Abstract
Accurate beliefs about partners’ viral suppression are important for HIV prevention and care. We fit multilevel mixed effects logistic regression models to examine associations between partners’ viral suppression beliefs and objective HIV RNA viral load tests, and whether relationship dynamics were associated with accurate viral suppression beliefs over time. Male couples (N=266 couples) with at least one HIV-positive partner on antiretroviral therapy completed five assessments over two years. Half of the 407 HIV-positive partners were virally suppressed. Of the 40% who had inaccurate viral load beliefs, 80% assumed their partner was suppressed. The odds of having accurate viral load beliefs decreased over time (OR=0.83; p=.042). Within-couple differences in dyadic adjustment (OR=0.66; p<.01) and commitment (OR=0.82; p=.022) were negatively associated with accurate viral load beliefs. Beliefs about a partner's viral load may factor into sexual decision-making and social support. Couple-based approaches are warranted to improve knowledge of partners’ viral load.
Keywords: HIV/AIDS, viral suppression, partner beliefs, relationship dynamics, couples
Abstract
Creencias precisas de la supresión viral de la pareja de una persona son importantes para la prevención y atención del VIH. Nos usamos una regresión logística multinivel modelos de efectos mixtos para examinar las asociaciones entre las creencias acerca de la supresión viral y pruebas objetivas de la carga viral de ARN del VIH de la pareja de una persona, y si dinámica de la relación se asociaron con preciso las creencias supresión viral con el tiempo. Pareja hombres (N = 266 parejas) con al menos una pareja con VIH en terapia antirretroviral completado cinco evaluaciones de más de dos años. La mitad de las 407 parejas con VIH había conseguido una supresión viral. Del 40% que tenía creencias inexactas de carga viral, el 80% asume su pareja fue suprimida. Dentro de parejas diferencias de ajuste diádico (OR=0.66; p<.01) y compromiso (OR=0.82; p=.022) se asociaron negativamente con las carga viral creencias. Las creencias sobre la carga viral de la pareja pueden tener en cuenta en la toma de decisiones sexuales y el apoyo social. Las probabilidades de tener creencias precisas de carga viral disminuyó con el tiempo (OR=0.83; p=.042). Los enfoques que utilizan las parejas están justificadas y puedan mejorar el conocimiento de la carga viral de la pareja de una persona.
Introduction
HIV-positive individuals with an undetectable viral load are significantly less likely to transmit HIV to their sexual partners (1-3). Growing recognition of the prevention benefits of HIV treatment, coupled with the rapid expansion of antiretroviral therapy (ART), has prompted the adoption of new HIV risk reduction approaches that incorporate viral load information—a process referred to as “viral sorting” (4). Viral sorting is one of many HIV risk-reduction strategies used among men who have sex with men (MSM) to prevent the transmission of HIV through condomless anal intercourse (CAI) (5). Among same-sex male couples, discussions of viral load are relatively common (6) and partners incorporate this information into decisions to engage in CAI (4, 5, 7, 8). Studies of men in serodiscordant relationships suggest that CAI is more likely to occur when HIV-positive partners are perceived to have an undetectable viral load (7, 8). In comparison to partner perceptions, actual viral load test results from the HIV-positive partner's perspective may be less important in decision-making around CAI. A study on gay men in Sydney found that for HIV-negative partners, the belief that a partner had an undetectable viral load was associated with CAI; however, viral load test results indicating an undetectable viral load were not associated with HIV-positive men's reports of CAI (8). Indeed, a meta-analysis examining studies conducted across many different samples concluded that patients on ART did not exhibit increased sexual risk behavior even if they were virally suppressed (9).
If heightened sexual risk is more related to partner beliefs than actual viral load test results, it would be important to assess the accuracy of these beliefs. Correct beliefs about a partner's viral suppression are needed to make informed, and potentially safer, decisions around sexual behavior. Other research on seroadaptive strategies among MSM suggests that even if men know their sex partners, they may practice “seroguessing” based on assumptions of a partner's HIV status rather than “serosorting”, which involves direct discussions of a partner's HIV status (10). In the case of viral sorting, relying on assumptions as opposed to disclosed information may undermine the effectiveness of these practices. Furthermore, viral load test results are dynamic and can change over time. Failure to disclose new viral load test results could contribute to outdated and thus inaccurate beliefs about a partner's viral load.
Even if viral information is routinely disclosed, couple communication is a complex process that requires both a transmitter and a receiver with the opportunity for error on both sides of the interaction (11). Indeed, there is evidence to suggest that partner beliefs about viral load could be inaccurate. Studies on heterosexual couples conducted outside of the U.S. have found that perceptions of a partner's HIV status and actual serostatus are often mismatched (12) and that there can be discrepancies between partners about whether HIV status disclosure has occurred (13-15). Yet, little research, if any, has examined the accuracy of partner beliefs about viral suppression and whether these beliefs change as treatment and relationship trajectories evolve over time.
Beyond the implications for HIV transmission, having accurate knowledge of partner viral suppression is important for the provision of social support related to HIV care and treatment. Social relationships can enhance medication adherence by reminding, prompting, aiding, and supporting the patient, assisting the patient in expressing feelings, and by offering feedback that reinforces treatment success (16). Among people living with HIV, social support is associated with better mental health (17-19), higher ART adherence (20-22), and slower disease progression (23). For male couples affected by HIV, partners provide an important source of social support (24, 25), which may be associated with higher ART adherence than similar support received from non-partners (26). In addition, it has been shown that HIV interventions emphasizing or including elements of partner social support are relatively efficacious at increasing ART adherence (27, 28).
The intensity and type of social support provided by partners may hinge on having accurate beliefs about health information such as viral load. According to the interdependence model of communal coping (29), couple members’ perceptions of a health threat may serve as a cue to action that elicits behavior change through the process of “transformation of motivation” (30)—or the movement from a self-centered orientation to one that is more pro-relationship and health-enhancing. When couples undergo this process, they would be more likely to engage in “communal coping” efforts by working collaboratively together to reduce the threat of a particular health issue. For example, men who assume their partner is virally suppressed could provide less social support (e.g., less communal coping) than if they believed their partner was unsuppressed and more ill. Thus, the failure or delay to act based on inaccurate knowledge of a partner's viral suppression may result in missed opportunities to provide social support, with the potential to improve partner health and well-being.
An important set of factors influencing the accuracy of partner beliefs about viral suppression may be relationship dynamics. Relationship dynamics such as intimacy, trust, communication, satisfaction, and commitment affect the dyadic capacity for successful coordination of health behaviors related to HIV (31). In other areas of HIV research, couples-based studies have shown that relationship dynamics such as trust, satisfaction, and commitment have been associated with CAI (32-35), use of sexual agreements (36, 37), and viral suppression (38). The interdependence model of communal coping (29) also posits that positive relationship dynamics such as satisfaction and commitment foster a transformation of motivation, which facilitates the process of communal coping. If communal coping occurs, we would expect partners to hold highly accurate beliefs about each other's HIV health status so that they can effectively support each other and cope with the threat of HIV together. While we do not measure communal coping in this study, we hypothesize that positive relationship dynamics (e.g., higher intimacy) will be positively associated with accurate beliefs about a partner's viral load. New information on relationship dynamics and beliefs about partner viral suppression could inform couples-based interventions to enhance partner social support as a pathway to optimal health.
