Abstract
The glutamate transporter GLT-1 (also called EAAT2 in humans) plays a critical role in regulating extracellular glutamate levels in the central nervous system (CNS). In Alzheimer’s disease (AD),EAAT2 loss is associated with neuropathology and cognitive impairment. In keeping with this, we have reported that partial GLT-1 loss (GLT-1+/−) causes early-occurring cognitive deficits in mice harboring familial AD AβPPswe/PS1ΔE9 mutations. GLT-1 plays important roles in several molecular pathways that regulate brain metabolism, including Akt and insulin signaling in astrocytes. Significantly, AD pathogenesis also involves chronic Akt activation and reduced insulin signaling in the CNS. In this report we tested the hypothesis that GLT-1 heterozygosity (which reduces GLT-1 to levels that are comparable to losses in AD patients) in AβPPswe/PS1ΔE9 mice would induce sustained activation of Akt and disturb components of the CNS insulin signaling cascade. We found that partial GLT-1 loss chronically increased Akt activation (reflected by increased phosphorylation at serine 473), impaired insulin signaling (reflected by decreased IRβ phosphorylation of tyrosines 1150/1151 and increased IRS-1 phosphorylation at serines 632/635 –denoted as 636/639 in humans), and reduced insulin degrading enzyme (IDE) activity in brains of mice expressing familial AβPPswe/PS1ΔE9 AD mutations. GLT-1 loss also caused an apparent compensatory increase in IDE activity in the liver, an organ that has been shown to regulate peripheral amyloid-β levels and expresses GLT-1. Taken together, these findings demonstrate that partial GLT-1 loss can cause insulin/Akt signaling abnormalities that are in keeping with those observed in AD.
Keywords: Amyloid-β, EAAT2, excitotoxicity, GLT1, metabolism, SLC1A2
INTRODUCTION
The amino acid glutamate plays a crucial role in excitatory neurotransmission in the central nervous system (CNS). However, once released, extracellular glutamate must be efficiently cleared in order to maintain spatial and temporal resolution of synaptic signaling and to prevent neuronal and synaptic excitotoxicity [1–3]. In the brain, this critical function is carried out by a family of five high-affinity Na+-dependent glutamate transporters referred to as GLAST, GLT- 1, EAAC1, EAAT4, and EAAT5 (also known as EAAT1 though EAAT5, respectively). Among these transporters, GLT-1 is especially important in cortex and hippocampus where it is responsible for clearing 80–90% of the released extracellular glutamate [1, 2, 4, 5]. The biological significance of GLT-1 is underscored by findings that GLT-1 knockout (−/−) mice develop lethal seizures accompanied by significant hippocampal neuron loss [1]. GLAST, EAAC1, and EAAT4 deficiency are also functionally significant. However, these knockout mice display less severe phenotypes than GLT-1 deficient mice [6–9].
Further supporting the essential role GLT-1 plays in regulating extracellular glutamate levels, loss of EAAT2 (the human homolog of GLT-1) has been reported in patients manifesting a range of neurological diseases or CNS insults including Huntington’s disease [10, 11], traumatic brain injury [12], and amyotrophic lateral sclerosis [13–15]. A number of laboratories have also recently reported that EAAT2 is significantly reduced in AD by as much as 50% [16–19].
In a previous study, we reported that GLT-1 heterozygous (GLT-1+/−) mice harboring compound familial AD mutations of the amyloid-β protein precursor (AβPPswe) and Presenilin 1 (PS1ΔE9) displayed early-occurring cognitive deficits compared to AβPPswe/PS1ΔE9 mice with normal wild-type GLT-1 (GLT-1+/+) levels [20]. These findings demonstrate that partial GLT-1 loss is capable of unmasking early-occurring cognitive disturbances brought about by expression of the AD-related AβPPswe/PS1ΔE9 transgene [20].
The mechanisms by which GLT-1 loss might contribute to AD-related cognitive disturbances are currently unknown. While there was a significant increase in the ratio of Aβ42/Aβ40 in GLT-1 (+/−)/AβPPswe/PS1ΔE9 mice, total AβPP levels and amyloid plaque levels were not affected by GLT-1 heterozygosity [20]. Thus, amyloid plaque burden appears to be a less likely explanation for the behavioral impairments exhibited by GLT-1 (+/−)/AβPPswe/PS1ΔE9 mice.
Similarly, the potential for overt excitotoxicity due to partial GLT-1 loss is also unlikely. In contrast to GLT-1 knockout mice, GLT-1 heterozygous mice appear phenotypically normal [1]. While Aβ42 exposure significantly slows the rate at which GLT-1 clears synaptically released glutamate [21], thereby increasing glutamate spread, we have recently shown that it does not prevent extracellular glutamate concentrations from returning to basal levels that are estimated to be in the low nanomolar range [22]. Such findings argue against the idea that the consequences of Aβ-mediated GLT-1 loss are due to chronic basal changes in extracellular glutamate concentrations.
In addition to the importance of GLT-1 in protecting against glutamate-induced toxicity [1–3], there is evidence that GLT-1 also plays an important role in CNS metabolism. This is supported by data showing that GLT-1 regulates activity-dependent glucose utilization [23, 24] and GLT-1 expression is regulated by Akt and insulin stimulation [25–27]. In this regard, a number of studies have reported increased Akt phosphorylation, as well as altered insulin receptor and insulin receptor substrate 1 (IRS-1) expression and function in AD patients [28–34].
Such findings prompted us to test the hypothesis that partial GLT-1 loss in AβPPswe/PS1ΔE9 mice would alter Akt and insulin-sensitive signaling proteins in vivo. Herein, we report that GLT-1 heterozygosity increased Akt phosphorylation, which was accompanied by alterations in the phosphorylation states of the insulin receptor β-subunit (IRβ) and IRS-1. We also found decreased insulin degrading enzyme (IDE) activity in the CNS. These findings are in keeping with those reported in AD patients and other AD animal models [28, 31, 35–38].
MATERIALS AND METHODS
Animals
AβPPswe/PS1ΔE9 (AβPP/PS1) hemizygous mice (line 85) maintained on a B6C3F1/J background [39] and GLT-1 heterozygous (GLThet) mice maintained on a C57BL/6 background [1] were mated in order to generate F1 offspring littermates with the genotypes: GLT-1wt/non-transgenic (GLTwt/nTg), GLT-1het/non-transgenic (GLThet/nTg), GLT-1wt/AβPPswe/PS1ΔE9 (GLTwt/AβPP/PS1), and GLT-1het/AβPPswe/PS1ΔE9 (GLThet/AβPP/PS1). GLT-1 heterozygous mice were a kind gift from Dr. Kohichi Tanaka at Tokyo Medical and Dental University, Tokyo, Japan. Male mice were fed a standard chow diet and given ad libitum access to food and water. All experiments were performed in accordance with procedures approved by the VAPSHCS Institutional Animal Care and Use Committee.
