Abstract
Objective
The purpose of this study was to explore communication barriers as independent predictors and potential mediators of variation in clinical recognition of diabetic peripheral neuropathy (DPN).
Methods
In this cross-sectional analysis, we estimated the likelihood of having a DPN diagnosis among 4,436 patients with DPN symptoms. We controlled for symptom frequency, demographic and clinical characteristics, and visit frequency using a modified Poisson regression model. We then evaluated 4 communication barriers as independent predictors of clinical documentation and as possible mediators of racial/ethnic differences: difficulty speaking English, not talking to one’s doctor about pain, limited health literacy, and reports of suboptimal patient-provider communication.
Results
Difficulty speaking English and not talking with one’s doctor about pain were independently associated with not having a diagnosis, though limited health literacy and suboptimal patient-provider communication were not. Limited English proficiency partially attenuated, but did not fully explain, racial/ethnic differences in clinical documentation among Chinese, Latino and Filipino patients.
Conclusions
Providers should be encouraged to talk with their patients about DPN symptoms, and health systems should consider enhancing strategies to improve timely clinical recognition of DPN among patients who have difficult speaking English. More work is needed to understand persistent race/ethnic differences in diagnosis.
Diabetic distal peripheral neuropathy (DPN) is a common complication that affects over 5.5 million people with diabetes and approximately 50% of people who have had diabetes for 10 or more years.1 An estimated 20% of patients with DPN experience neuropathic symptoms, such as pain, a burning sensation, “pins and needles”, or numbness of both lower extremities.2 These symptoms are associated with lower quality of life, limited mobility, depression, and social dysfunction across the life course of patients with diabetes.2-6 While symptoms are often treatable and ameliorable, DPN is often under-diagnosed and undertreated in primary care settings.7
Clinicians rely on patient reports of symptoms, including pain type, severity, and impact on daily living, as well as diagnostic tests to diagnose symptomatic DPN.8 However, patients may be reluctant to report symptoms to their doctors due to the unusual nature and combination of symptoms (e.g., numbness, itching, tingling, crawling) and because of the competing demands of increasingly time-constrained primary care visits.9-12 Racial and ethnic minorities may be at increased risk for both underreporting of symptoms and under diagnosis of chronic pain conditions like DPN, particularly when language and other communication barriers are present. Specific communication barriers that contribute to these differences include limited language proficiency, inadequate health literacy and cultural beliefs that make some patients less likely to discuss pain symptoms.13,14
Research into variation in clinical detection of DPN among highly diverse cohorts or patients and communication factors that may explain such differences are needed. Identifying communication barriers that impede the clinical detection of DPN and their role as potential mediators of potential race/ethnic differences in pain reporting and outcomes is an important first step in improving detection and treatment. In this paper, we explore variation in clinical recognition of DPN symptoms, as indicated by the absence of a documented diagnosis of DPN in the electronic health record (EHR), and communication factors as independent predictors of this variation. We further evaluate whether the presence of communication barriers mediates variation in diagnosis by race or ethnicity.
Research Design and Methods
The setting for this study was Kaiser Permanente Northern California (KPNC), a large integrated health care delivery system serving more than 3 million people. This study was conducted as part of the Diabetes Study of Northern California (DISTANCE) and its ancillary, the Diabetes & Aging Study. DISTANCE surveyed a race-stratified, random sample of patients with diabetes to understand social disparities in diabetes care and outcomes.15
Data from DISTANCE were linked to clinical and administrative data from the Kaiser Permanente Diabetes Registry of Northern California, a database of Kaiser Permanente members diagnosed with diabetes since 1971 and the EHR at KPNC. Among the 20,188 KPNC members who responded to the DISTANCE survey (response rate 62%), the sample was restricted to the five largest race/ethnicity subgroups (i.e., those who self-identified as African American or black, white, Chinese, Latino or Hispanic, or Filipino). Respondents with any lower extremity amputation were excluded due to the high probability that it was influenced by the condition of primary interest, DPN.1,2 Among the remaining 15,847 respondents, 12,681 or 80% answered a question regarding the presence of DPN symptoms and their responses will be the focus of this analysis. This study focused on the 4,436 adult diabetes patients who reported having symptoms consistent with DPN on the survey.
Outcome measure
The primary outcome of interest for this study was clinical documentation of DPN, defined as the presence of at least one inpatient or two outpatient diagnoses for peripheral neuropathy (ICD9: 356.0, 356.9, 357.2) in the medical record during the 24 months before or after the survey administration date.16 Also included were ICD 9 codes for related conditions that may mimic DPN in symptomatology, such as neuralgic amyotrophy, lesion of the plantar nerve, and neuralgia neuritis and radiculitis unspecified (ICD9: 250.60-250.63; 337.0, 337.01, 337.1, 337.9, 349.9, 353.0, 353.1, 354.1, 354.8, 354.9, 355.0, 355.2, 355.3, 355.4, 355,6, 355.79, 335.8, 335.9, 713.5, 723.4, 729.2). This dichotomous measure served as the primary outcome for the regression models.
Measures of Communication Barriers
Barriers to patient-provider communication were assessed in four ways. First, the behavior of discussing pain with one’s doctor was represented by a survey question asking whether the patient discussed pain with their doctor in the prior 12 months. The question did not refer to DPN specifically, but to pain of any kind. Seventy one percent of patients with symptoms answered the following question: “During the past 12 months, have you ever discussed problems with pain with your doctor or health care provider?”
