Abstract
This paper deals with the antioxidant and antimicrobial activity, total phenolic content and concentrations of flavonoids of Equisetum telmateia extracts. Total phenolic content was determined with Folin-Ciocalteu reagent and it ranged between 129.0 to 262.7 mg GA/g. The concentration of flavonoids in various extracts of E. telmateia was determined using spectrophotometric method with aluminum chloride and obtained results varied from 112.6 to 199.8 mg RU/g. Antioxidant activity was monitored spectrophotometrically and expressed in terms of IC50 (µg/ml), and its values ranged from 33.4 to 982.2 µg/ml. The highest phenolic content, concentrations of flavonoids and capacity to neutralize DPPH radicals were found in the acetone extract. In vitro antimicrobial activity was determined using microdilution method. Minimum inhibitory concentration (MIC) and minimum microbicidal concentration (MMC) were also determined. Testing was performed on 22 microorganisms, including 15 strains of bacteria (standard and clinical strains) and 7 species of fungi. There were statistically significant differences in activity between the extracts of E. telmateia. Different effects were noticed against the bacteria and the methanol extract appeared to be most efficient. All the extracts showed significant antibacterial activity against G+ bacteria and weak to moderate activity against other microorganisms.
Keywords: great horsetail, Equisetum telmateia, antimicrobial activity, antioxidative, phenols, flavonoids
Introduction
Great horsetail, (Equisetum telmateia Ehrh., fam. Equisetaceae) is a herbaceous perennial plant that throughout its life cycle exists as a pale yellowish non-photosynthetic spore-bearing fertile stem, produced in early spring. Green photosynthetic sterile stems are produced in late spring and die down in late autumn. They are heavily branched, with whorls of 14-40 branches up to 20 cm long, 1-2 mm diameter and unbranched, emerging from the axils of a ring of bracts. It inhabits damp and wet areas generally near streams, rivers and wetlands of Europe, western Asia, northwest Africa and north America (Equisetum, 1970[5]).
Medicinal plants belonging to the genus Equisetum are very often used in traditional medicine - for tea and other therapeutic products. They are highly efficient in treating urinary tract infection, cardiovascular diseases, respiratory tract infection and medical skin conditions. Among the most common species of this genus is Field Horsetail, E. arvense (Willfort, 1997[26]). In recent years, many researches have proven that inorganic acids, salts, phenolic acids, flavonoids, alkaloids and volatile components are major biologically active compounds with diuretic, antiseptic, anodyne, cardiac, carminative, galactagogue, vulnerary, diaphoretic, neuroprotective, antiucerogenic, anticancer, antimicrobial and antioxidative properties (Correia et al., 2005[3]; Pourmorad et al., 2006[13]; Mimica-Dukic et al., 2008[11]; Gürbüz and Yeşilada, 2008[6]; Rassouli et al., 2009[15]; Štajner et al., 2009[19]).
Phenolic compounds, especially flavonoids from plants, are a very important group of natural compounds with antioxidant and antimicrobial activity with application in medicine and pharmacy (Cushnie and Lamb, 2005[4]). In the food industry they are very important as natural preservatives without the adverse effects found in synthetic substances. Antimicrobial preservatives inhibit the growth of microbes such as bacteria and fungi, whereas antioxidant preservatives inhibit the oxidation of fats, lipids and other food ingredients (Abd El-aal and Halaweish, 2010[1]; Merkl et al., 2010[9]; Singh et al., 2010[17]).
Relevant literature review has shown very little data about the biological activity of great horsetail. Therefore, the purpose of this study was to determine total soluble phenolic content and concentrations of flavonoids using spectrophotometric methods and to evaluate E. telmateia as a new potential source of natural antioxidant and antimicrobial activity applying in vitro methods.
Materials and Methods
Plant material
In August 2009 aerial sterile stems of E. telmateia were collected from natural populations in the region of Kragujevac city in Central Serbia: (position: 43°59′N 20°53′E, altitude: 210.00 m, exposition: E, habitat: stream banks). Voucher specimens of E.telmateia Ehrh. were confirmed and deposited at the Herbarium of the Deparment of Biology and Ecology, Faculty of Science, University of Kragujevac. Collected plant material was air-dried in darkness at ambient temperature (20 oC). Dried plant material was cut up and stored in tightly sealed dark containers until needed.