Western blot analysis
Protein lysates were prepared for western blotting using methods described previously with a few modifications [20]. Briefly, frozen cortical brain or liver tissue was homogenized in 5 volumes (w/v) of lysis buffer (50 mM Tris, 150 mM NaCl, pH 8.0), sonicated, and centrifuged at approximately 20,000× g for 5 min at 4°C. The pellet and supernatant were divided to prepare crude membrane and soluble fractions, respectively. For the soluble fraction, to analyze phospho-Akt/Akt, phosph-IRS-1/IRS-1, phospho-mTOR/mTOR, phospho-p70 S6 kinase/p70 S6 kinase, and IDE, the 20,000× g supernatant was further centrifuged at approximately 135,000× g for 1 h at 4°C. The resulting supernatant was used as the soluble fraction. For the crude membrane fraction, to analyze phospho-IRβ/IRβ, the 20,000×g pellet was resuspended in lysis buffer containing 1% Triton X-100, briefly sonicated, and centrifuged at 20,000× g for 30 min at 4°C. The resulting supernatant was used as the membrane fraction. The lysates were stored at −80°C. Lysate protein concentrations were determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL).
Protein lysates were solubilized in Laemmli sample buffer, loaded onto 4–20% SDS-polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA), electrophoresed, and transferred onto PVDF membranes (Millipore, Billerica, MA). Membranes were blocked in 5% (w/v) non-fat dried milk dissolved in Tris-buffered saline (20 mM Tris, 140 mM NaCl, pH 7.4) with 0.03% Tween-20 and subsequently incubated with specific primary antibodies against phospho-insulin receptor β-subunit (pIRβ, Tyr1150/1151), total insulin receptor β-subunit (IRβ), phospho-Akt (pAkt, Ser473), total Akt, phospho-IRS-1 (pIRS-1, detects Ser632/635 in mice and Ser636/639 in humans), total IRS-1, phospho-mTOR (p-mTOR, Ser2448), total mTOR, phospho-p70 S6 kinase (p-p70 S6K, Thr389), total p70 S6 kinase, IDE, or pyruvate kinase overnight at 4°C. All primary antibodies were obtained from Cell Signaling Technology (Danvers, MA) with the exceptions of pan-specific antibodies to IRβ and IDE, which were obtained from Millipore, and anti-pyruvate kinase that was obtained from Rockland Immunochemicals (Gilbertsville, PA). Western blots were then incubated in the corresponding horseradish peroxidase-conjugated anti-rabbit IgG, anti-mouse IgG, or anti-goat IgG secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and were developed using enhanced chemiluminescence (ECL) (GE Healthcare, Piscataway, NJ) and an ImageQuant LAS 4000 (GE Healthcare). Densitometry analyses were performed using ImageQuant TL software (GE Healthcare). Band intensities of phosphorylated proteins were normalized to band intensities of corresponding total protein levels. For each protein examined (Akt, IRβ, mTOR, p70 S6K, and IRS-1) the ratios of phospho- epitope/total protein were normalized to the GLTwt/nTg group as 100%.
IDE activity
The degradation of insulin was measured using a trichloroacetic acid (TCA) solubility assay in accordance with previously established methods [37, 40]. Briefly, bovine serum albumin was added to siliconized tubes to prevent adsorption of the substrate. Soluble fraction protein lysates (20 µg) of brain (cortex) or liver were then incubated with [125I]-insulin (Phoenix Pharmaceuticals, Inc., Burlingame, CA) in phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) for 90 min (brain) or 45 min (liver) at 22°C (reaction volume 100 µl). The reaction was terminated by adding 300 µl of cold 13.3% TCA. After chilling for 15 min at 4°C, the samples were centrifuged at approximately 15,000× g for 10 min, 200µl of supernatant was removed and the radioactivity was counted using a gamma counter (Model 41600 HE; ICN Biomedicals, Costa Mesa, CA).To obtain a baseline level of degradation, negative controls were obtained for each sample by adding a 10,000-fold excess of non-radiolabeled insulin. For degradation counts, the negative control count was subtracted from the sample count and the activity data for each genotype was normalized to the GLTwt/nTg group.
Statistics
Data are presented as means ± standard error of the mean (SEM) and analyzed using standard analysis of variance (ANOVA) methods. Planned comparison Helmert tests [41, 42] were performed upon a statistically significant ANOVA result (p ≤ 0.05) to test the a priori hypothesis that wild-type, non-transgenic mice would be different from GLT-1 heterozygous and transgenic AβPPswe/PS1ΔE9 mice. Statistical analyses were carried out using SPSS software (IBM, Armonk, NY).
RESULTS
Sustained Akt activation, as reflected by increased levels of phosphorylation at Ser473, has been reported in brains of AD patients [28–30, 32]. To examine whether partial GLT-1 loss in the context of AD-related pathology alters CNS Akt signaling in vivo, we measured phospho-Akt (Ser473) and total Akt levels in immunoblots of protein lysates prepared from cortex of a cohort of mice comprised of four experimental groups: (i) GLTwt/nTg; (ii) GLThet/nTg; (iii) GLTwt/AβPP/PS1; and (iv) GLThet/AβPP/PS1. Previously we reported that GLT-1 heterozygosity caused significant behavioral impairments that were more prominent at 6 months than at 9 months of age; and which corresponded to a significant increase in the ratio of Aβ42/Aβ40 at 6, but not at 9 months of age [20].
GLT-1 heterozygosity and AβPP/PS1 transgene expression activate Akt
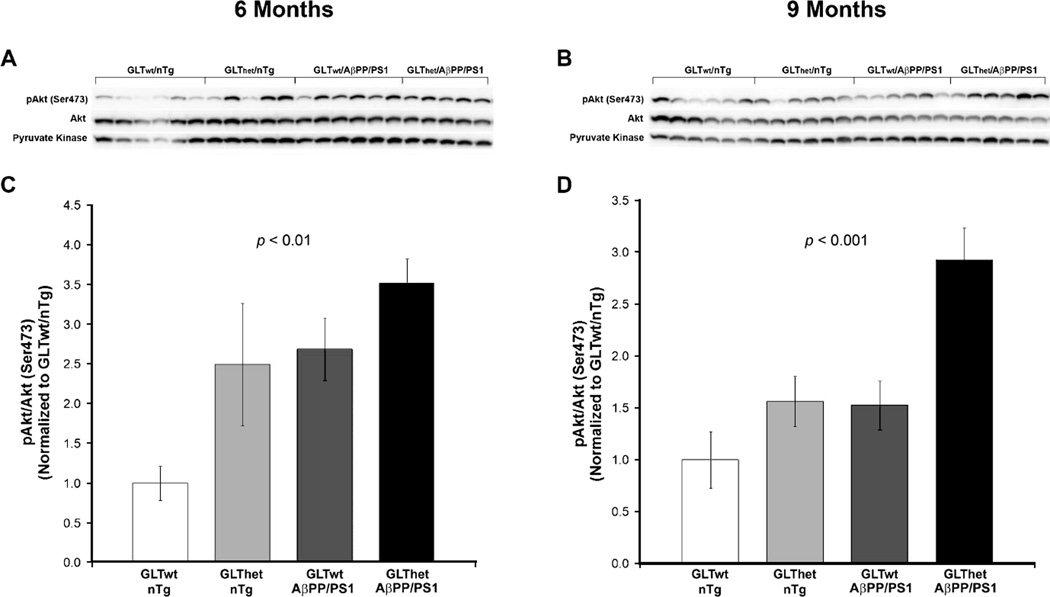
At 6 months (Fig. 1A, C) we found a statistically significant overall difference among genotypes in the ratios of phospho-Akt (Ser473) to total Akt protein (F[3,18] = 5.666, p <=0.006). A Helmert specific comparisons test [41, 42] confirmed that phospho-Akt/Akt ratios were significantly lower in GLTwt/nTg mice compared to the other three groups (p < 0.001). Similarly, at 9 months (Fig. 1B, D) there was a statistically significant overall difference among genotypes in phospho-Akt/Akt ratios (F[3,20] = 9.551, p < 0.001). Among the 9-month cohort, Helmert analysis revealed that phospho-Akt/Akt ratios were significantly lower in GLTwt/nTg mice compared to the other three groups (p < 0.004). However, at 9 months this difference in Akt phosphorylation among the GLThet/nTg and GLTwt/AβPP/PS1 groups was not as pronounced as at 6 months (Fig. 1B, D).