Second, an indicator of whether the patient reported difficulty communicating in English was included. This was measured based on a validated question asking if the patient had difficulty speaking or understanding English: always, often, sometimes, rarely or never). Eighty-five percent of patients with symptoms answered this question. Patients who answered always, often, or sometimes were categorized as having LEP.
Third, health literacy was assessed based on a previously validated 3-item brief health literacy scale. From this scale, a single item, “How confident are you filling out medical forms by yourself?”, was used to assess health literacy.17-21 Specifically, patients were classified as having limited health literacy if they reported being somewhat, a little or not at all confident and health literate if they reported being quite a bit or extremely confident in their ability to understand health information. Previous publications using our study population have demonstrated the utility and validity of this scale in predicting outcomes that would be expected to be associated with inadequate health literacy.17,19, 21-24
Quality of patient-provider communication was assessed using the 4-point communication composite score for the well-known Consumer Assessment of Healthcare Providers and Systems (CAHPS) version 2.0 survey,25 based on patient responses to at least two out of four questions regarding the frequency with which their provider 1) listened carefully to what they had to say, 2) explained things in a way they could understand, 3) showed respect for what the patient had to say or 4) spent enough time with the patient. These questions were combined into a summary score with a best possible value of 100 and a minimum value of 0. For the 77% of subjects that answered these questions, we created a dichotomous indicator of communication quality to indicate optimal communication (i.e., score of 100) or suboptimal communication (i.e., score < 100). Three previous publications have demonstrated the utility and validity of these items and dichotomization of responses as worded in the DISTANCE survey in predicting outcomes that would be expected to be associated with patient-provider communication.17-19
Race/Ethnicity
Race/ethnicity was the exposure of interest. Racial and ethnic subgroups were identified using self-reported categories from the DISTANCE survey: Chinese, African American, Latino/Hispanic, white or Filipino. Other racial and ethnic subgroups, including those reporting mixed race, were excluded due to small sample size.
Potential mediators
Clinical and Demographic Charcateristics
The frequency of DPN symptoms was assessed using a previously validated, single item question in the DISTANCE Survey: “Over the past 4 weeks, how often have you had pins and needles, numbness, burning or a tingling sensation in both your feet?”Response options regarding symptom frequency were: 5 to 7 days a week; 3 to 4 days a week; 1 to 2 days a week; 1 to 3 days a month; or never or rarely. The statistical models predicting the likelihood of a diagnosis were restricted to those who reported having symptoms at least one day per month.
Clinical status was classified based on a combination of self-report and EHR data. Several measures of diabetes severity that are known risk factors for peripheral neuropathy were included:self-reported duration of diabetes in years (<5, 5-9, 10 years or more), an indicator for whether the last observed (prior to survey date) hemoglobin A1c (HbA1c) was greater than 9%, and evidence of any pharmacy dispensing of insulin. We controlled for BMI (underweight<18.5, overweight=25-30; obese>30) as recorded in the EHR, using normal weight (BMI=18.5-25) as the referent group. Also included was an indicator for whether the total number of outpatient visits was above or below the median for all patients in the cohort to account for opportunities for diagnostic assessment and for possible competing demands for health care services.
Based on previous reports of a high degree of concurrent chronic pain and depression among patients with diabetes in our own5,6 and other26studies, an indicator for the presence of depressive symptoms reported in the survey was also included in the model. Depressive symptoms were defined as having a score of 10 or greater on the previously validated 8-item Patient Health Questionnaire depression scale (PHQ-8).27
Patients were classified into three age groups (<50, 50-65, 65+ years), sex, and educational achievement based on self-report (<high school graduate, high school graduate or equivalent, some college, college graduate).
Statistical Analysis
Baseline differences in sociodemographic and clinical characteristics for patients with and without DPN symptoms were evaluated using t tests and chi-square tests. Among the subset reporting symptoms, the likelihood of clinical documentation of DPN as described above was assessed. Given that clinical documentation of DPN is not a rare event, we estimated a modified Poisson regression model to evaluate predictors of this dichotomous outcome.28 The likelihood ratio statistic was used to assess model fit and multiple imputation to address missing covariates due to survey non-response to several categorical measures (e.g., difficulty understanding or speaking English).29 The multiple imputed data set (20 imputations) was created using the multivariate normal model and Markov Chain Monte Carlo approach. Variables included in the model were the above described measures of patient demographic, clinical characteristics, and frequency of contact with the health care system.
Crude differences in detection by race and ethnicity were first evaluated. Next each of the communication barriers was separately added to the basic model to evaluate them as mediators of the race/ethnic differences and/or independent predictors of the outcome. Due to a high degree of correlation between the communication measures, we did not include them together in a single model. The impact of the inclusion of these measures to the basic model was assessed through evaluation of changes in the effect size and statistical significance of the other covariates to evaluate whether the communication measures explained variation in fixed attributes (i.e., race/ethnicity).30 Model fit was assessed using the Information Criterion.31
All statistical analyses were conducted in SAS v9.3.32 This study was approved by the Institutional Review Board at Kaiser Foundation Research Institute.
Results
Characteristics of Respondents with and without DPN Symptoms
Our cohort of 12,681 adult diabetes patients included: 31% self-identified as white, 8% Chinese, 22% African American or black, 24% Latino and 15% Filipino (Table 1). Most patients (82%) were over the age of 50 and men and women were equally represented. Comparing patients with (4,436) and without (8,245) DPN symptoms, symptomatic patients were significantly more likely to be white, 50 or older, and less likely to have graduated college. Symptomatic patients had greater diabetes severity, including higher average BMI, longer duration of diabetes, a higher proportion of patients with A1c above 9.0%, and evidence of insulin use. Depressive symptoms were more commonly reported among symptomatic patients and they had a high average number of office visits. Patients with symptoms were also more likely to report inadequate health literacy (26.2% vs. 21.9%), and were more likely to talk with their doctor about pain (56.6% vs. 43.7%). Nearly half (46.2%) of patients reporting current symptoms had a diagnosis in the EHR compared to only 17% of those without current symptoms.
