Abstract
The present study deals with the isolation and partial purification of bioactive compounds from the crude methanol extracts of the leaves of Ageratum houstonianum (Asteraceae). The quantification and the identification of compounds in the crude extract and active bands isolated by preparative TLC were accomplished using GC-MS analysis. The most important compounds identified in the crude extract and active bands (AB-1 and AB-2) were 6-acetyl-7-methoxy-2, 2-dimethylchromene, hexadecanoic acid and squalene, respectively. Crude extract and active bands (AB-1 and AB-2) were investigated for their antibacterial activity against Staphylococcus epidermidis, Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Escherichia coli, Enterobacter aerogenes, Klebsiella pneumoniae, and Pseudomonas aeruginosa. The crude extract, AB-1 and AB-2 showed maximum zone of inhibition (10-13 mm) against Staphylococcus epidermidis, however, the antibacterial potential of active bands was slightly higher as compared to the crude extract. Dose-dependant increase in antioxidant potential was noticed in crude extract as well as with both active bands measured by DPPH free radicals, ion chelation and total antioxidants capacity. Our study reports various bioactive compounds in the leaves of the A. houstonianum with significant antioxidant and antibacterial potential.
Keywords: Ageratum sp., antibacterial, antioxidants, bioactive compounds, GC-MS
Introduction
The increase in bacterial resistance against the present drugs used in traditional therapy caused the emergence of new antibacterial entities that can be explored from a high number of natural sources such as plant materials to fight microbial diseases (Eloff, 1998[16]; Chariandy et al., 1999[12]). The functional properties - such as antibacterial, antifungal and insecticidal - of essential oils and plant extracts are widely used in processed food preservation, pharmaceuticals, cosmetics, alternative medicine and natural therapies (Bakkali et al., 2008[2]). A large number of plants about 25,000 species belonging to the family Asteraceae are rich in secondary metabolites with biological activity (Okunade, 2002[26]). Various studies have been carried out on some of Asteraceae plants, e.g. Achillea sp., Eupatorium sp., Tridax sp., Vernonia sp. and Ageratum sp. The pharmacological properties, such as anti-inflammatory, antioxidant, antispasmodic and anti-hemorrhoidal of the aerial parts of different species of the genus Achillea have been reported. The extracts of leaves of Eupatorium sp. possess both antibacterial and antimalarial activity. Tridax sp. is commonly used in Indian traditional medicine as anticoagulant, hair tonic, insect repellent, anti-diarrhea, and anti-dysentery (Candan et al., 2003[11]; Thakong, 1999[35]; Saraf et al., 1991[31]; Abubakar et al., 2011[1]; Kasturi et al., 1973[19]). Traditionally, Ageratum sp. is used as antidysenteric, in skin and wound diseases, in the treatment of leprosy and purulent ophthalmia. They are also used in treatment of pneumonia by rubbing them on the chest of the patient (Shirwaikar et al., 2003[32]; Begum and Nath, 2000[4]).
Oxidative stress results due to the imbalance of formation and neutralization of prooxidants, which is one of the major cause of today's diseases such as cancer, atherosclerosis, cardiovascular diseases, ageing and inflammatory diseases (Braca et al., 2002[10]; Maxwell, 1995[21]). The defense mechanism that includes enzymes such as superoxide dismutase (SOD) and catalase, or compounds such as ascorbic acid, tocopherol and glutathione are sometimes fail to protect cell components from oxidative damage (Niki et al., 1994[24]). Therefore, the supplementation of antioxidants are vital to combat oxidative damage. In this direction much attention has been given in search of ethnomedicines with strong antioxidant properties (Bibhabasu et al., 2008[6]). The antioxidant potential of Vitex negundo has been reported by employing various established in vitro systems and the presence of many polyphenolic compounds, terpenoids, glycosidic iridoids and alkaloids (Prakash et al., 2007[28]).
A large number of constituents has been identified by GC-MS analysis of the essential oil of A. conyzoides and other species also but pharmacological activities and chemical characterization of the most significant metabolites from A. houstonianum responsible for the medicinal properties have not been done (Ekundayo et al., 1998[15]). In recent years the interest for the characterization of organic compounds from plants has been developed. The GC-MS is an ideal technique for qualitative and quantitative analysis of volatile and semivolatile compounds (Iordache et al., 2009[17]). Therefore, the authors have attempted to isolate the bioactive compounds, evaluate the bioactive potential and characterize them by GC-MS analysis.
Materials and Methods
Plant material
Plants of A. houstonianum, growing wild in Pharmacy Herbal Garden, Integral University, Lucknow, were collected in January, 2010. The authenticity of the plant was confirmed by Dr. Tariq Husain, Senior Scientist, National Botanical Research Institute (NBRI), Lucknow, India and voucher specimens are maintained at the NBRI herbarium.
