Abstract
Social subordination in macaques is a well-established model to study the adverse effects of psychosocial stress on a number of health outcomes, including stress-induced eating. The present analysis was conducted to empirically define a meal among free-feeding female rhesus monkeys and to examine the roles of meal patterning (e.g., meal size, meal frequency, and snacking patterns) in findings from a previous study demonstrating that psychosocial stress increases overall caloric intake among subordinate animals with access to a highly palatable diet. Results indicate that all animals, regardless of social status, consumed more frequent meals, larger meals, and more calories in the form of snacks when a highly palatable diet was available. Additional findings suggest that subordinate animals consumed significantly larger meals compared to their dominant counterparts regardless of the dietary environment. Additionally, subordinate females with a history of exposure to the palatable diet consumed significantly more snack calories than both dominant and subordinate animals without previous exposure to the palatable diet when these females were returned to a standard laboratory diet. These findings illustrate how small changes in meal patterns can lead to significant increases in total caloric intake, which if prolonged, could promote the emergence of an obese phenotype.
Keywords: Psychosocial stress, Diet, Meal patterns, Emotional feeding, Obesity, Monkeys
Introduction
While the ultimate determinant of body weight and fat accumulation is excess caloric intake relative to energy expenditure (Bray et al., 2012; Sacks et al., 2009), the emergence of an obese phenotype is influenced by the complex interaction of genetic, metabolic, and environmental factors (Ogden, Yanovski, Carroll, & Flegal, 2007). A great deal of experimental work, both animal and human, has explored the biological effects of modifying the macro-nutrient content of diets (Helies et al., 2005; Klaus, 2005; Melhorn et al., 2010a; Petro et al., 2004; Rolls, 2009; Rolls, Drewnowski, & Ledikwe, 2005; Schoeller & Buchholz, 2005; Surwit et al., 1995). Others have taken this a step further to explore the idea that the frequency of food intake can influence body composition (Bellisle, McDevitt, & Prentice, 1997; Fabry & Tepperman, 1970).
Although the notion that between-meal snacking or increased eating frequency can reduce hunger to promote weight loss or weight maintenance is widely accepted (Chapelot et al., 2006; Drewnowski, Cohen, Faust, & Grinker, 1984; Fabry, Hejl, Fodor, Braun, & Zvolankova, 1964; Fabry & Tepperman, 1970), some epidemiological evidence suggests that snacking or increased eating frequency, rather than increased meal size, is a major culprit in the recent surge in obesity (Cutler, Glaeser, & Shapiro, 2003; de Graaf, 2006; Koletzko & Toschke, 2010). Further, the bulk of existing evidence from controlled laboratory studies in humans indicates minimal improvement in appetite control as a function of increased eating frequency (Bellisle et al., 1997; Leidy & Campbell, 2011).
The mixed findings of epidemiological studies of meal patterning in humans are likely due in part to variations in how meals and snacks are defined (Gregori, Foltran, Ghidina, & Berchialla, 2011; Johnson & Anderson, 2010) as well as the studies’ reliance on self-reports of dietary intake from 24-h recalls (Fabry et al., 1964; Howarth, Huang, Roberts, Lin, & McCrory, 2007; Jahns, Siega-Riz, & Popkin, 2001; Kant & Graubard, 2006; Nicklas et al., 2004; Zizza, Siega-Riz, & Popkin, 2001) and/or food diaries (Jahns et al., 2001; Newman, O'Connor, & Conner, 2007; O'Connor, Jones, Conner, McMillan, & Ferguson, 2008; Pearcey & de Castro, 2002; Zizza et al., 2001). Despite attempts to create universal definitions of meals versus snacks for use in scientific literature, a consensus has not been reached. Instead, studies have differentially defined meals and snacks based on the nutrient profile of the food(s) consumed, the timing of food consumption, or the perception of the study subject (Johnson & Anderson, 2010). Further, while dietary assessment methods provide estimates of the distribution of caloric intake throughout a specified period of time, recall methods are limited by dependence on memory and cooperation of the subject as well as communication skills of the interviewer, and food diaries can induce changes in dietary patterns through the actual process of recording food intake (Johnson, 2002). Additionally, a consistent finding with all dietary assessment methods is that under-reporting of energy intake is more prevalent among obese subjects compared to lean subjects (Johnson, 2002).
Animal models have proven extraordinarily useful for defining satiety and orexigenic signals that regulate food intake (Atkinson, 2008; Woods & D'Alessio, 2008). These studies also allow exploration of social, emotional, and environmental factors that affect feeding as well as modifying effects of the macronutrient composition of the diet (Cottone, Sabino, Steardo, & Zorrilla, 2009; Melhorn et al., 2010b; Tamashiro, Nguyen, & Sakai, 2005; Warne, 2009). However, most studies have looked at average caloric intake over a specific experimental period whereas only a few have attempted to define how various interventions affect meal patterns (Cottone, Sabino, Nagy, Coscina, & Zorrilla, 2007; Fekete et al., 2007; Tabarin et al., 2007; Zorrilla, Inoue, Valdez, Tabarin, & Koob, 2005b).
Social subordination in macaques is a well-established model to study the adverse effects of psychosocial stress on a number of phenotypes (Gust et al., 1991; Kaplan & Manuck, 2004; Kaplan et al., 1996; Morgan et al., 2002; Paiardini et al., 2009). Socially subordinate females receive more aggression from higher-ranking group mates and in turn must emit submissive behaviors, a defining feature of subordination, to terminate these interactions (Bernstein, 1976; Bernstein & Gordon, 1974; Bernstein, Gordon, & Rose, 1974; Shively & Kaplan, 1984). Subordinates have less control over their environment (Abbott et al., 2003; Silk, 2002) and, as a consequence of continual harassment, show dysregulation of the limbic–hypothalamic–pituitary–adrenal axis characterized by reduced glucocorticoid negative feedback (Jarrell et al., 2008; Paiardini et al., 2009; Shively, 1998; Shively, Laber-Laird, & Anton, 1997; Wilson, Pazol, Legendre, Fisher, & Chikazawa, 2005; Wilson et al., 2008).
The social subordination model in female macaques has recently been utilized to explore stress-induced eating (Arce, Michopoulos, Shepard, Ha, & Wilson, 2010; Michopoulos, Toufexis, & Wilson, 2012a), which has not been fully elucidated using other animal or human models (Greeno & Wing, 1994). Indeed, subordinate female macaques demonstrate increased visceral obesity and elevated risk for cardiovascular disease when fed a high-fat diet (Shively, Register, & Clarkson, 2009). Subordinate females also consume significantly more calories each day, on average, compared to dominant females when a high-fat, sugary diet is available (Arce et al., 2010). Further, while significant status differences in caloric intake have not been detected among naïve animals maintained on a standard laboratory diet, an interesting effect of diet history has emerged whereby subordinate animals with previous exposure to palatable diets demonstrate sustained hyperphagia once returned to a standard laboratory diet (Arce et al., 2010; Michopoulos et al., 2012a).
While these studies support the relationship between chronic social stress exposure and greater caloric intake (Dallman et al., 2003; Tamashiro et al., 2007; Tomiyama, Dallman, & Epel, 2011), very few studies have examined the microstructure of ingestive behavior under stressful conditions (Melhorn et al., 2010b; Varma, Chai, Meguid, Gleason, & Yang, 1999). It is unclear whether subordinate macaques with current or previous exposure to a rich dietary environment eat larger meals, consume more calories between predefined meals, or simply eat continuously. A clear assessment of the pattern of food intake relative to periods of energy expenditure could help identify biological and environmental signals that initiate and sustain excess caloric intake.