Table 1.
Characteristics of Adult Diabetes Patients with and without Peripheral Neuropathy, DISTANCE (n=12,681)*
|
All
N=12,681 |
Did not report
symptoms N=8245 |
Reported symptoms
N=4436 |
|
|---|---|---|---|
| Race and Ethnicity* | |||
| White | 3917 (30.9) | 2341 (28.4) | 1576 (35.5) |
| Chinese | 1055 (8.3) | 832 (10.1) | 223 (5.0) |
| African American | 2763 (21.8) | 1750 (21.2) | 1013 (22.8) |
| Latino | 3080 (24.3) | 2058 (25.0) | 1022 (23.0) |
| Filipino | 1866 (14.7) | 1264 (15.3) | 602 (13.6) |
| Agegroup* | |||
| <50 | 2232 (17.6) | 1561 (18.9) | 671 (15.1) |
| 50-64 | 6287 (49.6) | 4001 (48.5) | 2286 (51.5) |
| 65+ | 4162 (32.8) | 2683 (32.5) | 1479 (33.3) |
| Gender* | |||
| Female | 6359 (50.2) | 4073 (49.4) | 2286 (51.3) |
| Male | 6322 (49.9) | 4172 (50.6) | 2150 (48.5) |
| Education* | |||
| Did not graduate high school |
1857 (14.6) | 1196 (14.5) | 661 (14.9) |
| High School Graduate, no college |
3604 (28.4) | 2325 (28.2) | 1279 (28.8) |
| Some College | 3331 (26.3) | 2081 (25.2) | 1250 (28.2) |
| College Graduate+ | 3632 (28.6) | 2468 (29.9) | 1164 (26.2) |
| Missing | 257 (2.0) | 175 (2.1) | 82 (1.9) |
|
BMI (Mean (standard
deviation))* |
31.5 (7.2) | 31.0 (7.0) | 32.3 (7.4) |
| Missing | N=2220 | N=1409 | N=811 |
| Diabetes Severity | |||
|
Average Duration of
Diabetes (yrs) (Mean (standard deviation))* |
9.6 (8.1) | 9.0 (7.8) | 10.7 (8.6) |
| Missing | N=55 | N=37 | N=18 |
| Most Recent A1c | |||
| >7.0* | 5534 (43.6) | 3438 (41.7) | 2096 (47.3) |
| >9.0* | 1441 (11.4) | 841 (10.2) | 600 (13.5) |
| Missing | 1175 (9.3) | 796 (9.7) | 379 (8.5) |
| Any Insulin Use* | |||
| Yes | 2251 (17.8) | 1170 (14.2) | 1081 (24.4) |
| No | 10430 (82.3) | 7075 (85.8) | 3355 (75.6) |
|
Depressive Symptoms
(PHQ8≥10)* |
|||
| Yes | 1262 (10.0) | 578 (7.0) | 684 (15.4) |
| No | 8571 (67.6) | 5884 (71.4) | 2687 (60.6) |
| Missing | 2848 (22.5) | 1783 (21.6) | 1065 (24.0) |
| Medicaid Insured* | |||
| Yes | 177 (1.4) | 83 (1.0) | 94 (2.1) |
| No | 12504 (98.6) | 8162 (99.0) | 4342 (97.9) |
|
Number of outpatient
visits* |
|||
| Above median† | 6591 (52.0) | 3975 (48.2) | 2616 (59.0) |
| Below median | 6090 (48.0) | 4270 (51.8) | 1820 (41.0) |
| Communication Measures | |||
|
Difficulty with English
(Speaking/Understanding)* |
|||
| Yes | 1626 (12.8) | 1067 (12.9) | 559 (12.6) |
| No | 9273 (73.1) | 6078 (73.7) | 3195 (72.0) |
| Missing | 1782 (14.1) | 1100 (13.3) | 682 (15.4) |
|
Inadequate Health
Literacy* |
|||
| Yes | 2967 (23.4) | 1807 (21.9) | 1160 (26.2) |
| No | 7851 (61.9) | 5278 (64.0) | 2573 (58.0) |
| Missing | 1863 (14.7) | 1160 (14.1) | 703 (15.9) |
|
Patient-Provider
Communication* |
|||
| CAHPS score=100 | 4374 (34.5) | 3064 (37.2) | 1310 (29.5) |
| CAHPS score < 100 | 5397 (42.6) | 3305 (40.1) | 2092 (47.2) |
| Missing | 2910 (23.0) | 1876 (22.8) | 1034 (23.3) |
|
Discusses Pain with
Provider* |
|||
| Yes | 6110 (48.2) | 3601 (43.7) | 2509 (56.6) |
| No | 2255 (17.8) | 1605 (19.5) | 650 (14.7) |
| MIssing | 4316 (34.0) | 3039 (36.9) | 1277 (28.8) |
|
Diabetic Peripheral
Neuropathy Diagnosis* |
|||
| Yes | 3462 (27.3) | 1413 (17.1) | 2049 (46.2) |
| No | 9219 (72.7) | 6832 (82.9) | 2387 (53.8) |
Column percents presented, but may not sum to 100 due to rounding.
p-value < 0.05, Chi-square test for categorical variables or F-test for continuous variables
Median was 8 visits during the 12 months pre-baseline period (range: 0-254).