Preparation of plant extracts
Dried leaf powder (1 gm) was homogenized in 10 ml methanol (HPLC grade, Merck, India) and was extracted on a rotatory shaker in an Erlenmeyer flask at 40 rpm overnight (Daayf et al., 1997[14]). The crude extracts were then filtered through Whatman No. 1 filter paper and concentrated in vacuum at 40 °C using a rotary evaporator. The concentrated extract was then dried aseptically with the help of drier.
Isolation of the bioactive molecule
The methanol extract thus obtained was redissolved in 1 ml methanol. For the TLC analysis, 25 μl of the extract was loaded on the TLC (Silica gel 60) plates (Merck, Germany). The solvent ratio used for the separation of the compounds was Chloroform: methanol (4:1, v:v). UV-transillumination of the plates at 365 nm revealed seven bands. These bands were eluted separately with a minimum amount of methanol. All seven bands were bioassayed for antibacterial potential against Staphylococcus epidermidis (NCIM 2493) (National Chemical Laboratory, Pune, India) by disc diffusion method on Mueller-Hinton (MH) agar (Bauer et al., 1966[3]). Further preperative TLC was used to pooled-up the amount of active bands but band AB-1 and AB-2 were selected for further studies. Active bands (AB-1 and AB-2) were scrapped off from the silica gel and eluted in methanol (HPLC grade, Merck, India) followed by centrifugation at 10,000 rpm. Clear supernatant was then dried aseptically and subjected to antibacterial and antioxidant assays. GCMS analysis was done to characterize these active bands.
GC-MS analysis
For the identification of metabolites showing antibacterial and antioxidant potentials, the samples were subjected to GC-MS analysis. The sample (1 μl) was injected into a RTX-5 column (60 m X 0.25 mm i.d., film thickness 0.25 µm) of GC-MS (model GC-MS-QP-2010 plus, Shimadzu Make). Helium was used as carrier gas at a constant column flow 1.2 ml/min at 173 kpa inlet pressure. Temperature programming was maintained from 10 0°C to 200 °C with constant rise of 5 °C/min and then held isothermal at 200 °C for 6 min; further the temperature was increased by 10 °C/min up to 290 °C and again held isothermal at 290 °C for 10 min. The injector and ion source temperatures were 270 °C and 250 °C, respectively. The crude and active bands (AB-1) and (AB-2) (2 mg/ml) dissolve in methanol (HPLC grade, Merck, India) were injected with a split ratio of 1:10. Mass spectra were taken at 70 eV; a scan interval of 0.5 s and fragments from 40 to 950 Dalton. The final confirmation of constituents was made by computer matching of the mass spectra of peaks with the Wiley and National Institute Standard and Technology (NIST) libraries mass spectral database.
Antibacterial assay
Staphylococcus epidermidis (NCIM 2493), Staphylococcus aureus (NCIM 2079), Bacillus subtilis (NCIM 2920), Bacillus cereus (NCIM 2156), Escherichia coli (NCIM 2065), Enterobacter aerogenes (NCIM 5139), Klebsiella pneumoniae (NCIM 2957), and Pseudomonas aeruginosa (NCIM 5029) were used as test bacteria in the present study. For antibacterial testing fresh inoculum was prepared for each bacterium and incubated at 37 ± 2 °C for 24 h. The inoculum was adjusted with nutrient broth to obtain turbidity comparable to that of MacFarland 0.5 standard (108 CFU/ml) according to NCCLS (NCCLS, 1997[23]). The cell suspension in each case was swabbed onto MH agar with the help of a sterile cotton swab and incubated at 37 ± 2 °C for 20 minutes. Thereafter, the Whatman No. 1 filter paper discs impregnated with 10 μL of each extract either crude or active bands (AB-1 and AB-2) prepared in 50 % DMSO (40 mg/ml), was placed on the plates (DMSO was used as a control) and incubated at 37 ± 2 °C for 18 h. All of the experiments were performed in triplicate and the results (millimeters of the inhibition zone) were expressed as mean values.
Determination of free radical scavenging activity
The free radical scavenging activity of methanol crude extract and active bands (AB-1 and AB-2) was measured by using DPPH radical (Williams et al., 1995[37]; Miliauskas et al., 2004[22]). DPPH radicals have strong absorption maximum at 515 nm which decreases due to the reduction by antioxidants. The DPPH solution in methanol (6 × 10-5 M) was prepared freshly, and 3 mL of this solution was mixed with 100 μL of various concentration (0-0.5 mg/ml) of crude extract or TLC-eluted compounds. The samples were incubated for 20 min at 37 °C in a water bath, and then the decrease in absorbance at 515 nm was measured (Ae). A blank sample containing 100 μL of methanol in the DPPH solution was prepared and its absorbance was measured (Ab). All tests and analyses were run in triplicates and the results obtained were averaged.
Radical scavenging activity was calculated using the following formula:
% inhibition = [(Ab-Ae)/Ab] × 100
where Ab = absorbance of the blank sample, and Ae = absorbance of the methanol extract.
