Abstract
Introduction
Reliable characterization of the nicotine content and emissions from electronic cigarettes (ECIGs) is crucial for product regulation. Understanding nicotine delivery, and therefore efficacy and abuse potential, from ECIG products requires quantifying the total nicotine contained or emitted, as well as the partitioning between its free-base and protonated forms. To date, studies reporting nicotine content and emissions of ECIGs have not addressed whether the reported values correspond to the total nicotine or only one of its forms, making the reported results difficult to compare across studies, or to correlate against blood exposure measurements. In this study we investigate whether nicotine in ECIGs is indeed present in more than one form, whether measurements are affected by sampling media, and report a validated method for determining total, free-base (Nic) and protonated nicotine (NicH+) in ECIG liquids and aerosol emissions.
Methods
We developed an analytical method based on liquid-liquid extraction coupled with GC analysis to assess the respective amounts of Nic and NicH+. The method was first verified on pH-controlled solutions (5 < pH < 10) and then was applied to several ECIG liquids and aerosols generated using a smoking machine.
Results
The method showed high repeatability and efficiency, and the results were in agreement with theoretical predictions based on measured pH of the standard nicotine solutions. ECIG liquids and aerosols contained both Nic and NicH+, and their relative proportions varied widely. Free-base nicotine was found to account for 18-95% of the total nicotine depending on the product in question.
Conclusions
The wide variation in nicotine partitioning across products suggests that studies of nicotine delivery from ECIGs should account for this factor. A convenient method for analyzing nicotine fractions in electronic cigarettes has been demonstrated.
Keywords: Nicotine, electronic cigarette, analytical method, free-base nicotine, aerosol
1. Introduction
Electronic cigarettes (ECIGs) are rapidly gaining in popularity around the globe. This phenomenon is likely driven by a number of factors, including the perception that, in comparison to conventional cigarettes, they present a safer method for obtaining nicotine [1, 2]. This perception stems from the fact that ECIGs are electrically powered devices that heat and vaporize a nicotine-containing flavored liquid to produce an inhalable aerosol, without involving combustion and presumably much of the exposure to combustion-related toxicants such as CO, PAH, and nitric oxide [3]. that are endemic to conventional tobacco products. ECIGs liquids do not contain tobacco but rather a nicotine solution in propylene glycol or glycerol (or a mixture of both) with flavors to make the taste attractive [4, 5]. In summary, the central concept behind “vaping” is volatilization of nicotine into an aerosol of liquid droplets and vapors that will be delivered to the “vaper’s” body [6].
Nicotine has two basic nitrogen groups in its chemical structure (pka1 = 3.12, pka2 = 8.02), and thus it can exist in three forms (namely free-base (Nic), monoprotonated (NicH+) or diprotonated salt (NiCH2+) depending on the pH of the matrix [7-9]. This intrinsic characteristic is quite important since it controls the “bioavailability” of nicotine [10]. In particular, Nic is more volatile than its protonated forms and it is thought to be the only one that diffuses through epithelial tissues in human body [11, 12]. Thus, all else being equal, an ECIG with a higher proportion of Nic will deliver a larger effective nicotine dose to the user.
Though widely recognized as a critical variable in the site of absorption and pharmacokinetics of nicotine [13], quantification of Nic in tobacco products and smoke has been a challenging task. Several methods have been developed to estimate the amount of Nic in aerosols [12, 14], (and references therein). Drawbacks associated with these methods range from the presence of interfering acid/base additives in the matrix, the lack of specificity of the solvent in liquid-liquid extraction, and poor reproducibility.
In fact, studies to date of nicotine content in ECIG solutions or aerosols have not attempted to report information about nicotine forms. Goniewicz et al. used a modification of the NIOSH 2551 method to quantify total nicotine in cartridges [15]. The efficiency of that method is limited by the ability of ethyl acetate to solubilize all forms of nicotine. Other methods rely on methanol or isopropanol to extract total nicotine for quantification [15, 16], or on converting all forms of nicotine to its free base or protonated forms by adding ammonia or acidic solutions, respectively [17][18], and then analyzing the resulting sample for total nicotine content. More recently, a liquid-liquid extraction method using NaOH in methanol and dichloromethane to extract total nicotine followed by GC (gas chromatography) analysis was reported [19]. In summary, while a number of analytical approaches have been used to study total nicotine in ECIGs, none provides the ability to fractionate nicotine into its forms. As a result, we know little about the free-base fraction of ECIG nicotine, a variable that is critical to understanding the potential effects of inhaled ECIG aerosols.