Non-Communication Factors Associated with Clinical Documentation of DPN
Compared to whites, being Chinese [RR=0.66 (0.52,0.82))], Latino [RR=0.86 (0.77,0.95)], and Filipino [RR=0.68 (0.59,0.78)) were associated with a substantially lower likelihood of having a recorded diagnosis of DPN. Clinical recognition did not differ between African American [RR= 0.99 (0.91,1.07) and whites. Adjustment for all the communication factors and other potential mediators did not explain those observed differences; being Chinese [RR 0.66 (0.52, 0.82)], Latino [RR 0.86 (0.77, 0.95)] and Filipino [RR 0.68 (0.59, 0.78)] were still associated with a reduced likelihood of having a recorded diagnosis.
Other patient level factors associated with having a recorded diagnosis included greater symptom frequency [3-4 days per week: Relative Risk 1.27 (95% Confidence Interval:1.10, 1.48); 5-7 days per week: RR 1.62; (1.43, 1.83)], older age [50-64: RR 1.27 (1.10,1.47); 65+: RR 1.46 (1.27, 1.71)], insulin use [RR1.15 (1.06, 1.25)], longer duration of diabetes [5-9 years: RR 1.16 (1.03,1.31); 10 years or more: RR 1.31 (1.17,1.47)], and more frequent physician visits [RR 1.41 (1.28, 1.55)] (Table 2).
Table 2.
Estimated Risk Ratio for Receiving a Clinical Diagnosis of Diabetic Peripheral Neuropathy among Adults Diabetes Patients with Symptom, DISTANCE (n=4,436)*
| Characteristics | Unadjusted Risk Ratio (95% Confidence Interval) |
Partially adjusted**
Model Risk Ratio (95% Confidence Interval) |
|---|---|---|
| Race/Ethnicity (ref white) | ||
| African American | 0.88 (0.81,0.96) | 0.99 (0.91,1.07) |
| Chinese | 0.53 (0.42,0.67) | 0.66 (0.52,0.82) |
| Latino | 0.72 (0.65,0.79) | 0.86 (0.77,0.95) |
| Filipino | 0.56 (0.48,0.64) | 0.68 (0.59,0.78) |
| Age (ref < 50) | ||
| Age 50-64 | 1.40 (1.20,1.64) | 1.27 (1.10,1.47) |
| Age 65+ | 1.72 (1.48,2.01) | 1.46 (1.27,1.71) |
| Male (ref = female) | 0.95 (0.88,1.03) | 0.97 (0.9,1.05) |
| Education (ref = no high school diploma) | ||
| High School Diploma | 1.0 (0.88,1.14) | 0.95 (0.84,1.07) |
| Some College | 1.0 (0.88,1.14) | 0.98 (0.87,1.11) |
| College Graduate | 1.12 (0.98,1.27) | 1.11 (0.98,1.25) |
| Covariates | ||
| Symptom frequency (ref = 1-3 times/month) | ||
| 1-2 days/week | 1.08 (0.91,1.28) | 1.04 (0.89,1.22) |
| 3-4 days/week | 1.4 (1.20,1.64) | 1.27 (1.10,1.48) |
| 5-7 days/week | 1.86 (1.64,2.11) | 1.61 (1.43,1.83) |
| Insulin Use (ref= no) | 1.47 (1.36,1.58) | 1.15 (1.06,1.25) |
| Duration (ref = <5 years) | ||
| Duration 5-9 years | 1.2 (1.05,1.36) | 1.16 (1.03,1.31) |
| Duration 10 years or more | 1.56 (1.39,1.75) | 1.31 (1.17,1.47) |
| HbA1c >9.0 (ref ≤ 9.0) | 0.99 (0.88,1.11) | 1.07 (0.95,1.20) |
| BMI (ref-normal weight) | ||
| Underweight (<18.5) | 1.38 (0.99,1.94) | 1.15 (0.76,1.73) |
| Overweight (25-30) | 0.94 (0.81,1.10) | 0.94 (0.81,1.09) |
| Obese (>30) | 1.04 (0.90,1.21) | 0.99 (0.86,1.14) |
| Depression (ref PHQ9<10) | 1.14 (1.03,1.26) | 1.06 (0.98,1.15) |
| Medicaid (ref = no) | 1.24 (1.0,1.55) | 1.17 (0.97,1.41) |
|
Above median number of outpatient visits (ref below
median) |
1.63 (1.48,1.79) | 1.41 (1.28,1.55) |
Imputed missing values for all covariates
Partially adjusted model included all covariates except for the communication variables of interest.
Communication Barriers as Independent Predictors of Variation
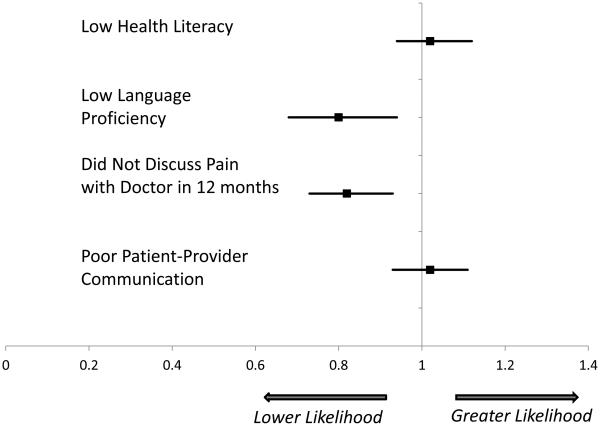
The results of our evaluation of the separate communication factors as independent predictors and as potential mediators of racial and ethnic differences are presented in Table 3. Limited English language proficiency [RR 0.80 (0.68, 0.94)] and not talking with one’s doctor about pain [RR 0.82 (0.73, 0.93)] were independently associated with the absence of clinical documentation. We found no evidence of an independent relationship between health literacy and patient-reported quality of communication with the physician on documentation of DPN.
