Summary
Lactobacillus plantarum WCFS1 is one of the best studied Lactobacilli, notably as its genome was unravelled over 12 years ago. L. plantarum WCFS1 can be grown to high densities, is amenable to genetic transformation and highly robust with a relatively high survival rate during the gastrointestinal passage. In this review, we present and discuss the main insights provided by the functional genomics research on L. plantarum WCFS1 with specific attention for the molecular mechanisms related to its interaction with the human host and its potential to modify the immune system, and induce other health‐related benefits. Whereas most insight has been gained in mouse and other model studies, only five human studies have been reported with L. plantarum WCFS1. Hence NCIMB 8826 (the parental strain of L. plantarum WCFS1) in human trials as to capitalize on the wealth of knowledge that is summarized here.
Introduction
There continues to be significant interest in lactic acid bacteria (LAB) that contribute to our quality of life by preserving and fortifying foods, producing flavours and texture, and providing health benefits (De Vos, 2011). Hence, recent years have seen the production of a panoply of publications that address these attributes in LAB with most of the applications relating to Lactobacillus spp. that are used in functional foods. While there are over 100 different Lactobacillus species that now all have been characterized at the genomic level (Sun et al., 2015), only few have been studied in detail and developed into paradigms, as is the case with many biotechnological systems. In 2003, the complete 3.3 Mb genome of Lactobacillus plantarum WCSF1, a single colony of the human saliva isolate L. plantarum NCIMB 8826, was published as the first genome of a Lactobacillus species (Kleerebezem et al., 2003). This was well before the genomes of other well‐studied Lactobacillus spp. were reported, such as those from L. acidophilus NCFM (Altermann et al., 2005) and L. rhamnosus GG (Morita et al., 2009) that are widely marketed as probiotics (Saxelin et al., 2005). Currently, there are 26 genome sequences of different L. plantarum strains available in the NCBI database. There is a high degree of gene content variation among L. plantarum strains (Molenaar et al., 2005). Due to the presence of its genome sequence, its flexible growth properties and high transformation efficiency with newly developed genetic tools (Pavan et al., 2000; Kleerebezem et al., 2003; Cohen et al., 2006; Yang et al., 2015), L. plantarum WCSF1 has been extensively studied, up to the level of genome scale modelling and growth optimization (Teusink et al., 2005, 2006). In retrospect, these were exactly the attributes why this particular strain was selected for genome sequencing a dozen years ago (Kleerebezem et al., 2003). In this review, we present an overview of the main insights that the molecular research on L. plantarum WCSF1 has provided in relation to the interaction with the host and potential health benefit for humans. Moreover, we indicate a variety of avenues that can be followed up for future industrial applications of this strain and summarize suggestions further research that is needed for such applications.
From genome to function
The initial genome sequence of L. plantarum WCFS1 was based on Sanger dideoxy‐sequencing (Kleerebezem et al., 2003) and has been revised by next generation sequence analysis on an Illumina platform, providing a genome sequence predicted to code for 3042 proteins (18 pseudogenes) and 83 RNA‐encoding genes (Siezen and van Hylckama Vlieg, 2011).
The genome contains two large regions, one of approximately 150 kb (at position 2.70–2.85 Mb) and one of 190 kb (at position 3.10–3.29 Mb) that are variable in many other L. plantarum strains and have been termed life style islands, since they code for a total of 293 genes that are mainly involved in sugar degradation. (Molenaar et al., 2005). Furthermore, L. plantarum WCFS1 contains three plasmids, including two small ones, pWCFS101 and pWCFS102, that are rolling‐circle replicating plasmids with an unclear function and a size of 1917 and 2365 bp respectively. A third plasmid, pWCFS103 has a size of 36 069 bp, the capacity for conjugative transfer, and encodes genes involved in heavy‐metal resistance (cadmium and arsenate) and NADH oxidase activity (Van Kranenburg et al., 2005).
The genome of L. plantarum WCFS1 has been well annotated not only by automated methods but also by detailed manual curation. However, there is still a large fraction (approximately 30%) of genes for which no function can be predicted. Moreover, as is the case with all genomes, in some cases the annotated genes are not correctly predicted and a combination of genetic and physiological experiments is needed to demonstrate the functionality of a gene. Due to the high transformation efficiency of L. plantarum WCFS1 (De Vos, 2011), a variety of useful inactivation systems (Lambert et al., 2007), and controlled expression platforms (such as NICE; Pavan et al., 2000), a great number of isogenic mutants have been generated in L. plantarum WCFS1 or NCIMB 8826. Many of these are relevant for its growth, cell shape or surface properties and its interactions with the environment – hence these are listed here and some of these are discussed further (see Table 1). Apart from the genetic systems, a useful set of high throughput tools have been applied in recent years, varying from various microarray platforms, RNAseq approaches and advanced proteomics (Molenaar et al., 2005; Bron et al., 2006; Cohen et al., 2006). This allowed detailed phenotypic analysis of mutants, functional studies of overexpressed genes, and the evaluation of genome‐wide expression in response to environmental cues. Many of these approaches have been instrumental in not only confirming the predicted function of genes but also defining new and relevant properties, notably for gastrointestinal (GI) tract survival and interactions with food, other bacteria and the host, as will be discussed below.
Table 1.
Overview of relevant Lactobacillus plantarum WCFS1 mutants, the involved gene and their phenotypes, classified according to their gene function. Some mutants with mutations in homologous genes and similar phenotypes are combined
| Gene(s) | Locus | Gene function | Affected phenotype | Reference |
|---|---|---|---|---|
| Substrate utilization & respiration | ||||
| melA | lp_3485 | α‐Galactosidase | Melibiose utilization | Lambert et al. (2007) |
| lacS2 | lp_3468 | Sugar permease | ND | Lambert et al. (2007) |
| cydA | lp_1125 | Subunit cytochrome (bd type) | Oxidative respiration | Brooijmans et al. (2009) |
| narG | lp_1497 | Subunit nitrate reductase | Nitrate respiration | Brooijmans et al. (2009) |
| rpoN | lp_0787 | Sigma factor 54 | Global expression | Stevens et al. (2010) |
| manR | lp_0585 | Mannose operon regulator | Mannose utilization | Stevens et al. (2010) |
| manIIC | lp_0576 | Mannose transport | Mannose utilization | Stevens et al. (2010) |
| ccpA | lp_2256 | Carbon control protein | Glucose repression | Zotta et al. (2012) |
| lpdB | lp_0271 | Gallate decarboxylase | Tannine utilization | Jiménez et al. (2013) |
| lpdC | lp_2945 | Gallate decarboxylase | Tannine utilization | Jiménez et al. (2013) |
| Quorum sensing & bacteriocin production | ||||
| lamA | lp_3580 | Response regulator | QS/EPS/biofilm production | Sturme et al. (2005) |
| lamR | lp_3087 | Response regulator | QS/EPS/biofilm production | Fujii et al. (2008) |
| plnGHSTUVWX | lp_0423‐0430 | QS pheromone & transport | QS pheromone production | Meijerink et al. (2010) |
| plnEFI | lp_0419‐0423 | ABC transporter | Plantaricin transport | Meijerink et al. (2010) |
| plnABCD | lp_0415‐0418 | Plantaricin & QS module | Plantaricin A production | Maldonado‐Barragán et al. (2009) |
| Stress response and intestinal tract survival | ||||
| bsh1 | lp_3538 | Choloyl glycin hydrolase | Bile resistance | Lambert et al. (2007) |
| bsh2‐bsh3‐bsh4 | lp_0067‐lp_3362‐lp_2572 | Penicillin acylase | Acylase activity | Lambert et al. (2008a) |
| ctsR | lp_1018 | Class III stressor | Stress/control ftsH expression | Fiocco et al. (2009) |
| ftsH | lp_0547 | Chaperone protease | Stress resistance | Fiocco et al. (2009) |
| hsp 18.55 | lp_3352 | Heat shock protein | Membrane fluidity | Capozzi et al. (2011) |
| Cell surface proteins & host interaction | ||||
| lp_0373 | lp_0373 | Cell Surface protein | ND | Pretzer et al. (2005) |
| msa | lp_1229 | Mannose‐specific adhesion | Agglutination | Pretzer et al. (2005) |
| srtA | lp_0514 | Sortase | Protein anchoring | Pretzer et al. (2005) |
| lp_2940 | lp_2940 | Cell surface protein | Mouse GI tract passage | Bron et al. (2007) |
| lp_1164 | lp_1164 | Cellobiose EII transporter | Mouse GI tract passage | Bron et al. (2007) |
| lp_3055 | lp_3055 | Copper transporter | Mouse GI tract passage | Bron et al. (2007) |
| napA3 | lp_2827 | Na/H antiporter | In vitro GI tract survival | Van Bokhorst‐van de Veen et al. (2012a) |
| pbp2A | lp_1413 | Penicillin‐binding protein | In vitro GI tract survival | Van Bokhorst‐van de Veen et al. (2012a) |
| lp_1699 | lp_1699 | AraC regulator | In vitro GI tract survival | Van Bokhorst‐van de Veen et al. (2012a) |
| Cell shape or surface properties modulation | ||||
| dltD | lp_2016 | D‐alanine transfer | Charged techoic acids | Grangette et al. (2005) |
| alr | lp_0523 | Alanine racemase | Cell envelope integrity | Palumbo et al. (2004) |
| acm2 | lp_2645 | N‐acetyl glucosaminidase | Autolysin cell separation | Rolain et al. (2012) |
| lytA | lp_3421 | D,L endopeptidase | Cell shape and integrity | Rolain et al. (2012) |
| lys2 | lp_3093 | N‐acetylmuramidase | Cell separation | Rolain et al. (2012) |
| tagF1‐tagF2 | lp_0268‐ lp_0269 | Glycerol phosphate transferase | Wall techoic acid modification | Tomita et al. (2013) |
| cps1A | lp_1177 | SPS production | Reduced SPS and rhamnose level | Remus et al. (2012) |
| cps2A | lp_1197 | SPS production | Reduced SPS levels | Remus et al. (2012) |
| cps3A‐cps4A | lp_1215‐ lp_2108 | SPS production | Reduced SPS levels | Remus et al. (2012) |
| gtfA | lp_1299 | Glycosyl transferase | Surface protein glycosylation | Lee et al. (2014) |
| gtfB | lp_1311 | Glycosyl transferase | Surface protein glycosylation | Lee et al. (2014) |
| oatA | lp_0856 | O‐acetyl transferase | Cell septation | Bernard et al. (2011) |
| oatB | lp_0925 | O‐acetyl transferase | Cell septation | Bernard et al. (2011) |
GI, Gastrointestinal; EPS, Extracellular polymeric substance; SPS, Surface Poly Saccharide; QS, Quorum Sensing; ND, not detected.