In this work, we report a novel and convenient analytical method for determining nicotine distribution into Nic and NicH+ in ECIG solutions and aerosols. The method is verified against standard nicotine solutions at various pHs, and is used to study a variety of electronic cigarette refill liquids, pre-filled cartridges, and aerosols. We also show that theoretical calculations of nicotine partitioning that are based on pH and pKa values of the pyrrolidine group cannot be always used to predict the Nic/NicH+ distribution in electronic cigarette products and aerosols.
2. Materials and Methods
In this work we developed a novel separation method to quantify Nic and NicH+ using gas chromatography coupled with mass spectrometry (GC-MS). The method exploits the relative difference in solubility of Nic and NicH+ in toluene and water to separate these two nicotine forms into discrete phases, which can be conveniently sampled and introduced into a gas chromatograph. To externally validate the analytical method, the experimental values of Nic and NicH+ distribution of systematically varied pH nicotine solutions were compared to the theoretical predictions based on the pH and pKa values of the pyrrolidine ring.
Using the new analytical method, we quantified the nicotine fractions in several ECIG liquids and smoking machine-generated ECIG aerosols of various products. Results were compared to theoretical predictions of nicotine partitioning based on measured pH. Moreover, applying this method, we investigated potential artifacts arising from various aerosol-sampling filters (glass fiber, Teflon, and quartz).
2.1. Materials
Prefilled ECIG cartridges of the Vapor for Life (V4L), V2, and Blu brands in various nicotine concentrations were procured from US internet vendors as was a sample of ECIG liquid refill solution (My Freedom Smoke Do It Yourself, 100 mg/ml). HPLC grade toluene (CAS registry number 108-88-3) was obtained from Aldrich. Pure nicotine (CAS registry number 54-11-5) was purchased from Acros. Hexadecane (CAS registry number 544-76-3) from Aldrich was used as internal standard (IS). Glass fiber, quartz and Teflon filters were purchased from Pall Corporation, Whatman International Ltd and SterliTech, respectively.
2.2. pH measurements
The pH of the prefilled cartridge and DIY liquids was measured using a Starter 3100 OHAUS pH-meter. The method consists of dissolving the e-liquid in a known amount of de-ionized water and then measuring its pH.
2.3. Liquid-liquid extraction
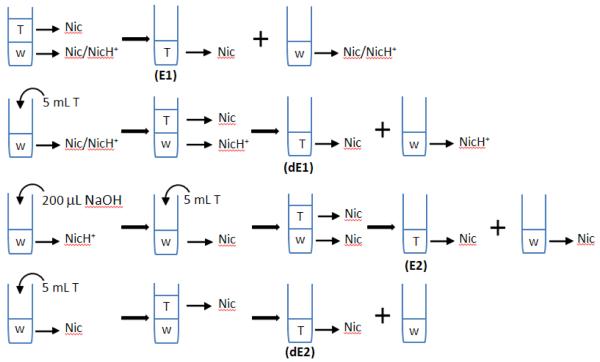
Samples were extracted from ECIG cartridges and standard nicotine solutions, as well as from filters used to collect the total particulate matter generated when the ECIGs were puffed using a smoking machine (described below). To extract samples from the ECIG cartridges, the wool filler was removed from each prefilled cartridge, transferred into a 1 mL plastic syringe and then squeezed into a glass vial. Three hundred microliters of the obtained liquid was dissolved in 5 mL of water and then 5 ml of toluene was added (Figure 1) and the mixture was shaken for 30 min. Then the mixture was allowed to separate into toluene and aqueous phases. The toluene phase (denoted as “E1” in Figure 1), which is capable of dissolving only Nic, was removed from the sample and then introduced into a GC-MS for quantification against hexadecane as internal standard (IS). To verify that all Nic has been extracted, the extraction step was repeated by adding an additional 5 ml of toluene and repeating the above steps, resulting in a second toluene sample (denoted “dE1”). After separating toluene from water as before, 200 μL of NaOH solution (1N) was added to the aqueous layer to convert NicH+ contained in it into Nic. Then 5 mL of toluene was added to the sample and previously described steps used to generate “E1” (and “dE1”) were repeated to generate “E2” (and “dE2”) samples. Thus “E1” (and “dE1”) contain Nic that was in the free-base form in the original sample, while “E2” (and “dE2”) contain NicH+ that was in the protonated form in the original sample.