Table 3.
Assessment of whether communication mediates the likelihood of Receiving a Clinical Diagnosis of Diabetic Peripheral Neuropathy among Adults Diabetes Patients with Symptom, DISTANCE (n=4,436)*
| Characteristics | Partially adjusted (see table 2) Model Risk Ratio (95% Confidence Interval) |
Basic Model + Health Literacy (n=3733) |
Basic Model + Language Proficiency (n=3754) |
Basic Model + Discussed Pain (n=3159) |
Basic Model + Patient-Provider Communication (n=3402) |
|---|---|---|---|---|---|
|
Race/Ethnicity (ref
white) |
|||||
| African American | 0.99 (0.91,1.07) |
0.99 (0.91,1.07) |
0.99 (0.92,1.08) |
0.96 (0.88,1.06) |
0.99 (0.91,1.07) |
| Chinese |
0.66
(0.52,0.82) |
0.65
(0.52,0.82) |
0.70
(0.56,0.88) |
0.64
(0.50,0.83) |
0.66
(0.52,0.82) |
| Latino |
0.86
(0.77,0.95) |
0.85
(0.77,0.94) |
0.90 (0.81,1.00) |
0.82
(0.73,0.92) |
0.86
(0.77,0.95) |
| Filipino |
0.68
(0.59,0.78) |
0.68
(0.59,0.78) |
0.71
(0.61,0.82) |
0.60
(0.51,0.72) |
0.68
(0.59,0.78) |
| Age (ref < 50) | |||||
| Age 50-64 | 1.27 (1.1,1.47) |
1.27
(1.1,1.46) |
1.27
(1.10,1.47) |
1.21
(1.04,1.41) |
1.27
(1.10,1.47) |
| Age 65+ |
1.47
(1.27,1.71) |
1.47
(1.27,1.71) |
1.47
(1.27,1.71) |
1.39
(1.19,1.63) |
1.48
(1.27,1.71) |
| Potential Mediators | |||||
|
Inadequate Health
Literacy (ref = no) |
-- | 1.02 (0.94,1.12) |
-- | -- | -- |
|
Low English
Language Proficiency (ref= no) |
-- | -- |
0.80
(0.68,0.94) |
-- | -- |
|
Did not discuss pain
with doctor (ref = yes) |
-- | -- | -- |
0.82
(0.73,0.93) |
-- |
|
Poor patient-
provider communication (ref=perfect score) |
-- | -- | -- | -- | 1.02 (0.93,1.11) |
All models were adjusted for the same variables as in the partially-adjusted model from Table 2 (educational attainment, BMI, gender, symptom frequency, duration of diabetes, HbA1c>9%, depression, Medicaid status, and the number of outpatient visits). Imputed missing values for all covariates except the potential mediating variables (inadequate health literacy, low English language proficiency, did not discuss pain with doctor and poor patient-provider communication).
Communication Barriers as Mediators of Racial/Ethnic Differences in Diagnosis
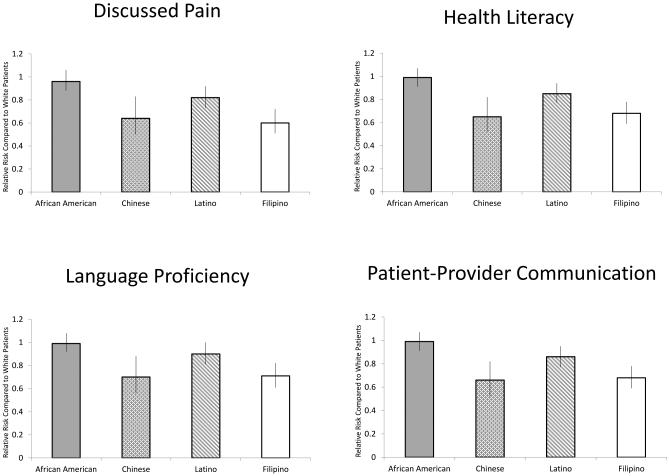
Adjustment for these communication factors did not substantively attenuate the above described variation by race/ethnicity, with the exception of language proficiency. Specifically, the effects of Chinese, Latino and Filipino race/ethnicity trended toward the null after adjustment for this factor. We also tested for, but did not find evidence of, interactions between race/ethnicity and the communication factors on DPN documentation.
Discussion
In a diverse cohort of diabetes patients, 35% (N=4,436) reported having symptoms indicating distal diabetic peripheral neuropathy. Diabetes patients with DPN symptoms had higher BMI and more outpatient visits relative to those without symptoms, which was similar to a previous study in a similar setting.33 DPN was clinically documented in the EHR for nearly half of patients who reported symptoms. Chinese, Filipino, and Latino were significantly less likely to have clinical documentation of DPN.