Fig. 1.
Akt phosphorylation is increased by partial loss of GLT-1 and expression of the AβPP/PS1 transgene and is more pronounced at 6 months of age. A, B) Western blots from 6 and 9 month animals, respectively. C, D) Indicating sustained activation of Akt, densitometric quantification of blots in (A) and (B) revealed a statistically significant increase in the ratio of phosphorylated Akt (Ser473) to total Akt in GLThet and AβPP/PS1 transgene mice compared to GLT-1wt/nTg mice at both 6 (C) and 9 (D) months of age (p ≤ 0.01 and p ≤ 0.001, respectively). Each lane represents one animal. Data are presented as normalized means ± SEM (n = 5–6 mice per group).
GLT-1 loss or AβPP/PS1 transgene expression suppresses insulin receptor activation
The findings in Fig. 1 demonstrate that GLT-1 heterozygosity and AβPPswe/PS1ΔE9 transgene expression caused prolonged activation of Akt. It has been reported that sustained Akt activation can lead to inhibition of insulin receptor autophosphorylation through negative feedback regulation [43–45]. In order to test whether sustained Akt elevation due to GLT-1 heterozygosity and/or AβPP/PS1 expression would be accompanied by reduced insulin receptor phosphorylation, we measured the levels of phosphorylated insulin receptor β-subunit (IRβ-Tyr1150/1151) and total IRβ by western blot analysis.
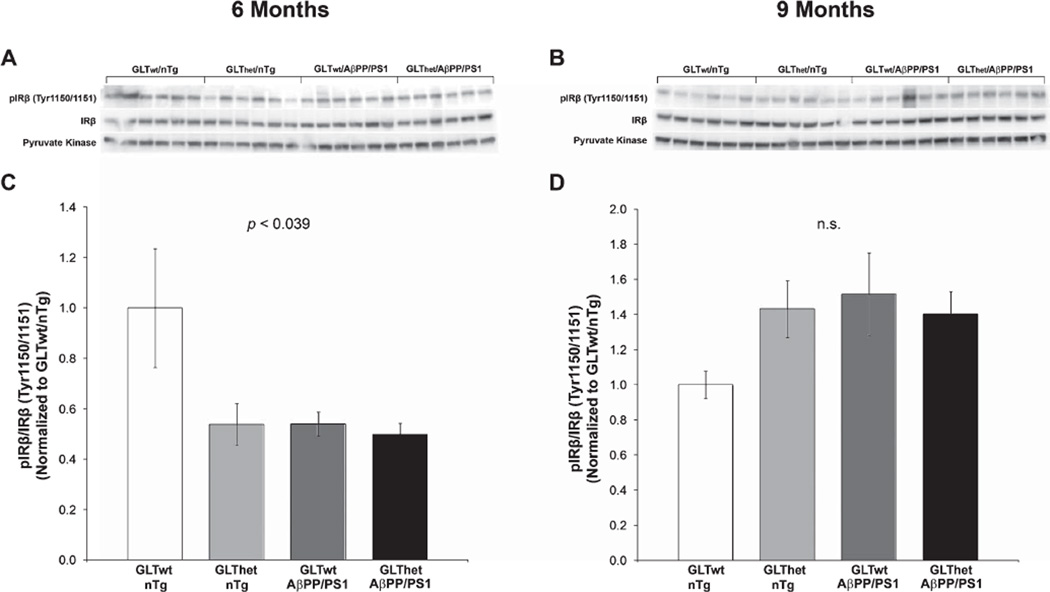
At 6 months (Fig. 2A, C) we found an overall difference among genotypes in the ratio of phospho-IRβ (Tyr1150/1151) to total IRβ protein (F[3,20] = 3.374, p ≤ 0.039). A Helmert analysis supported the conclusion that both GLT-1 heterozygosity and AβPP/PS1 transgene expression were sufficient to significantly suppress IRβ phosphorylation in all three groups compared to GLTwt/nTg mice (p ≤ 0.005). However, at 9 months there was no statistically significant difference among groups in the ratios of phospho-IRβ (Tyr1150/1151)/IRβ (F[3,20] = 2.042, n.s.). These findings indicate that at 6 months (a time point at which behavioral and Aβ42/Aβ40 abnormalities were most prominent [20]), partial GLT-1 loss or AβPP/PS1 transgene expression decreased tyrosine phosphorylation of the insulin receptor.
Fig. 2.
Insulin receptor autophosphorylation is reduced by partial loss of GLT-1 and AβPP/PS1 at 6 months of age. A, B) Western blots from 6 and 9 month animals, respectively. C, D) Indicating reduced insulin receptor activation, densitometric quantification of blots in (A) and (B) revealed a statistically significant decrease (p ≤ 0.039) in the ratio of Tyr1150/1151 phosphorylated IRβ to total IRβ in GLThet and AβPP/PS1 transgene mice compared to GLT-1wt/nTg mice at 6 (C), but not at 9 (D) months of age. Each lane represents one animal. Data are presented as normalized means ± SEM (n = 6 mice per group).
GLT-1 heterozygosity alters IRS-1, but not mTOR signaling
IRS-1 is a critical adapter protein that is responsible for transmitting signals from the insulin receptor to intracellular pathways [46]. IRS-1 is a key target for negative feedback regulation of insulin signaling [47, 48], and there is evidence that Akt activation may be in part responsible for regulation of IRS-1 [49].
