 7 and 14 days after injury (panels B, C and D). Antiallodynic effect of the selective P2Y1 receptor antagonist MRS2500 in rats previously (3 days) subjected to CCI (panel E). Effect of MRS2500 on CCI-induced up-regulation of P2Y1 receptors in DRG of rats previously (3 days) subjected to CCI (panel F). Antiallodynic effect of MRS2500 in rats previously (14 days) subjected to CCI (panel G). Effect of MRS2500 on CCI-induced up-regulation of P2Y1 receptors in DRG of rats previously (14 days) subjected to CCI (panel H). Behavioral and molecular data are expressed as mean ± S.E.M. for 6 and 3 animals, respectively. Data are expressed as the time course of the withdrawal threshold. * p < 0.05, ** p < 0.01 and *** p < 0.001, significantly different from the vehicle (Veh) or Sham (S) group, as determined by one (panels C and D) or two-way (panels E and G) analysis of variance followed by the Dunnett's test or Bonferroni test, respectively. Student t-test was used to compare 2 independent groups.
Figure 2.
Expression of P2Y1 receptors in DRG and spinal cord and effect of the selective P2Y1 receptor antagonist MRS2500 (MRS) in rats subjected to spared nerve injury (SNI). Time course of the withdrawal threshold observed after SNI (panel A). Expression of P2Y1 receptors in the ipsilateral (IL) dorsal root ganglia (DRG) and dorsal spinal cord (SC) in sham (S) and neuropathic rats at 1, 3, 7 and 14 days after injury (panels B, C and D). Antiallodynic effect of MRS2500 in rats previously (3 days) subjected to SNI (panel E). Effect of MRS2500 on SNI-induced up-regulation of P2Y1 receptors in DRG of rats previously (3 days) subjected to SNI (panel F). Antiallodynic effect of MRS2500 in rats previously (14 days) subjected to SNI (panel G). Effect of MRS2500 on SNI-induced up-regulation of P2Y1 receptors in DRG of rats previously (14 days) subjected to SNI (panel H). Behavioral and molecular data are expressed as mean ± S.E.M. for 6 and 3 animals, respectively. Data are expressed as the time course of the withdrawal threshold and 50% threshold withdrawal against time curve (AUC). * p < 0.05, ** p < 0.01 and *** p < 0.001, significantly different from the vehicle (Veh) or Sham (S) group, as determined by one (panels C and D) or two-way (panels E and G) analysis of variance followed by the Dunnett's test or Bonferroni test, respectively. +p < 0.05, ++ p<0.01 and +++ p<0.001, significantly different form the SNI + Veh group. Student t-test was used to compare 2 independent groups.
Figure 3.
Expression of P2Y1 receptors in DRG and spinal cord and effect of the selective P2Y1 receptor antagonist MRS2500 (MRS) in rats subjected to spinal nerve ligation (SNL). Time course of the withdrawal threshold observed after SNL (panel A). Expression of P2Y1 receptors in the ipsilateral (IL) dorsal root ganglia (DRG) and dorsal spinal cord (SC) in sham (S) and neuropathic rats at 1, 3, 7 and 14 days after injury (panels B, C and D). Antiallodynic effect of MRS2500 in rats previously (3 days) subjected to SNL (panel E). Effect of MRS2500 on SNL-induced up-regulation of P2Y1 receptors in DRG of rats previously (3 days) subjected to SNL (panel F). Antiallodynic effect of the selective P2Y1 receptor antagonist MRS2500 in rats previously (14 days) subjected to SNL (panel G). Behavioral and molecular data are expressed as mean ± S.E.M. for 6 and 3 animals, respectively. Data are expressed as the time course of the withdrawal threshold and 50% threshold withdrawal against time curve (AUC). * p < 0.05, ** p < 0.01 and *** p < 0.001, significantly different from the vehicle (Veh) or Sham (S) group, as determined by one (panels C and D) or two-way (panels E and G) analysis of variance followed by the Dunnett's test or Bonferroni test, respectively. +p < 0.05, significantly different form the SNI + Veh group. Student t-test was used to compare 2 independent groups.