Given this background literature and theory, the objectives of this study were: (1) to evaluate the extent to which partner beliefs about viral suppression correspond with objective biomarkers of viral load from blood tests; (2) to assess whether the accuracy of partner beliefs about viral suppression changes over time; (3) to identify which relationship dynamics may affect the accuracy of beliefs about a partner's viral suppression; and (4) to determine whether associations between relationship dynamics and accurate beliefs about a partner's viral suppression depends on couple HIV status.
Methods
Study Procedures
The data come from the “Duo Project”, which is a longitudinal study on same-sex male couples with at least one partner who is HIV-positive and on an acknowledged ART regimen. Data collection spanned two years for each couple, starting in January 2009. Participants were recruited from the San Francisco Bay Area in the United States (U.S.) using passive recruitment strategies and participant and provider referrals. Flyers were posted in clinics, community bulletin boards, AIDS service organizations, and at other community-based organizations. Media ads were placed in publications targeting HIV-positive and gay/bisexual men. Interested participants could contact study staff for more information on the study. Male couples were eligible for the study if the couple met the following criteria: (1) in a primary relationship, which was defined as “currently (for at least 3 months) in a relationship with someone you feel committed to above anyone else and with whom you have had a sexual relationship”; (2) at least 18 years old; (3) born male and currently identify as male; (4) has at least one partner who is HIV-positive and on an acknowledged ART regimen for at least 30 days; (5) English-speaking; and (6) able and willing to provide informed consent.
Each partner was screened separately over the phone to assess eligibility and if both partners were eligible, couples were scheduled for an in-person interview at the study research center. Both partners were required to attend the assessment appointments together, however, they were separated during data collection. Data were collected using a combination of Computer Assisted Personal Interviewing (CAPI) and Audio Computer Assisted Self Interviewing (ACASI) methods, which optimize data integrity through the reduction of data entry errors while minimizing the effects of social desirability bias (39). Couples were asked to participate in a total of five assessments, occurring every six months. At each assessment, participants were asked about relationship dynamics (e.g., commitment, satisfaction, intimacy) and perceptions of their partner's viral load. All HIV-positive participants had blood drawn for viral load tests at baseline, 12-months, and 24-months.
Ethical approval was obtained from the Committee on Human Research at the University of California, San Francisco. Written informed consent was obtained from all participants. Each partner of the couple was paid US $50 for each survey completed and HIV-positive participants were paid an additional $10 for providing a blood sample.
Measures
Background and Control Variables
Given the literature on couples and HIV (32, 38), we controlled for the following relationship factors as potential confounders: cohabitation status, relationship length, and couple HIV status. For example, it is possible that couples who are together longer might have more positive relationship dynamics and may also have better knowledge about their partner's HIV health information—which could confound the association between relationship dynamics and accurate beliefs about a partner's viral suppression. Relationship length was assessed by asking participants, “How long have you and your partner been together as a couple?” (calculated in months). We computed the average relationship length using both partners’ accounts. Cohabitation status was a binary variable assessed by the participants, “Are you currently living with your partner?” (yes/no). Couple HIV status was a binary variable computed using self-reported HIV status from both partners (discordant/concordant). Because HIV-negative men did not have confirmatory HIV tests performed, we relied on self-reported HIV status to compute couple HIV status (HIV-positive men's HIV status was directly confirmed through ART verification and indirectly confirmed via viral load testing).
Explanatory Variables (Relationship Dynamics)
We used validated scales to capture six relationship factors: relationship satisfaction, dyadic adjustment, relationship commitment, intimacy, equality, and constructive communication. Coefficient alphas were computed for the baseline sample of 407 HIV-positive men with viral load information. For all scales, higher scores indicate more positive relationship dynamics. Relationship satisfaction was measured using the 4-item Couples Satisfaction Index (40). Men were asked their level of agreement with statements such as “I have a warm and comfortable relationship with my partner” (α = 0.93). Response options ranged from 0 (not true at all) to 5 (completely true). Dyadic adjustment was measured using the 6-item Dyadic Adjustment Scale (41, 42), which taps into perceptions of how well things are going in the relationship and how often the partners confide in each other, laugh together, and calmly discuss matters. Men were asked questions such as: “In general, how often do you think that things between you and your partner are going well?” (α = 0.83). Response options ranged from 0 (never) to 5 (all of the time). Relationship commitment, intimacy, and equality were assessed with an adapted set of scales from Kurdek's work with couples (43). Relationship commitment was a 4-item scale consisting of statements such as “I am committed to maintaining my relationship with my partner”. Response options ranged from 1 (not at all true) to 9 (extremely true) (α = 0.95). Intimacy was a 6-item scale consisting of statements such as “I spend as much time as possible with my partner” (α = 0.72). Response options ranged from 0 (not at all true) to 9 (extremely true). Equality was an 8-item scale consisting of statements such as “My partner and I have equal power in the relationship” (α = 0.92). Response options ranged from 0 (not at all true) to 9 (extremely true). Constructive communication was measured using the 5-item constructive communication subscale of the Communications Patterns Questionnaire (44). Men were asked their level of agreement with statements such as “When a problem or issue arises, both of us try to discuss the problem” (α = 0.74). Response options ranged from 1 (very unlikely) to 9 (very likely).
For each explanatory variable, we computed the couple-level means and differences to separately estimate between-couple and within-couple effects and to reduce bias that would otherwise occur if we were to assume these two effects were equivalent for a given explanatory variable (45, 46). In particular, couple-level differences were computed using the absolute value of the difference between both partners’ scores. We hypothesized that a greater difference between partners (regardless of which partner has a higher/lower value) would be negatively associated with accurate beliefs about a partner's viral suppression and that a higher couple-level mean would be positively associated with accurate beliefs about a partner's viral suppression.
Dependent Variable (Accurate Partner Beliefs about Viral Suppression)
HIV RNA viral load tests were performed using the COBAS® AmpliPrep/COBAS® TaqMan® HIV test kit (Roche Molecular Systems, Inc.), which has a threshold for undetectability of ≤ 48 copies/ml. Viral suppression was dichotomized as detectable versus undetectable using this cutoff value. A binary variable was used to capture accurate partner beliefs about viral suppression using viral load tests and partner reports. Partner reports were captured with the question, “Was your partner's last viral load detectable or undetectable?” Response options included: detectable, undetectable, or refuse to answer. Very few men refused to answer the question. If the blood test indicated the participant was virally suppressed and their partner reported they had an undetectable viral load, accurate partner beliefs was coded as “1”. Similarly, if the blood test indicated that the participant was not virally suppressed and their partner reported that they had a detectable viral load, accurate partner beliefs was coded as a “1”. If blood test results and partner reports did not match, accurate partner beliefs was coded as “0”.