Chemicals
Organic solvents and sodium hydrogen carbonate were purchased from „Zorka pharma“ Šabac, Serbia. Gallic acid, rutin hydrate, chlorogenic acid and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were obtained from Sigma Chemicals Co., St Louis, MO, USA. Folin-Ciocalteu phenol reagent and aluminium chloride hexahydrate (AlCl3) were purchased from Fluka Chemie AG, Buchs, Switzerland. Mueller-Hinton broth was purchased from Liofilchem, Italy, while Sabouraud dextrose broth was obtained from Torlak, Belgrade. Doxycycline antibiotic was purchased from Galenika A.D., Belgrade, and fluconazole antifungal, was from Pfizer Inc., USA. All other solvents and chemicals were of analytical grade.
Preparation of plant extracts
Plant material (10 g) was transferred to dark-coloured flasks with 200 ml of each solvent (methanol, ethyl-acetate, acetone) and stored at room temperature. After 24 h the contents were filtered through Whatman No. 1 filter paper and the residue was re-extracted with equal volume of solvents. After 48 h the process was repeated. The combined supernatants were evaporated to dryness under vacuum at 40 oC in the rotary evaporator. The obtained extracts were kept in sterile sample tubes and stored in refrigerator at 4 °C.
Determination of total phenolic content of the plant extracts
The total phenolic content was determined using a spectrophotometric method (Singleton et al., 1999[18]). The reaction mixture was prepared by mixing 0.5 ml of methanolic solution (1 mg/ml) of extract, 2.5 ml of 10 % Folin-Ciocalteu reagent dissolved in water and 2.5 ml 7.5 % NaHCO3. The samples were incubated at 45 °C for 15 min. The absorbance was determined at λmax = 765 nm. The samples were prepared in triplicate and the mean value of absorbance was obtained. Blank was concomitantly prepared with methanol instead of extract solution. The same procedure was repeated for the gallic acid and the calibration line was construed. The total phenolic content was expressed in terms of gallic acid equivalent (mg of GA/g of extract).
Determination of flavonoid concentrations of the plant extracts
The concentration of flavonoids was determined using spectrophotometric method (Quettier-Deleu et al., 2000[14]). The sample contained 1 ml of methanolic solution of the extract in the concentration of 1 mg/ml and 1 ml of 2 % AlCl3 solution dissolved in methanol. The samples were incubated at room temperature for an hour. The absorbance was determined at λmax = 415 nm. The samples were prepared in triplicate and the mean value of absorbance was obtained. The same procedure was repeated for the rutin and the calibration line was construed. The concentration of flavonoids in extracts was expressed in terms of rutin equivalent (mg of RU/g of extract).
Evaluation of DPPH scavenging activity
The ability of the plant extract to scavenge DPPH free radicals was assessed using the method described by Tekao et al. (1994[22]), adopted with suitable modifications from Kumarasamy et al. (2007[8]). The stock solution of the plant extract was prepared in methanol to achieve the concentration of 1 mg/ml. Dilutions were made to obtain concentrations of 500, 250, 125, 62.5, 31.25, 15.62, 7.81, 3.90, 1.99, 0.97 µg/ml. Diluted solutions (1 ml each) were mixed with 1 ml of DPPH methanolic solution (80 µg/ml). After 30 min in darkness at room temperature (23 °C) the absorbance was recorded at 517 nm. The control samples contained all the reagents except the extract. The percentage inhibition was calculated using the equation: % inhibition = 100 x (A control - A sample)/A control), whilst IC50 values were estimated from the % inhibition versus concentration sigmoidal curve, using a non-linear regression analysis. The data were presented as mean values ± standard deviation (n = 3).