3. Discussion
We have observed that intrathecal administration of MRS2500 diminishes tactile allodynia in 3 models of neuropathic pain in the rat. The effects of this drug were mainly observed in the development (1-3 days) and, to a lesser extent, maintenance (14 days) of neuropathic pain. Since this drug is a selective P2Y1 receptor antagonist (Hechler et al., 2006), our data suggest that spinal P2Y1 receptors participate in the development of neuropathic pain induced by CCI, SNI and SNL. To our knowledge, this is the first study demonstrating that spinal P2Y1 receptors blockade reduces development of neuropathic pain in the rat. In support of our study, local peripheral administration of the selective P2Y1 receptor antagonists MRS2500 or MRS2179 reduces ischemia-, formalin- and carrageenan-induced nociception (Seo et al., 2011; Kwon et al., 2014a; Barragán-Iglesias et al., 2015). Conversely, our results disagree with one study showing that activation of P2Y1 receptors leads to antinociception as intraperitoneal administration of the selective P2Y1 receptor agonist MRS2365 produces antiallodynic effects in rats subjected to partial sciatic nerve ligation (Andó et al., 2010). This discrepancy could be due to the administration route (intrathecal versus intraperitoneal). After systemic administration, a drug reaches multiple sites of action (peripheral, intrathecal and supraspinal). Particularly, supraspinal sites of action could lead to opposite actions to what is observed in the spinal cord, peripheral terminals or immune cells. However, more research is needed in order to clarify this point.
We also have shown here that P2Y1 receptors are expressed in DRG and dorsal spinal cord of naïve rats. Our results concur with previous studies indicating that P2Y1 receptor mRNA or protein is found in rat DRG and spinal cord (Nakamura and Strittmatter, 1996; Xiao et al., 2002; Ruan and Burnstock, 2003; Kobayashi et al., 2006; Tsuchihara et al., 2009; Kwon et al., 2014a; Barragán-Iglesias et al., 2015). Particularly, P2Y1 receptors are expressed in peptidergic and non-peptidergic C-type sensory neurons (Ruan and Burnstock, 2003). Furthermore, these receptors have been found in laminae I–II and III–V of the dorsal horn (Kobayashi et al., 2006). Interestingly, CCI, SNI and SNL up-regulated P2Y1 receptors expression in the ipsilateral DRG, but not spinal cord, 1 or 3 days after nerve injury. As stated in results, SNI produced the greatest enhancement of protein expression compared to CCI and SNL. Differences could be due to the severity of the nerve injury induced in the SNI model as nerves are ligated and axotomized, whereas in the other models nerves are only ligated. Previous studies have reported that CCI or axotomy increases P2Y1 receptors mRNA expression in DRG 2 to 28 days after ligation (Xiao et al., 2002; Tsuchihara et al., 2009) suggesting that P2Y1 receptors have a role in the development and maintenance of neuropathic pain. Our data disagree with these studies in the time course of the P2Y1 receptors mRNA expression. Differences could be due to techniques used (western blot versus in situ hybridization). Notwithstanding, our data in 3 different models indicate that P2Y1 receptors protein expression in DRG has a peak during the development (1-3 days), but not maintenance, of neuropathic pain.
To further reinforce the participation of spinal P2Y1 receptors in the development of neuropathic pain in rats, we demonstrated that intrathecal administration of the P2Y1 receptor antagonist diminishes nerve injury-induced tactile allodynia and P2Y1 receptor up-regulation in DRG at 1-3 days. Reinforcing this, intrathecal injection of MRS2500 only produced a small antiallodynic effect while it did not modify expression of P2Y1 receptors in DRG or spinal cord at 14 days. These data strongly suggest that spinal P2Y1 receptors participate in the development of neuropathic pain. Interestingly, others have reported that intrathecal administration of the P2Y1 receptor antagonist MRS2179 decreased P2Y1 receptor mRNA and phosphorylated extracellular signal-regulated kinases (p-ERK1/2) protein expression in the spinal dorsal horn and DRG of rats with bone cancer pain (Chen et al., 2012). Furthermore, local peripheral injection of the P2Y1 receptor antagonist MRS2500 blocked carrageenan-induced up-regulated TRPV1 expression and phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK), while the P2Y1 receptor agonist MRS2365 enhanced TRPV1 expression and p38 MAPK phosphorylation in DRG from naïve rats (Kwon et al., 2014a). Based on this, it is tempting to speculate that inhibition of P2Y1 receptors may block the induction of p38 MAPK phosphorylation and reduce pro-inflammatory cytokines leading to down-regulation of the purinergic receptors and antinociception.