Accordingly, this study was conducted to examine the roles of meal patterning in findings from a previous study demonstrating that psychosocial stress increases overall caloric intake among subordinate animals both in the presence of a highly palatable diet and once returned to a standard laboratory diet (Michopoulos et al., 2012a). Specifically, the goals of the present analysis were (1) to empirically define a “meal” among free-feeding female rhesus monkeys based on a minimum energy content and a minimum threshold time interval between feeding bouts, and (2) to assess and compare meal size, meal frequency, and between-meal snacking among dominant and subordinate animals within two different dietary environments.
Methods
Study subjects were ovariectomized, adult female rhesus monkeys (n = 38) who were socially housed at the Yerkes National Primate Research Center. Subjects were housed in adjacent indoor–outdoor enclosures measuring 3.8 m by 3.8 m by 3.8 m in social groups consisting of five or six animals each (4–5 females and 1 male). Social groups were established approximately 3 years prior to the study using previously described methods (Jarrell et al., 2008). These animals formerly served as subjects in NIH-funded studies to determine the effects of psychosocial stress, induced by social subordination, on a number of behavioral, metabolic, and reproductive outcomes (Collura, Hoffman, & Wilson, 2009; Jarrell et al., 2008; Michopoulos, Berga, Kaplan, & Wilson, 2009; Michopoulos, Checchi, Sharpe, & Wilson, 2011; Michopoulos, Toufexis, & Wilson, 2012b; Michopoulos & Wilson, 2011; Michopoulos et al., in 2012a) that required brief hormone replacement therapy with estradiol and/or progesterone. For the present study, females were untreated and had not received hormone replacement therapy for approximately 8 months. The Emory University Institutional Animal Care and Use Committee approved all procedures in accordance with the Animal Welfare Act and the US Department of Health and Human Services “Guide for the Care and Use of Laboratory Animals.”
As previously described (Michopoulos et al., 2012a), females were studied for two, 2-week dietary phases separated by a 3-week washout period when animals were maintained on standard laboratory chow and food intake was not monitored. One phase consisted of a choice between a low caloric density diet (LCD; 3.43 kcal/g, 12% fat, 18% protein, and 4.14% sugar carbohydrate and 65.9% fiber carbohydrate; Purina #5038) and a more palatable, high caloric density diet (HCD; 3.73 kcal/g, 36% fat, 18% protein, 16.4% sugar carbohydrate and 29.6% fiber-starch carbohydrate; Purina Typical American Diet #L50R). The other phase consisted of access to the LCD only. Although palatability is a subjective concept, the HCD is regarded as more palatable because all animals show a clear, statistically significant preference for the HCD when the diet is presented alongside the standard LCD (Michopoulos et al., 2012a). This criterion is typically employed as an objective indicator of diet palatability (Araujo & Milgram, 2004). The order of dietary phases was counterbalanced so that half of the females received the choice condition first while the other half received the no choice, LCD-only condition first. For the present analysis, data from all animals (n = 38) during both diet conditions were used to establish parameters of meals. Once these parameters were defined, data were analyzed to determine status differences in meal parameters as a function of diet condition, LCD-only versus choice. Because previous results indicated that a history of exposure to a rich dietary environment, when both a HCD and LCD are available, affects subsequent intake of a LCD in subordinate but not dominant females (Michopoulos et al., 2012a), status differences in meal parameters during the LCD only phase were evaluated with respect to HCD history.
Food intake for each subject was recorded continuously using previously validated automated feeders (Wilson et al., 2008). Two feeders were placed on each housing unit. During the choice condition, one contained LCD while the other dispensed HCD. At the midpoint of the choice trial, the diets in the feeders were switched to control for feeder-specific preferences. Crystal Tag RF microchips were implanted subcutaneously in both wrists of each animal. When an animal placed its hand in a feeder, a Datamars reader surrounding the food dispenser detected the microchip and sent a signal to a remote computer that identified the monkey and triggered the delivery of a single food pellet. Data were recorded on a Unix server via an Ethernet connection. Previous data show that dominant females do not restrict subordinate animals’ access to the feeders and rarely (≈1% of the time) take food that subordinate females obtained (Wilson et al., 2008). This system allowed for continual quantification of caloric intake of individual monkeys embedded in social groups. Following convention (Kaplan, Adams, Clarkson, & Koritnik, 1984), females ranked 1 and 2 were classified as dominant while females ranked 3 through 5 were considered subordinate for the present analysis.
Statistical analyses
Feeding data were processed using BioDAQ Data Viewer© (New Brunswick, NJ). A range of inter-meal intervals (IMI) and minimum meal sizes (kcal) was generated by defining meals along a continuum ranging from consumption of a single food pellet to all food intake within a 24-h period. A brute force analysis was then performed to evaluate 3120 meal parameters, generated from all possible combinations of 120 IMI (in seconds) – ranging from 60 s to 7200 s (increasing in increments of 60 s) – and 26 kilocalorie values – ranging from 5.25 kcal to 140 kcal (increasing by increments of 5.25 kcal) using the collapsed values from all feeding conditions. Utilizing statistical methods from previous work with rats (Zorrilla et al., 2005), the first derivative values of these 3120 meal parameters were plotted, and the subset of these values that demonstrated visual homogeneity in classification of meals was identified. The resulting subset consisted of 1450 meal parameters, generated from all possible combinations of 58 time intervals, ranging from 60 to 3480 s, and 25 kilocalorie values, ranging from 5.25 kcal to 131.25 kcals. This subset was further collapsed into 29 even, contiguous matrices, each containing 50 data points. An absence of statistically significant differences among these matrices was verified using one-way ANOVA in SPSS.
Of these non-significantly differing sub matrices, the IMI and minimum kcal values of the median matrix were chosen as an appropriate meal categorization across feeding conditions as defined by their first derivative values. The validity of this assumption was tested using the previously validated method of plotting the resulting log-transformed frequency distribution to assess the accuracy of meal grouping and breakpoints (Zorrilla et al., 2005). In addition, the contiguously adjacent lower and upper matrices of the identified median matrix were also assessed using log-transformed frequency distributions. The previously referenced analysis of meal patterns among rats specified that a unimodal distribution of log-transformed meal frequencies signifies appropriate breakpoints in meal patterning, whereas a bimodal or otherwise heterogeneous distribution of post-meal intervals suggests that breakpoints are inappropriately splitting or merging “meals” (Zorrilla et al., 2005a). For each potential meal definition, the log transformed inter-meal interval was plotted for both collapsed and stratified (choice and LCD-only) dietary conditions across 24-h days and split into 12-hour diurnal and nocturnal phases. The low frequency of night feeding among rhesus monkeys resulted in a regular, prolonged inter-meal interval leading to a slight bimodal or truncated distribution during all dietary conditions when diurnal and nocturnal feeding were combined. Thus, a meal definition was considered inappropriate if a trimodal or otherwise heterogeneous distribution was observed in the 24-h analysis or if a bimodal distribution was observed during the diurnal and nocturnal analyses in one or more of the dietary conditions. The resulting empirically validated meal definition was then applied to evaluate descriptive meal characteristics including meal size (average kilocalories per meal), meal number (average number of meals per day), snack size (average daily kilocalories consumed outside of statistically defined meals), and the relative contributions of the two diets to both meal and snack intake as a function of social status, diet condition, and order of HCD presentation using multivariate ANOVA performed in SPSS. Results are presented as the mean ± standard error, and analyses were performed using log10 transformed data to normalize variances. P-values <0.05 were considered significant.