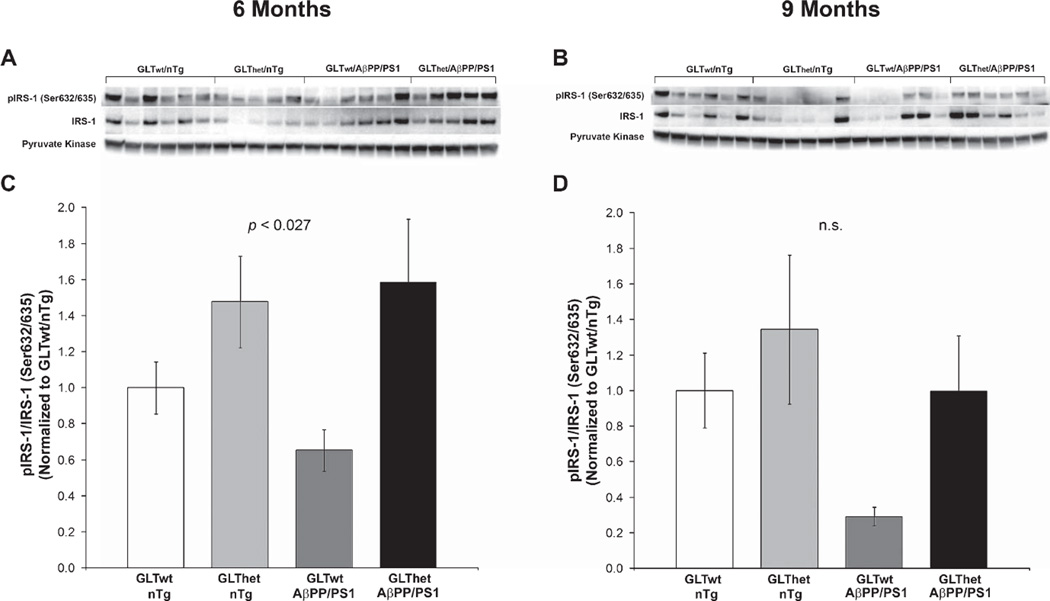
At 6 months (Fig. 3C, D), we found a statistically significant difference among the four groups in the ratio of phospho-IRS-1(Ser632/635)/IRS-1 (F[3,18] = 3.885, p ≤ 0.027). Phospho-IRS-1/IRS-1 ratios were significantly elevated by GLT-1 heterozygosity, whether or not the animals expressed an AβPP/PS1 transgene (GLT-1: F[1,18] = 10.183, p ≤ 0.005; AβPP: F[1,18] = 0.296, n.s.). While there appeared to be a similar trend of elevated IRS-1 Ser632/635 phosphorylation among the GLT-1het animals (Fig. 3B, D), phospho-IRS-1/IRS-1 ratios in the 9-month-old animals were not statistically significant (F[3,20] = 2.445, n.s.) and neither was the outcome of a two-factor analysis of GLT-1 genotype and AβPP/PS1 transgene status (GLT-1: F[1,20] = 3.442, n.s.; AβPP: F[1,20] = 3.486, n.s.).
Fig. 3.
IRS-1 serine phosphorylation is increased by partial loss of GLT-1 at 6 months of age. A, B) Western blots from 6 and 9 month animals, respectively. C, D) Indicating reduced IRS-1 signaling, densitometric quantification of blots in (A) and (B) revealed a statistically significant increase (p ≤ 0.027) in the ratio of Ser632/635 phosphorylated IRS-1 to total IRS-1 in mice with partial loss of GLT-1, but not animals with an AβPP/PS1 transgene at 6 months of age (C). Phospho-IRS-1/IRS (Ser632/635) ratios were not significantly different in 9-month-old mice (D). Each lane represents one animal. Data are presented as normalized mean ± SEM (n = 5–6 mice per group).
Negative feedback regulation of IRS-1 after prolonged Akt activation has been suggested to occur through mammalian target of rapamycin (mTOR), a downstream target of Akt [49]. To address this possibility, we assessed mTOR activity by measuring phosphorylation at Ser2448. For further confirmation of any changes in mTOR activity, we also measured phosphorylation of p70 S6 kinase (p70 S6K-Thr389), a downstream target of mTOR often used as an indicator of mTOR signaling [50, 51]. We found no statistically significant changes in the ratios of phospho-mTOR (Ser2448) to total mTOR or phospho-p70 S6K (Thr389) to total p70 S6K at either 6 months (mTOR: means ± SEM and (N) = 1.00 ± 0.13 (6), 1.14 ± 0.40 (5); 1.67 ± 0.26 (6), 1.24 ± 0.12 (5); F[3,18] = 1.469, n.s.; p70 S6K: 1.00 ± 0.25 (6), 0.59 ± 0.14 (5), 0.53 ± 0.12 (6), 0.65 ± 0.08 (5); F[3,18] = 1.649, n.s.; GLTwt/nTg, GLThet/nTg, GLTwt/AβPP/PS1, and GLThet/AβPP/PS1, respectively) or at 9 months of age (mTOR: means ± SEM and (N) = 1.00 ± 0.34 (6), 0.94 ± 0.27 (6); 1.05 ± 0.36 (6), 1.53 ± 0.41 (6); F[3,20] = 0.618, n.s. p70 S6K: 1.00 ± 0.50 (6), 1.50 ± 0.49 (6), 1.05 ± 0.27 (6), 2.05 ± 0.71 (6); F[3,20] = 0.897, n.s.; GLTwt/nTg, GLThet/nTg, GLTwt/AβPP/PS1, and GLThet/AβPP/PS1, respectively). These findings argue against the idea that partial GLT-1 loss influenced mTOR signaling.
GLT-1 regulates IDE activity
Brain insulin signaling changes that are in general keeping with those reported herein have been argued to underlie reduced IDE expression in AD, which also degrades Aβ [33, 37, 38, 52, 53]. In addition, decreased phosphorylation of IRβ (Tyr1150/1151) is also associated with reduced IDE activity in mice with impaired insulin signaling due to a high fat diet [36]. In order to address whether GLT-1 heterozygosity and/or AβPP/PS1 transgene expression alters IDE activity in the brain, we measured the levels of TCA-soluble counts after incubation of cortical protein lysates with [125I]-insulin. These counts were normalized to counts obtained from samples incubated with excess non-radiolabeled insulin. Table 1 shows that partial loss of GLT-1, but not AβPP/PS1 transgene expression, significantly reduced IDE activity and that IDE activity levels did not differ significantly between 6- and 9-month-old mice (GLT-1: F[1,40] = 6.930, p ≤ 0.012; AβPP: F[1,40] = 0.704, n.s.; Age: F[1,40] = 0.902, n.s., respectively). Table 1 also shows that IDE protein levels measured by western blot were not statistically different at either 6 or 9 months of age (GLT-1: F[1,38] = 2.030, n.s.; AβPP: F[1,38] = 0.370, n.s.: Age, F[1,38] = 2.029, n.s.).