Total antioxidant activity
Spectrophotometric method was used for the determination of total antioxidant activity (Shirwaikar et al., 2003[33]). Various concentrations (0-8 µg/ml) of the crude extracts and active bands (AB-1 and AB-2) were prepared separately in methanol. The reaction mixture contained 1 ml of the extract and 2 ml of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The mixture was incubated on water bath at 95 °C for 90 min and after cooling at room temperature, the absorbance of reaction mixture was measured at 695 nm against blank. Ascorbic acid was used as the standard and total antioxidant capacity was expressed in terms of ascorbic acid equivalents. All tests were run in triplicates and averaged.
Ion chelating activity
Ion chelating activity was evaluated by the standard colorimetric method (Benzie and Szeto, 1999[5]). The reaction mixture contained 0.5 ml phenanthroline (0.05 %) in methanol, 1 ml ferric chloride (200 μM) and 1 ml different concentrations (0-8 µg/ml) of the test compound. The reaction mixture was further incubated at room temperature for 10 min and the absorbance of the sample was read at 510 nm. Ascorbic acid was used as the standard and ion chelating activity was expressed as ascorbic acid equivalents. All tests and analyses were run in triplicates and the results obtained were averaged.
Statistical analysis
Statistical analysis was carried out using the SPSS software (SPSS Inc., version 7.0).
Results
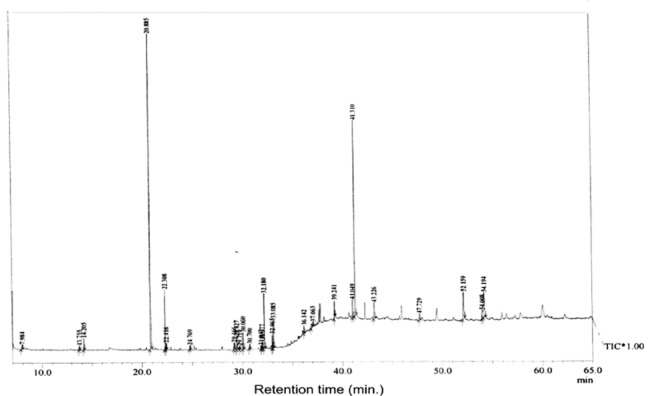
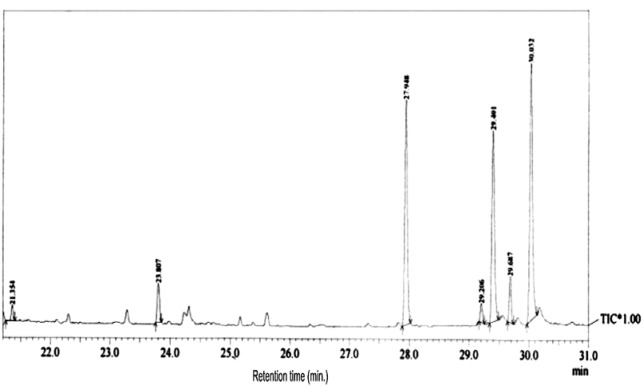
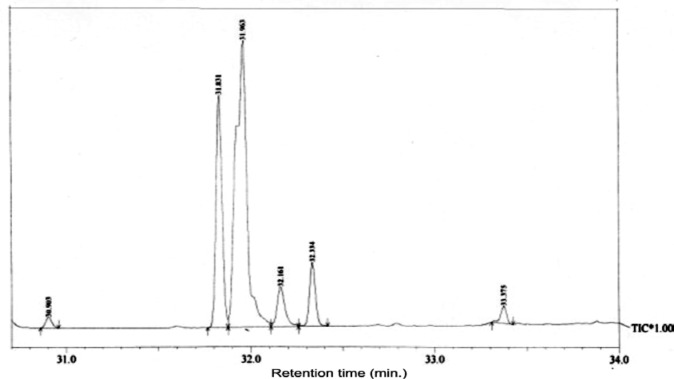
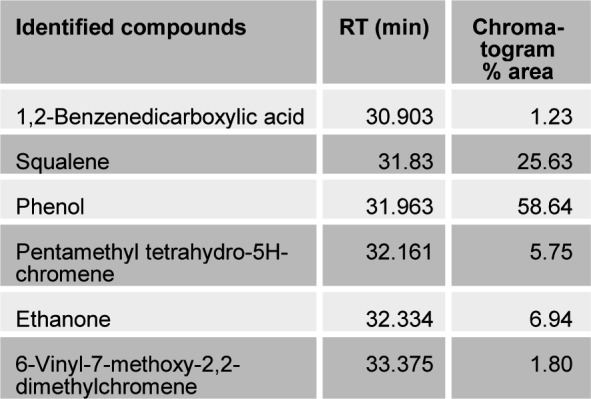
The present study tried to evaluate the bioactive potential of partially purified compounds isolated by preparative TLC from the leaves of Ageratum sp. and their characterization by GC-MS analysis. The methanol crude extract of leaves of Ageratum houstonianum showed antibacterial activity against Staphylococcus epidermidis, Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, and Enterobacter aerogenes whereas, no activity was noticed against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa (Table 1(Tab. 1)). The disc (400 μg) of crude extract exhibited maximum zone of inhibition (10 mm) against S. epidermidis followed by B. cereus (9 mm), E. aerogenes (9 