Results
Defining meal parameters
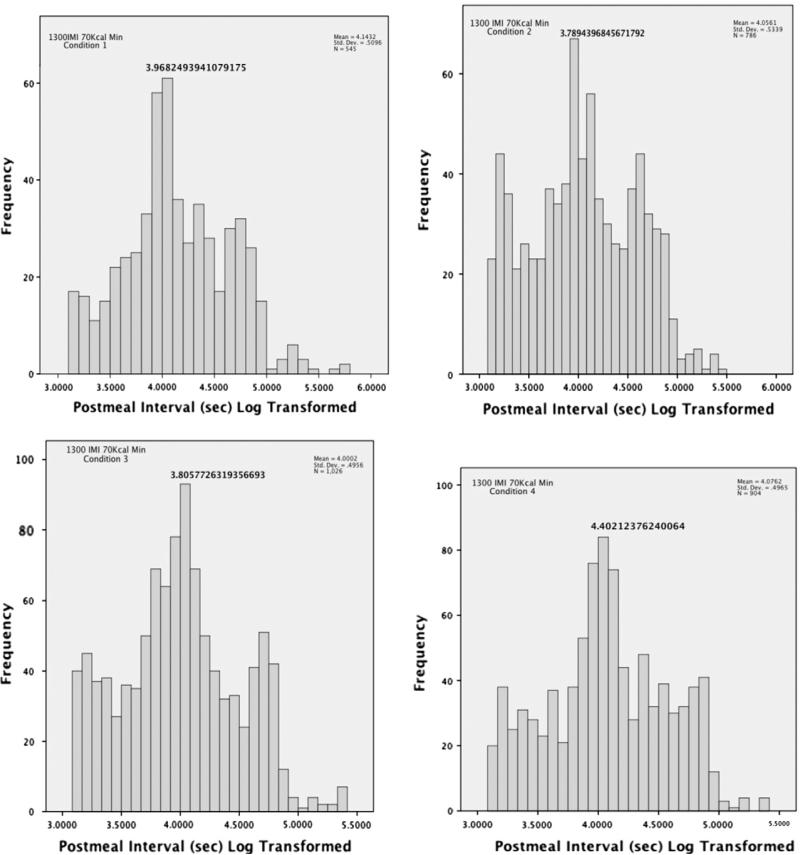
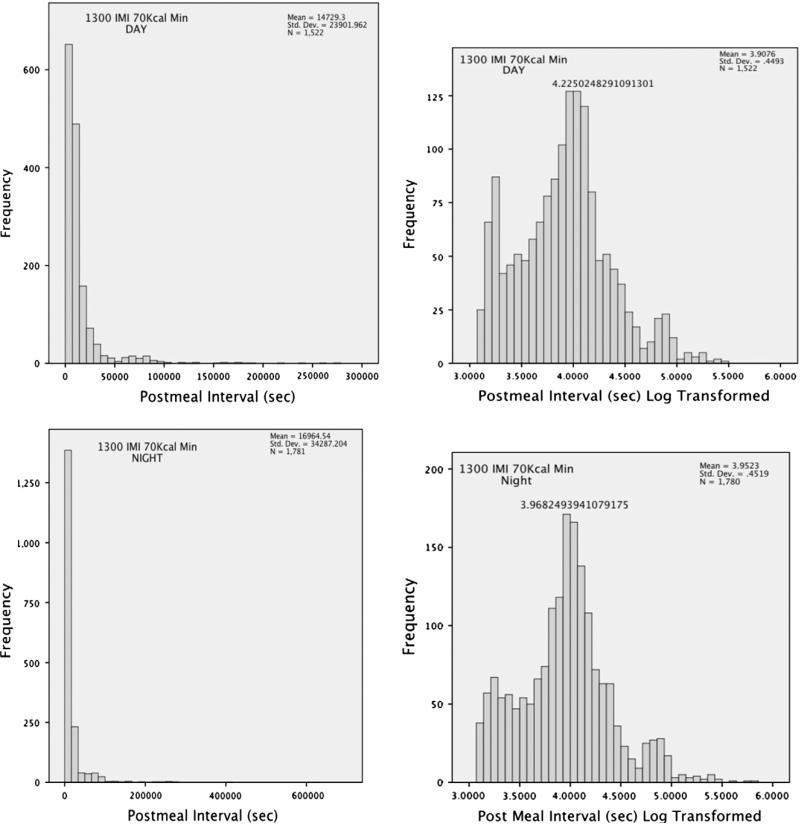
The three potential meal definitions identified using the brute force analysis and first derivative plots were: (1) a minimum of 40 kcal, separated from a previous feeding bout by a minimum time interval of 1300 s; (2) a minimum of 70 kcal, separated from a previous feeding bout by a minimum time interval of 1300 s; and (3) a minimum of 100 kcal, separated from a previous feeding bout by a minimum of 1600 s. All three definitions produced appropriate distributions of log-transformed meal frequencies when food intake data were collapsed across dietary conditions. However, definition 1 produced a largely heterogeneous distribution of log-transformed meal frequencies during the LCD-only condition, and definition 3 produced a trimodal distribution of log transformed meal frequencies during the choice condition. Definition 2 produced a consistent, predominantly unimodal distribution across all diet conditions when diurnal and nocturnal feeding distributions were combined (Fig. 1). A separate analysis of day and night of all four conditions collapsed showed a pronounced unimodal distribution during both diurnal and nocturnal time periods with a slight peak at the briefest time periods during the diurnal phase and none during the nocturnal phase (Fig. 2). The linear distribution of diurnal and nocturnal feeding distributions (Fig. 2, left) showed that time between meals was longer at night though the log-transformed the distributions were uniform (Fig. 2, right). Due to the consistent validation of meal distribution across dietary conditions and during collapsed and split time phases, a meal was defined as any feeding bout that resulted in ingestion of a minimum of 70 kcal, separated from a previous feeding bout by a minimum time interval of 1300 s as initially determined through a brute force first derivative analysis.
Fig. 1.
Logarithmic post-meal time interval distributions during the 2-week counterbalanced dietary conditions when meals were defined as ingestion of a minimum of 70 kcal, separated from a previous feeding bout by a minimum time interval of 1300 s. Condition 1 represents the initial 2-week LCD-only phase among animals with no history of HCD exposure. Condition 2 illustrates the subsequent 2-week choice phase. Condition 3 represents the 2-week choice phase among animals that received this condition first. Condition 4 represents the 2-week LCD-only phase among animals with previous exposure to HCD. Based on this definition, each condition produced primarily unimodal distributions with slight right tail oriented bimodal or truncated distributions indicative of an extended break in feeding due to the absence of nocturnal feeding.
Fig. 2.
Linear (left) and logarithmic (right) frequency distributions of 12 h diurnal (0700–1900 h) and nocturnal (1900–0700 h) feeding for all four feeding conditions collapsed. Linear frequency distributions show a greater post-meal interval during nocturnal feeding while log transformed distributions show uniform, unimodal distributions. The presence of snacking is represented by a slight modality of the briefest post meal intervals during diurnal feeding that is not present during nocturnal feeding.
Effects of the dietary environment and status on meal patterns
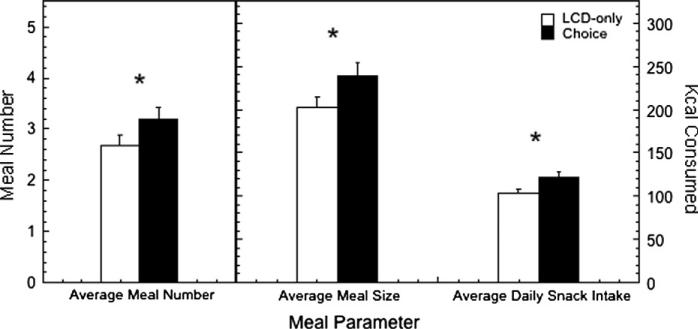
Based on this meal definition, these parameters were used to assess differences in meal size, meal number, and snack size (or the total calories consumed outside of defined meals) during the LCD-only and choice conditions as a function of status and the dietary environment. As reported previously (Michopoulos et al., 2012a), all animals consume more total calories each day during the choice dietary condition, with subordinate females consuming significantly more daily calories than dominant animals. Examination of meal parameters (Fig. 3) shows that during the choice dietary condition, females consumed significantly more meals (F1,36 = 5.86, p = 0.021), significantly larger meals (F1,36 = 5.48, p = 0.023), and significantly more calories from snacks (F1,36 = 6.39, p = 0.016) regardless of status.