Data Analysis
One-way frequency tables and measures of central tendency were generated to characterize the sample at baseline. For the main analysis, we used a hierarchical modeling approach. The data structure was hierarchical at three levels: time (level 1), within individuals (level 2), and within couples (level 3). Starting with the couple-level, we computed the Intraclass Correlation Coefficient (ICC) for each relationship dynamic to assess the degree of similarity between the two dyad members. ICCs can also be used to further justify the use of a multi-level approach with hierarchical data. The value ranges from 0 to 1, with a higher ICC indicating that individuals within dyads are more similar in their relationship dynamic than any other two individuals in the study (47). The ICC was computed using a one-way ANOVA with the couple identifier as the grouping variable. The ICCs were non-zero and statistically significant with the exception of commitment, which was marginally significant (p=.059). Overall, the value and significance of the ICCs (shown in Table 1) indicated the presence of shared variance at the couple-level and justified the use of a multi-level model to account for dyadic clustering.
Table 1.
Baseline characteristics of 407 HIV-positive men with viral load tests from the Duo Project
| Variable | |
|---|---|
| Demographic characteristics | %, Mean (SD) |
| Age (years) | 45.5 (10.0) |
| Race | |
| Black | 17.7 |
| White | 54.1 |
| Other | 28.2 |
| Latino ethnicity | 18.9 |
| Income level (per year) | |
| <$10,000 | 23.3 |
| $10,000-19,999 | 30.7 |
| $20,000-29,999 | 10.8 |
| >$30,000 | 35.2 |
| Employed | 42.3 |
| Relationship characteristics | |
| Living together | 79.9 |
| Relationship duration (years) | 6.3 (6.5) |
| Seroconcordant positive | 71.5 |
| Both on ART | 53.8 |
| Relationship dynamics | Within-Couple Mean (SD) | Within-Couple Difference (SD) | ICC |
|---|---|---|---|
| Relationship satisfaction (range: 0-5) | 3.7 (0.9) | 0.9 (0.8) | 0.40* |
| Closeness (dyadic adjustment) (range: 0-6) | 3.7 (0.7) | 0.7 (0.6) | 0.35* |
| Commitment (range: 1-9) | 8.0 (1.2) | 1.2 (1.6) | 0.15† |
| Intimacy (range: 1-9) | 6.3 (1.1) | 1.4 (1.2) | 0.25* |
| Equality (range: 1-9) | 7.0 (1.4) | 1.4 (1.3) | 0.35* |
| Constructive communication (range: 1-9) | 5.1 (0.6) | 0.8 (0.7) | 0.37* |
ICC=Intraclass correlation coefficient.
p<.10
p<.05
Moving on to the longitudinal structure of the dyadic data, we tested for associations between six continuous explanatory variables (satisfaction, dyadic adjustment, commitment, intimacy, equality, and constructive communication) and the binary outcome of accurate partner beliefs. We used random effects models to investigate the accuracy of partner beliefs over time. In comparison to standard regression, random effects models allow for the inclusion of one or more random effects to quantify the within-individual variability across repeated measurements (48). Random effects can include random intercepts, which estimate the variability in individuals’ initial beliefs about a partner's viral suppression, and random slopes, which estimate the variability of individuals’ trajectories of beliefs about a partner's viral suppression over time. While it is feasible to have a random slope for individuals nested within visits (level 2), the random slopes for the couple-level (level 3) are constrained to be equal across all dyads. Dyads do not have enough lower-level units (i.e., dyad members) to allow the slopes to vary from dyad to dyad (47).
To aid in model selection, we fit two sets of models for each of the six explanatory variables: (1) models with a random slope for individuals across time and separate random intercepts for the couple-level and individual-level; (2) models with random intercepts only (no random slopes). The models were fit using multilevel mixed effects logistic regression, which included the couple-level means and differences for the explanatory variable, and the visit number (as a proxy for time). We computed AIC and BIC statistics for the two sets of models (random intercept plus slopes vs. random intercept-only) for each of the six explanatory variables and examined the differences in values. All six models that included the random slope for time produced higher AIC and BIC statistics, indicating poorer model fit in the Stata software program and a preference for the intercept-only models (49). Therefore, the final models included only random intercepts for the couple-level and individual-level. The visit number (time) was entered as a fixed effect in all models. For the bivariate models, we fit six separate models for each relationship dynamic by including couple-level means and differences and the fixed effect for time. For the adjusted models, we included 12 explanatory variables (couple-level means and differences corresponding to the six relationship dynamics), a fixed effect for time, and three potential confounding variables (relationship length, cohabitation, and couple HIV status). All models were fit in Stata 13.1 using the melogit command with robust standard errors clustering on the couple identifier.
Prior to fitting the final models, we evaluated whether accurate beliefs about a partner's viral suppression differed by couple HIV status—as these differences could have implications for HIV prevention. We fit a model with 12 explanatory variables (couple-level means and differences), a fixed effect for time, a main effect for couple HIV status, and 12 interaction terms between each explanatory variable and couple HIV status. Then we performed a global test of association for the interaction terms using the Wald test (50). Because there was no evidence of any significant interactions (χ2(12)=12.62; p=0.397), we presented the results for the main effects model only.
Additional checks were performed to evaluate multi-collinearity among all predictor variables and the level of missing data at baseline, 12-months, and 24-months. The variance inflation factor (VIF) for all variables was well below the recommended cutoff of 10 (51), indicating that multi-collinearity was not an issue. There was no missing data on any explanatory or control variables for those who participated in each study visits. Only a few HIV-positive men had missing viral load information because they did not receive a blood draw (for example, at baseline, 5 participants had missing viral loads). There were missing data for the outcome across time due to attrition (see sample sizes in Table 2); however, one of the advantages of a multilevel approach with estimation performed via maximum likelihood is the ability to handle unbalanced data by using all available data in the analysis under the missing at random (MAR) assumption (52).
Table 2.
Viral load test results, partner beliefs, and accurate partner beliefs about viral suppression at three study visits of the Duo Project
| Visit 1 | Visit 2 | Visit 3 | |
|---|---|---|---|
| Total sample (N) | 407 | 319 | 263 |
| Virally suppressed (blood test), % | 49.9 | 51.1 | 49.4 |
| Virally suppressed (partner belief), % | 74.2 | 84.9 | 89.7 |
| Accurate partner belief about viral suppression, % | 60.0 | 55.5 | 53.6 |
| For those who were inaccurate, did they believe partner was suppressed? % | 80.4 | 88.0 | 93.4 |
| Seroconcordant couples (N) | 291 | 227 | 192 |
| Virally suppressed (blood test), % | 45.0 | 48.9 | 49.0 |
| Virally suppressed (partner belief), % | 72.9 | 84.1 | 90.1 |
| Accurate partner belief about viral suppression, % | 59.8 | 53.3 | 54.7 |
| For those who were inaccurate, did they believe partner was suppressed? % | 84.6 | 87.7 | 95.4 |
| Serodiscordant couples (N) | 116 | 92 | 71 |
| Virally suppressed (blood test), % | 62.1 | 56.5 | 50.7 |
| Virally suppressed (partner belief), % | 77.6 | 87.0 | 88.7 |
| Accurate partner belief about viral suppression, % | 60.3 | 60.9 | 50.7 |
| For those who were inaccurate, did they believe partner was suppressed? % | 69.6 | 88.9 | 88.6 |
Results
Of the 532 men who participated in the study at baseline, a subset of HIV-positive men (407 men nested within 266 couples) supplied outcome data by having a viral load test result available to compare against their partner's perception. Characteristics of the full baseline sample of 532 men are presented elsewhere (53). For the 407 HIV-positive men in our analysis, the majority were on ART at baseline (91%). The men were, on average, middle-aged (mean=45.5 years; SD=10.0), and many were predominantly white (54.1%) and non-Latino (81.1%) (see Table 1). The men had relatively low levels of income (64.8% had an annual income of less than $30,000), which may reflect high unemployment rates in the sample (57.7%). The majority of men were living with their partner (79.9%) and had been together on average for 6.3 years (SD=6.5; range: 3 months to 33 years). Most men were in an HIV-positive seroconcordant relationship (71.5%) and in approximately half of the couples, both partners were on ART (53.8%). For the six relationship dynamics, the within-couple means and differences are presented in Table 1.