mm), B. subtilis (8 mm) and S. aureus (8 mm). Based on these results methanolic crude extract of leaves was used to detect the bioactive compounds through TLC. The TLC results indicate the presence of seven bands under ultra violet light (365 nm) illumination (Figure 1(Fig. 1)). Out of these, two active bands (AB-1) and (AB-2) with Rf value - 0.20 and 0.48, respectively were selected to evaluate the bioactive potential based on their good antibacterial activity against S. epidermidis (data not shown). Further, through preparative TLC the compounds of both active bands were pooled separately and subjected to antibacterial, antioxidant and G-MS analysis. The yields of compounds were 25 µg/mg and 62.5 µg/mg of crude extract for AB-1 and AB-2, respectively. The GC-MS analysis of crude and active band as shown in Figures 2-4(Fig. 2)(Fig. 3)(Fig. 4) indicates the presence of different components. The major components present in the methanolic crude extract of leaves of A. houstonianum were diazoprogesterone (RT: 20.88), 6-acetyl-7-methoxy-2,2-dimethylchromene (RT: 22.30), 9,12-octadecadienoate (RT: 32.18), stigmast-5-en-3-ol (RT: 52.15) and 3,7-dimethyl-2,6-octadienyl acetate (RT:54.19) (Table 2(Tab. 2)). Seven components were identified in active band (AB-1), out of which hexadecanoic acid (RT: 27.94) and 3'-Acetyllycopsamine (RT: 29.40) were the major compounds (Table 3(Tab. 3)). Six components were detected in the active band (AB-2), however, squalene (RT: 31.83) and phenol (RT: 31.96) were the major components (Table 4(Tab. 4)).
Table 1. Antibacterial activity of crude extracts and active bands (AB-1 and AB-2) isolated by preparatory TLC.
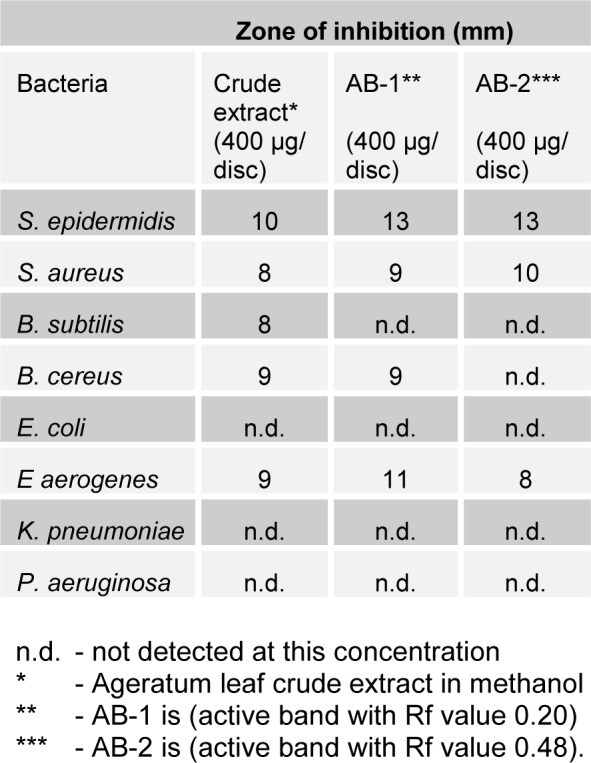
Figure 1. TLC separation of methanolic crude extract of leaves of A. houstonianum using chloroform:methanol (4:1) as mobile phase, visualized under UV light 365 nm.
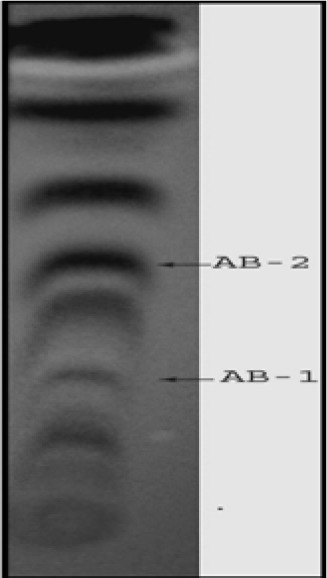
Figure 2. Figure 2: GC-MS chromatogram of methanolic crude extract of A. houstonianum leaves.
Figure 3. GC-MS chromatogram of Active Band (AB-1) isolated from crude A. houstonianum leaves.
Figure 4. GC-MS chromatogram of Active Band (AB-2) isolated from crude A. houstonianum leaves.
Table 2. Bioactive compounds identified in the methanolic crude extract of the leaves of A. houstonianum by GC-MS.