Microorganism test
The antimicrobial activities of acetone, ethyl acetate and methanol extracts were tested against 22 microorganisms including 15 strains of bacteria (standard strains: Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, Pseudomonas aeruginosa ATCC 27853, Bacillus subtilis ATCC 6633, Bacillus pumilus NCTC 8241 and clinical strains: Escherichia coli, Staphylococcus aureus, Enterococcus faecalis, Pseudomonas aeruginosa, Proteus mirabilis, Sarcina lutea, Salmonella enterica, Bacillus subtilis and Bacillus cereus); seven species of fungi: Penicillium italicum PMFKG-F29, Trichothecium roseum PMFKG-F32, Botrytis cinerea PMFKG-F33; Aspergillus niger ATCC 16404; Candida albicans (clinical isolate); Rhodotorula sp. PMFKG-F27 and Saccharomyces boulardii PMFKG-P34. All clinical isolates were a generous gift from the Institute of Public Health, Kragujevac. The other microorganisms were provided from the collection of the Microbiology Laboratory, Faculty of Science, University of Kragujevac.
Suspension preparation
Bacterial and yeast suspensions were prepared according to the direct colony method. The turbidity of the initial suspension was adjusted to 0.5 McFarland standard (Andrews, 2005[2]). The initial bacterial suspension contains about 108 colony forming units (CFU)/ml and yeast suspension contains 106 CFU/ml. 1:100 dilutions of initial suspension were additionally prepared into sterile 0.85 % saline. The suspensions of fungal spores were prepared by a gentle stripping of the spore from the slopes with growing aspergilli. The resulting suspensions were 1:1000 diluted in sterile 0.85 % saline.
Microdilution method
The antimicrobial activity was tested by determining the minimum inhibitory concentration (MIC) and minimum microbicidal concentration (MMC) using a microdilution method with resazurin (Sarker et al., 2007[16]). The 96-well plates were prepared by dispensing 100 μl of nutrient broth, Mueller-Hinton broth for bacteria and Sabouraud dextrose broth for fungi, into each well. A 100 μl from the stock solution of tested extracts (concentration of 80 mg/ml) was added into the first row of the plate. Then, twofold, serial dilutions were performed by using a multichannel pipette. The obtained concentration range was from 40 to 0.0781 mg/ml. The method is described in detail in the previous paper (Vasić et al., 2010[24]).
Doxycycline and fluconazole were used as a positive control. A solvent control test was performed to study an effect of 10 % DMSO on the growth of a microorganism. It was observed that 10 % DMSO did not inhibit the growth of a microorganism. Also, in the experiment, the concentration of DMSO was additionally decreased because of the twofold serial dilution assay (the working concentration was 5 % and lower). Each test included growth control and sterility control. All tests were performed in duplicate and MICs were constant.
Statistical analysis
The data were presented as the means ± standard deviations where appropriate. All statistical analyses were performed using SPSS package. Mean differences were established by Student's t-test. Data were analyzed using one-way analysis of variance (ANOVA). In all cases P values <0.05 were considered statistically significant.
Results and Discussion
Total phenolic content, total flavonoid content and antioxidant activity
Three extracts were prepared from whole sterile stems of E. telmateia using different solvents (methanol, acetone, ethyl acetate) in order to examine the total phenolic content, flavonoid concentrations, free radical scavenging activity and in vitro antimicrobial activity. Various solvents were used for the extraction of active substances of diverse polarities. Previous studies have demonstrated the effectiveness of such solvents (Stanković et al., 2010[20]).
Total phenolics were determined by the Folin-Ciocalteu method and are shown in Table 1(Tab. 1).
Table 1. Total phenolic content1 and flavonoid concentrations of E. telmateia extracts.