Fig. 3.
Meal ± SEM of meal number, meal size in kcals, kcals consumed outside of defined meals (“snacks”) for all females during the LCD-only condition and the choice condition when both LCD and HCD were available. An asterisk indicates the difference between the two dietary conditions were significant.
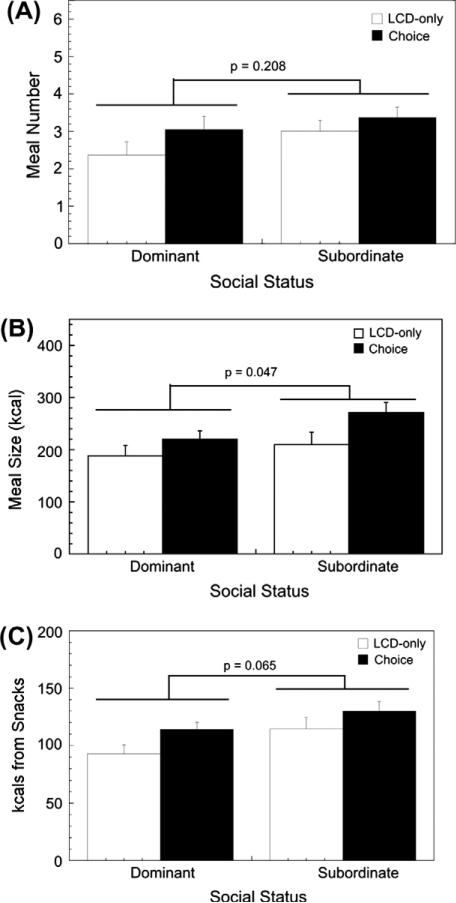
Social status had a significant effect on specific meal parameters independent of diet condition. As illustrated in Fig. 4A, there was no main effect of status on the number of daily meals (F1,36 = 1.64, p = 0.208), and this was not affected by an interaction with diet condition (F1,36 = 0.40, p = 0.532). In contrast, subordinates consumed significantly larger meals, on average, compared to dominant females (Fig. 4B; F1,36 = 4.22, p = 0.047), and this pattern was unaffected by diet condition (F1,36 = 0.77, p = 0.386). Furthermore, while subordinate animals ingested more calories between defined meals (Fig. 4C), the difference was not significant (F1,36 = 3.62, p = 0.065), and this social status pattern of snacking was not significantly affected by diet condition (F1,36 = 0.17, p = 0.684).
Fig. 4.
Mean ± SEM meal parameters for dominant and subordinate females during the LCD-only condition and the choice condition when both LCD and HCD were available. Panel A shows meal number, panel B shows meal size in kcals, and panel C shows kcals consumed outside of defined meals (“snacks”).
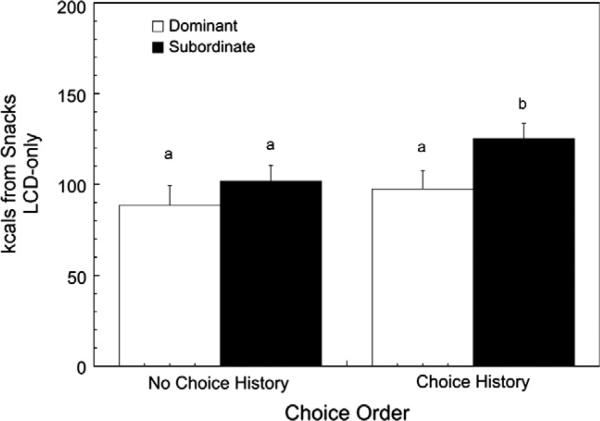
For the present study, the order of quantification of caloric intake during the LCD-only condition was counterbalanced such that LCD consumption was quantified in 18 females (7 DOM, 11 SUB) prior to any exposure to the HCD while LCD intake was quantified in 20 females (8 DOM, 12 SUB) following the dietary choice condition. We previously reported that a history of exposure to highly palatable food significantly increases average daily LCD in-take in subordinate but not dominant females when returned to a LCD-only condition (Michopoulos et al., 2012a). Considering the effect of diet order on meal parameters, there was a significant interaction of status by diet condition by diet order on snack intake (F1,34 = 4.83, p = 0.035) but not meal number (F1,34 = 0.12, p = 0.730) or meal size (F1,34 = 1.53, p = 0.224). As illustrated in Fig. 5, following the choice dietary condition, subordinate females (n = 12) with a history of HCD exposure consumed significantly more calories outside of meals compared to dominant animals (n = 8) in the same condition as well as dominant or subordinate animals with no history of HCD intake.
Fig. 5.
Mean ± SEM kcals consumed outside of defined meals (“snacks”) for dominant and subordinate females during the LCD-only condition prior to any exposure to the dietary choice condition (“No choice history”) or following exposure to the choice condition (“Choice history”). Different letters indicate groups are significantly different from one another.
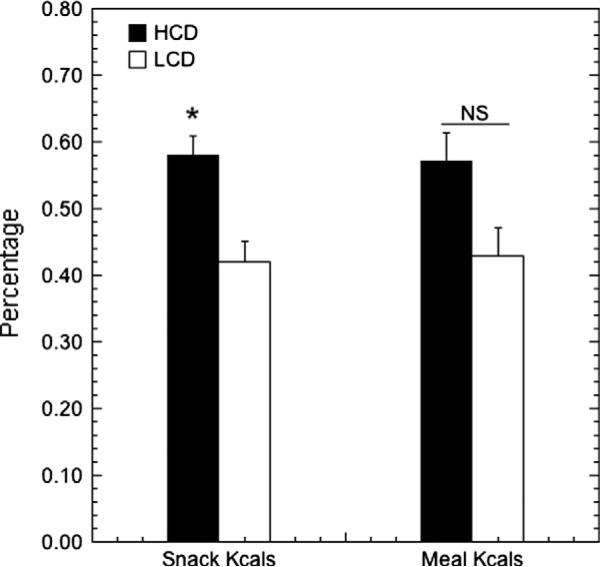
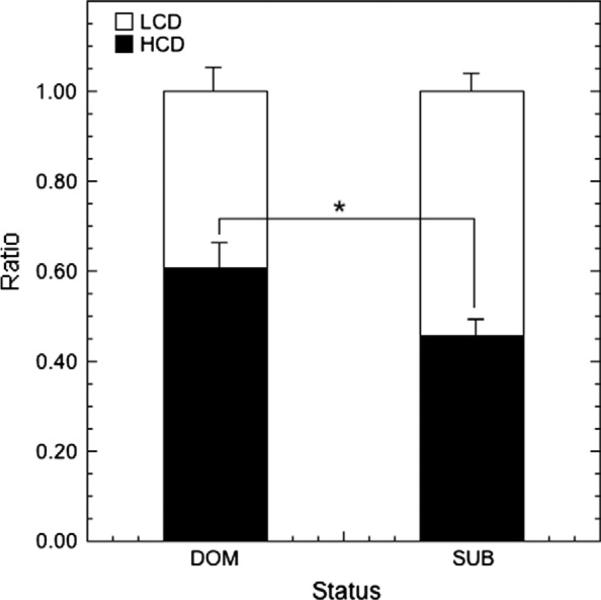
During the choice condition, significantly more meals were mixed (49.5%) compared to LCD-only (19.5%) or HCD-only (31%; F2,37 = 11.970, p < 0.001). This was not influenced by status (F1,37 = 0.464, p = 0.500). A final analysis evaluated the relative contributions of the two diets to snack intake and meal intake during the choice condition. Animals consumed a significant majority (Fig 6; F1,35 = 6.51, p = 0.015) of snack calories from HCD (57.9 ± 3.1%) versus LCD (42.1 ± 3.1%). This effect of diet on snack preference was not modified by status (F1,35 = 2.42, p = 0.129). When total caloric intake from meals was analyzed, a diet preference did not reach statistical significance (Fig 6; F1,35 = 2.74, p = 0.107) nor was there an effect of status (F1,35 = 1.35, p = 0.253). However, when caloric intake was evaluated strictly within mixed meals, there was significant status by diet interaction (Fig 7; F1,35 = 4.665, p = 0.038) with subordinates consuming a majority of calories within mixed meals from LCD (54.3 ± 4.7%) and dominants consuming a majority of calories from HCD (60.6 ± 5.4%).