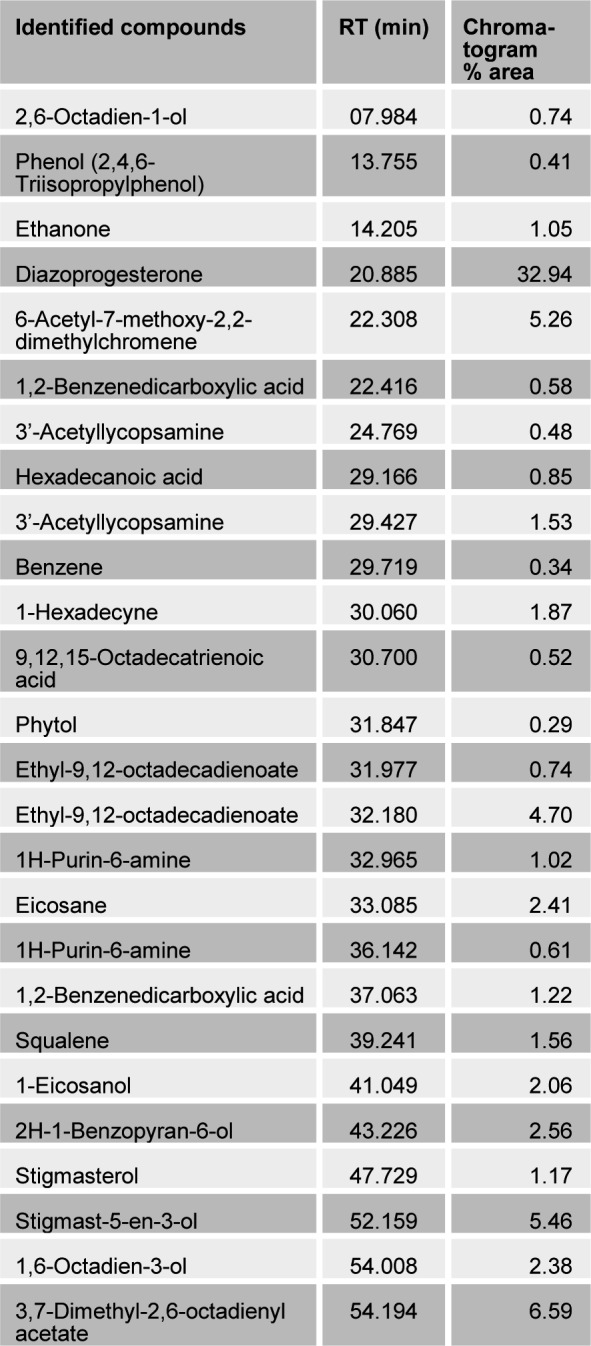
Table 3. Bioactive compounds identified in the active band (AB-1) isolated from methanolic crude extract of the leaves of A. houstonianum by GC-MS.
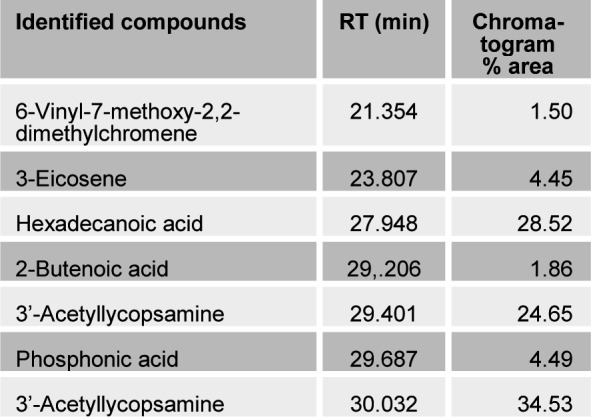
Table 4. Bioactive compounds identified in the active band (AB-2) isolated from methanolic crude extract of the leaves of A. houstonianum by GC-MS.
The results from Table 1(Tab. 1) shows the antibacterial potential of active bands AB-1 and AB-2 when tested against test bacteria. The disc prepared from active band (AB-1) showed maximum inhibition zone (13 mm) against S. epidermidis followed by E. aerogenes (11 mm), S. aureus (9 mm) and B. cereus (9 mm) while active band AB-2 showed inhibitory zone of 13, 10 and 8 mmm against S. epidermidis, S. aureus and E. aerogenes, respectively.
