Summary
Molecular methods to investigate functional groups in microbial communities rely on the specificity and selectivity of the primer set towards the target. Here, using rapid sand filters for drinking water production as model environment, we investigated the consistency of two commonly used quantitative PCR methods to enumerate ammonia‐oxidizing bacteria (AOB): one targeting the phylogenetic gene 16S rRNA and the other, the functional gene amoA. Cloning‐sequencing with both primer sets on DNA from two waterworks revealed contrasting images of AOB diversity. The amoA‐based approach preferentially recovered sequences belonging to Nitrosomonas Cluster 7 over Cluster 6A ones, while the 16S rRNA one yielded more diverse sequences belonging to three AOB clusters, but also a few non‐AOB sequences, suggesting broader, but partly unspecific, primer coverage. This was confirmed by an in silico coverage analysis against sequences of AOB (both isolates and high‐quality environmental sequences). The difference in primer coverage significantly impacted the estimation of AOB abundance at the waterworks with high Cluster 6A prevalence, with estimates up to 50‐fold smaller for amoA than for 16S rRNA. In contrast, both approaches performed very similarly at waterworks with high Cluster 7 prevalence. Our results highlight that caution is warranted when comparing AOB abundances obtained using different qPCR primer sets.
Introduction
Investigation of environmental microbial communities by molecular techniques has, over the last decades, demonstrated large advantages, primarily by overriding the limitation associated with microbial cultivation in the laboratory. Although so‐called ‘open format’ methods exist which do not require a priori sequence information from the community of interest, much of the routine monitoring techniques of microbial communities, such as qPCR, are ‘closed‐format’ (i.e. require a priori sequence information) (Zhou et al., 2015). These ‘closed‐format’ methods typically depend on the ability of a primer set for unbiased amplification of all (or most) of the target sequences without amplifying non‐target sequences. Primer sets are designed based on the current information from nucleotide databases and require occasional validations as new sequences from the environment are added.
To study bacterial functional groups, primer sets may either target a relevant functional gene or, if the function is performed by one or a few taxa, the phylogenetic 16S rRNA gene. This is the case for the ammonia‐oxidizing bacteria (AOB), which predominantly belong to the beta‐subclass of Proteobacteria and carry a functional amoA, which codes for a subunit of the ammonia monooxygenase (Norton, 2011). AOB are key players in the first step of nitrification in many natural and engineered systems. To quantify this group, researchers routinely employ qPCR targeting amoA or group‐specific 16S rRNA gene sequences (Junier et al., 2010). However, as these two approaches are not typically compared, it is unclear whether they are equally good at estimating AOB abundance.
We attempted this comparison, using biomass extracted from biological rapid sand filters (RSF) used for production of potable water from groundwater. One of the key roles of these filters is ammonium removal, which is mediated by ammonia‐oxidizing prokaryotes (AOP) (van der Wielen et al., 2009). Occasional failures in ammonium removal call for better understanding and monitoring of AOP communities, which require accurate molecular quantification methods.
Here, we investigate the consistency of two molecular approaches for quantifying AOB: one targeting a phylogenetic gene (16S rRNA) and the other, a functional gene (amoA). We tested two commonly applied AOB‐specific primer sets: CTO189a/b/c – RT1r for 16S rRNA (Kowalchuk et al., 1997; Hermansson and Lindgren, 2001) and amoA1f ‐ amoA2r (Rotthauwe et al., 1997). These primer sets were evaluated for coverage and specificity both in silico and on DNA extracted from biomass from two RSFs. The difference in primer pair selectivity, combined with the compositional differences of the AOB guild across sand filter communities, had a major effect on the quantification outcome.
Results & Discussion
Two molecular approaches targeting the phylogenetic 16S rRNA and functional (amoA) genes were applied to investigate AOB abundance in pre‐filter and after‐filter units at three Danish waterworks; Islevbro, Sjælsø, and Langerød. The abundance of the AOB 16S rRNA genes ranged from ca. 3 × 106 to ca. 3 × 108 copies/g drained wet sand across RSF units and waterworks (Fig. 1). The abundance estimates were similar between replicate after‐filters within waterworks and indicated some expected within‐plant patterns. Notably, at Sjælsø waterworks, AOB were one‐order of magnitude more abundant in the pre‐ than in the after‐filter units and, in Islevbro after‐filters, a clear two order of magnitude decline of AOB abundance was noted with depth. The enumeration of amoA revealed similar AOB distributional trends within and across pre‐ and after‐filters. However, the abundance of amoA was consistently lower (ca. 50‐fold) than that of 16S rRNA gene at both Islevbro and Sjælsø waterworks (Fig. S1). This difference becomes even larger considering that the genomes of betaproteobacterial ammonia‐oxidizers typically contain a single ribosomal operon (Stoddard et al., 2014) but frequently multiple (1–3) amo operons (Norton et al., 2002). This inconsistency between the two abundance estimates was not observed at Langerød waterworks.