Gastrointestinal tract survival
L. plantarum NCIMB 8826, the parental strain of L. plantarum WCFS1, shows high survival capacity in the human GI tract. After a single oral dose of 1.5 × 1010 CFU ml−1, the survival of L. plantarum NCIMB 8826 was 7%, which was much higher than that of L. fermentum KLD and Lactococcus lactis MG 1363 that showed an ileal survival of 0.5% and 1%, respectively (Vesa et al., 2000). On day 7 of a 1‐week ingestion period, L. plantarum NCIMB was found at high concentrations of 108 CFU g−1 in faecal samples (Vesa et al., 2000). In addition, L. plantarum WCFS1 was readily obtained from the ileal effluents of ileostoma patients fed an oral dose (Marco et al., 2010). Another study demonstrated that L. plantarum WCFS1 in healthy human volunteers survived the in vivo GI tract passage, as a 100‐ to 1000‐fold increased level of L. plantarum WCFS1 compared with other L. plantarum strains could be recovered from faecal samples until 3–4 days after administration (Van Bokhorst‐van de Veen et al., 2012b). The relative survival rate of L. plantarum WCFS1 under conditions mimicking the GI‐tract passage was high compared with other L. plantarum strains (e.g. a difference in survival of 7 log10 CFU ml−1 compared with L. plantarum CECT4646). However, the human volunteer trials confirmed an earlier study (Vesa et al., 2000) and indicated that L. plantarum WCFS1 is a passenger in the GI tract, and not an effective intestinal colonizer as found for various other Lactobacilli (Douillard and de Vos, 2014). It should be stressed that only the lumen was analysed, whereas the bacteria could have colonized the intestinal epithelium. Furthermore, during the GI passage the microorganism is able to exert its effect on the physiological and immunological systems of the host. For instance, the well‐studied probiotic strain L. rhamnosus GG, which has been subscribed to be a very good mucus adhering Lactobacillus strain due to mucus‐binding pili (spaCBA‐srtC1), is also only able to temporary colonize the gut (up to 1 week; Goldin et al., 1992; Alander et al., 1999; Segers and Lebeer, 2014). It is assumed that the majority of Lactobacilli are passengers in the GI tract, rarely exceeding 1% of the total number of bacteria (Douillard and de Vos, 2014).
The first hurdle a consumed bacterium encounters when entering the GI tract is the acidic stomach. An in vitro GI tract survival model, in which bacteria were exposed for 1 h to gastric juice containing pepsin and lipase at a pH of approximately 2.5, and subsequently subjected to pH‐neutralizing pancreatic juice containing pancreatin and bile salts, L. plantarum WCFS1 proved to be robust, with a 3‐log decrease in viable cells (Van Bokhorst‐van de Veen et al., 2012a). The gastric juice exerted the highest impact on L. plantarum WCFS1 survival, demonstrated by a million‐fold decrease in living cells, whereas the condition resembling the small intestines hardly affected the survival (Van Bokhorst‐van de Veen et al., 2012a). Another oro‐gastric‐intestinal tract model demonstrated that survival of L. plantarum WCFS1 is unaffected by the initial oro‐gastric stress, however, the viability decreased significantly when pH was downshifted to approximately 2.0 (Bove et al., 2013). There are several mechanisms upregulated in response to low‐pH conditions, for instance increased proton export by F0F1‐ATPase to retain a proper intracellular pH. Furthermore, the gastric stress was associated with an increased expression of the chaperone genes dnaK, groEL, clpB and clpE, small heat shock proteins hsp1, hsp2 and hsp3. In addition, the expression of the adhesion factors mucus‐binding (mub) and mannose‐specific adhesion (msa), and that of the operon plnEFI, an ABC transporter involved in plantaricin production, was increased in response to gastric stress (Table 1; Bove et al., 2013). The expression levels of three genes (pbp2A, napA3 and lp_1669) were negatively correlated with in vitro GI‐survival and encode a penicillin‐binding protein 2A, an Na+/H+‐antiporter and an AraC family regulator (see Table 1) that may control the expression of surface polysaccharide production respectively (Van Bokhorst‐van de Veen et al., 2012a).
After survival of the stomach passage, an ingested bacterium reaches the duodenum, where it encounters a variety of stressful conditions, including the presence of conjugated bile salts. Not only do these bile salts disperse and absorb fat, these compounds also function as surfactants and disrupt the cells membrane integrity, generate free radicals, and when protonated, can lower the intracellular pH (Bron et al., 2004a; Van Bokhorst‐van de Veen et al., 2012b). Bile salts are deconjugated by bacterial bile salt hydrolases (Bsh) and reabsorbed in the colon. L. plantarum WCFS1 contains four genes originally annotated as bile salt hydrolases (bsh1, bsh2, bsh3 and bsh4; Table 1). However, only Bsh1 was shown to have Bsh activity and can be considered as the major Bsh while Bsh3, 2 and 4 were only able to hydrolyse penicillin V and penicillin G (Lambert et al., 2008a,b). However, under selective conditions, Bsh1, Bsh3 and Bsh4 could be able to hydrolyse bile salts (Table 1). It is suggested that Bsh plays a role in bile detoxification, GI persistence, serve a nutritional role and induce membrane alterations (Begley et al., 2006).
An artificial GI tract environment, consisting of 0.1% oxgall, a bovine bile salt, only marginally affected growth of L. plantarum WCFS1 but its morphology was severely changed, for instance into a less smooth surface (Bron et al., 2004a). L. plantarum reacted on this physiological stress by upregulating the expression of proteins involved in bile export (lp_0085, lp_2564 and lp_3160) and four oxidoreductases and a redox protein to restore the oxidative and redox imbalance (Bron et al., 2004a). In addition, the genes lp_0237 and lp_0775 were found to be bile‐inducible. Overall, 62 and 28 open‐reading frames are up‐ or downregulated respectively. Among the upregulated genes are the oxidative stress‐associated glutathione reductase and the metC‐cysK operon (Bron et al., 2006). By reducing the expression of non‐essential membrane proteins, the cell might compensate for the bile‐induced loss of membrane integrity (Bron et al., 2006). Proteomic and transcriptomic analysis revealed that L. rhamnosus GG and L. casei BL23 also have a reduced expression of proteins involved in cell wall function in response to bile stress, suggesting this is a more common trait in LAB (Douillard and de Vos, 2014). L. plantarum WCFS1 seems to have a large array of response mechanisms to bile salts. Since apparently only the morphology is altered and not the viability, WCFS1 is able to efficiently cope with this stressful environment. The hydrolysis of bile salts is associated with lowering of serum cholesterol as well as mucin production (Lambert et al., 2008a). Recently, Bsh in the GI tract have been implied in providing specific signals that reduce weight gain in mice after a high fat diet (Joyce et al., 2014).
Although the colonic lumen, where the majority of the microbiota resides, is deprived of oxygen, oxidative stress can be a problem as the mucosal surface is more oxygen‐rich. In addition, a high osmolarity predominates in the colon (Kleerebezem et al., 2010). L. plantarum WCFS1 has multiple strategies to respond to oxidative stress, like manganese, glutathione, ascorbate, pyruvate, flavonoids, carotenoids and the action of peroxidases. L. plantarum WCFS1 was found to respond to oxidative stress using thioredoxin (TRX), the only active thiol‐reducing system in this strain. Not all Gram‐positive bacteria can synthesize the antioxidant glutathione, therefore, the TRX system is essential for this organism (Serrano et al., 2007). The expression of the trxA2 and trxB1 genes was increased following oxidative stress and trxB2 in combination with trxA2 were involved in reductive stress and a temperature shift (Serrano et al., 2007). The trxB1 gene codes for a thioredoxin reductase, which regenerates oxidized TXR (Arnér and Holmgren, 2000).