Table 1.
In brain, partial loss of GLT-1 reduced IDE activity, but not IDE protein expression
| Group | IDE Activitya | IDE Protein Expressionb | ||||||
|---|---|---|---|---|---|---|---|---|
| 6 Months | 9 Months | 6 Months | 9 Months | |||||
| GLTwt/nTg | 1.00 ± 0.08 | 1.00 ± 0.10 |
p <0.012 (GLT-1 +/− vs GLT-1 +/+) |
1.00 ± 0.04 | 1.00 ± 0.08 | n.s. (GLT-1 +/− vs GLT-1 +/+) |
||
| GLTwt/AβPP/PS1 | 1.04 ± 0.09 | 0.87 ± 0.06 | 0.86 ± 0.08 | 0.94 ± 0.06 | ||||
| GLThet/nTg | 0.82 ± 0.07 | 0.86 ± 0.03 | 1.00 ± 0.01 | 1.01 ± 0.08 | ||||
| GLThet/AβPP/PS1 | 0.85 ± 0.17 | 0.71 ± 0.06 | 1.01 ± 0.13 | 1.07 ± 0.08 | ||||
IDE activity was measured by the level of trichloroacetic acid-soluble counts after incubation of lysates with [125I]-insulin and were normalized to samples incubated with excess non-radiolabeled insulin. Data were then normalized to the mean of the GLTwt/nTg group defined as 1. A three-way analysis of variance (ANOVA) revealed that partial GLT-1 (GLT-1+/+ versus GLT-1+/−) loss caused a significant decrease in IDE activity (p < 0.012), while the effects of AβPP/PS1 transgene expression (AβPP/PS1 versus non-transgenic) and age (6 versus 9 months) were not statistically significant. Data are presented as mean±standard error of the mean (SEM). n = 6 mice per group.
IDE protein expression was measured by Western blot densitometry and normalized with respect to actin immunoreactivity reprobed on the same blot. As with IDE activity, IDE protein levels were normalized with respect to the mean of the GLTwt/nTg group defined as 1. A three-way ANOVA revealed no statistically significant differences for any factors (GLT-1 genotype, AβPP/PS1 expression, or age). Data are presented as mean±standard error of the mean (SEM). n = 5–6 mice per group.
These results indicate that partial GLT-1 loss significantly reduced the enzymatic activity of cortical IDE, an enzyme that has been found to be reduced in AD [38, 52], even at early stages of the disease [53]. This also raises an interesting question as to why overall amyloid levels were not elevated in these mice [20] in that IDE degrades both Aβ40 and Aβ42 [54]. While there are multiple proteases capable of catabolizing Aβ [55], another robust means of clearing CNS Aβ involves Aβ transiting the blood-brain barrier into the peripheral circulation followed by rapid degradation in the liver [56–59]. Both IDE and GLT-1 are highly expressed in liver [60, 61]. In addition, the liver has been shown to regulate Aβ clearance from the brain by affecting circulating peripheral Aβ levels [59].
To determine whether partial GLT-1 loss influenced IDE activity in the liver, we measured TCA-soluble counts after incubation of liver protein lysates with [125I]-insulin. Again, these counts were normalized to counts obtained from samples incubated with excess non-radiolabeled insulin. Table 2 shows that partial GLT-1 loss, but not expression of the AβPP/PS1 transgene, caused a marked, significant increase in IDE activity in the liver (GLT-1: F[1,36] = 42.717, p < 0.001; AβPP: F[1,36] = 3.457, n.s.; Age: F[1,36] = 1.882, n.s.). Liver IDE protein levels were not significantly different with respect to genotype factor (GLT-1: F[1,36] = 1.103, n.s.; AβPP: F[1,36] = 0.252, n.s.), but were overall higher in the older mice (Age: F[1,36] = 5.115, p ≤ 0.030).
Table 2.
In liver, partial loss of GLT-1 increased IDE activity, but not IDE protein expression
| Group | IDE Activitya | IDE Protein Expressionb | ||||||
|---|---|---|---|---|---|---|---|---|
| 6 Months | 9 Months | 6 Months | 9 Months | |||||
| GLTwt/nTg | 1.00 ± 0.28 | 1.00 ± 0.95 |
P <0.001 (GLT-1 +/− vs GLT-1 +/+) |
1.00 ± 0.10 | 1.00 ± 0.03 | n.s. (GLT-1 +/− vs GLT-1 +/+) |
||
| GLTwt/AβPP/PS1 | 0.49±0.08 | 0.86±0.25 | 1.11 ±0.17 | 1.38±0.09 | ||||
| GLThet/nTg | 4.14 ± 0.72 | 2.08 ± 0.22 | 1.04±0.15 | 1.14±0.09 | ||||
| GLThet/AβPP/PS1 | 3.10 ± 0.44 | 1.65 ± 0.21 | 0.70 ± 0.12 | 1.20 ± 0.05 | ||||
IDE activity was measured by the level of trichloroacetic acid-soluble counts after incubation of lysates with [125I]-insulin and were normalized to samples incubated with excess non-radiolabeled insulin. Data were then normalized to the mean of the GLTwt/nTg group defined as 1. A three-way analysis of variance (ANOVA) revealed that partial GLT-1 (GLT-1+/+ versus GLT-1+/−) loss caused a significant increase in IDE activity (p < 0.001), while the effects of AβPP/PS1 transgene expression (AβPP/PS1 versus non-transgenic) and age (6 versus 9 months) were not statistically significant. Data are presented as mean±standard error of the mean (SEM). n = 2–9 mice per group.
IDE protein expression was measured by Western blot densitometry and normalized with respect to pyruvate kinase immunoreactivity reprobed on the same blot. As with IDE activity, IDE protein levels were normalized with respect to the mean of the GLTwt/nTg group defined as 1. A three-way ANOVA (GLT-1 genotype, AβPP/PS1 expression, and age) revealed a statistically significant difference between the two ages. Upon further analysis, a two-way ANOVA (GLT-1 genotype and AβPP/PS1 expression) of 6 month data revealed no significant differences, while at 9 months there was only a significant effect of AβPP/PS1 expression (p < 0.019). Data are presented as mean±standard error of the mean (SEM). n = 2–9 mice per group.
These results provide additional evidence that GLT-1 regulates IDE activity. Moreover, they also suggest the possibility that elevated IDE activity in the liver could compensate for impaired CNS IDE activity by facilitating systemic Aβ clearance.
DISCUSSION
Glutamate is the primary excitatory neurotransmitter in the brain and is essential for normal CNS activity. The significance of glutamatergic signaling is underscored by estimates that as much as 80% of the metabolic energy consumed by the CNS is related to glutamate cycling [62]. This indicates that even at rest, glutamate cycling places a sustained metabolic burden on brain cells that require highly efficient and dynamic regulatory systems in order to prevent glutamate-induced CNS dysfunction. In keeping with the critical importance of rapidly clearing this amino acid, glutamate transporters are highly expressed in the brain where, for example, GLT-1 represents approximately 1% of the total tissue protein in hippocampal CA1 stratum radiatum [5].