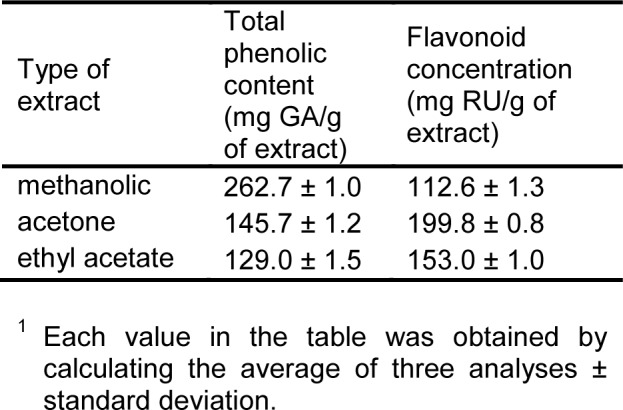
The total phenolic content was expressed as gallic acid equivalents and ranged from 129.0 to 262.7 mg GA/g. The total phenolic content was high in all the extracts from E. telmateia, among which the methanolic extract (262.7 mg GA/g) contained the highest concentration of phenolic compounds. Analyzing the results of total phenolic content in all the extracts, it was noticed that the highest concentration of phenolic compounds in the extracts were obtained using the solvents of high polarity. Other authors reported that, high solubility of phenols in polar solvents provides high concentration of these compounds in the extracts obtained using polar solvents for the extraction (Zhou and Yu, 2004[27]). The results indicate that methanol is the best solvent for extracting phenolic compounds from E. telmateia. Thus, high polar solvents should be used for this purpose.
The concentration of flavonoids in various extracts of E. telmateia was determined using spectrophotometric method with AlCl3. The content of flavonoids was expressed as rutin equivalent. The amount of flavonoids identified in the tested extracts is shown in Table 1(Tab. 1). The concentration of flavonoids in these plant extracts ranged from 112.6 to 199.8 mg RU/g. High concentrations of flavonoids were measured in acetone extracts. The concentration of flavonoids in plant extracts depends on the polarity of solvents used in the extract preparation (Min and Chun-Zhao, 2005[12]). Based on the obtained values of the concentration of flavonoids in the examined extracts of E. telmateia, it was found that the highest concentration of these compounds was in the extracts obtained using solvents of moderate polarity.
The antioxidant activity of the plant extracts of E. telmateia was determined using methanol solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH) reagent. DPPH method has also been used to quantify antioxidants in complex biological systems in recent years and it is based on the reduction of methanolic solution of the colored free radical DPPH by a free radical scavenger. Scavenging activity was measured as the decrease in absorbance by the extract samples versus a DPPH standard solution.
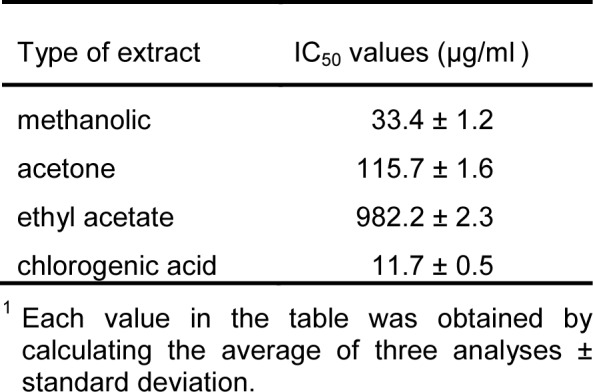
The antioxidant activity of three different extracts from E. telmateia is expressed in terms of IC50 (µg/ml) values. Also, the values were obtained for chlorogenic acid as the reference substance and compared with the values of the antioxidant activity of E. telmateia. The summary of obtained IC50 values of antioxidant activity of the extracts is given in Table 2(Tab. 2). The obtained values for antioxidant activity examined by DPPH radical scavenging activity ranged from 33.4 to 982.2 µg/ml. The largest capacity in neutralization of DPPH radicals was measured in the methanol extract from E. telmateia, which neutralized 50 % of free radicals in a very small concentration (33.4 µg/ml). In measuring total phenolic content, methanolic extract showed the highest values. Based on these results, each extract of E. telmateia showed a phenol concentration-dependent scavenging effect. Numerous investigations of the antioxidant activity of plant extracts have confirmed a high linear correlation between the values of total phenolic content and antioxidant activity (Katalinić et al., 2006[7]).
Table 2. Antioxidant (DPPH scavenging) activity1 of investigated plant extracts and standard substance presented as IC50 values (µg/ml).
Flavan-3-ol, kaempferol and phenolic acid derivates were also identified in previous investigations on E. telmateia. Several studies demonstrated strong antioxidant activity of these phenolic compounds (Correia et al., 2005[3]; Teffo et al., 2010[21]).