Low English language proficiency was independently associated with the absence of clinical documentation, as was not talking with one’s doctor about pain. While English proficiency slightly attenuated racial and ethnic differences in diagnosis, neither of these communication factors fully explained differences in DPN documentation by race/ethnicity. At the time these data were collected, interpreter and translation services were available in this setting, including in person services, phone services, and translated educational materials. However, additional services and programs related to the qualification of bilingual staff and the implementation of real-time interpreter services via remote video have been implemented in recent years.34
In addition to identifying substantive race/ethnic differences, these findings suggest that some, but not all communication factors may influence the likelihood of clinical recognition of DPN across subgroups. For example, we did not find an association between patient-reported communication quality and clinical documentation of DPN. A previous study using data from the DISTANCE survey found a positive association between patient-provider communication and medication adherence among diabetes patients.19 Our findings may suggest that the quality of communication has a different and potentially larger impact post-diagnosis than on the likelihood of clinical recognition of DPN.19 Clinical risk factors such as symptom frequency and the frequency of physician visits were more strongly associated with the outcome, suggesting that diseases that require a patient to tell the story may be under-detected relative to those that manifest in ways that are obvious to the clinician (e.g., physical signs).
This may be the first study to show differences in rates of clinical recognition of DPN by race and ethnicity. In particular, our finding of substantially lower rates of clinical recognition of DPN among Chinese, Latino and Filipino patients was enabled by the highly diverse nature of the DISTANCE cohort. However, the included communication barriers, some of which were modestly predictive, failed to explain observed differences in diagnosis by patient attributes of race/ethnicity. Moreover, no evidence of interaction effects was found, suggesting there may be other, unobserved factors that account for the social or cultural differences in detection of DPN.
Several study limitations should be taken into account. Causal inferences are not possible given the cross sectional design. The absence of a recorded clinical diagnosis in the EHR may reflect a failure to record, but not necessarily a failure to detect diabetic peripheral neuropathy. This measurement error may have caused us to underestimate rates of detection, but should not bias our estimates of the differential effect of social factors and communication on the likelihood of having a recorded diagnosis if failure to diagnose is similar across patient characteristics. While it is possible that some patients reporting DPN-like symptoms actually had other co-occurring pain conditions such as arthritis, the similarity of our symptom-based prevalence estimate to other reports of DPN suggest that such misattribution was rare. In addition, a conservative approach of including several additional ICD 9 codes to cover conditions that may have a similar presentation to DPN was employed. Similarly, DPN could be present in the absence of any patient awareness (e.g., numbness only) and this may impact the likelihood of clinical recognition and may differ by race/ethnicity.
Multiple imputation was used to account for missing data with the knowledge that the likelihood of missing covariates may have been related to patient characteristics and the likelihood of the outcome. These results were robust to sensitivity analyses that included modeling in a missing category and use of complete case analysis. However, the analysis did not control for possible non-response bias in communication related questions in the DISTANCE survey in which patients who elected not to answer questions about communication may have been systematically different in statistically significant ways. In addition, patients may have received a diagnosis outside our window of observation of two years before and after the symptom report. However, reliance on an EHR and the stable nature of this Kaiser membership make it less likely that diagnoses were missed.
Finally, because the data used were not explicitly collected for the purpose of identifying predictors of DPN, the models could not directly specify the temporal relationships between the communication variables (e.g., communicating with one’s doctor about pain) and the diagnosis of DPN.
Conclusions
Health care costs attributed to DPN are estimated to be between $5 and $15 billion per year, accounting for nearly one-third of all diabetes related costs.1 While DPN is not curable, progression can be slowed through improved glycemic control 3,35 and long term impact may be reduced through the effective treatment of symptoms.36,37 Untreated symptoms are associated with higher health services use and costs among patients with DPN.2,3,36 In addition, patients with distal DPN are at a much greater risk of lower extremity infection and amputation.38
These findings suggest that strategies to improve DPN recognition and treatment should be sensitive to both communication barriers and potential under-recognition of DPN among Chinese, Latinos and Filipino patients. Health systems should explore opportunities to reduce gaps in communication, specifically enhancing the propensity of patients to discuss pain, and alleviating language barriers between patients and providers. Competing demands in the clinic setting may impede efforts to collect information about DPN or its symptoms during routine visits.39 To address this challenge, the health care system in which this study was conducted recently instituted routine screening of diabetes patients for DPN by medical assistants while patients wait for a doctors’ appointment. Alternatively, several studies have reported success in the use of patient-focused technology (e.g., interactive voice response, web portals) to facilitate gathering of information on patient symptoms between physician visits across diverse patient populations, including those who do not speak English.40 Two of the authors (ASA, MJ) are conducting a cluster randomized controlled trial funded by the Patient Centered Outcome Research Institute (www.pcori.gov) to test the effectiveness of collectinginformation about DPN symptoms and treatment experiences directly from patients and feeding results back to their health care providers to facilitate treatment initiation or modification.[ CE-1304-7250]
Addressing communication gaps between clinicians and patients with diabetes and providers is likely to improve DPN recognition and outcomes. Regardless of the method employed, system level interventions should aim to standardize and systematize the collection of patient symptom reports, as well as to improve diagnostic strategies. A key goal of the enhanced National CLAS Standards is to provide more specific guidance to health care systems in addressing the specific communication needs of vulnerable populations, including those who do not speak English, in order to facilitate the translation of these strategies across diverse care settings.41 This study found limited evidence that addressing communication with patients with limited English proficiency may reduce the amount of under-diagnosed DPN in certain subgroups of patients. Given the inability of this study to explain most of the differences by race/ethnicity, future studies should work toward elucidating and addressing the causes of those differences.
Figure 1.
Relationship between Communication Barriers and the Likelihood of Receiving a Clinical Diagnosis of Diabetic Peripheral Neuropathy
Figure 2.