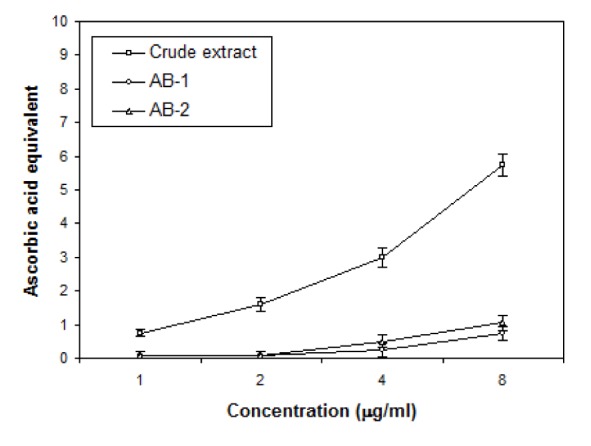
For evaluation of antioxidant properties of crude extract and TLC eluted compounds of Ageratum sp. three assays have been used: DPPH radical scavenging, Total antioxidant and Ion chelation assays and the results are shown in Figures 5(Fig. 5), 6(Fig. 6) and 7(Fig. 7). DPPH was reduced with the addition of crude and active bands in a concentration dependent manner (Figure 5(Fig. 5)). Crude extract was the most effective radical scavenger with the inhibition of 55.8 % at 0.5 mg/ml while the scavenging activity of active bands AB-1 and AB-2 was lower as 42.3 % and 39.05 % inhibition was noticed at 0.5 mg/ml, respectively. The results of total antioxidant capacities of crude and partially purifed compounds based on the formation of phosphomolybdenum complex are shown in Figure 6(Fig. 6). In this assay, crude extract possessed more antioxidant potential with 9.4 µg equivalent of ascorbic acid at 8 µg/ml concentration than the partially purified compounds which showed only 1 and 2.5 µg equivalent of ascorbic acid at 8 µg/ml concentration. The ion chelation activity was studied by interaction of crude or partially purifed compounds with ferrous-o-phenanthroline complex (Figure 7(Fig. 7)). Similar to this the crude extract has more ion chelation capacity than the active bands.
Figure 5. Effects of crude extract and active bands (AB-1 and AB-2) isolated through TLC from leaves of A. houstonianum on the scavenging of DPPH. Values are mean ± SE of three replicates.
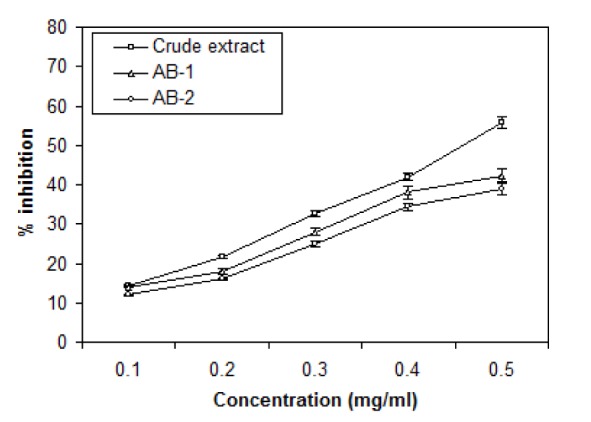
Figure 6. Total antioxidant activity of crude extract and active bands (AB-1 and AB-2) isolated through TLC from leaves of A. houstonianum *expressed in terms of ascorbic acid equivalent (µg/ml). Values are mean ± SE of three replicates.
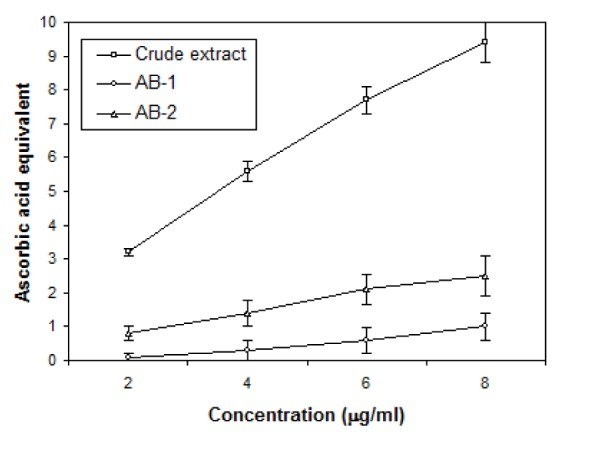
Figure 7. Ion chelation activity of crude extract and (AB-1 and AB-2) bands isolated through TLC from leaves of A. houstonianum *expressed in terms of ascorbic acid equivalent (µg/ml). Values are mean ± SE of three replicates.
Discussion
The present study was aimed to explore the potential bioactive substances from plant origin. Methanol is a polar solvent that has ability to extracts most of the bioactive compounds such as flavonoids, tannins and others (Niño et al., 2006[25]). Recently, strong antibacterial and antifungal activity of methanolic extracts of bark of Saraca Indica has been reported (Sainath et al., 2009[30]).
The antibacterial results showed that both crude extract and active bands (AB-1 and AB-2) were relatively more effective against the Gram-positive bacteria as compared to the Gram-negative bacteria. These results are in agreement with the earlier studies done with other plant species of Asteraceae family (Boukamcha et al., 2004[8]; Oueslati et al., 2004[27]). Similar pattern of antibacterial sensitivity was also reported with the extract of plant Rhaponticum acaule DC (Boussaada et al., 2008[9]). The higher resistance of the Gram-negative bacteria could be due to the complexity of the cell wall of this group of microorganisms. Indeed, the external membrane of Gram-negative bacteria renders highly hydrophilic surfaces whereas the negative charge of the surface of the Gram-positive cell wall may reduce their resistance to antibacterial compounds (Smith-Palmer et al., 1998[34]; Ultee et al., 1999[36]). This relative difference in the potential may be due to presence of several hydrophobic compounds in the crude extract, AB-1 and AB-2 of A. houstonianum leaves.