Involvement of GLT-1 in metabolically significant brain functions
A growing body of evidence indicates that GLT-1 plays important roles in regulating pathways that govern the metabolic status of the CNS. Modulation of activity-dependent glucose utilization is regulated by both GLT-1 and GLAST where it has been shown that partial glutamate transporter loss can impair glutamate-evoked, but not basal glucose uptake in astrocytes [23]. Also, recent findings show that synaptic activity regulates co-localization of mitochondria with GLT-1 at sites of glutamate uptake [63]. Significantly, a number of studies show that treatments which activate the IR/IRS-1/Akt signaling cascade [32, 64] can regulate GLT-1 expression levels [25–27]. For example, Li and colleagues [27] have reported that viral vectors expressing constitutively active Akt transgenes increase GLT-1 expression in primary cultured astrocytes. Interestingly, in this latter example altering Akt activity modulated GLT-1 expression, whereas in our experiments Akt signaling was altered by GLT-1 heterozygosity. This apparent distinction is likely due to differences between in vitro and in vivo experimental conditions, where GLT-1 expressing cells interact within synaptic networks, neurovascular elements, and other CNS systems in more complex ways than can reasonably be approached with isolated astrocytes in culture. More importantly, both the in vitro [27] data and our in vivo findings independently demonstrate that Akt and GLT-1 interact functionally in a significant metabolic pathway that likely accommodates both feed-forward and feedback responses evoked by glutamatergic signals.
GLT-1 is expressed at low levels in the pancreas [65], thereby raising the question whether some aspects of GLT-1 function might be related to pancreatic GLT-1 endocrine and/or exocrine activity. However, recent studies using mice with a brain-specific GLT-1 deficiency demonstrate that GLT-1 loss in the brain alone is sufficient to account for the primary phenotypes associated with total systemic GLT-1 loss [1, 66]. Moreover, GLT-1 expression levels in the pancreas are considered too low to be functionally significant in clearing extracellular pancreatic glutamate [66]. The animals used in this study were committed to biochemical analyses before we appreciated there may be GLT-1 mediated changes in insulin signaling. Therefore, we do not know whether GLT-1 heterozygous animals displayed peripheral insulin resistance. However, this possibility is unlikely as the mice were maintained on normal mouse chow, and more importantly, there were no significant differences in body weight among the genotype groups [20].
Involvement of GLT-1 in AD-related pathology
At 6 months of age, but not at 9 months, the mice studied in this report exhibited a significant increase in the ratio of Aβ42/Aβ40 among GLT-1 heterozygotes compared to animals with normal, wild-type GLT-1 levels [20]. While this increase was modest, this raises the possibility that Aβ42 levels might have contributed to the overall pattern of altered Akt, IRβ, and IRS-1 phosphorylation, which collectively was more pronounced in the 6-month-old animals than in the 9-month-old mice. Other investigators have reported that exposing primary cultured neurons to oligomeric Aβ42 (also known as amyloid-β-derived diffusible ligands, or ADDLs) increases phospho-Akt (Ser473) with an accompanying reduction in phospho-IRβ (Tyr1150/1151) levels [35]. In addition, glutamate exposure (simulating synaptic glutamate release) and treatments that stimulate neuronal glutamate release also reduced phospho-IRβ (Tyr1150/1151) [35]. These data are consistent with our findings which show that both partial GLT-1 loss and expression of an AβPPswe/PS1 ΔE9 transgene increases Akt (Ser473) phosphorylation; and is associated with a similar reduction in IRβ (Tyr1150/1151) phosphorylation.
We and others have reported that GLT-1 is significantly reduced and aberrantly expressed in both mild cognitively impaired and late-stage AD patients [16–19]. Such losses would not be expected to prevent restoration of basal extracellular glutamate levels following synaptic glutamate release [22, 67]. However, partial glutamate transporter loss has a significant impact on the spread of synaptically released glutamate that can degrade pathway specificity of synaptic transmission and plasticity [68, 69]. In keeping with this, we have recently reported that exposing hippocampal slices to oligomeric Aβ42 reduces GLT-1 activity, thereby approximately doubling the time required to clear endogenous, synaptically released glutamate by specifically reducing GLT-1 activity [21].
In this report, we found that partial GLT-1 loss, but not AβPP/PS1 mutant transgene expression, reduced IDE activity levels in the brain (Table 1). Other studies have shown that reduced CNS IDE activity and protein levels are associated with impaired insulin signaling and are also associated with AD pathology in humans and animal models of AD [33, 37, 38, 52, 53, 70, 71]. Whether GLT-1-specific reductions in IDE activity are driven by changes in Akt, IRβ, or IRS-1, or whether reduced IDE activity might cause the observed insulin signaling changes, cannot be discerned from the data reported herein. The finding that partial GLT-1 loss reduces IDE activity provides additional independent evidence supporting the conclusion that insulin-related disturbances are caused by GLT-1 loss.
In conjunction with reduced brain IDE activity, GLT-1 loss also caused a marked increase in IDE activity in the liver, which expresses high levels of GLT-1 in zone 3 hepatocytes [65, 72]. While we found a significant increase in the ratio of Aβ42/Aβ40 in these mice at 6 months of age, we did not find any other significant changes in Aβ accumulation between the GLTwt/AβPP/PS1 and GLThet/AβPP/PS1 groups at either 6 or 9 months [20]. CNS Aβ is efficiently cleared into the peripheral circulatory system, which is often referred to as a peripheral Aβ sink [56, 73]. We and others have shown that the liver functions as a critical systemic Aβ drain [58, 59, 74, 75]. Importantly, peripheral Aβ clearance by the liver can regulate CNS Aβ clearance [59]. Thus, it is possible that the increased hepatic IDE activity associated with GLT-1 heterozygosity could potentially help compensate for decreased IDE activity in the brain by increasing the dynamics of peripheral Aβ clearance mediated by the liver.
GLT-1 loss as an early factor in AD pathology
We found that partial GLT-1 loss had more pronounced effects on Akt, IRβ, and IRS-1 signaling at 6 months than at 9 months of age. This outcome is consistent with the behavioral impairments manifested by these mice [20], thereby strengthening the idea that GLT-1 loss can drive cognitive impairments via mechanisms that involve early-occurring insulin/Akt signaling defects.
Overall, these findings are in keeping with the idea that GLT-1 loss primarily influences AD-related neuropathology early on in the pathogenic process, prior to later time points when amyloid deposition becomes a more dominant pathological factor in these AβPP/PS1 transgenic mice that accumulate Aβ at higher rates after 6 months [39].