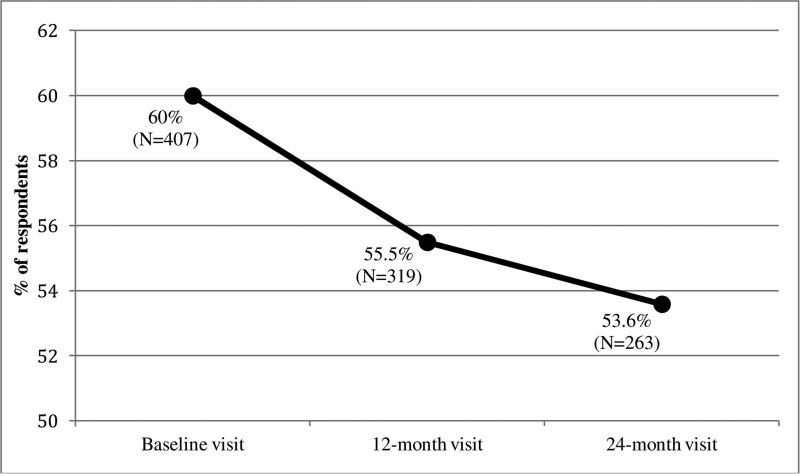
Table 2 presents the descriptive statistics for the viral load test results, partner beliefs about viral load, and accurate partner beliefs across the three study visits. For the sample of 407 HIV-positive men, rates of viral suppression (as measured by blood tests) were relatively constant across time—with approximately 50% of the sample being virally suppressed. Partners of the HIV-positive men were more likely to believe the men were virally suppressed, which also increased over time (from 74.2% at baseline to 89.7% at the 24-month visit). Thus, using both viral load test results and partner beliefs to measure accurate partner beliefs, the results show a steady decrease in accuracy over time (from 60.0% at baseline to 53.6% at the 24-month visit; see Figure 1 and Table 3). Among the men who were incorrect about their partner's viral suppression at baseline, 80.4% of these men had assumed their partner was virally suppressed when they were not. The incorrect belief that a partner was virally suppressed became more prevalent over time with 93.4% of men incorrectly assuming their partner was virally suppressed at the 24-month visit.
Figure 1.
Percentage of HIV-positive men with partners who had accurate beliefs about their viral suppression over three study visits of the Duo Project
Table 3.
Odds ratios and 95% CIs from multilevel mixed effects logistic regression models predicting accurate partner beliefs about viral suppression (N=412 individuals; 265 couples; 989 observations)
| Predictor variable | OR | 95% CI | p | AOR | 95% CI | p |
|---|---|---|---|---|---|---|
| Fixed effect for time | 0.83 | 0.69-0.99* | 0.042 | 0.79 | 0.66-0.96* | 0.016 |
| Confounding variables (adjusted models only) | ||||||
| Relationship duration | -- | -- | -- | 1.00 | 1.00-1.01† | 0.079 |
| Cohabitation | -- | -- | -- | 1.14 | 0.74-1.75 | 0.562 |
| Couple HIV status | -- | -- | -- | 0.98 | 0.65-1.47 | 0.912 |
| Relationship dynamics (couple-level means) | ||||||
| Relationship satisfaction | 1.01 | 0.84-1.23 | 0.908 | 1.36 | 0.84-2.20 | 0.216 |
| Dyadic adjustment | 0.91 | 0.72-1.16 | 0.461 | 0.84 | 0.49-1.44 | 0.532 |
| Commitment | 0.87 | 0.74-1.02† | 0.081 | 0.80 | 0.63-1.01† | 0.062 |
| Intimacy | 1.03 | 0.90-1.18 | 0.681 | 1.09 | 0.91-1.31 | 0.385 |
| Equality | 0.92 | 0.80-1.05 | 0.218 | 0.80 | 0.62-1.01† | 0.065 |
| Constructive communication | 1.00 | 0.88-1.15 | 0.948 | 1.12 | 0.91-1.39 | 0.281 |
| Relationship dynamics (couple-level differences) | ||||||
| Relationship satisfaction | 0.85 | 0.67-1.07 | 0.169 | 1.05 | 0.78-1.42 | 0.736 |
| Dyadic adjustment | 0.66 | 0.50-0.87** | 0.003 | 0.69 | 0.49-0.98* | 0.038 |
| Commitment | 0.82 | 0.70-0.97* | 0.022 | 0.87 | 0.73-1.04 | 0.125 |
| Intimacy | 0.99 | 0.85-1.14 | 0.852 | 1.08 | 0.91-1.31 | 0.385 |
| Equality | 0.88 | 0.75-1.04 | 0.128 | 0.94 | 0.78-1.11 | 0.447 |
| Constructive communication | 0.96 | 0.82-1.13 | 0.663 | 1.08 | 0.90-1.28 | 0.417 |
OR=Odds Ratio. AOR=Adjusted Odds Ratio. CI=Confidence Interval. Six bivariate models were fit for each relationship dynamic by including couple-level mean and differences, and a fixed effect for time. A single multivariate model was fit for all variables listed.
p<.10
p<.05
p<.01
***p<.001.
Table 2 also presents the corresponding results for HIV-positive men with viral load tests who were in HIV-positive seroconcordant and serodiscordant relationships. At baseline, rates of viral suppression were higher among the men in serodiscordant than HIV-positive seroconcordant relationships (45.0% versus 62.1%; F(1, 262)=9.2; p<.01). However, this difference was attenuated over time such that by the 24-month visit, the rates of viral suppression between the two groups were essentially the same (49.0% versus 50.7%; F(1, 169)=0.06; p=.809). HIV-negative partners in serodiscordant relationships were not any more or less likely to believe their partners were virally suppressed—at any visit—as compared to HIV-positive partners in seroconcordant relationships (see Table 2 for percentages; all F-statistics indicated non-significant differences between the two groups).
To assess whether accurate partner beliefs changed over time, we included a fixed effect for time in the multilevel mixed effects regression models. In line with the downward trend shown in Figure 1, the bivariate multilevel regression models showed that the odds of correctly assessing a partner's viral suppression decreased by 17% over time (OR=0.83; 95% CI: 0.69-0.99; p=.042; see Table 3). When the fixed effect of time was included in each of the six bivariate models for each relationship dynamic, the ORs for the fixed effect of time ranged from 0.81-0.83 and were all statistically significant at the p<.05 level (results not shown in tables for sake of brevity). This association held after controlling for other variables in the multivariate model (AOR=0.79; 95% CI: 0.66-0.96; p=.016).