Fig. 6.
Mean ± SEM percentage of HCD vs. LCD kilocalories contributed to total snack kilocalories and total meal kilocalories. An asterisk indicates a statistically significant preference for one diet over the other.
Fig. 7.
Mean ± SEM ratio of kilocalories contributed to mixed meals by HCD vs. LCD among dominant and subordinate females. An asterisk indicates a statistically significant difference between proportions of the two diets based on status.
Discussion
The present study utilized statistical methods validated by a previous analysis in rats (Zorrilla et al., 2005a) to define meals in free-feeding, female rhesus monkeys. The resulting definition – any feeding bout that resulted in ingestion of a minimum of 70 kilocalories, separated from a previous feeding bout by a minimum time interval of 1300 s – was then applied to assess meal patterns under conditions of chronic psychosocial stress induced by social subordination. The results of this analysis demonstrated a significant effect of the dietary environment on meal patterns regardless of status, as access to a highly palatable diet led to larger meal size, greater meal number, and greater caloric intake between meals among all animals in the choice condition. However, subordinate animals consumed significantly larger meals relative to dominant animals in both dietary conditions, and subordinate animals with a history of exposure to a highly palatable diet consumed significantly more snack calories than dominant animals as well as subordinate animals without previous exposure to the palatable diet. The animals showed a significant preference for HCD in the form of snacks during the choice condition regardless of status or order. No significant preference for either diet emerged within total meals, but mixed meals were consumed more frequently than LCD- or HCD-only meals. When ratios of HCD to LCD were analyzed within mixed meals only, there was significant status by diet interaction with dominants consuming a greater proportion of HCD and subordinates consuming a greater proportion of LCD within mixed meals. The reason for this status difference is unknown. It is unlikely that pattern is attributed to feeder access as all animals could free feed at any time, and we have previously shown dominant females do not block access to feeders containing highly palatable food (Wilson et al., 2008). It must be emphasized that while the proportion of calories from the two diets varied by status during mixed meals, subordinate animals were consuming significantly larger meals with regard to caloric content. These findings illustrate how small changes in meal patterns can induce significant increases in total caloric intake, which if prolonged, could promote the emergence of an obese phenotype.
To date, studies assessing the microstructure of ingestive behavior in response to stress are relatively rare. One such study utilizing the visible burrow system (VBS), a rodent model of chronic social stress, reported that meal patterns of dominant male rats did not differ from controls when animals were maintained on standard rat chow, but subordinate male rats had reduced meal frequency during the social group housing (Melhorn et al., 2010b). Interestingly, subordinate rats in this study demonstrated increased meal size during a recovery period on a similar low caloric density laboratory diet (Melhorn et al., 2010b). Another study examined the effects of surgical stress on chow intake in rats and reported post-stress decreases in both meal number and meal size compared to pre-stress conditions (Varma et al., 1999). However, it is well established that acute or relatively short-term stress reduces food intake (Dallman et al., 2003), and examinations of the effect of chronic stress generally report increased food-intake when calorically dense, palatable foods are available (Warne, 2009). In fact, evidence suggests that a palatable diet is essential to initially elicit stress-induced hyperphagia (Arce et al., 2010; Hagan et al., 2002; Wilson et al., 2008). Recent findings also indicate that previous exposure to a palatable diet may promote stress-induced hyperphagia when a healthier diet is available (Arce et al., 2010; Michopoulos et al., 2012a). Additionally, the current finding that introduction of a highly palatable diet altered meal parameters in all animals is not surprising given that previous research in rats has demonstrated an increase in meal size in a high-fat dietary environment in the absence of chronic stress (Melhorn et al., 2010a).
It is important to note that the results of any study assessing the microstructure of feeding patterns are contingent upon the employed definition of a “meal” or feeding bout. While some previous studies have arbitrarily chosen meal criteria, the present study set out to empirically derive meal parameters through brute force, data-driven analyses of feeding patterns among rhesus monkeys. The employed methodology was informed by previous work involving nocturnal feeding data obtained from rats (Zorrilla et al., 2005a). However, there are notable differences between the referenced and utilized methodologies. Specifically, Zorilla et al. did not systematically vary the minimum meal size. Rather, the minimum meal sized was fixed at a low value, and the inter-meal interval was systematically varied. Under the cited procedure, almost all food intake was captured within meals, and there was no separate category for snacks. While the cited methodology was appropriate for nocturnal rats whose meal size and post-meal intervals appear to fit a single peak distribution, validation analyses in which the minimum inter-meal interval was systematically varied across five time values (300, 840, 1300, 1600, and 1800 s) for three separate, fixed, minimum meal sizes (5.25, 40, and 70 kcal) validate the initially chosen meal parameters while disqualifying alternative meal parameters suggesting the employed methodology was in fact appropriate for the present study population. Specifically, fixing the minimum meal size at 5.25 kcal or 40 kcal produced inconsistent feeding frequency distributions for one or more feeding conditions when day, night, and 24-h distributions were compared for each minimum inter-meal interval. Additionally, while a minimum meal size of 70 kcal produced consistent frequency distributions during each dietary condition regardless of time splits, a slight bimodal distribution emerged during the briefest minimum inter-meal intervals, which was particularly pronounced when animals were exposed to the choice condition, suggesting that these animals engage in snacking behavior particularly when given access to palatable food. As an aside, one rare study that attempted to empirically differentiate meals from snacking in humans approached the task in the manner of the present investigation by varying combinations of minimum meal size requirements in addition to minimum inter-meal intervals (de Castro, 1993).
The present findings are strengthened by the use of an ethologically relevant model of chronic psychosocial stress exposure and the direct quantification of caloric intake in socially-housed female rhesus monkeys; however, the study is not without limitations. Caloric intake in the presence of a high-fat, sugary diet was assessed over the course of just 2 weeks, and this short-term exposure period was not adequate to induce significant increases in body weight (Michopoulos et al., 2012a). While we hypothesize that prolonged exposure to a rich dietary environment would result in significant weight gain, particularly among subordinates, it is possible that meal patterns and total caloric intake may return to baseline profiles when the HCD is no longer novel. Therefore, more long-term studies must be conducted to assess how stress and the dietary environment may interact to promote the emergence of an obese phenotype. Additionally, animals utilized for this study were ovariectomized and were not receiving hormone replacement during these experiments. Both estrogen and progesterone are known to affect appetite and meal size (Asarian & Geary, 2006; Buffenstein, Poppitt, McDevitt, & Prentice, 1995; Gray & Greenwood, 1982). Thus, the effects of female hormones on feeding patterns were eliminated and must be considered when evaluating the external validity of our results. Finally, because this study was conducted with nonhuman primates, cognitive influences such as deliberate versus indiscriminant snacking and conscious dietary restraint could not be explored. Nonetheless, findings from these studies with female rhesus monkeys are extremely valuable because longitudinal studies of this magnitude cannot be conducted in human populations due to the limitations of current dietary assessment tools (Johnson, 2002) and the environmental and behavioral confounders present in human populations (Marmot & Wilkinson, 2006).