Figure 1.
Schematic diagram for the presented extraction procedure to separate Nic from NicH+. “T” stands for toluene and “w” for water
2.4. Preparation of standard nicotine solutions at different pHs
For external validation, the extraction method was used to evaluate the distribution of Nic and NicH+ at different pHs and to check the conformity of the method with the calculated distribution based on the pH and the pyrrolidine pKa value of 8.02. For this purpose, five samples of standard nicotine (STD) solution (100 mg/mL) at pH of 5.16 - 9.66 were prepared. Experimental results were then compared to the theoretical calculations and the correlation between experimental % NicH+ versus predicted % NicH+ was evaluated.
2.5. Preparation of cartridge e-liquid solutions
The analytical method was also applied to random cartridges of different brands with different flavoring composition. Solutions were prepared as described in the Materials section. Five different cartridges of pHs varying from 6.30 to 8.74 were tested. V4L at pH 6.30 was unflavored while all the other cartridges were flavored.
2.6. GC-MS conditions
The GC-MS analysis was performed on Thermo-Finnigan Trace GC-2000 Polaris QMS equipped with a binary pump and a thermostatically controlled AI 3000 autosampler. Separation was achieved with a SLB™-5MS (30 m × 0.25 mm × 0.25 μm film thickness) fused silica capillary column purchased from Sigma Aldrich. Splitless injection mode of 1 μL was utilized. The mobile phase was helium gas with 1 mL/min flow rate. The injector temperature was set at 250 °C. The oven temperature program was: 70 °C for 2 min, 20 °C/min to 230 °C and hold for 1 min. The total run time was 11 min, and solvent delay time was 4 min. Analyses were performed using one target ion (the main peak) as follows: nicotine m/z = 84, IS m/z = 57. Nicotine in the samples was quantified using a calibration curve of standard solutions (50-1500 ppm).
2.7. Aerosol generation and sampling
A custom-designed digital puff production machine at the American University of Beirut was used to generate ECIG aerosols from the cartridges (see Talih et al., in press, for details). Puff topography (puff duration, interpuff interval, and flow rate) was selected to represent an experienced e-cigarette user (4s puff duration and 10s inter-puff duration) with a puffing flow rate of 1 L/min [20-22]. The ECIG was powered using a 3.3 v regulated DC power supply. Total particulate matter was collected by drawing the ECIG aerosol generated in 15 puffs puff through a filter located at the ECIG mouthpiece outlet. To investigate potential interferences due to filter components, we used three types of filters (glass fiber, quartz or Teflon filter). Each filter was suspended in 5 mL of water and then the same liquid-liquid extraction steps described above were repeated.
3. Results
3.1. pH measurements of e-liquid solutions
pH measurements of different pre-filled cartridges showed an average pH of ~8 as shown in Table 1. Similar results were recently reported by Stepanov and Fujioka for two brands out of four tested in their study (the other two brands exhibited alkaline pH of ~ 9). Being close to the pKa value of the pyrrolidine ring, these pH values indicate that the e-liquid solutions represent a buffer medium where Nic and its conjugated NicH+ co-exist. The pH of a “Do It Yourself” (DIY) solution (100 mg/mL), which is known to be composed of Nic, was measured for comparison. For the DIY, the measured pH value of 9.7 indicates that nicotine is predominantly present in the free-base form.
Table 1.
pH measurements of DIY and V4L cartridge solutions of different concentrations. The liquid of the cartridge was soaked in 10 mL of water.
| Sample* | Nicotine (mg) | pH±Stdev |
|---|---|---|
| V4L (n=6) | 4 | 7.8±0.3 |
| V4L (n=3) | 18 | 7.4±0.6 |
| V4L (n=5) | 24 | 8.0±0.3 |
| V4L (n=6) | 36 | 8.3±0.2 |
| DIY (n=3) | 100mg/ml | 9.7±0.0 |
n denotes the number of tested samples
3.2. Analytical Method Validation
A standard calibration curve was prepared by extracting a range of concentrations (50-1500 ppm) of standard nicotine solutions following the aforementioned method. The linearity of the method was assessed by a regression line drawn for a standard calibration curve of the peak areas of Nic/IS versus concentrations. The correlation coeffcient (R2) was 0.999. The recovery of the method, estimated from the extraction of Nic standard solutions of pH 9.7 at three different concentrations (50, 200, 400 ppm), was 89 ± 4.6 %. It is important to note that the recovery of the method was found to be lower at concentrations below the reported range of the calibration curve. Furthermore, the repeatability of the method was examined by quantifying six replicate samples of three different concentrations. These samples were subjected to the whole extraction procedure and the relative standard deviation (%RSD) within each concentration, as a measure of repeatability, was found to be ≤ 5%. The method was also used for label verification, for example, for the unflavored V4L cartridges labeled with 18 mg/mL nicotine content and average pH of 6.3 (± 0.68), the concentrations of nicotine collected from E1, dE1, E2 and dE2 were 0.75, 0.13, 6.15 and 0.74 mg/mL respectively. The total nicotine concentration amounted to 7.77 mg/mL which is evidently different than the label.