We hypothesized that positive relationship dynamics would be positively associated with accurate partner beliefs and conversely, greater discrepancies between partners on these relationship dynamics would be negatively associated with accurate partner beliefs. The multilevel mixed effects regression results for the bivariate analysis showed that greater couple-level differences in dyadic adjustment (OR=0.66; 95% CI: 0.50-0.87; p<.01) and commitment (OR=0.82; 95% CI: 0.70-0.97; p=.022) were associated with a decreased odds of having accurate partner beliefs (Table 3). After controlling for other variables, the association for couple-level differences in dyadic adjustment remained statistically significant and negatively associated with accurate partner beliefs (AOR=0.69; 95% CI: 0.49-0.98; p=0.038). Relationship length, cohabitation, and couple HIV status were not significantly associated with accurate partner beliefs in the multivariate model.
Discussion
While knowledge of a partner's viral load status is important for HIV-risk reduction and for social support for HIV care and treatment, it remains unclear whether partner beliefs about viral suppression are accurate. To our knowledge, the present study is the first to address this issue. First, we found that primary partners were frequently inaccurate, with a tendency to overestimate that their partner was virally suppressed. More importantly, when partners were inaccurate, the majority (around 80% at baseline) assumed their partner was virally suppressed when they were not. We also found that accuracy about a partner's viral suppression declined over the two-year follow-up period. Together, these findings highlight the critical need for couple-based interventions to increase accurate partner knowledge about viral suppression.
Several explanations for these findings are possible. HIV-positive men may disclose their viral load status early on in the relationship while developing positive relationship dynamics and negotiating sexual risk reduction practices, but not revisit these discussions later after trust and commitment have been established. While the net change in viral suppression for the entire sample was negligible, intra-individual change was relatively common. From baseline to the 12-month follow-up visit, 119 out of 407 men had a change in viral load status (approximately half of the 119 men went from unsuppressed to suppressed). From the 12-month to 24-month follow-up visit, an additional 43 men had a change in viral load status. If these changes were not re-communicated to partners, it is understandable that partners could report outdated information. It is also possible that sexual activity with a primary partner changed (e.g., not having sex) such that it was not necessary to re-assess or communicate about a partner's viral load. Other longitudinal research on gay couples has found that CAI decreases over time, which may be attributed, in part, to decreases in sexual frequency as the relationship continues (32). Finally, optimistic beliefs about ART may also lead partners to overestimate the likelihood of a partner's viral suppression as a function of their time spent on ART—a process that may be best labeled as “viral guessing” rather than “viral sorting”. Several studies have shown that people living with HIV often hold optimistic beliefs that ART reduces the risk of HIV transmission (54, 55) and such beliefs have been linked to engaging in condomless sex with HIV-negative or unknown status partners (55). Future studies are needed to investigate the sources of error on both sides of the dyadic interaction, which could be used to tailor couple-based intervention efforts aimed at reducing HIV transmission risk with primary and casual partners.
We also found that couples with greater within-couple differences in dyadic adjustment and commitment had lower odds of accurate beliefs about partner viral suppression. In accordance with interdependence theory, effective communal coping strategies occur when couples hold congruent health beliefs and goals (29). While we did not find any associations between constructive communication and beliefs about viral suppression, it stands to reason that incongruence between partners’ in their perceptions of relationship quality may be indicative of communication difficulties about health-related issues. Alternatively, couples members who hold incongruent perceptions of relationship quality may be less invested in the process of viral sorting; and therefore, partners may be more likely to possess inaccurate knowledge about viral suppression. Thus, future research is warranted to better understand whether and how partner communication is associated with partner beliefs about viral suppression.
Finally, while we expected that HIV-negative partners would have more accurate beliefs about their partner's viral suppression because of the risk for HIV, we did not find differences by couple HIV serostatus. These findings suggest that associations between discrepancies in relationship dynamics and accurate beliefs about partner viral suppression exist for all of these couples, regardless of couple HIV serostatus. While HIV-negative men may be motivated by protection, they may be equally motivated by concerns for their partner's health—at levels similar to men in HIV-positive seroconcordant relationships. Another explanation for a lack of difference by couple HIV status may relate to the type of partnerships studied. In primary partnerships, both partners may be more comfortable with their couple HIV status if they are in longer term, more committed relationships as compared to other types of relationships (e.g., casual partnerships). Qualitative research on gay couples in the U.S. illustrates that HIV serodiscordant couples often come up with an acceptable level of risk based on the underlying dynamics of their relationships such as trust and feeling safe (56). Because our sample consisted of men in primary partnerships with high levels of commitment, the majority of whom lived together and had been together for years, couple serostatus may play less of a role in risk-reduction practices such as viral sorting. Viral sorting is also likely to be one of many HIV risk-reduction strategies used by MSM male couples, and there may be subtle differences between serodiscordant and seroconcordant couples regarding the number and types of risk-reduction strategies employed (5).
To the contrary, we might expect that HIV-positive partners in seroconcordant relationships would hold more accurate beliefs about each other's viral suppression, compared to partners in serodiscordant relationships, so that they can jointly monitor and support each other with regards to healthy living (57, 58). If the threat of HIV is perceived as greater in HIV concordant positive couples, interdependence theory (29) would suggest that partners would be more likely to undergo a transformation of motivation and engage in communal coping around HIV infection and ART adherence support. HIV-positive partners may also be better able to understand and communicate about their viral load because it is within their own direct experience. However, we did not find higher levels of accurate beliefs among these men. It is possible that the lack of differences in accurate beliefs by couple HIV status is attributed to a combination of both shared and divergent motivations for knowing a partner's viral load. Future qualitative studies are needed to characterize the range of motivations for engaging in viral sorting, under which scenarios these practices are employed, and how partner type may shape the frequency and priority given to viral sorting.
Several limitations are noteworthy. First, because this was a study on primary partners from the San Francisco Bay Area, we cannot generalize to other types of couples, contexts, and geographic regions. The relatively high prevalence of inaccurate beliefs in this study comes from a sample of men in primary relationships in which levels of communication, trust, and disclosure about private health information may be higher than for other types of couples (e.g., casual sexual relationships). For example, a study among MSM engaged in three types of partnerships with varying degrees of commitment (one-night stands, multiple-time sexual partners, and regular sex buddies) found that men reported more serosorting with regular sex buddies than with casual partners such as one-night stands (59). If the frequency of viral sorting is lower among men in non-committed relationships, these men may be even less accurate about their sexual partner's viral suppression and may be at higher risk for HIV infection (although this risk may be offset by generally lower levels of CAI with casual or secondary partners (34, 60) and for those who do engage in CAI, they may be aware of their partner's viral suppression). Despite this, we believe our findings are relevant to many gay men. Other US-based studies have found that primary partnerships are common among gay men (61, 62). Further, research shows that HIV transmission among MSM is most likely to occur from primary sex partners as compared to casual-type partners (62).