Conclusion
Our data indicate that intrathecal injection of a P2Y1 receptor antagonist blunts neuropathy-induced tactile allodynia during development and, to a lesser extent, maintenance of neuropathic pain. Moreover, P2Y1 receptors are expressed in DRG and dorsal spinal cord while nerve injury up-regulates P2Y1 receptors in DRG but not in the spinal cord. Our data suggest that the spinal P2Y1 receptors participate in the development and, to a lesser extent, maintenance of neuropathic pain.
4. Experimental procedure
4.1. Animals
Previous experiments in our conditions found no differences between male and female rats (Caram-Salas et al., 2007). Thus, all experiments in this study were performed on female Wistar rats (140–180 g). Animals were obtained from our own breeding facilities or from Taconic (Hudson, NY) and had free access to food and drinking water. All experiments followed the Guidelines on Ethical Standards for Investigation of Experimental Pain in Animals (Zimmerman, 1983) and the Mexican regulation (NOM-062-ZOO-1999). The Institutional Ethics Committee or Institutional Animal Care and Use Committee approved all experiments (Protocol #s 042-13, 14-04).
4.2. SNL, CCI and SNI models of neuropathic pain
Animals used in the three models were anesthetized with a mixture of ketamine (50 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). In the SNL model, spinal L5-L6 nerves were exposed and tightly ligated with 6-0 silk suture distal to the dorsal root ganglion as previously reported (Kim and Chung, 1992). On the other hand, CCI model was performed according as in Bennett and Xie (1988). Briefly, the sciatic nerve was exposed and ligated with four loosely tied chromic gut ligatures around the sciatic nerve at 1 mm intervals. Finally, SNI model was performed according to the original report (Decosterd and Woolf, 2000). The procedure comprised a ligation and axotomy of the tibial and common peroneal nerves leaving the sural nerve intact.
For sham-operated rats, nerves were exposed but not ligated or axotomized. Tactile allodynia in all models was determined according to a reported method (Chaplan et al., 1994). For this purpose, rats were transferred to clear plastic, wire mesh-bottomed cages and allowed to acclimatize for 30–40 min. Von Frey filaments (Stoelting, Wood Dale, IL) were used to determine the 50% paw withdrawal threshold using the up-down method (Dixon, 1980). Allodynia was considered to be present when paw withdrawal thresholds were less than 4 g.
4.3. Intrathecal administration
Intrathecal administration of vehicle or MRS2500 (10 μl) at days 1, 3 or 14 following SNL, CCI or SNI was conducted by direct lumbar puncture in the L5-L6 intervertebral space with a 30-gauge needle following the method of Mestre (Mestre et al., 1994). This procedure was performed under isoflurane anesthesia to permit rapid recovery of the animals.
4.4. Drugs
(1R*,2S*)-4-[2-Iodo-6-(methylamino)-9H-purin-9-yl]-2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester tetraammonium salt (MRS2500) was purchased from Tocris Bioscience (Ellisville, MO). This purinergic drug was selected based on relevant receptor selectivity and efficacy (Hechler et al., 2006). The doses used in this study were chosen based in pilot experiments in our conditions. MRS2500 was dissolved in 0.9% saline.
4.5. Western blot analysis
Western blot analysis was used to determine the expression of P2Y1 receptors in the ipsilateral DRG and spinal cord. Naïve and ligated rats were sacrificed by decapitation. The L4-L6 DRGs and lumbar region of the dorsal spinal cord were dissected. Immediately, tissues were frozen and stored at −70°C. Tissues from individual animals were homogenized in ice-cold lysis buffer (in mM: 150 NaCl, 50 Tris–HCl, 5 EDTA), pH 7.4 for 30 min at 4°C. The protease inhibitors PMSF (1 mM), aprotinin (10 μg/mL), leupeptin (10 μg/mL), pepstatin A (10 μg/mL) and the surfactant 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO) were added to the lysis buffer immediately prior to use. The homogenate was then centrifuged (Eppendorf, Hamburg) at 14,000 rpm for 10 min to remove cellular debris. The resultant supernatant was used to measure protein concentration by the Bradford's method (Bio-Rad, Hercules, CA).