Conclusion
Effective dietary strategies for weight management are needed to address the current obesity epidemic, which affects approximately 36% of US adults (Flegal, Carroll, Kit, & Ogden, 2012). When attempting to extrapolate findings from this study to human populations, it is critical to realize that excess caloric intake may occur via several avenues, and energy expenditure is equally as important as energy intake in the energy balance equation. Thus, low eating frequency coupled with large portion sizes, high eating frequency with small portion sizes, or high eating frequency and large portion sizes could all potentially lead to positive energy imbalance. Simply put, weight-loss is achieved by ingesting fewer calories than are expended (Adams & Morgan, 1981; Bray et al., 2012; Sacks et al., 2009). However, energy density of the diet has emerged as an important factor influencing both short-term and long-term energy intake (Rolls, 2009). Additionally, as corroborated by our findings, it is plausible that the palatability of food can override homeostatic mechanisms of hunger and satiety to promote excess caloric intake via increased portion sizes and/or snacking as a result of hedonic motivation (Havermans, 2011), particularly during times of stress (Adam & Epel, 2007). Although, given the greater caloric content and measured preference for HCD, the present study does not permit assessment of the differential effects of energy density versus palatability in terms of their impact on appetitive profiles.
The demonstration that stress interacts with HCD exposure to increase total caloric intake via small changes in meal patterns highlights the insidious nature of the obesity epidemic. Indeed, the surge in obesity and shift in median weight that has been observed in recent decades, can be explained by a continuous, net caloric imbalance of just 100–150 kilocalories per day (Cutler et al., 2003). While the current study sheds light on how changes in the dietary environment and its interaction with psychosocial stress may have contributed to this phenomenon via larger meal sizes and increased eating frequency, future studies are necessary to investigate the reproducibility of these results, examine the impact of prolonged exposure to a HCD, and explore the potential neurobiological mechanisms underlying the observed hyperphagia among chronically stressed animals with access and/or previous exposure to a highly palatable diet.
Footnotes
Acknowledgements: The expert technical assistance of Jennifer Whitley, Shannon Bounar, Christine Marsteller, and Erin O'Sheil was greatly appreciated. We also thank Dr. Rebecca Herman for her assistance with data summarization. The collaborative efforts of Dr. Ed Ulman and Matt Williams at Research Diets for assistance with BioDAQ were greatly appreciated. This was funded by HD046501 (MW), MH081816 (DT) and in part RR00165. The Yerkes NPRC is accredited by AAALAC, International.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Hormones and Behavior. 2003;43(1):67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Adam TC, Epel ES. Stress, eating and the reward system. Physiology & Behavior. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. doi: S0031-9384(07)00127-8 [pii] 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Adams CE, Morgan KJ. Periodicity of eating. Implications for human food consumption. Nutrition Research. 1981;1:26. [Google Scholar]
- Araujo JA, Milgram NW. A novel cognitive palatability assessment protocol for dogs. [Research Support, Non-U.S. Gov't]. Journal of Animal Science. 2004;82(7):2200–2206. doi: 10.2527/2004.8272200x. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2006;361(1471):1251–1263. doi: 10.1098/rstb.2006.1860. http://dx.doi.org/10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce M, Michopoulos V, Shepard KN, Ha QC, Wilson ME. Diet choice, cortisol reactivity, and emotional feeding in socially housed rhesus monkeys. Physiology & Behavior. 2010;101(4):446–455. doi: 10.1016/j.physbeh.2010.07.010. doi: S0031-9384(10)00275-1 [pii] 10.1016/j.physbeh.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson TJ. Central and peripheral neuroendocrine peptides and signalling in appetite regulation. Considerations for obesity pharmacotherapy [Review]. Obesity Reviews. 2008;9(2):108–120. doi: 10.1111/j.1467-789X.2007.00412.x. doi: 10.1111/j.1467-789X.2007.00412. [DOI] [PubMed] [Google Scholar]
- Bellisle F, McDevitt R, Prentice AM. Meal frequency and energy balance [Review]. The British Journal of Nutrition. 1997;77(Suppl. 1):S57–S70. doi: 10.1079/bjn19970104. [DOI] [PubMed] [Google Scholar]
- Bernstein IS. Dominance, aggression and reproduction in primate societies. Journal of Theoretical Biology. 1976;60(2):459–472. doi: 10.1016/0022-5193(76)90072-2. 0022-5193(76)90072-2 [pii] [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP. The function of aggression in primate societies. American Scientist. 1974;62(3):304–311. [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP, Rose RM. Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatol (Basel) 1974;21(2):81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- Bray GA, Smith SR, de Jonge L, Xie H, Rood J, Martin CK, et al. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating. A randomized controlled trial [Comparative Study Randomized Controlled Trial Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.]. JAMA. The Journal of the American Medical Association. 2012;307(1):47–55. doi: 10.1001/jama.2011.1918. http://dx.doi.org/10.1001/jama.2011.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle. A retrospective analysis, with implications for appetite research. [Research Support, Non-U.S. Gov't Review]. Physiology & Behavior. 1995;58(6):1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- Chapelot D, Marmonier C, Aubert R, Allegre C, Gausseres N, Fantino M, et al. Consequence of omitting or adding a meal in man on body composition, food intake, and metabolism. [Randomized Controlled Trial Research Support, Non-U.S. Gov't]. Obesity. 2006;14(2):215–227. doi: 10.1038/oby.2006.28. http://dx.doi.org/10.1038/oby.2006.28. [DOI] [PubMed] [Google Scholar]
- Collura LA, Hoffman JB, Wilson ME. Administration of human leptin differentially affects parameters of cortisol secretion in socially housed female rhesus monkeys. Endocrine. 2009 doi: 10.1007/s12020-009-9250-7. http://dx.doi.org/10.1007/s12020-009-9250-7. [DOI] [PubMed]
- Cottone P, Sabino V, Nagy TR, Coscina DV, Zorrilla EP. Feeding microstructure in diet-induced obesity susceptible versus resistant rats. Central effects of urocortin 2. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Journal of Physiology. 2007;583(Pt 2):487–504. doi: 10.1113/jphysiol.2007.138867. http://dx.doi.org/10.1113/jphysiol.2007.138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology. 2009;34(1):38–49. doi: 10.1016/j.psyneuen.2008.08.010. doi: S0306-4530(08)00215-1 [pii] 10.1016/j.psyneuen.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler DM, Glaeser EL, Shapiro JM. Why have Americans become more obese? Journal of Economic Perspectives. 2003;17(3):93–118. [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity. A new view of “comfort food”. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. doi: 10.1073/pnas.1934666100 1934666100 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro JM. Genetic influences on daily intake and meal patterns of humans. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Physiology & Behavior. 1993;53(4):777–782. doi: 10.1016/0031-9384(93)90188-l. [DOI] [PubMed] [Google Scholar]