Figure 1.
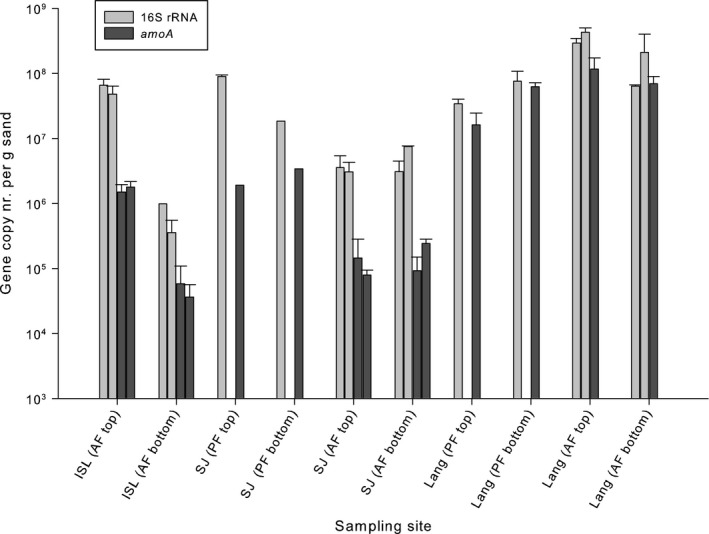
qPCR enumeration of ammonia‐oxidizing bacteria in the top (0–5 cm) and bottom (40–50 cm) layers of rapid sand filters units (pre‐filter ‐PF‐ and after‐filter‐AF) at three Danish waterworks: Islevbro (ISL), Sjælsø (SJ), and Langerød (Lang). The qPCR was performed either by targeting 16S rRNA (primers CTO189a/b/c –RT1r (Kowalchuk et al., 1997; Hermansson and Lindgren, 2001)) or amoA (primers amoA1f ‐ amoA2r, Rotthauwe et al., 1997) genes. When multiple filter units have been sampled at the same waterworks, the data are presented as multiple bars. See supplementary information for details on sampling, DNA extraction, and for qPCR conditions.
Islevbro and Langerød waterworks, showing, respectively, the largest and smallest differences between the two methods, were selected for cloning‐sequencing analysis in order to examine the selectivity and specificity of the two primer sets towards the AOB. The sequences of the clones recovered from Islevbro and Langerød waterworks (82 and 77 with 16S rRNA genes primers and 43 and 92 for amoA primers) were placed into maximum likelihood trees along reference sequences to tentatively classify them (Figs 2 and 3). Phylogenic analysis and classification of the 16S sequences were hindered by the very short length of the amplicon (75 bases). All partial amoA sequences and most of the 16S ones were tentatively identified as belonging to Betaproteobacterial AOB (Norton, 2011), but five 16S sequences (6%) were closely related to sequences that did not belong to AOB but to other Beta‐Proteobacteria. (Fig. 2, Table S2). These diverse non‐AOB sequences are thus false positives and indicate that the selectivity of the 16S rRNA primer set is imperfect, which might introduce overestimation of AOB abundance in some communities. We indeed note that these non‐AOB sequences were obtained from one of our clone libraries (Islevbro) but not the other. Imperfect specificity of primer sets targeting the 16S rRNA gene of betaproteobacterial AOB has already been noted (e.g., Mahmood et al., 2006). Most clones from Islevbro waterworks were tentatively assigned to Nitrosomonas Cluster 6A (N. oligotropha lineage), irrespective of the primer set. At Langerød waterworks, in contrast, the identity of the dominant AOB lineage changed depending on the molecular approach: the 16S rRNA‐ based approach identified AOB belonging to Cluster 6A as dominant, while the amoA‐based approach only retrieved sequences from the Nitrosomonas europaea/eutropha lineage (Cluster 7). While the difference between the diversity retrieved by the two primer sets could also originate from cloning bias (e.g. Palatinszky et al., 2011) we further explored the role of amplification bias.
Figure 2.