In addition, the phenolic contents of the extracts depend on the extraction solvent, and not only the phenolic content but also properties of these compounds contribute to the activites of different extracts. In order to extract active components, the most effective method involves the use of high-polar solvents.
Antimicrobial activity
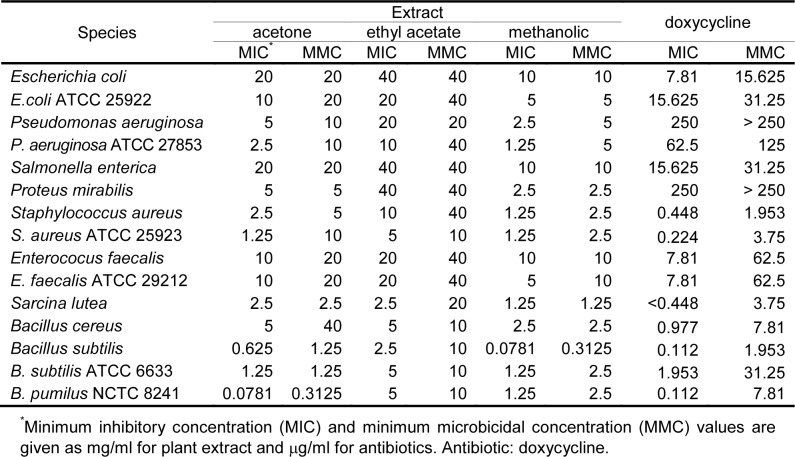
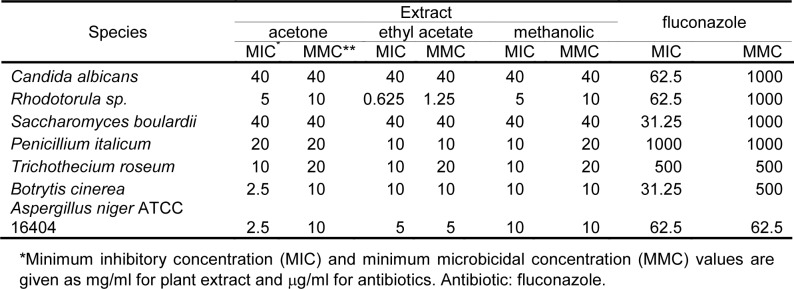
The in vitro results of antibacterial and antifungal activities of acetone, ethyl acetate and methanol extracts of E. telmateia are shown in Tables 3(Tab. 3) and 4(Tab. 4). For comparison, MIC and MMC values for doxycycline and fluconazole are also given in Tables 3(Tab. 3) and 4(Tab. 4). The solvent (10 % DMSO) did not inhibit the growth of the tested microorganisms.
Table 3. Antibacterial activities of acetone, ethyl acetate and methanolic extracts of E. telmateia against tested microorganisms based on microdilution method.
Table 4. Antifungal activities of acetone, ethyl acetate and methanolic extracts of E. telmateia against tested microorganisms based on microdilution method.
Antimicrobial activity of tested extracts was evaluated by determining MICs and MMCs of 22 species of microorganisms. MICs and MMCs values were in range from 0.0781 mg/ml to 40 mg/ml. The tested extracts showed different levels of antimicrobial activity depending on tested species. The intensity of antimicrobial action varied depending on the groups of microorganisms and the type of extracts.
In general, the tested extracts demonstrated selective and moderate antimicrobial activity, and showed stronger inhibitory effects against G+ bacteria than to other tested microorganisms (p <0.05). Statistically significant difference in activity between the extracts of E. telmateia was also observed (p <0.05). The methanol extract shows the highest activity, and ethyl acetate showed low activity. The difference in activity between extracts was seen at bacteria but not at fungi. All tested extracts demonstrated approximately similar activity in relation to the tested standard and clinical strains of bacteria. An exception was seen with B. subtillis, a clear difference can be seen in the methanol extracts.
The tested extracts showed high antibacterial activity against G+ bacteria, especially the species of the genus Bacillus (clinical isolates and standard strains). MICs values were in range from 0.0781 mg/ml to 5 mg/ml, and MMCs values were from 0.3125 mg/ml to 10 mg/ml. The acetone extract showed significant effect against B. pumilus NCTC 8241 with MIC 0.0781 mg/ml and MMC 0.3125 mg/ml, and the methanol extract had the same values of MIC and MMC against Bacillus subtilis.