3.3. Quantification of Nic and NicH+ in standard nicotine solutions (STD)
Table 2 shows the measured and predicted ratios of Nic/NicH+ in STD solutions prepared at different pHs in the lab. Predicted ratios were obtained using Henderson-Hasselbalch equation (pH = pKa + log[Nic]/[NicH+]). Therefore based on the value of pH and pka (of the pyrrolidine ring, pKa =8.02), STD solutions (100 mg/mL) at pH < 7 are expected to contain mainly NicH+, STD solutions at 7 < pH < 9 would consist of both forms of nicotine (Nic and NicH+) and in STD solutions at pH > 9 Nic predominates. Experimental results were in good agreement with the predicted Nic/NicH+ ratios. The correlation between the experimental versus calculated % of NicH+ gives an R2 of 0.999. These results indicate a high level of accuracy and precision for the analytical method.
Table 2.
pH measurements and percentages of Nic and NicH+ in standard nicotine solution assessed by the optimized extraction method. Results are compared to predicted values from pH measurement and pKa
| Sample (nicotine label) |
pH | % of predicted Nic/NicH+ |
% of measured Nic/NicH+ |
|---|---|---|---|
| STD(1) | 5.16 | 0.2/99.8 | 2.5/97.5 ± 0.09 |
| STD(2) | 7.52 | 24.0/76.0 | 27.8/72.2 ± 0.84 |
| STD(3) | 8.07 | 52.9/47.1 | 53.0/47.0 ± 1.43 |
| STD(4) | 8.55 | 72.2/22.8 | 79.2/20.8 ± 0.62 |
| STD(5) | 9.66 | 97.8/2.2 | 97.7/2.3 ± 0.27 |
3.4. Quantification of Nic and NicH+ in cartridge e-liquid
The analytical method was used to measure the different ratios of Nic/NicH+ for various e-liquid cartridge solutions. Unlike the case with the standard solutions described above, we found that prediction of nicotine partitioning based on measured pH was unreliable compared to the measured values, as shown in able 3. We suspect that pH measurement of the flavored e-cigarette liquids may be affected by flavor additives used in these liquids. The small difference between measured and calculated values shown in the unflavored (V4L) is due to minimal presence of additives. Interestingly, the measured Nic/NicH+ ratios for the tobacco flavored cartridges show different values across brands, and also different flavors gave different ratios within the same brand. These factors highlight the necessity for directly measuring nicotine fractions using an analytical method, rather than relying on pH measurement, when knowledge of nicotine partitioning is needed.
3.5. Quantification of Nic and NicH+ in ECIG aerosols
Results of the quantification of Nic/NicH+ in machine-generated ECIG aerosols are shown in Table 4 for the three sampling filter types examined. The results showed that only Nic was detected when samples were collected on glass fiber filters, whereas both forms of nicotine were detected on quartz and Teflon filters with similar ratios.
Table 4.
Ratio of Nic/IS peak area for Nic content in the aerosol of V4L cartridges assessed using the introduced analytical method. Each trial is repeated three times.
| Sample | Nic (%) | NicH+ (%) |
|---|---|---|
| Glass fiber filter | 100 | 0 |
| Teflon filter | 61.7 ± 4.8 | 37.5 ± 4.8 |
| Quartz filter | 68.7 ± 10.1 | 31.2 ± 10.1 |
4. Discussion
The presented analytical method shows a very good agreement between experimentally measured and calculated Nic/NicH+ ratios in standard nicotine solutions of known concentrations and pH. The weaker correlation between experimental results and calculated values of %Nic/NicH+ in e-liquid cartridges is attributed to the presence of other additives (e.g. flavors). Hence, attempts to calculate the Nic and NicH+ content in e-cigarette liquids based on pH measurements cannot be a method of choice especially when the pH is influenced by the presence of other components in the matrix.