Second, this was a longitudinal study and therefore participant loss-to-follow up could be a concern if those who participated were different from those who did not. For example, the results could be biased by the increased participation of higher-functioning couples who stayed together over the study period—which has been noted as a limitation in other couples studies on primary relationships (15, 63). However, our modeling approach was robust enough to account for missing data under the relatively mild MAR assumption and made use of all data available from participants at each visit, which would enhance generalizability. Third, one explanation for the tendency of partners to overestimate their partner's viral suppression may relate to the belief that taking ART and having an undetectable viral load are synonymous. Because the index partner was required to be on ART for this study, we cannot assess partner beliefs about viral suppression among men who are not on ART. Future qualitative research is needed to understand how these beliefs are formulated and the reasons for inaccurate beliefs within couples. Fourth, we would like to note a lack of specificity in the survey question on partner beliefs about viral load. Partners could refuse to answer the question, but could not indicate if they were uncertain about a partner's viral load and therefore those who did not know the results of their partner's last viral load test may have been more inclined to guess. We cannot assess the extent to which the men were told/know a partner's viral load test results versus those who did not know and guessed, which may partially explain the discrepancy found between viral load tests and partner beliefs. Fifth, our analysis also used the criteria of 48 copies/ml as a cutoff value for viral suppression (the level of detection for the assay used). In clinical practice, recent changes to definitions of viral suppression and virologic failure have prompted clinicians to use the less stringent criteria of 200 copies/ml (64, 65). If HIV-positive men were told their viral status based on the higher cutoff value and communicated this to their partners, it is possible that this may explain, in part, the differences between the viral load test results and partner perceptions. However, when we looked at the distribution of values for the viral load data at baseline, only 10% of men had a viral load within the range of 50-200 copies/ml and could have been misclassified in the worst-case scenario. Sixth, while we did not find any differences by couple HIV status, there is the possibility that we may have had limited power to detect a significant interaction due to differences in the group sizes for the two types of couples. Future studies with larger, equally weighted samples of seroconcordant and discordant men should attempt to replicate our findings.
Finally, it is important to point that the data from this paper were collected prior to the implementation of Treatment as Prevention (TasP) as policy in San Francisco (66) and therefore these issues could play out differently within couples today. As of 2013, an estimated 72% of people living with HIV in San Francisco were virally suppressed (67) and the time to viral suppression among newly diagnosed San Franciscans decreased significantly following the adoption of universal ART in 2010 (68). While Duo participants were recruited throughout the greater San Francisco Bay Area, including areas that have not implemented a TasP approach, the study population may be more likely to be virally suppressed today—surpassing the 50% viral suppression rate we found and more in line with what their partners believed (upwards of 73% believed that their partners were suppressed). In addition, partners may be more likely to believe their partners are suppressed due to a heightened awareness of TasP and viral suppression. Thus, discrepancies between actual partner viral load and partner perceptions may be less pronounced if the study was conducted today.
Limitations aside, the main strengths of this paper include the use of longitudinal dyadic data allowing for temporal assessment of beliefs and the application of objective biomarkers of viral load to compare with subjective partner beliefs. We highlight several important implications of the findings for HIV programming with gay men in primary partnerships. As HIV treatment gains momentum as an effective form of biomedical prevention (69), it will be critical to ensure that couples have the correct and up-to-date biomedical knowledge to effectively incorporate these messages into their toolbox of HIV risk-reduction strategies. For example, both partners need to be informed that viral load is subject to change over time and thus viral sorting is not a foolproof strategy in the absence of regular viral load testing. The findings also raise concerns about potential untapped social support within primary partnerships among men who incorrectly assume their partners are virally suppressed, which could be harnessed to improve medication adherence and treatment outcomes. Couples-based interventions that encourage viral sorting through routine disclosure of viral status and also build the necessary communication skills within couples will help to minimize viral-guessing practices that may be less effective at preventing HIV. Involving partners in other aspects of HIV care such as couples testing for HIV and in treatment literacy classes may help to generate an increased understanding of viral load information and awareness about a partner's viral status. Further, couples-based interventions that foster positive relationship dynamics to reduce discrepancies in relationship dynamics such as dyadic adjustment and commitment may provide a pathway to encourage effective communal coping around ART support. Such behavioral interventions for couples have been shown to be relatively efficacious at addressing other HIV-related outcomes such as sexual risk (70) and could be further adopted to target couple communication around HIV-related health information such as viral load.
Acknowledgements
We would also like to thank the men who participated in the study and the tireless efforts of the research staff. We would also like to thank John Sauceda for translation of the abstract into Spanish.
Funding: This study was funded by grants from the National Institute of Nursing Research (R01NR010187), the National Institute of Drug Abuse (K24DA037034), and the National Institute of Mental Health (T32MH019105; T32MH078788).
Footnotes
Compliance with Ethical Standards:
Conflict of Interest: Amy Conroy declares no conflict of interest. Kristi Gamarel declares no conflict of interest. Torsten Neilands declares no conflict of interest. Samantha Dilworth declares no conflict of interest. Lynae Darbes declares no conflict of interest. Mallory Johnson declares no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Siedner MJ, Musinguzi N, Tsai AC, et al. Treatment as long-term prevention: sustained reduction in HIV sexual transmission risk with use of antiretroviral therapy in rural Uganda. AIDS. 2014;28:267–71. doi: 10.1097/QAD.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen M, Chen Y, McCauley M, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodger A, Bruun T, Cambiano V, et al. HIV Transmission Risk Through Condomless Sex If HIV+ Partner On Suppressive ART: PARTNER Study. Conference on Retroviruses and Opportunistic Infections (CROI) on March 3-6, 2014 [Google Scholar]