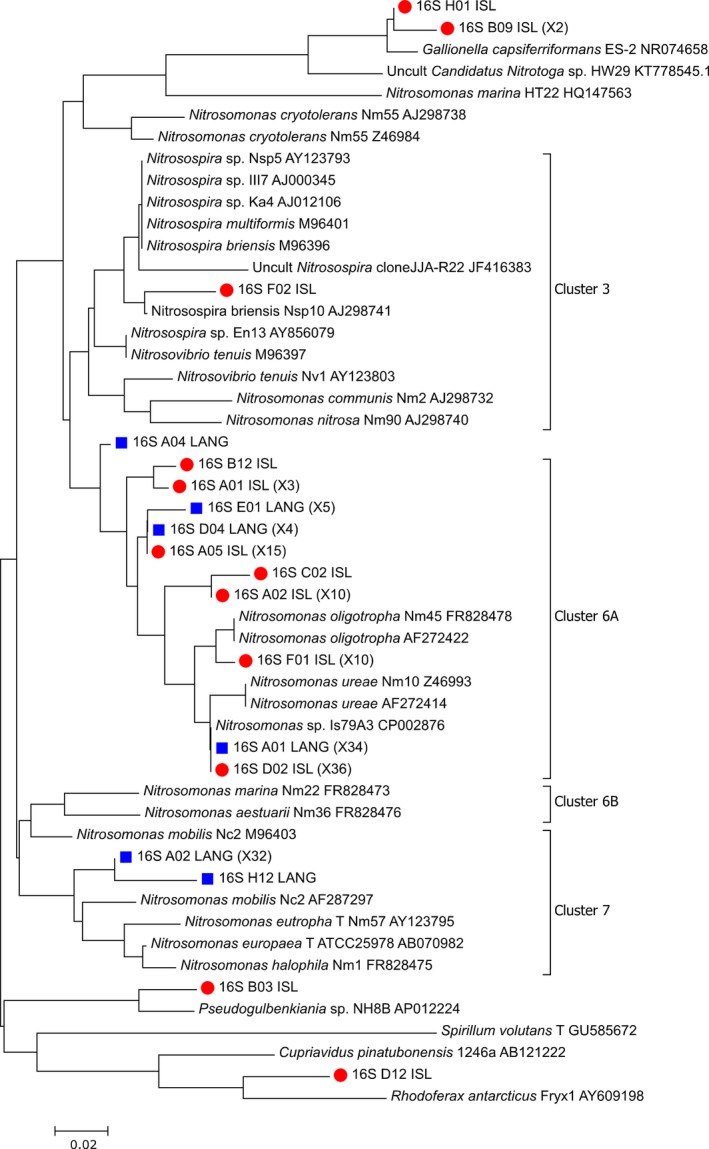
Neighbour‐joining tree of the 16S rRNA gene sequences cloned from after‐filter units at Islevbro (ISL, red circle) and Langerød (LANG, blue square) waterworks along with reference sequences. The cloning was performed with the same primer set as for the qPCR and the sequences have been submitted to DDBJ under accession number LC100143‐100160. The tree was created using the maximum likelihood function of Mega 7 software (Kumar et al., 2016) and relies on 41 variable positions. Multiple occurrences of identical sequences are presented in parentheses. The reference strains are presented with the accession numbers after their name.
Figure 3.
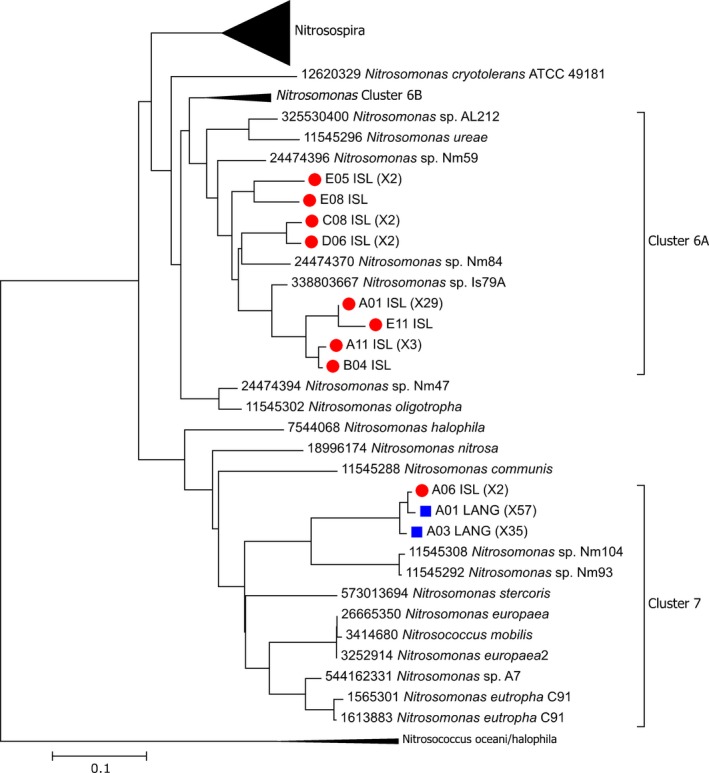
Neighbour‐joining phylogenetic tree inferred from AOB amoA gene sequences cloned from after filter‐units at Islevbro (ISL, red circle) and Langerød (LANG, blue square) waterworks and from reference sequences. The cloning was performed with the same primer set as for the qPCR. These sequence data have been submitted to DDBJ under accession number LC142697‐142707. The phylogenetic tree was created using the maximum likelihood function of Mega 7 software (Kumar et al., 2016) Multiple occurrences of highly similar sequences (≥ 99% similarity) are presented in parentheses. The reference strains are presented with the sequence identification numbers (GI) numbers in front of their name.