Moreover, the developed analytical method showed that vaped aerosols contain the two forms of nicotine. However, the quantified amounts of Nic and NicH+ could be affected by the collection medium. For example, all nicotine is transformed into Nic if collected on glass fiber filters (known to be basic), however, when collected on Teflon and Quartz filters around 65 % abundance of Nic was determined. The discrepancy between glass fiber filters and the other two filter types may stem from the fact that the glass fiber filters are made of borosilicate, (a base), which can therefore convert NicH+ into Nic. Teflon and quartz, by comparison, are neutral. Consequently, we feel confident to report that unflavored V4L (at this particular pH of the cartridge water extract) smoked under the mentioned conditions will deliver ~65 % of Nic and ~35 % of NicH+.
The free-base nicotine fraction in an e-cigarette aerosol likely influences nicotine delivery, and is therefore likely a key attribute when considering the efficacy and abuse liability of an electronic cigarette product. This study has demonstrated a convenient and reliable analytical method for determining the free-base and protonated nicotine fractions in e-cigarette products and aerosols. It has also demonstrated that prediction of nicotine fractionation using standard pH measurements can be unreliable when applied to commercial e-cigarette products. The extrapolation of the Nic/NicH+ ratio determined in the e-liquid to the amount in the aerosol phase warrants further investigation, especially that the aerosol appears to be enriched in free-base nicotine due to its higher volatility compared to the protonated forms.
5. Conclusion
An analytical method to assess the respective amounts of Nic and NicH+ in e-cigarette cartridges and aerosol was developed. The method validation showed good repeatability (% RSD ≤ 5%) and high recovery (89%) from e-liquids. Application of the method on standard nicotine solutions showed that Nic/NicH+ ratios are in good agreement with theoretical predictions based on pH and pKa value of the pyrrolidine group. On the other hand, it was also shown that pH measurement is unreliable for predicting the distribution of Nic and NicH+ in e-liquid cartridges, probably due to the interference of additives and flavors on the pH measurement. In addition, determination of nicotine fractionation in aerosols is highly sensitive to filter collection medium. Future work is recommended on the relation between Nic in the ECIG liquid to that of the resulting emissions, and, most importantly, to nicotine delivery to the user. The method presented in this work provides a means for doing so.
Table 3.
pH measurements and percentages of Nic and NicH+ in random e-liquid cartridges assessed by the described method and compared to prediction from pH and pKa values
| Sample (nicotine label) |
pH | % of predicted Nic/NicH+ |
% of measured Nic/NicH+ |
|---|---|---|---|
| V4L (unflavored) | 6.30 ± 0.68 | 5.3/94.7 ± 6.50 | 10.7/89.3 ± 12.36 |
| V4L (strawberry) | 8.11 ± 0.27 | 55.1/44.9 ± 14.87 | 92.2/7.8 ± 3.38 |
| Blu (tobacco) | 6.85 ± 0.11 | 6.5/93.5 ± 1.57 | 16.0/84.0 ± 3.05 |
| V2 (tobacco) | 8.63 ± 0.02 | 80.1/19.9 ± 0.89 | 97.6/2.4 ± 0.15 |
| V2 (menthol) | 8.74 ± 0.02 | 84.0/16.0 ± 0.65 | 90.3/9.7 ± 0.88 |
Cartridge brand
Acknowledgements
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Footnotes
Declaration of interests
The authors have no conflicts of interest to report.