Using a special in vivo expression technology system, a set of 72 genes of L. plantarum WCFS1 was identified whose promoters were specifically active during mouse GI tract passing (Bron et al., 2004b). Many of these genes were predicted to be involved in cell wall anchoring, exporters and metabolism. By inactivating a selection of these (Table 1), it was observed that the GI tract survival of the mutants Δlp_1164, Δlp_2940 and Δlp_3055 was decreased compared to the control strains, whereas the mutants Δlp_1403, Δlp_3281 and Δlp_3659 showed no differences (Bron et al., 2007). The gene at locus lp_1164 is suggested to encode a component of the cellobiose transport and could be involved in the host‐specific signalling, that at lp_2940 encodes an extracellular protein and that at lp_3055 is important in the copper homeostasis, as it is predicted to encode a copper‐transporting ATPase. These genes thus play an important role in the GI‐survival in mice and while the GI tract of mouse and human differ in architecture, pH and microbial composition, it is possible that these genes also play a role in the survival in the human system.
Administration of L. plantarum WCFS1 to a mouse model demonstrated that strains could be retrieved from faecal samples up to 7 days (Van Bokhorst‐van de Veen et al., 2013). The GI tract persistence could be increased to over 32 days when isolated faecal strains were re‐administered to the mice (Van Bokhorst‐van de Veen et al., 2013). The persistent strains all demonstrated a single nucleotide polymorphism (SNP) in genes coding for membrane associated proteins. The CFUs of L. plantarum in the stomach and small intestine remained high for at least 4 h after administration of 2 × 1010 CFU L. plantarum WCFS1 in mice but thereafter declined to background levels. This amount remains, however, at 1 × 109 CFU g−1 tissue for at least 8 h in the caecum and colon (Marco et al., 2007). A single intragastric gavage consisting of 1 × 109 CFU L. plantarum WCFS1 in GF mice on a chow or ‘western’ diet, showed colonization over the intestinal epithelium (Marco et al., 2009); remarkably, a significantly higher colonization in the colon and caecum was achieved when mice were on a chow diet. The host diets were associated with dramatically different transcription profiles. For instance, the western diet, consisting of mainly simple sugars, is restricting growth, demonstrated by 3 to 5‐times lower expression of genes involved in transcription, translation and nucleotide biosynthesis (Marco et al., 2009).
Interaction with food components
Current dietary recommendations include the consumption of fruits and vegetables. In addition to the vitamins and dietary fibre content of these products, it is a source of the polyphenol tannin. Tannins can form indigestible protein complexes and bind heavy metals. Tannins have also been associated with hepatotoxicity and cancer (Jiménez et al., 2013). On the other hand, tannins have antimicrobial properties; thereby potentially alter the gut composition. L. plantarum is so far the only tannin‐degrading Lactobacillus species found in humans and contains tannase (tannin acyl hydrolase; Reverón et al., 2013). L. plantarum WCFS1 is able to hydrolyse tannin into glucose and gallic acid, a harmful and anti‐nutritional compound, which is decarboxylated by LpdB and LpdC (lp_0271 and lp_2945) that encode gallate decarboxylase activity (Jiménez et al., 2013). Other L. plantarum strains that are suggested to possess tannase‐activity are L. plantarum CNRZ 1228, CNRZ 184, ATCC 8014 and ATCC 14917 (Osawa et al., 2000). Culturing of L. plantarum WCFS1 in the presence of tannic acid induces significantly higher expression of persistence and survival genes copA, lp_2940, ram2 and argG that are highly induced in the GI tract in response to high osmolarity and bile in mice and humans (Reverón et al., 2013). L. plantarum WCFS1 is thus able to respond to these toxic compounds as well as use these as an energy source, thereby selectively stimulating its growth.
Plant cell walls contain many phenolic compounds which, when released, have shown to have several beneficial effects on the host (e.g. anti‐inflammatory and anti‐oxidants; Esteban‐Torres et al., 2013). Feruloyl esterase (FE; lp_0796), an enzyme involved in the release of these compounds would be able to release these beneficial compounds. Unfortunately, an efficient transport system for feruloyl esters appeared to be lacking in L. plantarum WCFS1 as it was unable to hydrolyse any of the extracellular model substrates (Esteban‐Torres et al., 2013); cell extracts could, however, partially hydrolyse methyl ferulate and methyl p‐coumarate, demonstrating that the enzyme Lp_0769 is functional and is likely to be released upon lysis of L. plantarum WCFS1. Whether the activity of FE in L. plantarum WCFS1 is of significance remains to be determined, as other strains such as L. fermentum NCIMB 5221 and L. fermentum 11976 have superior FE‐activity and already demonstrate potential health effects (Bhathena et al., 2009; Tomaro‐Duchesneau et al., 2012).
L. plantarum WCFS1 encodes a p‐nitrobenzoate reductase (PnbA; encoded by lp_0050). The PnbA enzyme catalyses the reduction in nitroaromatics which are highly abundant food products due to several industrial processes (Guillen et al., 2009). These nitroaromatic compounds have been shown to be cytotoxic and mutagenic, therefore bacterial nitroreductases can have beneficial health effects on the host. PnbA is a highly selective reductase as it only reduces 4‐nitrobenzoate and 2,4‐dinitrobenzoate (Guillen et al., 2009).
Currently, many commercial products contain prebiotics, which are substances that selectively stimulate growth and/or activity of one or a limited number of bacteria and can thereby be beneficial to the host (Gibson et al., 2004). Short chain fructooligosaccharides (scFOS), a well‐studied prebiotic, is converted by L. plantarum WCFS1 by a sucrose phosphoenolpyruvate transport system, a β‐fructofuranosidase and a fructokinase (Saulnier et al., 2007). In vitro culturing indicated that scFOS are the optimal prebiotic substrates, although growth on scFOS was relatively slow. Detailed analysis showed that preferentially the trisaccharide 1‐ketose is used and its conversion was heterofermentative, since the end‐products are mainly lactate and acetate.
Interaction with other microorganisms
For successful GI tract survival, adaptation and response to environmental cues, L. plantarum needs sensing to react to other, mutualistic and competing, microorganisms. Gene expression depending on cell‐density is referred to as quorum sensing, and can significantly aid in the survival of the bacteria (Kleerebezem et al., 1997; Sturme et al., 2007). The quorum sensing systems are regulated by signal molecules, autoinducing peptides (AIPs) that are sensed by a two component systems (TCS), that include a histidine protein kinase (HPK) and a response regulator (RR; Sturme et al., 2005). The quorum‐sensing systems of L. plantarum WCFS1 have been well‐studied, notably the quorum‐sensing systems for the production of bacteriocins. L. plantarum WCFS1 possesses the pln locus that contains five operons; plnABCD, that encodes the AIP termed plantaricin A (plnA) which also is a bacteriocin, the HPK PlnB (plnB) and the two RRs PlnC and PlnD (Table 1; Sturme et al., 2007). L. plantarum WCFS1 shares this locus (to some extend) with L. plantarum C11, NC8 and J23 (Rojo‐Bezares et al., 2008). At a certain bacterial cell density, the plantaricin A concentration reaches a threshold, thereby activating the HPK PlnB and this subsequently phosphorylates the RRs PlnC an PlnD. The RRs regulate the transcription of all the genes involved in bacteriocin synthesis. In this way, there is a density‐dependent expression of plantaricin A. The operons plnEFI and plnJKLR encode the plantaricins EF and JK with their respective immune proteins (Rojo‐Bezares et al., 2008). The bacteriocins are subsequently transported and secreted by an ABC transporter and accessory proteins (PlnGH) encoded by the operon plnGHSTUVWXY, the role of which remains to be determined (Sáenz et al., 2009).
Bacteriocins play an important role in the competition with other microorganisms. Based on their characteristics, bacteriocins are distinguished into several classes. The antimicrobial peptides of L. plantarum WCFS1 can be classified into the non‐lantibiotic family (class II) and include the well‐studied plantaricin A, which is a class IIc bacteriocin, and the plantaricins EF and JK belonging to the class IIb two‐peptide bacteriocins (Table 1; Diep et al., 2009). The antimicrobial activity of plantaricin A has a relatively narrow spectrum and is significantly lower activity than that of the plantaricins EF and JK (Diep et al., 2009). The latter bacteriocins PlnEF and PlnJK are mostly active against Lactobacillus species and closely related Gram‐positive bacteria (e.g. L. plantarum, L. casei, L. sakei, L. curvatus, Pediococcus pentosaceus and P. acidilactici), whereas plantaricin A is effective against Lactobacillus species, such as L. casei, L. sakei, L. plantarum and L. viridescens (Diep et al., 2009). L. plantarum WCFS1 demonstrated bacteriocin production with a low minimum inhibitory concentration against Enterococcus faecalis CNRZ135, L. pentosus CECT4023T, L. plantarum CECT748T, Listeria innocua BL86/26 and P. pentosaceus FBB63. The bacteriocin production however depends on the inoculation size and is dependent on quorum sensing as described above. Hence, at low cell densities, the bacteriocin production is too low to inhibit growth of competing microorganisms (Maldonado‐Barragán et al., 2009). Moreover, the bacteriocins have not shown to be active against clinically relevant Gram‐positive pathogens, such as Staphylococcus aureus or Listeria monocytogenes, and therefore, their medical or biotechnological application remains limited.