We thus performed an in silico coverage analysis of the primer sets against sequences from AOB for which the entire priming region for forward and/or reverse primer was available, collected from RDP (Cole et al., 2014) or FunGene databases (Fish et al., 2013) for 16S rRNA and amoA respectively (number of sequences considered and their distribution into AOB clusters is presented in Table S1). For the 16S rRNA primer set, our analysis revealed a high coverage for both primers across all clusters (Fig. S2), with the forward primer having a slightly worse coverage than the reverse one (zero or one mismatch for more than 91% and 98% target sequences respectively).
The analysis of amoA primer set revealed a clear difference between Cluster 6A and the other clusters: the sequences assigned to the former presented, on average, more mismatches to both the forward and the reverse primers (Fig. S2). All Cluster 6A sequences presented at least two, and up to four, mismatches with the primer set while it barely exceeded one, on average, for the other clusters (Fig. S2). The good match between Cluster 7 sequences and the amoA primer set is consistent with the high amplification of N. europaea sequences reported in a recent qPCR comparative assay (Meinhardt et al., 2015). In contrast, the presence of an increasing number of mismatches between a primer and its binding site, in particular two and more mismatches, can significantly reduce the efficiency of PCR amplification (Bru et al., 2008). Therefore, we conclude from our in silico analysis that the amoA primer set would be, on average, less efficient at amplifying Cluster 6 sequences than Cluster 7 ones when both are present. This can explain the lack of detection of Cluster 6A with the amoA cloning‐sequencing at Langerød, where both clusters co‐dominated according to the 16S approach (Table S2). However, this co‐dominance of Cluster 6A and 7 did not result in incorrect quantification of AOB by the amoA qPCR, which performed similarly as that for the 16S rRNA gene (Fig. 1, Fig. S1). In Islevbro waterworks, cloning‐sequencing with the 16S primer set indicated a strong dominance of Cluster 6A, with Cluster 7 being undetectable. This low abundance of Cluster 7 probably allowed Cluster 6A to retain its high prevalence in the amoA clone library, in spite of its low PCR amplification efficiency. This low efficiency, caused by primer‐target mismatches, was, however, apparent in the drastic underestimation of AOB abundance with the amoA‐based qPCR at Islevbro (Fig. 1, Fig. S1).
Our in silico and experimental results highlight the importance of primer set selection for AOB quantification in complex microbial communities and demonstrate that results can be heavily primer‐ and community‐dependent. Thanks to its consistent coverage across AOB clusters, the primer set targeting the 16S rRNA gene performed better than the amoA one which amplifies Cluster 6A at low efficiency. This amoA primer set, although commonly used, is thus poorly adapted to analyse communities dominated by this type of AOB. The 16S rRNA gene‐qPCR presents the disadvantage that it can overestimate AOB abundance, although this problem was not detected in one of the waterworks and was modest in the other, with a few percent of non‐target sequences in the clone library. A recent comparison of 16S rRNA and amoA‐ based qPCR methods, using primer sets similar, but not identical, to those used here, in activated sludge communities (Baptista et al., 2014) revealed that the amoA‐based methods yielded abundance estimates very similar to those obtained with FISH, while the 16S rRNA qPCR ones were significantly lower. Their conclusion differs from that of this study and is likely due to compositional differences in the respective communities. Therefore, we recommend, along with (Meinhardt et al., 2015) to consider using multiple primer sets when exploring AOB in unknown communities. Inconsistencies between qPCR results would indicate that there is uncertainty in the abundance estimates and that these estimates should be checked with other methods (e.g., qFISH).
Supporting information
Data S1. Material and method.
Acknowledgements
We gratefully acknowledge the comments from three anonymous reviewers which contributed to improving the manuscript.