Further, concentration dependent effect of methanol crude extract was found to have more antioxidant activity than active bands (AB-1 and AB-2). The antioxidant potential of the crude extract and active bands (AB-1 and AB-2) may be attributed to differences in their chemical composition such as phenolic compounds, polyphenyl compounds like squalene, hexadecanoic acid and others (Kelly, 1999[20]; Cowan, 1999[13]).
Our GC-MS analysis of the crude extract and AB-1 and AB-2 showed presence of certain metabolites (Tables 2-4(Tab. 2)(Tab. 3)(Tab. 4)). The antibacterial activity of the crude extract might be due to the presence of 6-acetyl-7-methoxy-2,2-dimethylchromene. The present work also corroborates the earlier work in which the antimicrobial activities of 2,2-dimethylchromene derivatives, such as 6-(1-hydroxyethyl)-7,8-dimethoxy-2,2-dimethylchromene and 6-hydroxy-7,8-dimethoxy-2, 2-dimethyl chromene have been reported (Bodoprost and Rosemeyer, 2007[7]).
The bioactive potential of active band (AB-1) as observed in the present study could be due to the presence of hexadecanoic acid as the antioxidant and antimicrobial activities of this compound has been reported earlier (Ragasa et al., 1998[29]). While the antibacterial and antioxidant potentials of the active band (AB-2) could be attributed to the presence of squaline and phenols. Squalene, an isoprenoid from the group of polyphenyl compounds, is an intermediate metabolite in cholesterol synthesis. It has antioxidant, immunostimulating, hypolipidemic, cholesterol reducing, anticarcinogenic and antiinflammatory activity (Kelly, 1999[20]). Squalene also possesses antimicrobial activity, in particular, in relation to tuberculosis mycobacteria (Jimenez et al., 2005[18]). Phenolic compounds with less complex structures, such as catechol and coumarin, have also shown to exhibit bactericidal and fungicidal activities (Cowan, 1999[13]).
The presence of wide range of phytochemical constituents as observed in the partially purified bands indicates that further studies are required to pinpoint the role of various minor and major compounds in the antimicrobial and other biological activities. Thus, the present study reflects a hope for the development of novel agents of biomedical importance.
Acknowledgements
The authors are thankful to the Vice Chancellor, Integral University, Lucknow, India for providing necessary facilities for the research work. We also thank to Mr. Ajai Kumar, Advanced Instrumentation Facility, University Science Instrumentation Centre, JNU, New Delhi, for the GC-MS analysis of the sample.
References
- 1.Abubakar BA, Aliyu MM, Mikhail SA, Hamisu I, Adebayo OO. Phytochemical screening and antibacterial activities of Vernonia ambigua, Vernonia blumeoides and Vernonia oocephala (Asteraceae) Acta Poloniae Pharmaceutica Drug Res. 2011;68:67–73. [PubMed] [Google Scholar]
- 2.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils – a review. Food Chem Tox. 2008;46:446–75. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 3.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibilitiy testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 4.Begum D, Nath SC. Ethnobotanical review of medicinal plants used for skin diseases and related problems in Northeastern India. J Herbs Spices Med Plants. 2000;7:55. [Google Scholar]
- 5.Benzie IF, Szeto YT. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J Agric Food Chem. 1999;47:633–636. doi: 10.1021/jf9807768. [DOI] [PubMed] [Google Scholar]
- 6.Bibhabasu H, Santanu B, Nripendranath M. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complementary and Alternative Medicine. 2008;8:63. doi: 10.1186/1472-6882-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodoprost J, Rosemeyer H. Analysis of phenacylester derivatives of fatty acids from human skin surface sebum by reversed-phase HPTLC: chromatographic mobility as a function of physicochemical properties. Int J Mol Sci. 2007;8:1111–1124. [Google Scholar]
- 8.Boukamcha H, Ben Jannet H, Chriaa J, Mighri Z. Etude biologique et chimique de la plante Cardopatium amethystinum poussant en Tunisie. Structures de deux derivés benzaldehydiques. Identification de constituants de bands apolaires par CPG/S.M. J Soc Algérienne Chim. 2004;14:65–78. [Google Scholar]
- 9.Boussaada O, Ammar S, Saidana D, Chriaa J, Chraif I, Daami MZ, et al. Chemical composition and antimicrobial activity of volatile components from capitula and aerial parts of Rhaponticum acaule DC growing wild in Tunisia. Microbiol Res. 2008;163:87–95. doi: 10.1016/j.micres.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol. 2002;79:379–381. doi: 10.1016/s0378-8741(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 11.Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sokmen A, et al. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae) J Ethnopharmacol. 2003;87:215–20. doi: 10.1016/s0378-8741(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 12.Chariandy CM, Seaforth CE, Phelps RH, Pollard GV, Khambay BPS. Screening of medicinal plants from Trinidad and Tobago for antimicrobial and insecticidal properties. J Ethnopharmacol. 1999;3:265–270. doi: 10.1016/s0378-8741(98)00130-5. [DOI] [PubMed] [Google Scholar]