Gram-positive bacteria were more sensitive than gram-negative bacteria (Uzun et al. 2004[23]). The tested extracts did not affect the growth of clinical isolates and standard strains of G-bacteria or their activities were very low (MIC and MMC ranged from 2.5 mg/ml to 40 mg/ml). The exception was the methanol extract of the species P. aeruginosa ATCC 27853, where MIC value was 1.25 mg/ml.
The tested extracts showed low to moderate antifungal activity. The ethyl acetate extract showed a significant effect against Rhodotorula sp., where MIC was 0.625 mg/ml, and MMC was 1.25 mg/ml.
Very little data is currently available about antimicrobial activity of the plant E. telmateia. Uzun and co-authors (2004[23]) investigated petroleum ether and ethanol extracts of E. telmateia against different microorganisms by disc-diffusion method. Ethanol extract did not show antimicrobial activity, while petroleum ether extracts showed certain activity on S. aureus, S. epidermidis and Candida albicans, but they did not act against G-bacteria. Using the same method, Milovanović and co-authors (2007[10]), investigated hydro-alcoholic extract of E. telmateia which showed good antimicrobial activity against G- bacteria, and little influence on fungi, while it had no effect against S. aureus.
Previous studies support our research data to a great extent. Similarities and differences in obtained results can be explained by different quantity of active components in plants. Differences in the results related to some microorganisms can also be explained by different sensitivity of tested microorganisms, different locations from wich the plant came, different methods of testing and the solvents used .
This is the first study on antimicrobial activity of acetone, ethyl acetate and methanol extracts of E. telmateia. The results of our research indicate good antimicrobial activity of E. telmateia. Thus, E. telmateia should be considered a potential source of antimicrobial substances.
Conclusions
The results of our study suggest the great value of the species E. telmateia for use in phytotherapy, pharmacy and food industry. Therefore, the aerial sterile stems of this plant are natural sources of antioxidant substances of high importance. Also, it can be a potential source of antimicrobial substances.
Acknowledgements
This investigation was supported by the Ministry of Education and Science of the Republic of Serbia, grants No. III41010 and OI173032.
References
- 1.Abd El-aal HA, Halaweish FT. Food preservative activity of phenolic compounds in orange peel extracts (Citrus Sinensis L.) Lucrări Ştiinţifice - Seria Zootehnie. 2010;53:233–240. [Google Scholar]
- 2.Andrews JM. BSAC standardized disc susceptibility testing method (version 4) J Antimicrob Chemother. 2005;56:60–76. doi: 10.1093/jac/dki124. [DOI] [PubMed] [Google Scholar]
- 3.Correia H, Gonzalez-Paramas A, Amaral MT, Santos-Buelga C, Batista MT. Characterisation of polyphenols by HPLC-PAD-ESI/MS and antioxidant activity in Equisetum telmateia. Phytochem Anal. 2005;16:380–387. doi: 10.1002/pca.864. [DOI] [PubMed] [Google Scholar]
- 4.Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–56. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Equisetum. Flore de la Republique Socialiste de Serbie I, Acad. Serb. Sci. and Arts. Vukicevic E, Belgrade: 1970. p. 69. [Google Scholar]
- 6.Gürbüz I, Yeşilada E. In vivo anti-ulcerogenic activity of Equisetum telmateia Ehrh. Extracts used in Turkish folk medicine. Turk J Biol. 2008;32:259–263. [Google Scholar]
- 7.Katalinić V, Miloš M, Kulišić T, Jukić M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94:550–557. [Google Scholar]
- 8.Kumarasamy Y, Byres M, Cox PJ, Jasapars M, Nahar L, Sarker SD. Screening seeds of some Scottish plants for free-radical scavenging activity. Phytother Res. 2007;21:615–621. doi: 10.1002/ptr.2129. [DOI] [PubMed] [Google Scholar]