These data linking GLT-1 loss to insulin/Akt signaling defects correspond comparatively well with findings in AD patients. Nonetheless, a limitation of this animal model is that in AD GLT-1 loss in the brain is progressive over the course of disease [16] and in humans, presumably normal adult levels of GLT-1 are maintained for years prior to the onset of latent pathogenic processes. In the mice, genetically-mediated GLT-1 heterozygous mice express 50–60% of the GLT-1 wild-type levels from birth. It is possible this could contribute to complex interactions between GLT-1 heterozygosity and prolonged AβPP/PS1 transgene expression that could also influence the prominence of GLT-1 loss on cognition and Akt/insulin signaling at earlier, rather than later time points.
Summary
In conclusion, the findings in this report demonstrate for the first time that partial GLT-1 loss causes sustained Akt activation in vivo that is associated with feedback-related impairment of IR/IRS-1 signaling; and which together mimic the pattern of cognitive impairments in these mice. These results are consistent with prior findings that implicate Akt activity and insulin signaling in GLT-1 regulation. Taken collectively, these studies expand and strengthen the argument that GLT-1 plays an important role in regulating the metabolic status of the brain. In addition, these results suggest the possibility that GLT-1 dysfunction/loss which attends AD pathogenesis may be a contributing mechanism to disturbances in Akt and the IR/IRS-1 pathway that occur in AD [32–34].
Acknowledgments
This work was supported by funding from the Veteran’s Affairs Office of Research and Development Medical Research Service (DGC), an NIH Pharmacological Sciences training grant [5T32GM00775033] (KDM), an NIH institutional fellowship [T32 AG000258] (JSM), and services by the Cellular Functional Analysis Core of the University of Washington Diabetes Research Center (PO1 DK17047). Original GLT-1 (+/−) mice were graciously made availabe by Kohichi Tanaka (Tokyo Medical and Dental University).
Footnotes
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/14-2304r1).
REFERENCES
- 1.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 2.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 3.Jarzylo LA, Man HY. Parasynaptic NMDA receptor signaling couples neuronal glutamate transporter function to AMPA receptor synaptic distribution and stability. J Neurosci. 2012;32:2552–2563. doi: 10.1523/JNEUROSCI.3237-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haugeto O, Ullensvang K, Levy LM, Chaudhry FA, Honore T, Nielsen M, Lehre KP, Danbolt NC. Brain glutamate transporter proteins form homomultimers. J Biol Chem. 1996;271:27715–27722. doi: 10.1074/jbc.271.44.27715. [DOI] [PubMed] [Google Scholar]
- 5.Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: Chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang YH, Dykes-Hoberg M, Tanaka K, Rothstein JD, Bergles DE. Climbing fiber activation of EAAT4 transporters and kainate receptors in cerebellar Purkinje cells. J Neurosci. 2004;24:103–111. doi: 10.1523/JNEUROSCI.4473-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 1997;16:3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci. 1998;10:976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 9.Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 10.Arzberger T, Krampfl K, Leimgruber S, Weindl A. Changes of NMDA receptor subunit (NR1, NR2B) and glutamate transporter (GLT1) mRNA expression in Huntington’s disease–an in situ hybridization study. J Neuropathol Exp Neurol. 1997;56:440–454. doi: 10.1097/00005072-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Hassel B, Tessler S, Faull RL, Emson PC. Glutamate uptake is reduced in prefrontal cortex in Huntington’s disease. Neurochem Res. 2008;33:232–237. doi: 10.1007/s11064-007-9463-1. [DOI] [PubMed] [Google Scholar]
- 12.van Landeghem FK, Weiss T, Oehmichen M, von Deimling A. Decreased expression of glutamate transporters in astrocytes after human traumatic brain injury. J Neurotrauma. 2006;23:1518–1528. doi: 10.1089/neu.2006.23.1518. [DOI] [PubMed] [Google Scholar]
- 13.Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 14.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 15.Maragakis NJ, Dykes-Hoberg M, Rothstein JD. Altered expression of the glutamate transporter EAAT2b in neurological disease. Ann Neurol. 2004;55:469–477. doi: 10.1002/ana.20003. [DOI] [PubMed] [Google Scholar]
- 16.Woltjer RL, Duerson K, Fullmer JM, Mookherjee P, Ryan AM, Montine TJ, Kaye JA, Quinn JF, Silbert L, Erten-Lyons D, Leverenz JB, Bird TD, Pow DV, Tanaka K, Watson GS, Cook DG. Aberrant detergent-insoluble excitatory amino acid transporter 2 accumulates in Alzheimer disease. J Neuropathol Exp Neurol. 2010;69:667–676. doi: 10.1097/NEN.0b013e3181e24adb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob CP, Koutsilieri E, Bartl J, Neuen-Jacob E, Arzberger T, Zander N, Ravid R, Roggendorf W, Riederer P, Grunblatt E. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. J Alzheimers Dis. 2007;11:97–116. doi: 10.3233/jad-2007-11113. [DOI] [PubMed] [Google Scholar]
- 18.Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- 19.Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H, 3rd, Kraner SD, Norris CM. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009;29:12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mookherjee P, Green PS, Watson GS, Marques MA, Tanaka K, Meeker KD, Meabon JS, Li N, Zhu P, Olson VG, Cook DG. GLT-1 loss accelerates cognitive deficit onset in an Alzheimer’s disease animal model. J Alzheimers Dis. 2011;26:447–455. doi: 10.3233/JAD-2011-110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scimemi A, Meabon JS, woltjer RL, Sullivan JM, Diamond JS, Cook DG. Amyloid-beta 1–42 slows clearance of synaptically released glutamate by mislocalizing astrocytic GLT-1. J Neurosci. 2013;33:5312–5318. doi: 10.1523/JNEUROSCI.5274-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, Magistretti PJ, Pellerin L. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37:275–286. doi: 10.1016/s0896-6273(02)01170-4. [DOI] [PubMed] [Google Scholar]
- 24.Herard AS, Dubois A, Escartin C, Tanaka K, Delzescaux T, Hantraye P, Bonvento G. Decreased metabolic response to visual stimulation in the superior colliculus of mice lacking the glial glutamate transporter GLT-1. Eur J Neurosci. 2005;22:1807–1811. doi: 10.1111/j.1460-9568.2005.04346.x. [DOI] [PubMed] [Google Scholar]
- 25.Ji YF, Xu SM, Zhu J, Wang XX, Shen Y. Insulin increases glutamate transporter GLT1 in cultured astrocytes. Biochem Biophys Res Commun. 2011;405:691–696. doi: 10.1016/j.bbrc.2011.01.105. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Kihara T, Akaike A, Niidome T, Sugimoto H. PI3K/Akt/mTOR signaling regulates glutamate transporter 1 in astrocytes. Biochem Biophys Res Commun. 2010;393:514–518. doi: 10.1016/j.bbrc.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 27.Li LB, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, Robinson MB. Regulation of astrocytic glutamate transporter expression by Akt: Evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem. 2006;97:759–771. doi: 10.1111/j.1471-4159.2006.03743.x. [DOI] [PubMed] [Google Scholar]