Quorum sensing is also essential in the formation of biofilms. Biofilms render the bacteria less sensitive to antimicrobials due to reduced penetration and resistance mechanisms. In addition, bacteria are less susceptible due to a lower growth rate (Van der Veen and Abee, 2011). For instance, cells of L. plantarum WCFS1 in a mixed biofilm with L. monocytogenes were more resistant to disinfection treatments by benzalkonium chloride and peracetic acid than the single species biofilms (Van der Veen and Abee, 2011). The formation of biofilms with other species might be beneficial to the host as it may involve co‐aggregation with pathogens, thereby decreasing the colonization potential of the pathogens (Goh and Klaenhammer, 2010). Auto‐aggregation entails the aggregation of genetically identical cells, and can enhance the resistance to stress in the intestines (Hevia et al., 2013). Aggregation promoting factors (APFs) are extracellular proteins, highly expressed in the stationary phase, that are directly linked to the ability to co‐aggregate (Boris et al., 1997). In L. plantarum NCIMB 8826, the serine/threonine domain of the APF, D1, binds to (porcine) mucin III and is involved in auto‐aggregation as it has been found that L. plantarum loses its auto‐aggregative abilities when gene D1 is knocked‐out (Hevia et al., 2013). Moreover, gene D1 overproduction in L. lactis leads to aggregation. As L. plantarum WCFS1 has been derived from strain NCIMB 8826, it was not a surprise to find the gene D1 in the L. plantarum WCFS1 genome as Lp_0304, which has been annotated as an extracellular transglycosylase. However, gene D1 contained several SNPs as compared to the known sequence of Lp_0304 (Kleerebezem et al., 2003), either reflecting sequence errors or strain heterogeneity in NCIMB 8826, but it is likely that Lp_0304 also can bind mucus as it has the serine/threonine domain.
The accessory gene regulatory system performs a key role in biofilms formation and the lamBDCA operon of L. plantarum WCFS1 controls the expression of around 100 genes (Sturme et al., 2005). This system encodes a HPK (LamC) and the RR (LamA) that form a TCS, as well the AIP (LamD) and the export and modification protein (LamB). The expression of the lamBDCA operon seems to correlate with growth, as expression increased during the exponential phase. The AIP was found to be a novel cyclic thiolactone AIP that seems to control adherence, most likely via its effect on the expression of EPS operons (Sturme et al., 2005). The lamBDCA operon was found to be engaged in cross‐talk with another TCS encoded by the lamKR operon, as a lamA/lamR mutant demonstrated a highly reduced adherence to glass compared to a single mutant or wild‐type (Fujii et al., 2008). TCSs monitor and respond to environmental cues such as stress (Sturme et al., 2007). Quorum sensing is thus essential in the formation of biofilms, which renders L. plantarum WCFS1 less susceptible to external stressors due to restricted penetration.
Peptidoglycan hydrolases (PGHs) are major actors in cell division, cell wall turn over, autolysis and biofilm formation. By cleavage of the bacteria peptidoglycan, they may even play a role in host interaction by the release of muramyl‐peptides and PG fragments (Rolain et al., 2012). The genome of L. plantarum WCFS1 encodes for at least 12 PGHs, with N‐acetylglucosaminidase (Acm2) and γ‐d‐Glu‐mDAP muropeptidase (LytA) as the most pivotal proteins for physiology and morphogenesis (Rolain et al., 2012; Table 1). It has recently been observed that Acm2 is post‐translationally modified by glycosylation (Fredriksen et al., 2012) and this may further enhance the interaction with bacteria, contributing to biofilm formation.
Interactions with the host – epithelial barrier
Extracellular proteins, that together constitute the secretome, are involved in variable processes such as host‐adherence, recognition, degradation and uptake of luminal nutrients and transduction of signals (Buck et al., 2005). The genome of L. plantarum WCFS1 encodes 223 predicted extracellular proteins of which 81 are membrane anchored, 37 attached to the cell wall, 48 covalently bound through a lipobox and 57 have been predicted to be secreted (Boekhorst et al., 2006). Analysis of the secretome identified 12 adhesion factors; three contained a domain to adhere to collagen, one to chitin, one to fibronectin and seven to mucus. L. plantarum WCFS1 also contains a MUB domain, a domain that is unique for LAB and present in the MUB products of four genes (lp_1229, lp_3114, lp_3059 and lp_1643; Boekhorst et al., 2006; Fig. 1). These include lp_1229, which encodes the Msa (Table 1). Deletion of the msa gene resulted in loss of the ability of L. plantarum WCFS1 to agglutinate with yeast (Pretzer et al., 2005). When comparing to other Lactobacillus strains, 14 mucus‐binding proteins were identified in L. gasseri ATCC 33323, and 18 proteins with potential adhesive properties in L. acidophilus L‐92 (Douillard and de Vos, 2014); demonstrating the large repertoire of adhesive proteins within LAB.
Figure 1.
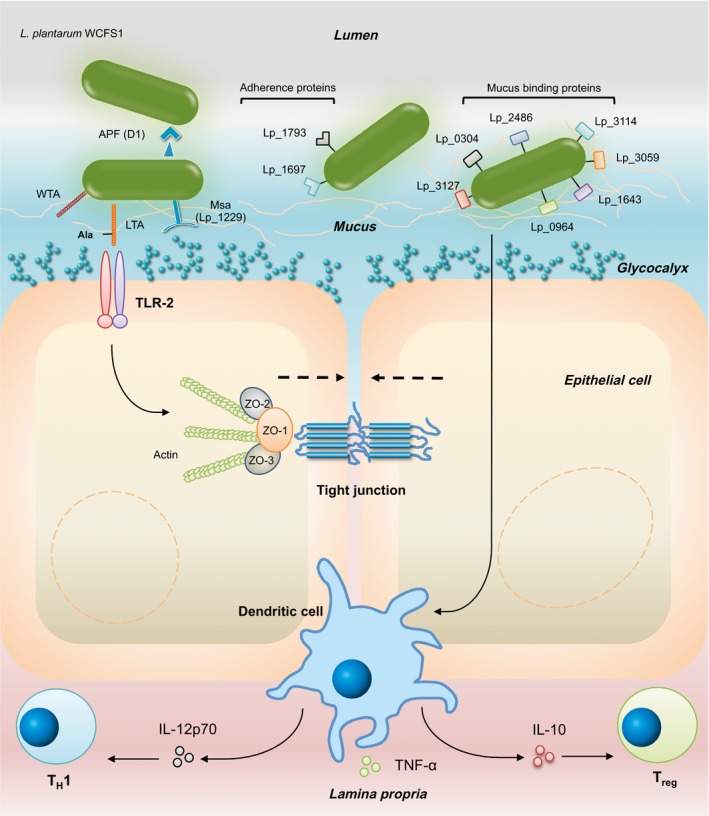
Putative proteins involved in the host–microbe interaction of Lactobacillus plantarum WCFS1. APF(D1), Aggregation promoting factor D1; WTA, Wall teichoic acid; LTA, Lipoteichoic acid; TLR, Toll‐like receptor; Msa, Mannose‐specific adhesion; ZO‐1, Zonulin‐1; ZO‐2, Zonulin‐2; ZO‐3, Zonulin‐3; IL, Interleukin; TNF, Tumour necrosis factor; TH1, T‐helper cell 1; Treg, Regulatory T cell.
L. plantarum encodes 32 proteins with a LPxTG‐motif, which are proteins that are covalently bound to the cell wall as they are recognized and cleaved by sortase A, the product of the srtA gene. In Gram‐positive pathogens, these LPXTG motif‐containing proteins are often virulence factors, and associated with functions as adhesion and receptors. In some cases, these proteins are post‐translationally glycosylated and are suggested to play a major role in cell‐to‐cell interaction (Fredriksen et al., 2013). O‐linked glycosylated extracellular proteins are of interest due to their matrix interaction. For instance, the L. plantarum WCFS1 major autolysin Acm2 and the MUB protein lp_1643 (see above), are O‐linked glycosylated (Fredriksen et al., 2013). Our current understanding of glycosylation in LAB is limited, but these glycoproteins likely play a role in the bacteria‐host interaction (Fredriksen et al., 2012; Tytgat and Lebeer, 2014).
An important site for bacteria‐host interaction is the GI epithelial cells that form a barrier between the body and the inside of the lumen of the gut. An increased or altered GI permeability of this barrier is associated with a variety of illnesses. It has been suggested that inflammatory bowel diseases (IBD) and irritable bowel syndrome (IBS) and IBS are characterized by infiltration of antigens due to mucosal barrier dysfunction and subsequent ongoing inflammation of the intestines (Barbara, 2006; Bruewer et al., 2006). The epithelial integrity is mainly controlled by tight junctions (TJs), which are multifunctional complexes of integral membrane proteins located at the apical parts of the epithelial cell (Schneeberger and Lynch, 2004). These structures interconnect the cells and include occludins, claudins and junction adhesion molecules. L. plantarum WCFS1 has the potential to enhance the intestinal integrity in cell lines through activation of toll‐like receptor (TLR)‐2, which is expressed on intestinal epithelial cells (Karczewski et al., 2010). In vitro activation of TLR‐2 transiently enhanced the epithelial resistance through zonula occludens 1 (ZO‐1) translocation (Cario et al., 2004). An experiment using a Caco‐2 human epithelial model demonstrated a significant translocation of ZO‐1 to the TJ‐region due to L. plantarum WCFS1 (Fig. 1). This was also observed in the duodenum of healthy individuals after short‐term (6‐h period) administration of L. plantarum WCFS1, suggesting that this also occurs in humans (Karczewski et al., 2010). In addition, the drop in trans epithelial electrical resistance (TER) induced by phorbol 12,13‐dibutyrate, which dislocates occludin and ZO‐1, was decreased in combination with L. plantarum WCFS1 (Karczewski et al., 2010). The enhanced barrier function is likely due to an altered TJ composition rather than an increase in TJ proteins, as transcription levels were not significantly altered by L. plantarum WCFS1 (Troost et al., 2008). Other L. plantarum strains were also able to prevent a reduction in TER in Caco‐2 cells when co‐cultured with pathogenic Escherichia coli strains (Ulluwishewa et al., 2011), indicating that L. plantarum can play a beneficial role in maintaining epithelial integrity.