Fifty micrograms of total protein were resolved by denaturing in 10% SDS–polyacrylamide gel electrophoresis and transferred to PVDF membranes. Membranes were blocked in 5% non-fat milk in PBS at pH 7.4 (in mM: 137 NaCl, 2.7 KCl, 10 Na2HPO4 and 2 KH2PO4) with 0.05% Tween-20 for 1 h. After that, they were washed and incubated overnight at 4°C in 5% non-fat dry milk/PBS containing goat anti-P2Y1 antibody (1:500, Santa Cruz Biotechnology, Cat # SC-15204, Dallas, Texas). Membranes were incubated for 1 h at room temperature in 1% non-fat milk/PBS containing the horseradish peroxidase-conjugated secondary antibody (Donkey anti-goat, Cat # 705-035-003, 1:10000, Jackson immunoresearch, West Grove, PA). Protein signal detection was achieved with the ECL chemiluminescence system (ECL plus, Amersham, UK). The next day, blots were stripped and incubated with a monoclonal antibody directed against β-actin (1:10000, Cat # MAB1501R, Millipore, Billerica, MA) or GAPDH (1:10000, Cat # 14C10, Cell Signaling, Danvers, MA), which were used as internal controls to normalize P2Y1 protein expression level. Scanning of the immunoblots was performed and the bands were quantified by densitometry using an image analysis program (Image Lab Software Version 5.2.1, Bio-Rad, Hercules, CA).
4.6. Experimental design
In order to assess the role of spinal P2Y1 receptors in the neuropathic pain induced by SNL, CCI or SNI, we determined the expression of this receptor at the ipsilateral DRG and dorsal spinal cord at 1, 3, 7 and 14 days after nerve injury. P2Y1 receptors expression reached a peak at 1 or 3 days after injury. Thus, administration of the P2Y1 receptor antagonist MRS2500 (19-190 ng) in neuropathic rats was carried out at the same times to determine the role of these receptors in the development of neuropathic pain. We also tested the effect of MRS2500 14 days after nerve injury in order to determine the role of these receptors in the maintenance of neuropathic pain. Moreover, to confirm the participation of the spinal P2Y1 receptors in the development and maintenance of neuropathic pain in rats, we measured the expression of P2Y1 receptors in presence and absence of MRS2500 at 3 and 14 days, respectively, after nerve injury.
4.7. Data analysis and statistics
All behavioral results are given as the mean ± S.E.M. for six animals per group. Curves were constructed by plotting the threshold for paw withdrawal as a function of time. An increase of 50% withdrawal threshold was considered as antiallodynia. For protein expression, all results are given as the mean relative intensity ± S.E.M. for 3 animals per group.
One- or two-way analysis of variance, followed by the Dunnett test was used to compare differences in more than 2 experimental groups. Student t-test was used to compare 2 experimental groups. Differences were considered to reach statistical significance when p < 0.05.
Acknowledgements
The authors greatly appreciate the bibliographic and technical assistance of B.Sc. Pilar Quinteros-Carrillo and M.Sc. Guadalupe C Vidal-Cantú, respectively. This work is part of the Ph.D. dissertation of Paulino Barragán-Iglesias. Graduate students Paulino Barragán-Iglesias, Jorge B. Pineda-Farias, Claudia Cervantes-Durán and Mariana Bravo-Hernández are Conacyt fellows. Partially supported by Conacyt (grant CB-2012/179294 to VG-S) and NIH (grant GM102575 to TJP).
Abbreviations
- ADP
Adenosine 5′- diphosphate.
- ATP
Adenosine 5′-triphosphate.
- CCI
Chronic constriction injury.
- CFA
Complete Freund's adjuvant.
- DRG
Dorsal root ganglia.
- ERK1/2
Extracellular signal-regulated kinases.
- MRS2500
(1R*,2S*)-4-[2-Iodo-6-(methylamino)-9H-purin-9-yl]-2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester tetraammonium salt.
- p38 MAPK
p38 mitogen-activated protein kinase.
- SNI
Spared nerve injury.
- SNL
Spinal nerve ligation.
- UDP
Uridine-5′-diphosphate.
- UTP
Uridine-5′-triphosphate.
Footnotes
Conflicts of interest statement The authors declare that they have no competing interests with respect to this manuscript.