Evaluation of Mediation of Race/Ethnicity as a Predictor of the Likelihood of Diagnosis by Specific Communication Barriers
Acknowledgements
The authors would also like to thank data consultants, Bruce Folck and Meng Lu at the Kaiser Permanente Division of Research, for assistance with data linkages. Dr. Adams conceptualized the work, interpreted the data, and drafted the manuscript. Dr. Parker conducted the analysis and edits/revised the manuscript. Drs. Moffet, Jaffe, Schillinger, Callaghan, Piette, Adler, Bauer and Karter contributed to the conceptualization of the paper, interpretation of the data, and revisions/edits to the manuscript. Dr. Karter also provided the data.
Funding for this study was provided by the National Institute of Diabetes, Digestive and Kidney Diseases [R01DK081796, RC1 DK086178, R01DK080726, R01DK65664, P30 DK092924], the Division of Diabetes Translation, Centers for Disease Control and Prevention [U58 DP002641]. This work was (partially) supported through a Patient-Centered Outcomes Research Institute (PCORI) Assessment of Prevention, Diagnosis, and Treatment Options Program Award [CE-1304-7250].
Footnotes
DISCLAIMER: All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
Data Access
We are not free to release participants' personal data under our promise to them regarding confidentiality. However, the DISTANCE study team is interested in collaborations with external researchers. DISTANCE investigators are particularly interested in comparing processes and outcomes in this insured population with uniform access to care to disparities observed in population-based samples, where quality and access to care vary widely by social strata. Requests for collaboration must be submitted to the director of the central coordinating center. Before collaborations can be initiated, proposals require review and approval by the Publications and Presentations (P&P) Committee. This committee was formed to: (i) Ensure accurate, uniform, timely and high quality reporting of DISTANCE findings; (ii) Preserve the scientific integrity of the study and (iii) Safeguard the rights and confidentiality of participants. For more information, please contact Howard.H.Moffet@kp.org.
References
- 1.Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26:1790–1795. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- 2.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in Type 2 diabetes. Diabetes Care. 2006;29:1518–1522. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 3.Sadosky A, Schaefer C, Mann R, Bergstrom F, Baik R, Parsons B, Nalamachu S, Nieshoff E, Stacey BR, Anschel A, Tuchman M. Burden of illness associated with painful diabetic peripheral neuropathy among adults seeking treatment in the US: results from a retrospective chart review and cross-sectional survey. Diabetes Metab Syndr Obes. 2013;6:79–92. doi: 10.2147/DMSO.S37415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain R, Jain S, Raison CL, Maletic V. Painful diabetic neuropathy is more than pain alone: examining the role of anxiety and depression as mediators and complicators. Curr Diab Rep. 2011;11:275–284. doi: 10.1007/s11892-011-0202-2. [DOI] [PubMed] [Google Scholar]
- 5.Laiteerapong N, Karter AJ, Liu JY, Moffet HH, Sudore R, Schillinger D, John PM, Huang ES. Determinants of quality of life in older adults with diabetes: The Diabetes & Aging Study. Diabetes Care. 2011;34:1749–1753. doi: 10.2337/dc10-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sudore RL, Karter AJ, Huang ES, Moffet HH, Laiteerapong N, Schenker Y, Adams A, Whitmer RA, Liu JY, Miao Y, John PM, Schillinger D. Symptom burden of adults with type 2 diabetes across the disease course: Diabetes & Aging Study. J Gen Intern Med. 2012;27(12):1674–81. doi: 10.1007/s11606-012-2132-3. DOI: 10.1007/s11606-012-2132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall GC, Carroll D, McQuay HJ. Primary care incidence and treatment of four neuropathic pain conditions: a descriptive study, 2002-2005. BMC Fam Pract. 2008;9:26. doi: 10.1186/1471-2296-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27:1458–1486. doi: 10.2337/diacare.27.6.1458. [DOI] [PubMed] [Google Scholar]
- 9.Maiman LA, Becker MH. The Health Belief Model: Origins and correlates in psychological theory. Health Educ Monogr. 1974;2:336–353. [Google Scholar]
- 10.Janz NK, Becker MH. Health Educ Q. Vol. 11. Spring; 1984. The Health Belief Model: A decade later; pp. 1–47. [DOI] [PubMed] [Google Scholar]
- 11.Herr K. Neuropathic pain: A guide to comprehensive assessment. Pain Management Nursing. 2004;5(Suppl 1):9–18. doi: 10.1016/j.pmn.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Abbo ED, Zhang Q, Zelder M, Huang ES. The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med. 2008 Dec;23(12):2058–2065. doi: 10.1007/s11606-008-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kalauokalani DA, Lasch KE, Myers C, Tait RC, Todd KH, Vallerand AH. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4:277–294. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 14.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain. 2009;10(12):1187–204. doi: 10.1016/j.jpain.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Moffet HH, Adler N, Schillinger D, Ahmed AT, Laraia B, Selby JV, Neugebauer R, Liu JY, Parker MM, Warton M, Karter AJ. Cohort Profile: The Diabetes Study of Northern California (DISTANCE)--objectives and design of a survey follow-up study of social health disparities in a managed care population. Int J Epidemiol. 2009;38:38–47. doi: 10.1093/ije/dyn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor PJ, Rush WA, Pronk NP, Cherney LM. Identifying diabetes mellitus or heart disease among health maintenance organization members: Sensitivity, specificity, predictive value, and cost of survey and database methods. Am J Man Care. 1998;4:335–342. [PubMed] [Google Scholar]