Lipoteichoic acid, major constituents of the cell wall of Gram‐positive bacteria (and suggested as equivalent of the Gram‐negative lipopolysaccharides (LPS)), are important molecules for interaction with TLR‐2. The inflammatory properties of LTA greatly depend on the decoration of this protein by ᴅ‐Ala. A L. plantarum NCIMB 8826 Dlt− mutant that results in LTA with significantly less incorporated ᴅ‐Ala units induces significantly less pro inflammatory cytokines when incubated with peripheral blood mononuclear cells (PBMCs) (Grangette et al., 2005). In addition, deletion of lp_2991, a repressor of the LTA glycosylation enzyme Gtca3, led to significant higher interleukin (IL)‐10, IL‐12p70 and tumour necrosis factor (TNF)‐α levels (Meijerink et al., 2010). In agreement, a ᴅ‐Ala mutant in L. rhamnosus GG or complete removal of LTA in L. acidophilus NCFM led to strongly reduced pro‐inflammatory responses (Segers and Lebeer, 2014). Effects on the epithelial barrier have not yet been investigated for these mutants.
Interaction with the host – immune systems
In vitro as well as in vivo research has shown immune modulatory capacities for L. plantarum WCFS1. Co‐culture of PBMCs with L. plantarum NCIMB 8826 showed significant increases in different markers of activated T cells (Dong et al., 2012). L. plantarum WCFS1 induces expression of different pro‐inflammatory cytokines as well as the anti‐inflammatory cytokine IL‐10 by PBMCs (Larché et al., 2003; Van Hemert et al., 2010; Dong et al., 2012), although the concentration of induced IL‐10 and IL‐12 were relatively low and moderate compared to other L. plantarum strains (Van Hemert et al., 2010). Co‐culture of immature monocyte derived dendritic cells (DCs) with L. plantarum WCFS1 activated the DCs and induced expression of the cytokines IL‐10, TNF‐α and the TH1 inducing cytokine IL‐12p70 (Larché et al., 2003; Smelt et al., 2012; Remus et al., 2013). A more IL‐10/IL‐12 cytokine profile would be beneficial in an allergic and autoimmune disorder; however, one should wonder whether these subtle changes will lead to significant effect in vivo. Genes of L. plantarum WCFS1 involved in immunomodulation include an N‐acetyl‐glucosamine/galactosamine phosphotransferase system, the LamBDCA quorum sensing system, components of the plantaricin (bacteriocin) biosynthesis and transport pathway, and transcription regulator lp_2991 (Meijerink et al., 2010; Van Hemert et al., 2010). However, the function of these genes is quite different and hence it is likely that different mechanisms underlie the observed phenotypes. Moreover, no human data are available as the mutants are generated by genetic modification (GMO), precluding human trials. Non‐GMO approaches as recently described for Lactobacillus rhamnosus GG and coupled to next generation sequencing may be used to overcome this and provide avenues for human trials to address cause–effect relations (Rasinkangas et al., 2014).
In healthy wild‐type mice, L. plantarum WCFS1 leads to an increase in the number of regulatory DCs and regulatory T cells in the spleen (Smelt et al., 2012). In the small intestine a decrease in the Th1/Th2 ratio was seen, whereas in the large intestine a more regulatory phenotype was induced (Smelt et al., 2013a; Fig. 1). Some of these effects were dependent on the D‐alanylation of teichoic acids, as the L. plantarum WCFS1 induced immune changes were not observed when the D‐alanylation negative mutant dltX‐D was used (Smelt et al., 2013b). Also a human cross‐over study with healthy volunteers indicated establishment of immune tolerance (Van Baarlen et al., 2009). The volunteers consumed L. plantarum WCFS1 every half an hour for 6 h and thereafter gene expression responses in the duodenal cells were investigated. Among the regulated genes were numerous genes involved in immune regulation. Although this extensive administration does not reflect a ‘real life’ setting, it provides insightful biological context (Van Baarlen et al., 2009). Induction of a regulatory phenotype can dampen inflammatory conditions, for instance, such as observed in UC. Indeed, in a murine TNBS‐induced colitis model, administration of NCIMB 8826 led to a dose‐dependent protection level in weak to moderate colitis (Foligné et al., 2006).
The effect of L. plantarum WCFS1 on the healthy intestinal mucosa transcriptional response was assessed after a short 1 and 6‐h exposure in human volunteers. In a randomized, placebo controlled, cross‐over study 15 healthy individuals were exposed to 1 × 1011 CFU L. plantarum WCFS1 after which duodenal samples were taken (Troost et al., 2008). A 1‐h exposure demonstrated an upregulation of genes involved in the complement pathway (Troost et al., 2008). At the same time, genes involved with lipid and fatty acid metabolism, and the major transcriptional regulators were downregulated. Initial contact between L. plantarum WCFS1 seems to down‐regulate the proliferation and there is a primary immune response induced to the microbial presence (Troost et al., 2008). In agreement, in a comparable set‐up using L. rhamnosus GG the mucosal response was characterized by induction of TH1 development (Van Baarlen et al., 2011). A prolonged exposure of 6 h is associated with upregulation of lipid/fatty acid metabolism and oxidative stress. In addition, genes involved in the antigen presentation are upregulated (Troost et al., 2008). These data suggest that the mucosa is initially alarmed, but after 6 h return to their non‐inflammatory proliferative state (Troost et al., 2008). No inflammatory signals were expressed at both time‐points.
Studies with L. plantarum WCSF1 have shown variable results in the area of allergic diseases, possibly due to the nature of the different antigens. L. plantarum NCIMB 8826 dampened the response of DCs derived from house dust mite allergic individuals stimulated with the dust mite allergen Der‐p1 (Pochard et al., 2005). However, in a mice study using a well‐established pathogen‐free mouse peanut sensitization model, administration of L. plantarum WCFS1 increased the peanut‐extract specific IgG1, IgG2, IgE and mouse mast cell protease‐1 levels in serum significantly (Meijerink et al., 2012).
Conclusions and future directions
During the last 10 years, a large number of mutants of L. plantarum WCFS1 have been made by scientists to investigate effects of single or multiple genes (Table 1). Most of these mutants have been studied in only one or a few screening assays and it would be interesting to investigate these mutants in other assays with a focus on host‐microbe interactions. As indicated above, non‐GMO mutants can now be generated and characterized much faster than before using high throughput sequencing (Rasinkangas et al., 2014; Derkx et al., 2014). Using these and other non‐GMO mutants in human studies, further insight into mechanisms of host‐microbe interaction could be obtained.
L. plantarum WCFS1 is unique because of the large amount of molecular studies performed with this strain. This has advanced our knowledge significantly and indicated several properties that could be further exploited, such as effects on epithelial barrier function, stimulation of Th1 cells, and the potential to remove cholesterol. An important safety aspect of probiotic strains is the absence of transferable antibiotic resistant carriers (Bories et al., 2008). No transferable antibiotic resistance genes have been identified in the genome of L. plantarum WCFS1 (Kleerebezem et al., 2003). Therefore, it is unlikely that the strain can transfer antibiotic resistance genes. The parental strain is of human origin, and L. plantarum has the Qualified Presumption of Safety (of the European Food Safety Authority, EFSA) and Generally Recognized as Safe (of the U.S. Food and Drug Administration, FDA) status. Current probiotics have an excellent safety profile (even in highly immunocompromised individuals), it is unlikely that safety issues around L. plantarum WCFS1 will arise (Van den Nieuwboer et al., 2014a,b, 2015). Nevertheless, investigators should remain aware of potential risks when administering high dosages to immune compromised individuals. Whether L. plantarum WCFS1 can be developed into a successful probiotic remains to be determined and clinical trials showing a health benefit will be necessary.
Microbial Biotechnology (2016) 9(4), 452–465
Funding Information SvH was supported by Winclove Probiotics BV. WMdV was supported by the European Research Council (ERC grant 250172 ‐ Microbes Inside), the Academy of Finland (grant number 141130) and the Gravity (SIAM 024.002.002) and Spinoza grants of the Netherlands Organization for Scientific Research (NWO). The authors declare no conflict of interest.