- de Graaf C. Effects of snacks on energy intake. An evolutionary perspective. [Review]. Appetite. 2006;47(1):18–23. doi: 10.1016/j.appet.2006.02.007. http://dx.doi.org/10.1016/j.appet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Cohen AE, Faust IM, Grinker JA. Meal-taking behavior is related to predisposition to dietary obesity in the rat. [Comparative Study Research Support, U.S. Gov't, P.H.S.]. Physiology & Behavior. 1984;32(1):61–67. doi: 10.1016/0031-9384(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Fabry P, Hejl Z, Fodor J, Braun T, Zvolankova K. The frequency of meals. Its relation to overweight, hypercholesterolaemia, and decreased glucose-tolerance. Lancet. 1964;2(7360):614–615. doi: 10.1016/s0140-6736(64)90510-0. [DOI] [PubMed] [Google Scholar]
- Fabry P, Tepperman J. Meal frequency. A possible factor in human pathology. [Review]. The American journal of clinical nutrition. 1970;23(8):1059–1068. doi: 10.1093/ajcn/23.8.1059. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szucs A, et al. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats. Intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Neuropsychopharmacology. 2007;32(5):1052–1068. doi: 10.1038/sj.npp.1301214. doi: 10.1038/sj.npp.1301214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010 [Comparative Study]. JAMA. The Journal of the American Medical Association. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. http://dx.doi.org/10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Gray JM, Greenwood MR. Time course of effects of ovarian hormones on food intake and metabolism. [Research Support, U.S. Gov't, P.H.S.]. The American Journal of Physiology. 1982;243(5):E407–412. doi: 10.1152/ajpendo.1982.243.5.E407. [DOI] [PubMed] [Google Scholar]
- Greeno CG, Wing RR. Stress-induced eating. Psychological Bulletin. 1994;115(3):444–464. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]
- Gregori D, Foltran F, Ghidina M, Berchialla P. Understanding the influence of the snack definition on the association between snacking and obesity. A review. [Review]. International Journal of Food Sciences and Nutrition. 2011;62(3):270–275. doi: 10.3109/09637486.2010.530597. http://dx.doi.org/10(3109/09637486), 2010, 530597. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain, Behavior, and Immunity. 1991;5(3):296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating. Key synergistic role of past caloric restriction and stress. Physiology & Behavior. 2002;77(1):45–54. doi: 10.1016/s0031-9384(02)00809-0. S0031938402008090 [pii] [DOI] [PubMed] [Google Scholar]
- Havermans RC. “You Say it's Liking, I Say it's Wanting”. On the difficulty of disentangling food reward in man. [Review]. Appetite. 2011;57(1):286–294. doi: 10.1016/j.appet.2011.05.310. http://dx.doi.org/10.1016/j.appet.2011.05.310. [DOI] [PubMed] [Google Scholar]
- Helies JM, Diane A, Langlois A, Larue-Achagiotis C, Fromentin G, Tome D, et al. Comparison of fat storage between Fischer 344 and obesity-resistant Lou/C rats fed different diets. [Comparative Study]. Obesity Research. 2005;13(1):3–10. doi: 10.1038/oby.2005.3. http://dx.doi.org/10.1038/oby.2005.3. [DOI] [PubMed] [Google Scholar]
- Howarth NC, Huang TT, Roberts SB, Lin BH, McCrory MA. Eating patterns and dietary composition in relation to BMI in younger and older adults. [Comparative Study Research Support, U.S. Gov't, Non-P.H.S.]. International Journal of Obesity. 2007;31(4):675–684. doi: 10.1038/sj.ijo.0803456. http://dx.doi.org/10.1038/sj.ijo.0803456. [DOI] [PubMed] [Google Scholar]
- Jahns L, Siega-Riz AM, Popkin BM. The increasing prevalence of snacking among US children from 1977 to 1996. The Journal of Pediatrics. 2001;138(4):493–498. doi: 10.1067/mpd.2001.112162. http://dx.doi.org/10.1067/mpd.2001.112162. [DOI] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiology & Behavior. 2008;93(4–5):807–819. doi: 10.1016/j.physbeh.2007.11.042. doi: S0031-9384(07)00483-0 [pii] 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RK. Dietary intake. How do we measure what people are really eating? Obesity Research. 2002;10(Suppl. 1):63S–68S. doi: 10.1038/oby.2002.192. http://dx.doi.org/10.1038/oby.2002.192. [DOI] [PubMed] [Google Scholar]
- Johnson GH, Anderson GH. Snacking definitions. Impact on interpretation of the literature and dietary recommendations. Critical Reviews in Food Science and Nutrition. 2010;50(9):848–871. doi: 10.1080/10408390903572479. http://dx.doi.org/10.1080/10408390903572479. [DOI] [PubMed] [Google Scholar]
- Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans. NHANES 1971–1975 to NHANES 1999–2002. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural]. The American Journal of Clinical Nutrition. 2006;84(5):1215–1223. doi: 10.1093/ajcn/84.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Koritnik DR. Psychosocial influences on female ‘protection’ among cynomolgus macaques. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Atherosclerosis. 1984;53(3):283–295. doi: 10.1016/0021-9150(84)90129-1. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA, Williams JK. Psychosocial factors, sex differences, and atherosclerosis. Lessons from animal models. Psychosomatic Medicine. 1996;58(6):598–611. doi: 10.1097/00006842-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease. A primate continuum. ILAR. 2004;45:89–115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- Klaus S. Increasing the protein. Carbohydrate ratio in a high-fat diet delays the development of adiposity and improves glucose homeostasis in mice. [Research Support, Non-U.S. Gov't]. The Journal of Nutrition. 2005;135(8):1854–1858. doi: 10.1093/jn/135.8.1854. [DOI] [PubMed] [Google Scholar]
- Koletzko B, Toschke AM. Meal patterns and frequencies. Do they affect body weight in children and adolescents? [Research Support, Non-U.S. Gov't Review]. Critical Reviews in Food Science and Nutrition. 2010;50(2):100–105. doi: 10.1080/10408390903467431. http://dx.doi.org/10.1080/10408390903467431. [DOI] [PubMed] [Google Scholar]
- Leidy HJ, Campbell WW. The effect of eating frequency on appetite control and food intake. Brief synopsis of controlled feeding studies. [Review]. The Journal of Nutrition. 2011;141(1):154–157. doi: 10.3945/jn.109.114389. http://dx.doi.org/10.3945/jn.109.114389. [DOI] [PubMed] [Google Scholar]
- Marmot MG, Wilkinson RG. Social determinants of health. 2nd ed. Oxford University Press; Oxford; New York: 2006. [Google Scholar]
- Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, et al. Acute exposure to a high-fat diet alters meal patterns and body composition. Physiology & Behavior. 2010a;99(1):33–39. doi: 10.1016/j.physbeh.2009.10.004. http://dx.doi.org/10.1016/j.physbeh.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, et al. Meal patterns and hypothalamic NPY expression during chronic social stress and recovery. [Research Support, N.I.H., Extramural]. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2010b;299(3):R813–822. doi: 10.1152/ajpregu.00820.2009. http://dx.doi.org/10.1152/ajpregu.00820.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biology of Reproduction. 2009;81(6):1154–1163. doi: 10.1095/biolreprod.109.079038. doi: biolreprod.109.079038 [pii] 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Checchi M, Sharpe D, Wilson ME. Estradiol effects on behavior and serum oxytocin are modified by social status and polymorphisms in the serotonin transporter gene in female rhesus monkeys. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Hormones and Behavior. 2011;59(4):528–535. doi: 10.1016/j.yhbeh.2011.02.002. http://dx.doi.org/10.1016/j.yhbeh.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Higgins M, Toufexis D, Wilson ME. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology. 2012b;37:1071–1085. doi: 10.1016/j.psyneuen.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Toufexis D, Wilson ME. Social stress interacts with diet history to promote emotional feeding in females. Psychoneuroendocrinology. 2012a doi: 10.1016/j.psyneuen.2012.02.002. http://dx.doi.org/10.1016/j.psyneuen.2012.02.002. [DOI] [PMC free article] [PubMed]