- 4.van den Boom W, Davidovich U, Witlox R, Stolte I. Frequent use of viral sorting by HIV-positive MSM: The consideration of viral load when deciding to engage in unprotected anal intercourse with HIV-positive and HIV-negative partners.. Presented at 10th Annual Aids Impact conference; Santa Fe, New Mexico. 2011. [Google Scholar]
- 5.Mitchell JW. HIV-Negative and HIV-Discordant Gay Male Couples’ Use of HIV Risk-Reduction Strategies: Differences by Partner Type and Couples’ HIV-Status. AIDS Behav. 2013;17:1557–69. doi: 10.1007/s10461-012-0388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horvath KJ, Smolenski D, Iantaffi A, Grey JA, Rosser BRS. Discussions of viral load in negotiating sexual episodes with primary and casual partners among men who have sex with men. AIDS Care. 2012;24(8):1052–5. doi: 10.1080/09540121.2012.668168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prestage G, Mao L, Kippax S, et al. Use of Viral Load to Negotiate Condom Use Among Gay Men in Sydney, Australia. AIDS Behav. 2009;13:645–51. doi: 10.1007/s10461-009-9527-0. [DOI] [PubMed] [Google Scholar]
- 8.Van de Ven P, Mao L, Fogarty A, et al. Undetectable viral load is associated with sexual risk taking in HIV serodiscordant gay couples in Sydney. AIDS. 2005;19:179–184. doi: 10.1097/00002030-200501280-00010. [DOI] [PubMed] [Google Scholar]
- 9.Crepaz N, Hart TA, Marks G. Highly Active Antiretroviral Therapy and Sexual Risk Behavior: A Meta-analytic Review. JAMA. 2004;292:224–36. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
- 10.Zablotska IB, Imrie J, Prestage G, et al. Gay men's current practice of HIV seroconcordant unprotected anal intercourse: Serosorting or seroguessing? AIDS Care. 2009;21:501–10. doi: 10.1080/09540120802270292. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery BM. The form and function of quality communication in marriage. Fam Relat. 1981:21–30. [Google Scholar]
- 12.Anglewicz PA, Bignami-Van Assche S, Clark S, Mkandawire J. HIV risk among currently married couples in rural Malawi: What do spouses know about each other? AIDS Behav. 2008;14(1):103–12. doi: 10.1007/s10461-008-9497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anglewicz P, Chintsanya J. Disclosure of HIV status between spouses in rural Malawi. AIDS Care. 2011;23(8):998–1005. doi: 10.1080/09540121.2010.542130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz DA, Kiarie JN, John-Stewart GC, et al. HIV testing men in the antenatal setting: understanding male non-disclosure. Int J STD AIDS. 2009;20(11):765–7. doi: 10.1258/ijsa.2009.009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conroy AA, Wong LH. How reliable are self-reports of HIV status disclosure? Evidence from couples in Malawi. Soc Sci Med. 2015;144:28–37. doi: 10.1016/j.socscimed.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meichenbaum D, Turk DC. Facilitating Treatment Adherence: A Practitioner's Guidebook. Plenum Press; New York: 1987. [Google Scholar]
- 17.Bekele T, Rourke SB, Tucker R, et al. Direct and indirect effects of perceived social support on health-related quality of life in persons living with HIV/AIDS. AIDS Care. 2013;25(3):337–46. doi: 10.1080/09540121.2012.701716. [DOI] [PubMed] [Google Scholar]
- 18.Huynh AK, Kinsler JJ, Cunningham WE, Sayles JN. The role of mental health in mediating the relationship between social support and optimal ART adherence. AIDS Care. 2013;25(9):1179–84. doi: 10.1080/09540121.2012.752787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeji F, Klipstein-Grobusch K, Newell ML, et al. Are social support and HIV coping strategies associated with lower depression in adults on antiretroviral treatment? Evidence from rural KwaZulu-Natal, South Africa. AIDS Care. 2014;26(12):1482–9. doi: 10.1080/09540121.2014.931561. [DOI] [PubMed] [Google Scholar]
- 20.Ammassari A, Trotta MP, Murri R, Castelli F, Narciso P, Noto P, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: Overview of published literature. JAIDS. 2002;31:S123–S7. doi: 10.1097/00126334-200212153-00007. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez JS, Penedo FJ, Antoni MH, et al. Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychol. 2004;23(4):413. doi: 10.1037/0278-6133.23.4.413. [DOI] [PubMed] [Google Scholar]
- 22.Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 2000;19(2):124. [PubMed] [Google Scholar]
- 23.Leserman J, Perkins DO, Evans DL. Coping with the threat of AIDS: the role of social support. American Journal of Psychiatry. Am J Psychiatry. 1992;149:1514. doi: 10.1176/ajp.149.11.1514. [DOI] [PubMed] [Google Scholar]
- 24.Wrubel J, Stumbo S, Johnson MO. Antiretroviral medication support practices among partners of men who have sex with men: A qualitative study. AIDS Patient Care STDS. 2008;22(11):851–8. doi: 10.1089/apc.2008.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wrubel J, Stumbo S, Johnson MO. Male same-sex couple dynamics and received social support for HIV medication adherence. J Soc Pers Relatsh. 2010;27(4):553–72. doi: 10.1177/0265407510364870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton MM, Razzano LA, Martin NB. The relationship between type and quality of social support and HIV medication adherence. J HIV/AIDS Soc Serv. 2007;6(1-2):39–63. [Google Scholar]
- 27.Davies G, Koenig LJ, Stratford D, Palmore M, Bush T, Golde M, et al. Overview and implementation of an intervention to prevent adherence failure among HIV-infected adults initiating antiretroviral therapy: Lessons learned from Project HEART. AIDS Care. 2006;18:895–903. doi: 10.1080/09540120500329556. [DOI] [PubMed] [Google Scholar]
- 28.Remien RH, Stirratt MJ, Dolezal C, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. AIDS. 2005;19:807–14. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 29.Lewis MA, McBride CM, Pollak KI, et al. Understanding health behavior change among couples: An interdependence and communal coping approach. Soc Sci Med. 2006;62:1369–80. doi: 10.1016/j.socscimed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Rusbult C, Lange PMAV. Interdependence, interaction, and relationships. Annu Rev Psychol. 2003;54(54):351–75. doi: 10.1146/annurev.psych.54.101601.145059. [DOI] [PubMed] [Google Scholar]
- 31.Karney BR, Hops H, Redding CA, et al. A Framework for Incorporating Dyads in Models of HIV-Prevention. AIDS Behav. 2010;14(2):189–203. doi: 10.1007/s10461-010-9802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darbes LA, Chakravarty D, Neilands TB, Beougher SC, Hoff CC. Sexual Risk for HIV Among Gay Male Couples: A Longitudinal Study of the Impact of Relationship Dynamics. Arch Sex Behav. 2014;43(1):47–60. doi: 10.1007/s10508-013-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell JW, Champeau D, Harvey SM. Actor–Partner Effects of Demographic and Relationship Factors Associated with HIV Risk Within Gay Male Couples. Arch Sex Behav. 2013;42(7):1337–45. doi: 10.1007/s10508-012-9985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell JW, Harvey SM, Champeau D, Seal DW. Relationship factors associated with HIV risk among a sample of gay male couples. AIDS Behav. 2012;16:404–11. doi: 10.1007/s10461-011-9976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoff CC, Chakravarty D, Beougher SC, Neilands TB, Darbes L. Relationship characteristics associated with sexual risk behavior among MSM in committed relationships. AIDS Patient Care STDS. 2012;26(12):738–45. doi: 10.1089/apc.2012.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez AM, Beougher SC, Chakravarty D, Neilands TB, Mandic CG, Darbes LA, Hoff CC. Relationship dynamics as predictors of broken agreements about outside sexual partners: Implications for HIV prevention among gay couples. AIDS Behav. 2012;16(6):1584–8. doi: 10.1007/s10461-011-0074-0. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell JW, Harvey SM, Champeau D, Moskowitz DA, Seal DW. Relationship Factors Associated with Gay Male Couples’ Concordance on Aspects of Their Sexual Agreements: Establishment, Type, and Adherence. AIDS Behav. 2012;16:1560–9. doi: 10.1007/s10461-011-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson MO, Dilworth SE, Taylor JM, et al. Primary relationships, HIV treatment adherence, and virologic control. AIDS Behav. 2012;16(6):1511–21. doi: 10.1007/s10461-011-0021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner C, Ku L, Rogers S, et al. Adolescent sexual behavior, drug use, and violence: increased reporting with computer survey technology. Science. 1998;280(5365):867–73. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- 40.Funk JL, Rogge RD. Testing the Ruler With Item Response Theory: Increasing Precision of Measurement for Relationship Satisfaction With the Couples Satisfaction Index. J Fam Psychol. 2007;21(4):572–83. doi: 10.1037/0893-3200.21.4.572. [DOI] [PubMed] [Google Scholar]