References
- Alander, M. , Satokari, R. , Korpela, R. , Saxelin, M. , Vilpponen‐Salmela, T. , Mattila‐Sandholm, T. , and von Wright, A. (1999) Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Environ Microb 65: 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altermann, E. , Russell, W.M. , Azcarate‐Peril, M.A. , Barrangou, R. , Buck, B.L. , McAuliffe, O. , et al (2005) Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. PNAS 102: 3906–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnér, E.S. , and Holmgren, A. (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267: 6102–6109. [DOI] [PubMed] [Google Scholar]
- Barbara, G. (2006) Mucosal barrier defects in irritable bowel syndrome. Who left the door open? Am J Gastroenterol 101: 1295–1298. [DOI] [PubMed] [Google Scholar]
- Begley, M. , Hill, C. , and Gahan, C.G. (2006) Bile salt hydrolase activity in probiotics. Appl Environ Microb 72: 1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, E. , Rolain, T. , Courtin, P. , Guillot, A. , Langella, P. , Hols, P. , and Chapot‐Chartier, M.P. (2011) Characterization of O‐acetylation of N‐acetylglucosamine a novel structural variation of bacterial peptidoglycan. J Biol Chem 286: 23950–23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhathena, J. , Martoni, C. , Kulamarva, A. , Urbanska, A.M. , Malhotra, M. , and Prakash, S. (2009) Orally delivered microencapsulated live probiotic formulation lowers serum lipids in hypercholesterolemic hamsters. J Med Food 12: 310–319. [DOI] [PubMed] [Google Scholar]
- Boekhorst, J. , Wels, M. , Kleerebezem, M. , and Siezen, R.J. (2006) The predicted secretome of Lactobacillus plantarum WCFS1 sheds light on interactions with its environment. Microbiology 152: 3175–3183. [DOI] [PubMed] [Google Scholar]
- Bories, G. , Brantom, P. , Brufau de Barbera, J.C.A. , Cocconcelli, P.S. , and Debski, B. (2008) Update of the criteria used in the assessment of bacterial resistance to antibiotics of human or veterinary importance. EFSA J 732: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boris, S. , Suarez, J.E. , and Barbes, C. (1997) Characterization of the aggregation promoting factor from Lactobacillus gasseri, a vaginal isolate. J Appl Microbiol 83: 413–420. [DOI] [PubMed] [Google Scholar]
- Bove, P. , Russo, P. , Capozzi, V. , Gallone, A. , Spano, G. , and Fiocco, D. (2013) Lactobacillus plantarum passage through an oro‐gastro‐intestinal tract simulator: carrier matrix effect and transcriptional analysis of genes associated to stress and probiosis. Microbiol Res 168: 351–359. [DOI] [PubMed] [Google Scholar]
- Bron, P.A. , Marco, M. , Hoffer, S.M. , Van Mullekom, E. , de Vos, W.M. , and Kleerebezem, M. (2004a) Genetic characterization of the bile salt response in Lactobacillus plantarum and analysis of responsive promoters in vitro and in situ in the gastrointestinal tract. J Bacteriol 186: 7829–7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron, P.A. , Grangette, C. , Mercenier, A. , De Vos, W.M. , and Kleerebezem, M. (2004b) Identification of Lactobacillus plantarum genes that are induced in the gastrointestinal tract of mice. J Bacteriol 186: 5721–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron, P.A. , Molenaar, D. , De Vos, W.M. , and Kleerebezem, M. (2006) DNA micro‐array‐based identification of bile‐responsive genes in Lactobacillus plantarum . J Applied Microbiol 100: 728–738. [DOI] [PubMed] [Google Scholar]
- Bron, P.A. , Meijer, M. , Bongers, R.S. , De Vos, W.M. , and Kleerebezem, M. (2007) Dynamics of competitive population abundance of Lactobacillus plantarum ivi gene mutants in faecal samples after passage through the gastrointestinal tract of mice. J Appl Microbiol 103: 1424–1434. [DOI] [PubMed] [Google Scholar]
- Brooijmans, R.J.W. , De Vos, W.M. , and Hugenholtz, J. (2009) Lactobacillus plantarum WCFS1 electron transport chains. Appl Environ Microb 75: 3580–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruewer, M. , Samarin, S. , and Nusrat, A.S.M.A. (2006) Inflammatory bowel disease and the apical junctional complex. Ann NY Acad Sci 1072: 242–252. [DOI] [PubMed] [Google Scholar]
- Buck, B.L. , Altermann, E. , Svingerud, T. , and Klaenhammer, T.R. (2005) Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl Environ Microb 71: 8344–8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzi, V. , Weidmann, S. , Fiocco, D. , Rieu, A. , Hols, P. , Guzzo, J. , and Spano, G. (2011) Inactivation of a small heat shock protein affects cell morphology and membrane fluidity in Lactobacillus plantarum WCFS1. Res Microbiol 162: 419–425. [DOI] [PubMed] [Google Scholar]
- Cario, E. , Gerken, G. , and Podolsky, D.K. (2004) Toll‐like receptor 2 enhances ZO‐1‐associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterol 127: 224–238. [DOI] [PubMed] [Google Scholar]
- Cohen, D.P. , Renes, J. , Bouwman, F.G. , Zoetendal, E.G. , E. Mariman, E. , de Vos, W.M. , and Vaughan, E.E. (2006) Proteomic analysis of log to stationary growth phase Lactobacillus plantarum cells and a 2‐DE database. Proteomics 6: 6485–6493. [DOI] [PubMed] [Google Scholar]
- De Vos, W.M. (2011) Systems solutions by lactic acid bacteria: from paradigms to practice. Microb Cell Fac 10: S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkx, P.M. , Janzen, T. , Sørensen, K.I. , Christensen, J.E. , Stuer‐Lauridsen, B. , and Johansen, E. (2014) The art of strain improvement of industrial lactic acid bacteria without the use of recombinant DNA technology. Microb Cell Fact 13: S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep, D.B. , Straume, D. , Kjos, M. , Torres, C. , and Nes, I.F. (2009) An overview of the mosaic bacteriocin pln loci from Lactobacillus plantarum . Peptides 30: 1562–1574. [DOI] [PubMed] [Google Scholar]
- Dong, H. , Rowland, I. , and Yaqoob, P. (2012) Comparative effects of six probiotic strains on immune function in vitro. Br J Nutr 108: 459–470. [DOI] [PubMed] [Google Scholar]
- Douillard, F.P. , and de Vos, W.M. (2014) Functional genomics of lactic acid bacteria: from food to health. Microb Cell Fac 13: S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban‐Torres, M. , Reverón, I. , Mancheño, J.M. , de las Rivas, B. , and Muñoz, R. (2013) Characterization of a feruloyl esterase from Lactobacillus plantarum . Appl Environ Microb 79: 5130–5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocco, D. , Collins, M. , Muscariello, L. , Hols, P. , Kleerebezem, M. , Msadek, T. , and Spano, G. (2009) The Lactobacillus plantarum ftsH gene is a novel member of the CtsR stress response regulon. J Bacteriol 191: 1688–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foligné, B. , Nutten, S. , Steidler, L. , Dennin, V. , Goudercourt, D. , Mercenier, A. , and Pot, B. (2006) Recommendations for improved use of the murine TNBS‐induced colitis model in evaluating anti‐inflammatory properties of lactic acid bacteria: technical and microbiological aspects. Digest Dis Sci 51: 390–400. [DOI] [PubMed] [Google Scholar]
- Fredriksen, L. , Mathiesen, G. , Moen, A. , Bron, P.A. , Kleerebezem, M. , Eijsink, V.G. , and Egge‐Jacobsen, W. (2012) The major autolysin Acm2 from Lactobacillus plantarum undergoes cytoplasmic O‐glycosylation. J Bacteriol 194: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksen, L. , Moen, A. , Adzhubei, A.A. , Mathiesen, G. , Eijsink, V.G. , and Egge‐Jacobsen, W. (2013) Lactobacillus plantarum WCFS1 O‐linked protein glycosylation: an extended spectrum of target proteins and modification sites detected by mass spectrometry. Glycobiology 23: 1439–1451. [DOI] [PubMed] [Google Scholar]
- Fujii, T. , Ingham, C. , Nakayama, J. , Beerthuyzen, M. , Kunuki, R. , Molenaar, D. , et al (2008) Two homologous Agr‐like quorum‐sensing systems cooperatively control adherence, cell morphology, and cell viability properties in Lactobacillus plantarum WCFS1. J Bacteriol 190: 7655–7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, G.R. , Probert, H.M. , Van Loo, J. , Rastall, R.A. , and Roberfroid, M.B. (2004) Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 17: 259–275. [DOI] [PubMed] [Google Scholar]
- Goh, Y.J. , and Klaenhammer, T.R. (2010) Functional roles of aggregation‐promoting‐like factor in stress tolerance and adherence of Lactobacillus acidophilus NCFM. Appl Environ Microb 76: 5005–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin, B.R. , Gorbach, S.L. , Saxelin, M. , Barakat, S. , Gualtieri, L. , and Salminen, S. (1992) Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig Dis Sci 37: 121–128. [DOI] [PubMed] [Google Scholar]
- Grangette, C. , Nutten, S. , Palumbo, E. , Morath, S. , Hermann, C. , Dewulf, J. , et al (2005) Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. PNAS 102: 10321–10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen, H. , Curiel, J.A. , Landete, J.M. , Muñoz, R. , and Herraiz, T. (2009) Characterization of a nitroreductase with selective nitroreduction properties in the food and intestinal lactic acid bacterium lactobacillus plantarum WCFS1. J Agr Food Chem 57: 10457–10465. [DOI] [PubMed] [Google Scholar]