Microbial Biotechnology (2016) 9(4), 519–524
Funding Information This work was supported by DW‐Biofilter, funded by the Danish Council for Strategic Research, and by Mermaid ‐ an Initial Training Network funded by the People Programme ‐ Marie Skłodowska‐Curie Actions‐ of the European Union's Seventh Framework Programme FP7/2007‐2013/ under REA grant agreement no. 607492.
References
- Baptista, J.D.C. , Lunn, M. , Davenport, R.J. , Swan, D.L. , Read, L.F. , Brown, M.R. , et al (2014) Agreement between amoA gene‐specific quantitative PCR and fluorescence in situ hybridization in the measurement of ammonia‐oxidizing bacteria in activated sludge. Appl Environ Microbiol 80: 5901–5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bru, D. , Martin‐Laurent, F. , and Philippot, L. (2008) Quantification of the detrimental effect of a single primer‐template mismatch by real‐time PCR using the 16S rRNA gene as an example. Appl Environ Microbiol 74: 1660–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, J.R. , Wang, Q. , Fish, J.A. , Chai, B. , McGarrell, D.M. , Sun, Y. , et al (2014) Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42: D633–D642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish, J.A. , Chai, B. , Wang, Q. , Sun, Y. , Brown, C.T. , Tiedje, J.M. , and Cole, J.R. (2013) FunGene: the functional gene pipeline and repository. Front Microbiol 4 http://dx.doi.org/10.3389/fmicb.2013.00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansson, A. , and Lindgren, P.E. (2001) Quantification of ammonia‐oxidizing bacteria in arable soil by real‐time PCR. Appl Environ Microbiol 67: 972–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junier, P. , Molina, V. , Dorador, C. , Hadas, O. , Kim, O.‐S. , Junier, T. , et al (2010) Phylogenetic and functional marker genes to study ammonia‐oxidizing microorganisms (AOM) in the environment. Appl Microbiol Biotechnol 85: 425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalchuk, G.A. , Stephen, J.R. , De Boer, W. , Prosser, J.I. , Embley, T.M. , and Woldendorp, J.W. (1997) Analysis of ammonia‐oxidizing bacteria of the beta subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR‐amplified 16S ribosomal DNA fragments. Appl Environ Microbiol 63: 1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. , and Tamura, K. (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood, S. , Freitag, T.E. , and Prosser, J.I. (2006) Comparison of PCR primer‐based strategies for characterization of ammonia oxidizer communities in environmental samples. FEMS Microbiol Ecol 56: 482–493. [DOI] [PubMed] [Google Scholar]
- Meinhardt, K.A. , Bertagnolli, A. , Pannu, M.W. , Strand, S.E. , Brown, S.L. , and Stahl, D.A. (2015) Evaluation of revised polymerase chain reaction primers for more inclusive quantification of ammonia‐oxidizing archaea and bacteria. Environ Microbiol Rep 7: 354–363. [DOI] [PubMed] [Google Scholar]
- Norton, J.M. (2011) Diversity and environmental distribution of ammonia‐oxidizing bacteria In Nitrification. Ward B.B., Arp D., and Klotz M.G. (eds). Washington, DC: American Society of Microbiology, pp. 39–55. [Google Scholar]
- Norton, J.M. , Alzerreca, J.J. , Suwa, Y. , and Klotz, M.G. (2002) Diversity of ammonia monooxygenase operon in autotrophic ammonia‐oxidizing bacteria. Arch Microbiol 177: 139–149. [DOI] [PubMed] [Google Scholar]
- Palatinszky, M. , Nikolausz, M. , Sváb, D. , and Márialigeti, K. (2011) Preferential ligation during TA‐cloning of multitemplate PCR products–a factor causing bias in microbial community structure analysis. J Microbiol Methods 85: 131–136. [DOI] [PubMed] [Google Scholar]
- Rotthauwe, J.H. , Witzel, K.P. , and Liesack, W. (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine‐scale analysis of natural ammonia‐oxidizing populations. Appl Environ Microbiol 63: 4704–4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard, S.F. , Smith, B.J. , Hein, R. , Roller, B.R.K. , and Schmidt, T.M. (2014) rrnDB: improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucleic Acids Res 43: D593–D598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wielen, P.W.J.J. , Voost, S. , and van der Kooij, D. (2009) Ammonia‐oxidizing bacteria and archaea in groundwater treatment and drinking water distribution systems. Appl Environ Microbiol 75: 4687–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , He, Z. , Yang, Y. , Deng, Y. , Tringe, S.G. , and Alvarez‐Cohen, L. (2015) High‐throughput metagenomic technologies for complex microbial community analysis: open and closed formats. MBio 6 DOI: 10.1128/mBio.02288‐14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Material and method.