- 13.Cowan M. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daayf P, Schmidt A, Belanger PR. Evidence of phytoalexin in cucumber leaves infected with powdery mildew following treatment with leaf extracts of Reynoutria Sacchalinensis. Plant Pathol. 1997;113:719. doi: 10.1104/pp.113.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekundayo O, Laakso I, Hiltunen R. Essential oil of Ageratum conyzoides. Planta Med. 1998;54:55–57. doi: 10.1055/s-2006-962336. [DOI] [PubMed] [Google Scholar]
- 16.Eloff JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants. J Ethnopharmacol. 1998;60:1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 17.Iordache A, Culea M, Gherman C, Cozar O. Characterization of some plant extracts by GC–MS. Rom Nuc Inst Meth Phy Res. 2009;267:338–42. [Google Scholar]
- 18.Jimenez A, Meckes M, Alvarez V. Secondary metabolites from Chamaedora tepejilote (Palmae) are active against Mycobacterium tuberculosis. Phytother Res. 2005;19:320–322. doi: 10.1002/ptr.1664. [DOI] [PubMed] [Google Scholar]
- 19.Kasturi TR, Thomas M, Abraham EM. Essential oil of Ageratum conyzoides. Isolation and structure of 2 new constituents. Indian J Chem. 1973;11:91–95. [Google Scholar]
- 20.Kelly GS. Squalene and its potential clinical uses. Altern Med Rev. 1999;4:29–36. [PubMed] [Google Scholar]
- 21.Maxwell SR. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–361. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- 22.Miliauskas G, Venskutonis PR, Van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–7. [Google Scholar]
- 23.NCCLS, National Committee For Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. Wayne, PA, USA: NCCLS; 1997. [Google Scholar]
- 24.Niki E, Shimaski H, Mino M. Antioxidantism-free radical and biological defense. Gakkai Syuppn Center Tokyo. 1994:3–16. [Google Scholar]
- 25.Niño J, Narváez DM, Mosquera OM, Correa YM. Antibacterial, antifungal and cytotoxic activities of eight Asteraceae and two Rubiaceae plants from Colombian biodiversity. Braz J Microbiol. 2006;37:566–570. [Google Scholar]
- 26.Okunade AL. Review Ageratum conyzoides L. (Asteraceae) Fitoterapia. 2002;73:1–16. doi: 10.1016/s0367-326x(01)00364-1. [DOI] [PubMed] [Google Scholar]
- 27.Oueslati MH, Ben Jannet H, Abreu PJM, Mighri Z. Alcohols tetrahydropyraniques antintibatériens de la plante Rhantherium suaveolens poussant dans le sud tunisien. J Soc Algérienne Chim. 2004;14:245–58. [Google Scholar]
- 28.Prakash T, Yamini B, Tripathi Antioxidant properties of different bands of Vitex negundo Linn. Food Chem. 2007;100:1170–1176. [Google Scholar]
- 29.Ragasa, CY, Tepora M, Rideout JA. Antimicrobial chromenes from Eupatorium toppingianum. ACGC Chem Res Com. 1998;7:54–61. [Google Scholar]
- 30.Sainath RS, Prathiba J, Malathi R. Antimicrobial properties of the stem bark of Saraca indica (Caesalpiniaceae) Eur Rev Med Pharmacol Sci. 2009;13:371–374. [PubMed] [Google Scholar]
- 31.Saraf S, Pathak AK, Dixit VK. Hair growth promoting activity of Tridax procumbens. Fitoterapia. 1991;62:495–498. [Google Scholar]
- 32.Shirwaikar A, Bhilegaonkar PM, Malini S, Kumar JS. The gastroprotective activity of the ethanol extract of Ageratum conyzoides. J Ethnopharmacol. 2003;86:117–121. doi: 10.1016/s0378-8741(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 33.Shirwaikar A, Govindrajan R, Rastogi S, Vijaykumar M, Rawat AKS, Ehlotra SM, et al. Studies on the antioxidant activities of Desmodium gangeticum. Biol Pharm Bull. 2003;26:1424–1427. doi: 10.1248/bpb.26.1424. [DOI] [PubMed] [Google Scholar]
- 34.Smith-Palmer A, Stewart J, Fyfe L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett App Microbiol. 1998;26:118–122. doi: 10.1046/j.1472-765x.1998.00303.x. [DOI] [PubMed] [Google Scholar]
- 35.Thakong KA. Study on the antimalarial constituents and chemical composition of Eupatorium odoratum (L.). Thesis M.Sc. (Pharmaceutical chemistry and Phytochemistry) Faculty of Graduate studies, Mahidol University; 1999. [Google Scholar]
- 36.Ultee A, Kets EPW, Smid EJ. Mechanisms of actions of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 1999;65:4606–10. doi: 10.1128/aem.65.10.4606-4610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams BW, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Tech. 1995;28:25–30. [Google Scholar]