- Hevia, A. , Martínez, N. , Ladero, V. , Álvarez, M.A. , Margolles, A. , and Sánchez, B. (2013) An extracellular serine/threonine‐rich protein from Lactobacillus plantarum NCIMB 8826 is a novel aggregation‐promoting factor with affinity to mucin. Appl Environ Microb 79: 6059–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez, N. , Curiel, J.A. , Reverón, I. , de las Rivas, B. , and Muñoz, R. (2013) Uncovering the Lactobacillus plantarum WCFS1 gallate decarboxylase involved in tannin degradation. Appl Environ Microb 79: 4253–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez, N. , Esteban‐Torres, M. , Mancheño, J.M. , de las Rivas, B. , and Muñoz, R. (2014) Tannin degradation by a novel tannase enzyme present in some Lactobacillus plantarum strains. Appl Environ Microb 80: 2991–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce, S.A. , MacSharry, J. , Casey, P.G. , Kinsella, M. , Murphy, E.F. , Shanahan, F. , et al (2014) Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. PNAS 111: 7421–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski, J. , Troost, F.J. , Konings, I. , Dekker, J. , Kleerebezem, M. , Brummer, R.J.M. , and Wells, J.M. (2010) Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol‐Gastr L 298: G851–G859. [DOI] [PubMed] [Google Scholar]
- Kleerebezem, M. , Quadri, L.E. , Kuipers, O.P. , and De Vos, W.M. (1997) Quorum sensing by peptide pheromones and two‐component signal‐transduction systems in Gram‐positive bacteria. Mol Microbiol 24: 895–904. [DOI] [PubMed] [Google Scholar]
- Kleerebezem, M. , Boekhorst, J. , van Kranenburg, R. , Molenaar, D. , Kuipers, O.P. , Leer, R. , et al (2003) Complete genome sequence of Lactobacillus plantarum WCFS1. PNAS 100: 1990–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleerebezem, M. , Hols, P. , Bernard, E. , Rolain, T. , Zhou, M. , Siezen, R.J. , and Bron, P.A. (2010) The extracellular biology of the lactobacilli. FEMS Microb Rev 34: 199–230. [DOI] [PubMed] [Google Scholar]
- Lambert, J.M. , Bongers, R.S. , and Kleerebezem, M. (2007) Cre‐lox‐based system for multiple gene deletions and selectable‐marker removal in Lactobacillus plantarum . Appl Environ Microb 73: 1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, J.M. , Bongers, R.S. , de Vos, W.M. , and Kleerebezem, M. (2008a) Functional analysis of four bile salt hydrolase and penicillin acylase family members in Lactobacillus plantarum WCFS1. Appl Environ Microb 74: 4719–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, J.M. , Siezen, R.J. , de Vos, W.M. , and Kleerebezem, M. (2008b) Improved annotation of conjugated bile acid hydrolase superfamily members in Gram‐positive bacteria. Microbiology 154: 2492–2500. [DOI] [PubMed] [Google Scholar]
- Larché, M. , Robinson, D.S. , and Kay, A.B. (2003) The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immun 111: 450–463. [DOI] [PubMed] [Google Scholar]
- Lee, I.C. , van Swam, I.I. , Tomita, S. , Morsomme, P. , Rolain, T. , Hols, P. , et al (2014) GtfA and GtfB are both required for protein O‐glycosylation in Lactobacillus plantarum . J Bacteriol 196: 1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado‐Barragán, A. , Ruiz‐Barba, J.L. , and Jiménez‐Díaz, R. (2009) Knockout of three‐component regulatory systems reveals that the apparently constitutive plantaricin‐production phenotype shown by Lactobacillus plantarum on solid medium is regulated via quorum sensing. Int J Food Microbiol 130: 35–42. [DOI] [PubMed] [Google Scholar]
- Marco, M.L. , Bongers, R.S. , De Vos, W.M. , and Kleerebezem, M. (2007) Spatial and temporal expression of Lactobacillus plantarum genes in the gastrointestinal tracts of mice. Appl Environ Microb 73: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco, M.L. , Peters, T.H. , Bongers, R.S. , Molenaar, D. , Van Hemert, S. , Sonnenburg, J.L. , et al (2009) Lifestyle of Lactobacillus plantarum in the mouse caecum. Environ Microbiol 11: 2747–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco, M.L. , de Vries, M.C. , Wels, M. , Molenaar, D. , Mangell, P. , Ahrne, S. , et al (2010) Convergence in probiotic Lactobacillus gut‐adaptive responses in humans and mice. ISME J 4: 1481–1484. [DOI] [PubMed] [Google Scholar]
- Meijerink, M. , Van Hemert, S. , Taverne, N. , Wels, M. , De Vos, P. , Bron, P.A. , et al (2010) Identification of genetic loci in Lactobacillus plantarum that modulate the immune response of dendritic cells using comparative genome hybridization. PLoS ONE 5: e10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijerink, M. , Wells, J.M. , Taverne, N. , Zeeuw Brouwer, M.L. , Hilhorst, B. , Venema, K. , and Bilsen, J. (2012) Immunomodulatory effects of potential probiotics in a mouse peanut sensitization model. FEMS Immunol Med Mic 65: 488–496. [DOI] [PubMed] [Google Scholar]
- Molenaar, D. , Bringel, F. , Schuren, F.H. , de Vos, W.M. , Siezen, R.J. , and Kleerebezem, M. (2005) Exploring Lactobacillus plantarum genome diversity by using microarrays. J Bacteriol 187: 6119–6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, H. , Toh, H. , Oshima, K. , Murakami, M. , Taylor, T.D. , Igimi, S. , and Hattori, M. (2009) Complete genome sequence of the probiotic Lactobacillus rhamnosus ATCC 53103. J Bacteriol 191: 7630–7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa, R. , Kuroiso, K. , Goto, S. , and Shimizu, A. (2000) Isolation of tannin‐degrading lactobacilli from humans and fermented foods. Appl Environ Microb 66: 3093–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo, E. , Favier, C.F. , Deghorain, M. , Cocconcelli, P.S. , Grangette, C. , Mercenier, A. , et al (2004) Knockout of the alanine racemase gene in Lactobacillus plantarum results in septation defects and cell wall perforation. FEMS Microbiol Lett 233: 131–138. [DOI] [PubMed] [Google Scholar]
- Pavan, S. , Hols, P. , Delcour, J. , Geoffroy, M.C. , Grangette, C. , Kleerebezem, M. , and Mercenier, A. (2000) Adaptation of the nisin‐controlled expression system in Lactobacillus plantarum: a tool to study in vivo biological effects. Appl Environ Microb 66: 4427–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochard, P. , Hammad, H. , Ratajczak, C. , Charbonnier‐Hatzfeld, A.S. , Just, N. , Tonnel, A.B. , and Pestel, J. (2005) Direct regulatory immune activity of lactic acid bacteria on Der p 1–pulsed dendritic cells from allergic patients. J Allergy Clin Immun 116: 198–204. [DOI] [PubMed] [Google Scholar]
- Pretzer, G. , Snel, J. , Molenaar, D. , Wiersma, A. , Bron, P.A. , Lambert, J. , et al (2005) Biodiversity‐based identification and functional characterization of the mannose‐specific adhesin of Lactobacillus plantarum . J Bacteriol 187: 6128–6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasinkangas, P. , Reunanen, J. , Douillard, F.P. , Ritari, J. , Uotinen, V. , Palva, A. , and de Vos, W.M. (2014) Genomic characterization of non‐mucus adherent derivatives of Lactobacillus rhamnosus GG reveals genes affecting pilus biogenesis. Appl Environ Microb 80: 7001–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remus, D.M. , van Kranenburg, R. , van Swam, I.I. , Taverne, N. , Bongers, R.S. , Wels, M. , et al (2012) Impact of 4 Lactobacillus plantarum capsular polysaccharide clusters on surface glycan composition and host cell signaling. Microb Cell Fact 11: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remus, D.M. , Bongers, R.S. , Meijerink, M. , Fusetti, F. , Poolman, B. , de Vos, P. , et al (2013) Impact of Lactobacillus plantarum sortase on target protein sorting, gastrointestinal persistence, and host immune response modulation. J Bacteriol 195: 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverón, I. , Rodríguez, H. , Campos, G. , Curiel, J.A. , Ascaso, C. , Carrascosa, A.V. , et al (2013) Tannic acid‐dependent modulation of selected Lactobacillus plantarum traits linked to gastrointestinal survival. PLoS ONE 8: e66473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo‐Bezares, B. , Sáenz, Y. , Navarro, L. , Jiménez‐Díaz, R. , Zarazaga, M. , Ruiz‐Larrea, F. , and Torres, C. (2008) Characterization of a new organization of the plantaricin locus in the inducible bacteriocin‐producing Lactobacillus plantarum J23 of grape must origin. Arch Microbiol 189: 491–499. [DOI] [PubMed] [Google Scholar]
- Rolain, T. , Bernard, E. , Courtin, P. , Bron, P.A. , Kleerebezem, M. , Chapot‐Chartier, M.P. , and Hols, P. (2012) Identification of key peptidoglycan hydrolases for morphogenesis, autolysis, and peptidoglycan composition of Lactobacillus plantarum WCFS1. Microb Cell Fact 11: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáenz, Y. , Rojo‐Bezares, B. , Navarro, L. , Díez, L. , Somalo, S. , Zarazaga, M. , et al (2009) Genetic diversity of the pln locus among oenological Lactobacillus plantarum strains. Int J Food Microbiol 134: 176–183. [DOI] [PubMed] [Google Scholar]