- Michopoulos V, Wilson ME. Body weight decreases induced by estradiol in female rhesus monkeys are dependent upon social status. [Research Support, N.I.H., Extramural]. Physiology & Behavior. 2011;102(3–4):382–388. doi: 10.1016/j.physbeh.2010.11.031. http://dx.doi.org/10.1016/j.physbeh.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys. Dopamine D2 receptors and cocaine self-administration. Nature Neuroscience. 2002;5(2):169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Newman E, O'Connor DB, Conner M. Daily hassles and eating behaviour. The role of cortisol reactivity status. Psychoneuroendocrinology. 2007;32(2):125–132. doi: 10.1016/j.psyneuen.2006.11.006. doi: S0306-4530(06)00201-0 [pii] 10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Nicklas TA, Morales M, Linares A, Yang SJ, Baranowski T, De Moor C, et al. Children's meal patterns have changed over a 21-year period. The Bogalusa Heart Study. [Comparative Study Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.]. Journal of the American Dietetic Association. 2004;104(5):753–761. doi: 10.1016/j.jada.2004.02.030. http://dx.doi.org/10.1016/j.jada.2004.02.030. [DOI] [PubMed] [Google Scholar]
- O'Connor DB, Jones F, Conner M, McMillan B, Ferguson E. Effects of daily hassles and eating style on eating behavior. Health Psychology. 2008;27(1 Suppl.):S20–31. doi: 10.1037/0278-6133.27.1.S20. doi: 2008-00684-004 [pii] 10.1037/0278-6133.27.1.S20. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–2102. doi: 10.1053/j.gastro.2007.03.052. doi: S0016-5085(07)00579-3 [pii] 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Paiardini M, Hoffman J, Cervasi B, Ortiz AM, Stroud F, Silvestri G, et al. T-cell phenotypic and functional changes associated with social subordination and gene polymorphisms in the serotonin reuptake transporter in female rhesus monkeys. Brain, Behavior, and Immunity. 2009;23(2):286–293. doi: 10.1016/j.bbi.2008.10.006. doi: S0889-1591(08)00391-7 [pii] 10.1016/j.bbi.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcey SM, de Castro JM. Food intake and meal patterns of weight-stable and weight-gaining persons. The American Journal of Clinical Nutrition. 2002;76(1):107–112. doi: 10.1093/ajcn/76.1.107. [DOI] [PubMed] [Google Scholar]
- Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL/6J mouse. [Comparative Study Research Support, Non-U.S. Gov't]. Metabolism. Clinical and Experimental. 2004;53(4):454–457. doi: 10.1016/j.metabol.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Rolls BJ. The relationship between dietary energy density and energy intake. Physiology & Behavior. 2009;97(5):609–615. doi: 10.1016/j.physbeh.2009.03.011. doi: S0031-9384(09)00122-X [pii] 10.1016/j.physbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls BJ, Drewnowski A, Ledikwe JH. Changing the energy density of the diet as a strategy for weight management. [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, P.H.S. Review]. Journal of the American Dietetic Association. 2005;105(5 Suppl. 1):S98–103. doi: 10.1016/j.jada.2005.02.033. http://dx.doi.org/10.1016/j.jada.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. [Comparative Study Multicenter Study Randomized Controlled Trial Research Support, N.I.H., Extramural]. The New England Journal of Medicine. 2009;360(9):859–873. doi: 10.1056/NEJMoa0804748. http://dx.doi.org/10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeller DA, Buchholz AC. Energetics of obesity and weight control. Does diet composition matter? [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, P.H.S. Review]. Journal of the American Dietetic Association. 2005;105(5 Suppl. 1):S24–28. doi: 10.1016/j.jada.2005.02.025. http://dx.doi.org/10.1016/j.jada.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biological Psychiatry. 1998;44(9):882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Shively C, Kaplan J. Effects of social factors on adrenal weight and related physiology of Macaca fascicularis. Physiology & Behavior. 1984;33(5):777–782. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biological Psychiatry. 1997;41(8):871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Clarkson TB. Social stress, visceral obesity, and coronary artery atherosclerosis in female primates. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.74. doi: oby200974 [pii] 10.1038/oby.2009.74. [DOI] [PubMed] [Google Scholar]
- Silk JB. Practice random acts of aggression and senseless acts of intimidation. The logic of status contexts in social groups. Evolutionary Anthropology. 2002;11:221–225. [Google Scholar]
- Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, et al. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. [Comparative Study Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Metabolism. Clinical and Experimental. 1995;44(5):645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- Tabarin A, Diz-Chaves Y, Consoli D, Monsaingeon M, Bale TL, Culler MD, et al. Role of the corticotropin-releasing factor receptor type 2 in the control of food intake in mice. A meal pattern analysis. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. European Journal of Neuroscience. 2007;26(8):2303–2314. doi: 10.1111/j.1460-9568.2007.05856.x. doi: 10.1111/j.1460-9568.2007.05856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Ostrander MM, Gardner SR, Ma LY, Woods SC, et al. Social stress and recovery. Implications for body weight and body composition. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2007;293(5):R1864–1874. doi: 10.1152/ajpregu.00371.2007. doi: 00371.2007 [pii] 10.1152/ajpregu.00371.2007. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Sakai RR. Social stress. From rodents to primates. Frontiers in Neuroendocrinology. 2005;26(1):27–40. doi: 10.1016/j.yfrne.2005.03.001. doi: S0091-3022(05)00003-8 [pii] 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Dallman MF, Epel ES. Comfort food is comforting to those most stressed. Evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.04.005. http://dx.doi.org/10.1016/j.psyneuen.2011.04.005. [DOI] [PMC free article] [PubMed]
- Varma M, Chai JK, Meguid MM, Gleason JR, Yang ZJ. Effect of operative stress on food intake and feeding pattern in female rats. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Nutrition. 1999;15(5):365–372. doi: 10.1016/s0899-9007(99)00033-7. [DOI] [PubMed] [Google Scholar]
- Warne JP. Shaping the stress response. Interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. [Review]. Molecular and Cellular Endocrinology. 2009;300(1–2):137–146. doi: 10.1016/j.mce.2008.09.036. http://dx.doi.org/10.1016/j.mce.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys. Social status effects on caloric consumption. Physiology & Behavior. 2008;94(4):586–594. doi: 10.1016/j.physbeh.2008.03.019. doi: S0031-9384(08)00094-2 [pii] 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Pazol K, Legendre A, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic–hypothalamic–pituitary–adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26(2) doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- Woods SC, D'Alessio DA. Central control of body weight and appetite. [Research Support, N.I.H., Extramural Review]. Journal of Clinical Endocrinology and Metabolism. 2008;93(11 Suppl. 1):S37–50. doi: 10.1210/jc.2008-1630. http://dx.doi.org/10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizza C, Siega-Riz AM, Popkin BM. Significant increase in young adults’ snacking between 1977–1978 and 1994–1996 represents a cause for concern! Preventive Medicine. 2001;32(4):303–310. doi: 10.1006/pmed.2000.0817. http://dx.doi.org/10.1006/pmed.2000.0817. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals. Structure of prandial food and water intake of rats. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2005;288(6):R1450–1467. doi: 10.1152/ajpregu.00175.2004. http://dx.doi.org/10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Valdez GR, Tabarin A, Koob GF. Leptin and post-prandial satiety. Acute central leptin more potently reduces meal frequency than meal size in the rat. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Psychopharmacology (Berl) 2005a;177(3):324–335. doi: 10.1007/s00213-004-1952-1. http://dx.doi.org/10.1007/s00213-004-1952-1. [DOI] [PubMed] [Google Scholar]