- 9.Merkl R, Hrbdkovb I, Filip V, Šmidrkal J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J Food Sci. 2010;28:275–9. [Google Scholar]
- 10.Milovanović V, Radulović N, Todorović Z, Stanković M, Stojanović G. Antioxidant, antimicrobial and genotoxicity screening of hydro-alcoholic extracts of five Serbian equisetum species. Plant Foods Hum Nutr. 2007;62:113–9. doi: 10.1007/s11130-007-0050-z. [DOI] [PubMed] [Google Scholar]
- 11.Mimica-Dukic N, Simin N, Cvejic J, Jovin E, Orcic D, Bozin B. Phenolic compounds in field horsetail (Equisetum arvense L.) as natural antioxidants. Molecules. 2008;13:1455–1464. doi: 10.3390/molecules13071455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min G, Chun-Zhao L. Comparison of techniques for the extraction of flavonoids from cultured cells of Saussurea medusa maxim. World J Microb Biotech. 2005;21:1461–1463. [Google Scholar]
- 13.Pourmorad F, Hosseinimehr SJ, Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotechnol. 2006;5:1142–1145. [Google Scholar]
- 14.Quettier-Deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, et al. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol. 2000;72:35–42. doi: 10.1016/s0378-8741(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 15.Rassouli MB, Nasari FG, Nikravesh MR, Moghimi A. Neuroprotective effects of Equisetum telmateia in rat. J Cell Mol Res. 2009;1:29–33. [Google Scholar]
- 16.Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42:321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh A, Sharma PK, Garg G. Natural products as preservatives. Int J Pharma Bio Sciences. 2010;1:601–612. [Google Scholar]
- 18.Singleton VL, Orthofer R, Lamuela RRM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 19.Štajner D, Popović BM, Čanadanović-Brunet J, Anačkov G. Exploring Equisetum arvense L., Equisetum ramosissimum L. and Equisetum telmateia L. as sources of natural antioxidants. Phytotherapy Res. 2009;23:546–50. doi: 10.1002/ptr.2682. [DOI] [PubMed] [Google Scholar]
- 20.Stanković MS, Topuzović M, Solujić S, Mihailović V. Antioxidant activity and concentration of phenols and flavonoids in the whole plant and plant parts of Teucrium chamaedrys L. var. glanduliferum Haussk. J Med Plant Res. 2010;4:2092–2098. [Google Scholar]
- 21.Teffo LS, Aderogba MA, Eloff JN. Antibacterial and antioxidant activities of four kaempferol methyl ethers isolated from Dodonaea viscosa Jacq. var. angustifolia leaf extracts. South African J Botany. 2010;76:25–29. [Google Scholar]
- 22.Tekao T, Watanabe N, Yagi I, Sakata K. A simple screening method for antioxidant and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci Biotechnol Biochem. 1994;58:1780–1783. [Google Scholar]
- 23.Uzun E, Sariyar G, Adsersen A, Karakoc B, Ötük G, Oktayoglu E, et al. Traditional medicine in Sakarya province (Turkey) and antimicrobial activities of selected species. J Ethnopharmacol. 2004;95:287–96. doi: 10.1016/j.jep.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Vasić GP, Glodjović VV, Radojević ID, Stefanović OD, Ćomić LjR, Djinović VM, et al. Stereospecific ligands and their complexes. V. Synthesis, characterization and antimicrobial activity of palladium(II) complexes with some alkyl esters of (S,S)-ethylenediamine-N,N’-di-2-propanoic acid. Inorg Chim Acta. 2010;363:3606–3610. [Google Scholar]
- 25.Willfort R. Gesundheit durch Heilkräuter. Erkennung, Wirkung und Anwendung der wichtigsten einheimischen Heilpflanzen. 27th. Verlag Trauner; 1997. [Google Scholar]
- 26.Willfort R. Gesundheit durch Heilkräuter. Erkennung, Wirkung und Anwendung der wichtigsten einheimischen Heilpflanzen. 27. ed. Verlag Trauner, 1997 [Google Scholar]
- 27.Zhou K, Yu L. Effects of extraction solvent on wheat bran antioxidant activity estimation. LWT - Food Sci Technol. 2004;37:717–721. [Google Scholar]