References
- [1].Ayers JW, Ribisl Kurt M., Brownstein John S. Tracking the Rise in Popularity of Electronic Nicotine Delivery Systems (Electronic Cigarettes) Using Search Query Surveillance. American Journal of Preventive Medicine. 2011;40(4):448–453. doi: 10.1016/j.amepre.2010.12.007. [DOI] [PubMed] [Google Scholar]
- [2].Adkison SE, et al. Electronic Nicotine Delivery Systems. American Journal of Preventive Medicine. 2013;44(3):207–215. doi: 10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zachary Cahn MS. Electronic cigarettes as a harm reduction strategy for tobacco control: A step forward or a repeat of past mistakes? Journal of Public Health Policy. 2011;32:16–31. doi: 10.1057/jphp.2010.41. [DOI] [PubMed] [Google Scholar]
- [4].Grana RA, Ling PM. “Smoking Revolution”. American Journal of Preventive Medicine. 2014;46(4):395–403. doi: 10.1016/j.amepre.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Farsalinos K, et al. Impact of Flavour Variability on Electronic Cigarette Use Experience: An Internet Survey. International Journal of Environmental Research and Public Health. 2013;10(12):7272–7282. doi: 10.3390/ijerph10127272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Williams M, et al. Metal and Silicate Particles Including Nanoparticles Are Present in Electronic Cigarette Cartomizer Fluid and Aerosol. PLoS ONE. 2013;8(3):e57987. doi: 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Perfetti TA. Structural Study of Nicotine Salts. Beitr. Tabakforsch. Int. 1983:43. [Google Scholar]
- [8].Lee Jeong-Min, G.-C.J., Lee John-Tae, Park Jin-Won, Kim Do-Yeon, Kim Hyo-Keun, Hwang Keon-Joong, Min Young-Keun. Changes in Unprotonated Nicotine Concentration in Cigarette Mainstream Smoke with Three Machin-Smoking Conditions. Journal of the Korean Society of Tobacco Science. 2006:136. [Google Scholar]
- [9].Graton J, et al. Site of Protonation of Nicotine and Nornicotine in the Gas Phase: Pyridine or Pyrrolidine Nitrogen? Journal of the American Chemical Society. 2002;124(35):10552–10562. doi: 10.1021/ja017770a. [DOI] [PubMed] [Google Scholar]
- [10].Reininghaus W. Bioavailability of nicotine. Philip Morris Research center USA; Richmond USA: 1994. [Google Scholar]
- [11].Pankow JF. A Consideration of the Role of Gas/Particle Partitioning in the Deposition of Nicotine and Other Tobacco Smoke Compounds in the Respiratory Tract. Chemical Research in Toxicology. 2001;14(11):1465–1481. doi: 10.1021/tx0100901. [DOI] [PubMed] [Google Scholar]
- [12].Pankow JF, et al. Percent Free Base Nicotine in the Tobacco Smoke Particulate Matter of Selected Commercial and Reference Cigarettes. Chemical Research in Toxicology. 2003;16(8):1014–1018. doi: 10.1021/tx0340596. [DOI] [PubMed] [Google Scholar]
- [13].Caldwell B, Sumner Walt, Crane Julian. A Systematic Review of Nicotine by Inhalation: Is There a Role for the Inhaled Route? Nicotine & Tobacco Research. 2012;(1127-1139) doi: 10.1093/ntr/nts009. [DOI] [PubMed] [Google Scholar]
- [14].Bao M, Joza Peter, Rickert William S., Lauterbach John H. An improved headspace solid-phase microextraction method for the analysis of free-base nicotine in particulate phase of mainstream cigarette smoke. Analytica Chimica Acta. 2010;663(1):49–54. doi: 10.1016/j.aca.2010.01.036. [DOI] [PubMed] [Google Scholar]
- [15].Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014;109(3):500–507. doi: 10.1111/add.12410. [DOI] [PubMed] [Google Scholar]
- [16].Trehy ML, et al. Analysis of Electronic Cigarette Cartridges, Refill Solutions, and Smoke for Nicotine and Nicotine Related Impurities. Journal of Liquid Chromatography & Related Technologies. 2011;34(14):1442–1458. [Google Scholar]
- [17].Etter J-F, Zäther E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013;108(9):1671–1679. doi: 10.1111/add.12235. [DOI] [PubMed] [Google Scholar]
- [18].Cheah NP, et al. Electronic nicotine delivery systems: regulatory and safety challenges: Singapore perspective. Tobacco Control. 2012:119–125. doi: 10.1136/tobaccocontrol-2012-050483. [DOI] [PubMed] [Google Scholar]
- [19].Hutzler C, et al. Chemical hazards present in liquids and vapors of electronic cigarettes. Archives of Toxicology. 2014;88(7):1295–1308. doi: 10.1007/s00204-014-1294-7. [DOI] [PubMed] [Google Scholar]
- [20].Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tobacco Control. 2011:103–106. doi: 10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
- [21].Farsalinos K, et al. Evaluation of Electronic Cigarette Use (Vaping) Topography and Estimation of Liquid Consumption: Implications for Research Protocol Standards Definition and for Public Health Authorities’ Regulation. International Journal of Environmental Research and Public Health. 2013;10(6):2500–2514. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Talih S, et al. Effects of User Puff Topography, Device Voltage, and Liquid Nicotine Concentration on Electronic Cigarette Nicotine Yield: Measurements and Model Predictions. 2014. In Press. [DOI] [PMC free article] [PubMed]