- 16.Hargraves JL, Hays RD, Cleary PD. Psychometric properties of the Consumer 351 Assessment of Health Plans Study (CAHPS) 2.0 adult core survey. Health Serv Res. 2003;38(6 Pt 1):1509–1527. doi: 10.1111/j.1475-6773.2003.00190.x. 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer AM, Schillinger D, Parker MM, Katon W, Adler N, Adams AS, Moffet HH, Karter AJ. Health literacy and antidepressant medication adherence among adults with diabetes: the diabetes study of Northern California (DISTANCE) J Gen Intern Med. 2013;28:1181–7. doi: 10.1007/s11606-013-2402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratanawongsa N, Karter AJ, Parker MM, Lyles CR, Heisler M, Moffet HH, Adler N, Warton EM, Schillinger D. Communication and medication refill adherence: the Diabetes Study of Northern California. doi: 10.1001/jamainternmed.2013.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam.Med. 2004;36:588–594. [PubMed] [Google Scholar]
- 20.Sarkar U, Schillinger D, Lopez A, Sudore R. Validation of self-reported health literacy questions among diverse english and spanish-speaking populations. JGIM. 2011;26:265–271. doi: 10.1007/s11606-010-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkar U, Karter AJ, Liu JY, Adler NE, Nguyen R, Lopez A, Schillinger D. The literacy divide: Health literacy and the use of an internet-based patient portal in an integrated health system-results from the diabetes study of northern california (distance) J Health Commun. 2010;15(Suppl 2):183–96. doi: 10.1080/10810730.2010.499988. 183-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar U, Karter AJ, Liu JY, Moffet HH, Adler NE, Schillinger D. Hypoglycemia is more common among type 2 diabetes patients with limited health literacy: The diabetes study of northern california (distance) JGIM. 2010;25:962–968. doi: 10.1007/s11606-010-1389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karter AJ, Subramanian U, Saha C, Crosson JC, Parker MM, Swain BE, Moffet HH, Marrero DG. Barriers to insulin initiation: The translating research into action for diabetes insulin starts project. Diabetes Care. 2010;33:733–735. doi: 10.2337/dc09-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer AM, Parker MM, Schillinger D, Katon W, Adler N, Adams AS, Moffet HH, Karter AJ. Associations between antidepressant adherence and shared decision-making, patient-provider trust, and communication among adults with diabetes: Diabetes Study of Northern California (DISTANCE) J Gen Intern Med. 2014 Apr 5; doi: 10.1007/s11606-014-2845-6. [ePub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krein SL, Heisler M, Piette D, Maki F, Kerr EA. The effect of chronic pain on diabetes patients' self-management. Diabetes Care. 2005;28:65–70. doi: 10.2337/diacare.28.1.65. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke K, Stine TW, Spitzer RL, Williams JB, Barry JT, Mokdad AH. J The PHQ-8 as a measure of current depression in the general population. Affect Disord. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 28.Rubin DB, Schenker N. Multiple imputation in health care databases: an overview and some applications. Stat Med. 1991;10:585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 29.Balsa AI, McGuire TG, Meredith LS. Testing for statistical discrimination in health care. HSR. 2005;40:227–252. doi: 10.1111/j.1475-6773.2005.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beal DJ. Information Criteria Methods for SAS for Multiple Linear Regression Models; SESUG Proceedings, Paper SA 05; (insert URL) [Google Scholar]
- 31.SAS Institute Inc. SAS Onlind Doc. SAS Institute Inc; Cary, NC: pp. 202–2006. Version 9. [Google Scholar]
- 32.Ritzwoller DP, Ellis JL, Korner EJ, Hartsfield CL, Sadosky A. Comorbidities, healthcare service utilization and costs for patients identified with painful DPN in a managed-care setting. Curr Med Res Opin. 2009 Jun;25(6):1319–28. doi: 10.1185/03007990902864749. [DOI] [PubMed] [Google Scholar]
- 33.Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6:CD007543. doi: 10.1002/14651858.CD007543.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaiser Permanente Institute for Health Policy Kaiser Permanente’s Qualified Bilingual Staff Model. Kaiser Permanente Policy Story. 2014;3(2) http://www.kpihp.org/wp-content/uploads/2014/05/KPStories-v3n2-KP-Bilingual-Model-FINAL.pdf. [Google Scholar]
- 34.Lindsay TJ, Rodgers BC, Savath V, Hettinger K. Treating diabetic peripheral neuropathic pain. Am Fam Physician. 2010;82:151–158. [PubMed] [Google Scholar]
- 35.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28(Suppl 1):8–14. doi: 10.1002/dmrr.2239. [DOI] [PubMed] [Google Scholar]
- 36.Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care. 1989;12:24–31. doi: 10.2337/diacare.12.1.24. [DOI] [PubMed] [Google Scholar]
- 37.Abbo ED, Zhang Q, Zelder M, Huang ES. The increasing number of clinical items addressed during the time of adult primary care visits. J Gen Intern Med. 2008;23:2058–2065. doi: 10.1007/s11606-008-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollak KI, Krause KM, Yarnall KS, Gradison M, Michener JL, Ostbye T. Estimated time spent on preventive services by primary care physicians. BMC Health Serv Res. 2008;8:245. doi: 10.1186/1472-6963-8-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostbye T, Yarnall KS, Krause KM, Pollak KI, Gradison M, Michener JL. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3:209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piette JD, Marinec N, Gallegos-Cabriales EC, Gutierrez-Valverde JM, Rodriguez-Saldaña J, Mendoz-Alevares M, Silveira MJ. Spanish-speaking patients' engagement in interactive voice response (IVR) support calls for chronic disease self-management: data from three countries. J Telemed Telecare. 2013;19:89–94. doi: 10.1177/1357633X13476234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Office of Minority Health US Department of Health and Human Services. The National CLAS Standards. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=2&lvlid=53.