- 41.Sabourin S, Valois P, Lussier Y. Development and validation of a brief version of the dyadic adjustment scale with a nonparametric item analysis model. Psycholological Assessment. 2005;17(1):15–27. doi: 10.1037/1040-3590.17.1.15. [DOI] [PubMed] [Google Scholar]
- 42.Spanier G. Measuring dyadic adjustment: new scales for assessing the quality of marriage and similar dyads. J Marriage Fam. 1976:15–28. [Google Scholar]
- 43.Kurdek L. Assessing multiple determinants of relationship commitment in cohabitating gay, cohabitating lesbian, dating heterosexual, and married heterosexual couples. Fam Relat. 1995:261–6. [Google Scholar]
- 44.Christensen A, Shenk JL. Communication, conflict, and psychological distance in nondistressed, clinic, and divorcing couples. J Consult Clin Psychol. 1991;59(3):458–63. doi: 10.1037//0022-006x.59.3.458. [DOI] [PubMed] [Google Scholar]
- 45.Allison P. Fixed effects regression methods for longitudinal data using SAS. SAS Institute; Cary, NC: 2005. [Google Scholar]
- 46.Neuhaus JM, Kalbfleisch JD. Between- and within-cluster covariate effects in the analysis of clustered data.”. Biometrics. 1998;54(2):638–45. [PubMed] [Google Scholar]
- 47.Kenny DA, Kashy DA, Cook WL. Dyadic data analysis. The Guilford Press; New York: 2006. [Google Scholar]
- 48.Petersen T. Analyzing Panel Data: Fixed- and Random-Effects Models. In: Hardy M, Bryman A, editors. Handbook of data analysis. Sage; Thousand Oaks: 2004. pp. 331–45. [Google Scholar]
- 49.Burnham KP, Anderson DR. Model selection and multimodel inference: A practical information-theoretic approach (second edition) Springer; New York: 2003. [Google Scholar]
- 50.Harrell FE. Regression modeling strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; New York: 2001. [Google Scholar]
- 51.Agresti A, Finlay B. Statistical Methods for the Social Sciences. 4th ed. Pearson Prentice Hall; Upper Saddle River, New Jersey: 2009. [Google Scholar]
- 52.Hox JJ. Multilevel Analysis: Techniques and Applications. Second Edition Routledge; New York: 2010. [Google Scholar]
- 53.Carrico A, Woolf-King S, Neilands T, Dilworth S, Johnson M. Stimulant use and HIV disease management among men in same-sex relationships. Drug Alcohol Depend. 2014;139:174–7. doi: 10.1016/j.drugalcdep.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalichman SC, Eaton L, Cain D, et al. Changes in HIV treatment beliefs and sexual risk behaviors among gay and bisexual men. Health Psychol. 2007;26(5):650–6. doi: 10.1037/0278-6133.26.5.650. [DOI] [PubMed] [Google Scholar]
- 55.Kalichman SC, Cherry C, Kalichman MO, et al. Sexual behaviors and transmission risks among people living with HIV: Beliefs, perceptions, and challenges to using treatments as prevention. Arch Sex Behav. 2015:1–10. doi: 10.1007/s10508-015-0559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beougher S, Chakravarty D, Garcia C, et al. Risks worth taking: safety agreements among discordant gay couples. AIDS Care. 2012;24(9):1071–7. doi: 10.1080/09540121.2011.648603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gamarel K, Comfort M, Wood T, Neilands T, Johnson M. A qualitative analysis of male couples coping with HIV: Disentangling the “we.”. J Health Psychol. 2015 doi: 10.1177/1359105315571975. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gamarel K, Revenson T. Dyadic adaptation to chronic illness: The importance of considering context in understanding couples’ resilience. In: Skerrett K, Fergus K, editors. Couples Resilience: Emerging Perspectives. Springer; Netherlands: 2015. pp. 83–105. [Google Scholar]
- 59.van den Boom W, Stolte I, Sandfort T, Davidovich U. Serosorting and sexual risk behaviour according to different casual partnership types among MSM: the study of one-night stands and sex buddies. AIDS Care. 2011;24(2):167–73. doi: 10.1080/09540121.2011.603285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell JW, Garcia L, Champeau D, Harvey SM, Petroll AE. HIV-negative seroconcordant gay male couples' attitudes, intentions, and perceived behavioral control for planned condom use within and outside of their relationships. Int J Sex Health. 2012;24(4):239–53. [Google Scholar]
- 61.Dodge B, Herbenick D, Tsung-Chieh F, et al. Sexual Behaviors of U.S. Men by Self-Identified Sexual Orientation: Results From the 2012 National Survey of Sexual Health and Behavior. J Sex Med. 2016;13(4):637–49. doi: 10.1016/j.jsxm.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan PS, Salazar L, Buchbinder S, Sanchez TH. Estimating the proportion of HIV transmissions from main sex partners among men who have sex with men in five US cities. AIDS. 2009:1153–62. doi: 10.1097/QAD.0b013e32832baa34. [DOI] [PubMed] [Google Scholar]
- 63.Conroy AA, McGrath N, Van Rooyen H, et al. Power and the Association with Relationship Quality in South African Couples: Implications for HIV/AIDS Interventions. Soc Sci Med. 2016;153(1-11) doi: 10.1016/j.socscimed.2016.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ribaudo H, Lennox J, Currier J, et al. An analysis of ACTG studies Paper presented at the 16th Conference on Retroviruses and Opportunistic Infection. Montreal, Canada: 2009. Virologic failure endpoint definition in clinical trials: Is using HIV-1 RNA threshold <200 copies/mL better than <50 copies/mL? [Google Scholar]
- 65.U.S. Department of Health and Human Services Administration HIV/AIDS Bureau Performance Measures: HIV Viral Load Suppression. 2013 http://hab.hrsa.gov/deliverhivaidscare/coremeasures.pdf.
- 66.Russell R. City endorses new policy for treatment of HIV. The New York Times; 2010. [February 4, 2016]. Available at http://www.nytimes.com/2010/04/04/us/04sftreatment.html. [Google Scholar]
- 67.SFDPH . HIV Epidemiology Annual Report. San Francisco Department of Public Health; San Francisco, CA: 2014. [Google Scholar]
- 68.Schwarcz S, Hsu LC, Scheer S. Disparities and Trends in Viral Suppression During a Transition to a “Test and Treat” Approach to the HIV Epidemic, San Francisco, 2008–2012. JAIDS. 2015;70:529–37. doi: 10.1097/QAI.0000000000000794. [DOI] [PubMed] [Google Scholar]
- 69.UNAIDS . 90-90-90: An ambitious treatment target to help end the AIDS epidemic. UNAIDS; Geneva, Switzerland: 2014. [Google Scholar]
- 70.Burton J, Darbes LA, Operario D. Couples-focused behavioral interventions for prevention of HIV: Systematic review of the state of evidence. AIDS Behav. 2010;14:1–10. doi: 10.1007/s10461-008-9471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]