REFERENCES
- Andó RD, Mehesz B, Gyires K, Illes P, Sperlagh B. A comparative analysis of the activity of ligands acting at P2X and P2Y receptor subtypes in models of neuropathic, acute and inflammatory pain. Br. J. Pharmacol. 2010;159:1106–1117. doi: 10.1111/j.1476-5381.2009.00596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragán-Iglesias P, Pineda-Farias JB, Cervantes-Durán C, Bravo-Hernández M, Rocha-González HI, Murbartián J, Granados-Soto V. Role of spinal P2Y6 and P2Y11 receptors in neuropathic pain in rats: possible involvement of glial cells. Mol. Pain. 2014;10:29. doi: 10.1186/1744-8069-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragán-Iglesias P, Méndoza-Garces L, Pineda-Farias JB, Solano-Olivares V, Rodríguez-Silverio J, Flores-Murrieta FJ, Granados-Soto V, Rocha-González HI. Participation of peripheral P2Y1, P2Y6 and P2Y11 receptors in formalin-induced inflammatory pain in rats. Pharmacol. Biochem. Behav. 2015;128:23–32. doi: 10.1016/j.pbb.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Krugel U, Abbracchio MP, Illes P. Purinergic signalling: From normal behaviour to pathological brain function. Prog. Neurobiol. 2011;95:229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Caram-Salas NL, Reyes-García G, Bartoszyk GD, Araiza-Saldaña CI, Ambriz-Tututi M, Rocha-González HI, Arreola-Espino R, Cruz SL, Granados-Soto V. Subcutaneous, intrathecal and periaqueductal grey administration of asimadoline and ICI-204448 reduces tactile allodynia in the rat. Eur. J. Pharmacol. 2007;573:75–83. doi: 10.1016/j.ejphar.2007.06.034. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang L, Zhang Y, Yang J. P2Y1 purinoceptor inhibition reduces extracellular signal-regulated protein kinase 1/2 phosphorylation in spinal cord and dorsal root ganglia: implications for cancer-induced bone pain. Acta Biochim. Biophys. Sin. (Shanghai) 2012;44:367–372. doi: 10.1093/abbs/gms007. [DOI] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Illes P. P2X receptors and nociception. Pharmacol. Rev. 2001;53:553–568. [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu. Rev.Pharmacol. Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, Jacobson KA, Gachet C. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2'-deoxyadenosine-3',5'-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J. Pharmacol. Exp. Ther. 2006;316:556–563. doi: 10.1124/jpet.105.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Donnelly-Roberts D, Namovic M, Hsieh G, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, Harris R, Medrano AP, Carroll W, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-740003 [N-(1-{[(cyanoimino)(5- quinolinylamino) methyl]amino}-2,2-dimethylpropyl)- 2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J. Pharmacol. Exp. Ther. 2006;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- Honore P, Kage K, Mikusa J, Watt AT, Johnston JF, Wyatt JR, Faltynek CR, Jarvis MF, Lynch K. Analgesic profile of intrathecal P2X3 antisense oligonucleotide treatment in chronic inflammatory and neuropathic pain states in rats. Pain. 2002;99:11–19. doi: 10.1016/s0304-3959(02)00032-5. [DOI] [PubMed] [Google Scholar]
- Horváth G, Gölöncsér F, Csölle C, Király K, Andó RD, Baranyi M, Koványi B, Máté Z, Hoffmann K, Algaier I, Baqi Y, Müller CE, Kügelgen IV, Sperlágh B. Central P2Y12 receptor blockade alleviates inflammatory and neuropathic pain and cytokine production in rodents. Neurobiol. Dis. 2014;70:162–178. doi: 10.1016/j.nbd.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Ivanov AA, de Castro S, Harden TK, Ko H. Development of selective agonists and antagonists of P2Y receptors. Purinergic Signal. 2009;5:75–89. doi: 10.1007/s11302-008-9106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Soneji DJ, Ekmann KM, Anderson CE, Molliver DC, Koerber HR. Purinergic receptor P2Y1 regulates polymodal C-fiber thermal thresholds and sensory neuron phenotypic switching during peripheral inflammation. Pain. 2012;153:410–419. doi: 10.1016/j.pain.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc. Natl. Acad. Sci. USA. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J. Comp. Neurol. 2006;498:443–454. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J. Neurosci. 2008;28:2892–2902. doi: 10.1523/JNEUROSCI.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Yamanaka H, Yanamoto F, Okubo M, Noguchi K. Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia. 2012;60:1529–1539. doi: 10.1002/glia.22373. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Yamanaka H, Noguchi K. Expression of ATP receptors in the rat dorsal root ganglion and spinal cord. Anat. Sci. Int. 2013;88:10–16. doi: 10.1007/s12565-012-0163-9. [DOI] [PubMed] [Google Scholar]