- Saulnier, D.M. , Molenaar, D. , de Vos, W.M. , Gibson, G.R. , and Kolida, S. (2007) Identification of prebiotic fructooligosaccharide metabolism in Lactobacillus plantarum WCFS1 through microarrays. Appl Environ Microb 73: 1753–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxelin, M. , Tynkkynen, S. , Mattila‐Sandholm, T. , and de Vos, W.M. (2005) Probiotic and other functional microbes: from markets to mechanisms. Curr Opin Biotech 16: 204–211. [DOI] [PubMed] [Google Scholar]
- Schneeberger, E.E. , and Lynch, R.D. (2004) The tight junction: a multifunctional complex. Am J Physiol‐Cell Ph 286: C1213–C1228. [DOI] [PubMed] [Google Scholar]
- Segers, M.E. , and Lebeer, S. (2014) Towards a better understanding of Lactobacillus rhamnosus GG‐host interactions. Microb Cell Fact 13: S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano, L.M. , Molenaar, D. , Wels, M. , Teusink, B. , Bron, P.A. , De Vos, W.M. , and Smid, E.J. (2007) Thioredoxin reductase is a key factor in the oxidative stress response of Lactobacillus plantarum WCFS1. Microb Cell Fact 6: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siezen, R.J. , and van Hylckama Vlieg, J.E. (2011) Genomic diversity and versatility of Lactobacillus plantarum, a natural metabolic engineer. Microb Cell Fact 10: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelt, M.J. , de Haan, B.J. , Bron, P.A. , van Swam, I. , Meijerink, M. , Wells, J.M. , et al (2012) L. plantarum, L salivarius, and L. lactis attenuate Th2 responses and increase Treg frequencies in healthy mice in a strain dependent manner. PLoS ONE 7: e47244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelt, M.J. , de Haan, B.J. , Bron, P.A. , van Swam, I. , Meijerink, M. , Wells, J.M. , et al (2013a) Probiotics can generate FoxP3 T‐cell responses in the small intestine and simultaneously inducing CD4 and CD8 T cell activation in the large intestine. PLoS ONE 8: e68952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelt, M.J. , de Haan, B.J. , Bron, P.A. , van Swam, I. , Meijerink, M. , Wells, J.M. , et al (2013b) The impact of Lactobacillus plantarum WCFS1 teichoic acid D‐alanylation on the generation of effector and regulatory T‐cells in healthy mice. PLoS ONE 8: e63099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, M.J.A. , Molenaar, D. , de Jong, A. , De Vos, W.M. , and Kleerebezem, M. (2010) sigma54‐Mediated control of the mannose phosphotransferase system in Lactobacillus plantarum impacts on carbohydrate metabolism. Microbiology 156: 695–707. [DOI] [PubMed] [Google Scholar]
- Sturme, M.H. , Nakayama, J. , Molenaar, D. , Murakami, Y. , Kunugi, R. , Fujii, T. , et al (2005) An agr‐like two‐component regulatory system in Lactobacillus plantarum is involved in production of a novel cyclic peptide and regulation of adherence. J Bacteriol 187: 5224–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturme, M.H. , Francke, C. , Siezen, R.J. , de Vos, W.M. , and Kleerebezem, M. (2007) Making sense of quorum sensing in lactobacilli: a special focus on Lactobacillus plantarum WCFS1. Microbiology 153: 3939–3947. [DOI] [PubMed] [Google Scholar]
- Sun, Z. , Harris, H.M. , McCann, A. , Guo, C. , Argimón, S. , Zhang, W. , et al (2015) Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat Commun 6: 8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teusink, B. , van Enckevort, F.H. , Francke, C. , Wiersma, A. , Wegkamp, A. , Smid, E.J. , and Siezen, R.J. (2005) In silico reconstruction of the metabolic pathways of Lactobacillus plantarum: comparing predictions of nutrient requirements with those from growth experiments. Appl Environ Microb 71: 7253–7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teusink, B. , Wiersma, A. , Molenaar, D. , Francke, C. , de Vos, W.M. , Siezen, R.J. , and Smid, E.J. (2006) Analysis of growth of Lactobacillus plantarum WCFS1 on a complex medium using a genome‐scale metabolic model. J Biol Chem 281: 40041–40048. [DOI] [PubMed] [Google Scholar]
- Tomaro‐Duchesneau, C. , Saha, S. , Malhotra, M. , Coussa‐Charley, M. , Al‐Salami, H. , Jones, M.L. , et al (2012) Lactobacillus fermentum NCIMB 5221 has a greater ferulic acid production compared to other ferulic acid esterase producing Lactobacilli. Int J probiotics prebiotics 7: 23–32. [Google Scholar]
- Tomita, S. , de Waard, P. , Bakx, E.J. , Schols, H.A. , Kleerebezem, M. , and Bron, P.A. (2013) The structure of an alternative wall teichoic acid produced by a Lactobacillus plantarum WCFS1 mutant contains a 1,5‐linked poly (ribitol phosphate) backbone with 2‐α‐d‐glucosyl substitutions. Carbohyd Res 370: 67–71. [DOI] [PubMed] [Google Scholar]
- Troost, F.J. , van Baarlen, P. , Lindsey, P. , Kodde, A. , de Vos, W.M. , Kleerebezem, M. , and Brummer, R.J.M. (2008) Identification of the transcriptional response of human intestinal mucosa to Lactobacillus plantarum WCFS1 in vivo. BMC Genom 9: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat, H.L. , and Lebeer, S. (2014) The sweet tooth of bacteria: common themes in bacterial glycoconjugates. Microbiol Mol Biol Rev 78: 372–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulluwishewa, D. , Anderson, R.C. , McNabb, W.C. , Moughan, P.J. , Wells, J.M. , and Roy, N.C. (2011) Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 141: 769–776. [DOI] [PubMed] [Google Scholar]
- Van Baarlen, P. , Troost, F.J. , van Hemert, S. , van der Meer, C. , de Vos, W.M. , de Groot, P.J. , et al (2009) Differential NF‐κB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. PNAS 106: 2371–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baarlen, P. , Troost, F. , van der Meer, C. , Hooiveld, G. , Boekschoten, M. , Brummer, R.J. , and Kleerebezem, M. (2011) Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. PNAS 108: 4562–4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bokhorst‐van de Veen, H. , Lee, I.C. , Marco, M.L. , Wels, M. , Bron, P.A. , and Kleerebezem, M. (2012a) Modulation of Lactobacillus plantarum gastrointestinal robustness by fermentation conditions enables identification of bacterial robustness markers. PLoS ONE 7: e39053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bokhorst‐van de Veen, H. , van Swam, I. , Wels, M. , Bron, P.A. , and Kleerebezem, M. (2012b) Congruent strain specific intestinal persistence of Lactobacillus plantarum in an intestine‐mimicking in vitro system and in human volunteers. PLoS ONE 7: e44588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bokhorst‐van de Veen, H. , Smelt, M.J. , Wels, M. , van Hijum, S.A. , de Vos, P. , Kleerebezem, M. , and Bron, P.A. (2013) Genotypic adaptations associated with prolonged persistence of Lactobacillus plantarum in the murine digestive tract. Biotechnol J 8: 895–904. [DOI] [PubMed] [Google Scholar]
- Van den Nieuwboer, M. , Brummer, R.J. , Guarner, F. , Morelli, L. , Cabana, M. , and Claassen, E. (2014a) The administration of probiotics and synbiotics in immune compromised adults: is it safe? Benef Microbes 6: 3–17. [DOI] [PubMed] [Google Scholar]
- Van den Nieuwboer, M. , Claassen, E. , Morelli, L. , Guarner, F. , and Brummer, R.J. (2014b) Probiotic and synbiotic safety in infants under two years of age. Benef Microbes 5: 45–60. [DOI] [PubMed] [Google Scholar]
- Van den Nieuwboer, M. , Brummer, R.J. , Guarner, F. , Morelli, L. , Cabana, M. , and Claassen, E. (2015) Safety of probiotics and synbiotics in children under 18 years of age. Benef Microbes 6: 615–630. [DOI] [PubMed] [Google Scholar]
- Van der Veen, S. , and Abee, T. (2011) Mixed species biofilms of Listeria monocytogenes and Lactobacillus plantarum show enhanced resistance to benzalkonium chloride and peracetic acid. Int J Food Microbiol 144: 421–431. [DOI] [PubMed] [Google Scholar]
- Van Hemert, S. , Meijerink, M. , Molenaar, D. , Bron, P.A. , de Vos, P. , Kleerebezem, M. , et al (2010) Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol 10: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kranenburg, R. , Golic, N. , Bongers, R. , Leer, R.J. , De Vos, W.M. , Siezen, R.J. , and Kleerebezem, M. (2005) Functional analysis of three plasmids from Lactobacillus plantarum . Appl Environ Microb 71: 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesa, T. , Pochart, P. , and Marteau, P. (2000) Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment Pharmacol Ther 14: 823–828. [DOI] [PubMed] [Google Scholar]
- Yang, P. , Wang, J. , and Qingsheng, Q. (2015) Prophage recombinases‐mediated genome engineering in Lactobacillus plantarum . Microb Cell Fact 14: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotta, T. , Ricciardi, A. , Guidone, A. , Sacco, M. , Muscariello, L. , Mazzeo, M.F. , et al (2012) Inactivation of ccpA and aeration affect growth, metabolite production and stress tolerance in Lactobacillus plantarum WCFS1. Int J Food Microbiol 155: 51–59. [DOI] [PubMed] [Google Scholar]