- Kwon SG, Roh DH, Yoon SY, Moon JY, Choi SR, Choi HS, Kang SY, Han HJ, Beitz AJ, Lee JH. Blockade of peripheral P2Y1 receptors prevents the induction of thermal hyperalgesia via modulation of TRPV1 expression in carrageenan-induced inflammatory pain rats: involvement of p38 MAPK phosphorylation in DRGs. Neuropharmacology. 2014a;79:368–379. doi: 10.1016/j.neuropharm.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Kwon SG, Roh DH, Yoon SY, Moon JY, Choi SR, Choi HS, Kang SY, Han HJ, Beitz AJ, Oh SB, Lee JH. Acid evoked thermal hyperalgesia involves peripheral P2Y1 receptor mediated TRPV1 phosphorylation in a rodent model of thrombus induced ischemic pain. Mol. Pain. 2014b;10:2. doi: 10.1186/1744-8069-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lu Z-Y, Yu L-H, Burnstock G, Deng X-M, Ma B. Inhibition of G protein-coupled P2Y2 receptor induced analgesia in a rat model of trigeminal neuropathic pain. Mol. Pain. 2014;10:21. doi: 10.1186/1744-8069-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptors activating molecules. Mol. Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Magni G, Merli D, Verderio C, Abbracchio MP, Ceruti S. P2Y2 receptor antagonists as anti-allodynic agents in acute and sub-chronic trigeminal sensitization: role of satellite glial cells. Glia. 2015;63:1256–1269. doi: 10.1002/glia.22819. [DOI] [PubMed] [Google Scholar]
- Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J. Pharmacol. Toxicol. Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Morán-Jimenez MJ, Matute C. Immunohistochemical localization of the P2Y1 purinergic receptor in neurons and glial cells of the central nervous system. Brain Res. Mol. Brain Res. 2000;78:50–58. doi: 10.1016/s0169-328x(00)00067-x. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: contribution to touch-induced impulse generation. Proc. Natl. Acad. Sci. USA. 1996;93:10465–10470. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan HZ, Burnstock G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem Cell Biol. 2003;120:415–426. doi: 10.1007/s00418-003-0579-3. [DOI] [PubMed] [Google Scholar]
- Seo HS, Roh DH, Kwon SG, Yoon SY, Kang SY, Moon JY, Choi SR, Beitz AJ, Lee JH. Acidic pH facilitates peripheral alphabetameATP-mediated nociception in rats: differential roles of P2X, P2Y, ASIC and TRPV1 receptors in ATP-induced mechanical allodynia and thermal hyperalgesia. Neuropharmacology. 2011;60:580–586. doi: 10.1016/j.neuropharm.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Sharp CJ, Reeve AJ, Collins SD, Martindale JC, Summerfield SG, Sargent BS, Bate ST, Chessell IP. Investigation into the role of P2X3/P2X2/3 receptors in neuropathic pain following chronic constriction injury in the rat: an electrophysiological study. Br. J. Pharmacol. 2006;148:845–852. doi: 10.1038/sj.bjp.0706790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J. Neurosci. 2008;28:4949–4956. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchihara T, Ogata S, Nemoto K, Okabayashi T, Nakanishi K, Kato N, Morishita R, Kaneda Y, Uenoyama M, Suzuki S, Amako M, Kawai T, Arino H. Nonviral retrograde gene transfer of human hepatocyte growth factor improves neuropathic pain-related phenomena in rats. Mol. Ther. 2009;17:42–50. doi: 10.1038/mt.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Waldo GL, Harden TK. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol. Pharmacol. 2004;65:426–436. doi: 10.1124/mol.65.2.426. [DOI] [PubMed] [Google Scholar]
- Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc. Natl. Acad. Sci. USA. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]