- 28.Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O’Connor R, O’Neill C. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem. 2005;93:105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- 29.Pei JJ, Khatoon S, An WL, Nordlinder M, Tanaka T, Braak H, Tsujio I, Takeda M, Alafuzoff I, Winblad B, Cowburn RF, Grundke-Iqbal I, Iqbal K. Role of protein kinase B in Alzheimer’s neurofibrillary pathology. Acta Neuropathol. 2003;105:381–392. doi: 10.1007/s00401-002-0657-y. [DOI] [PubMed] [Google Scholar]
- 30.Rickle A, Bogdanovic N, Volkman I, Winblad B, Ravid R, Cowburn RF. Akt activity in Alzheimer’s disease and other neurodegenerative disorders. Neuroreport. 2004;15:955–959. doi: 10.1097/00001756-200404290-00005. [DOI] [PubMed] [Google Scholar]
- 31.Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31:224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cholerton B, Baker LD, Craft S. Insulin, cognition, and dementia. Eur J Pharmacol. 2013;719:170–179. doi: 10.1016/j.ejphar.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol. 2014;88:548–559. doi: 10.1016/j.bcp.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22:246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- 36.Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 37.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta- protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook DG, Leverenz JB, McMillan PJ, Kulstad JJ, Ericksen S, Roth RA, Schellenberg GD, Jin LW, Kovacina KS, Craft S. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-epsilon4 allele. Am J Pathol. 2003;162:313–319. doi: 10.1016/s0002-9440(10)63822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: Evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 40.Bennett RG, Duckworth WC, Hamel FG. Degradation of amylin by insulin-degrading enzyme. J Biol Chem. 2000;275:36621–36625. doi: 10.1074/jbc.M006170200. [DOI] [PubMed] [Google Scholar]
- 41.Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. McGraw-Hill, Inc.; 1991. [Google Scholar]
- 42.Field A. Discovering Statistics Using SPSS. London: Sage Publications; 2005. [Google Scholar]
- 43.Morisco C, Condorelli G, Trimarco V, Bellis A, Marrone C, Condorelli G, Sadoshima J, Trimarco B. Akt mediates the cross-talk between beta-adrenergic and insulin receptors in neonatal cardiomyocytes. Circ Res. 2005;96:180–188. doi: 10.1161/01.RES.0000152968.71868.c3. [DOI] [PubMed] [Google Scholar]
- 44.Tian R. Another role for the celebrity: Akt and insulin resistance. Circ Res. 2005;96:139–140. doi: 10.1161/01.RES.0000156076.17807.1F. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Z, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huber HE, Duggan ME, Lindsley CW. Development of potent, allosteric dual Akt1 and Akt2 inhibitors with improved physical properties and cell activity. Bioorg Med Chem Lett. 2008;18:49–53. doi: 10.1016/j.bmcl.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 46.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: Insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 47.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 48.Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296:E581–E591. doi: 10.1152/ajpendo.90437.2008. [DOI] [PubMed] [Google Scholar]
- 49.Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci U S A. 2001;98:4640–4645. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci U S A. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 52.Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Abeta-degrading enzymes in Alzheimer’s disease. Brain Pathol. 2008;18:240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Z, Xiang Z, Haroutunian V, Buxbaum JD, Stetka B, Pasinetti GM. Insulin degrading enzyme activity selectively decreases in the hippocampal formation of cases at high risk to develop Alzheimer’s disease. Neurobiol Aging. 2007;28:824–830. doi: 10.1016/j.neurobiolaging.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Mukherjee A, Song E, Kihiko-Ehmann M, Goodman JP, Jr, Pyrek JS, Estus S, Hersh LB. Insulysin hydrolyzes amyloid beta peptides to products that are neither neurotoxic nor deposit on amyloid plaques. J Neurosci. 2000;20:8745–8749. doi: 10.1523/JNEUROSCI.20-23-08745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s Abeta peptide: The many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 56.Zlokovic BV. Clearing amyloid through the blood-brain barrier. J Neurochem. 2004;89:807–811. doi: 10.1111/j.1471-4159.2004.02385.x. [DOI] [PubMed] [Google Scholar]
- 57.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Ghiso J, Shayo M, Calero M, Ng D, Tomidokoro Y, Gandy S, Rostagno A, Frangione B. Systemic catabolism of Alzheimer’s Abeta40 and Abeta42. J Biol Chem. 2004;279:45897–45908. doi: 10.1074/jbc.M407668200. [DOI] [PubMed] [Google Scholar]
- 59.Marques MA, Kulstad JJ, Savard CE, Green PS, Lee SP, Craft S, Watson GS, Cook DG. Peripheral amyloid-beta levels regulate amyloid-beta clearance from the central nervous system. J Alzheimers Dis. 2009;16:325–329. doi: 10.3233/JAD-2009-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: Progress and potential. Endocr Rev. 1998;19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 61.Berger UV, Hediger MA. Distribution of the glutamate transporters GLT-1 (SLC1A2) and GLAST (SLC1A3) in peripheral organs. Anat Embryol (Berl) 2006;211:595–606. doi: 10.1007/s00429-006-0109-x. [DOI] [PubMed] [Google Scholar]
- 62.Raichle ME, Gusnard DA. Appraising the brain’s energy budget. Proc Natl Acad Sci U S A. 2002;99:10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jackson JG, O’Donnell JC, Takano H, Coulter DA, Robinson MB. Neuronal activity and glutamate uptake decrease mitochondrial mobility in astrocytes and position mitochondria near glutamate transporters. J Neurosci. 2014;34:1613–1624. doi: 10.1523/JNEUROSCI.3510-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20:665–679. doi: 10.1016/j.beem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 65.Meabon JS, Lee A, Meeker KD, Bekris LM, Fujimura RK, Yu CE, Watson GS, Pow DV, Sweet IR, Cook DG. Differential expression of the glutamate transporter GLT-1 in pancreas. J Histochem Cytochem. 2012;60:139–151. doi: 10.1369/0022155411430095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou Y, Waanders LF, Holmseth S, Guo C, Berger UV, Li Y, Lehre AC, Lehre KP, Danbolt NC. Proteome analysis and conditional deletion of the EAAT2 glutamate transporter provide evidence against a role ofEAAT2 in pancreatic insulin secretion in mice. J Biol Chem. 2014;289:1329–1344. doi: 10.1074/jbc.M113.529065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diamond JS, Jahr CE. Synaptically released glutamate does not overwhelm transporters on hippocampal astrocytes during high-frequency stimulation. J Neurophysiol. 2000;83:2835–2843. doi: 10.1152/jn.2000.83.5.2835. [DOI] [PubMed] [Google Scholar]
- 68.Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsvetkov E, Shin RM, Bolshakov VY. Glutamate uptake determines pathway specificity of long-term potentiation in the neural circuitry of fear conditioning. Neuron. 2004;41:139–151. doi: 10.1016/s0896-6273(03)00800-6. [DOI] [PubMed] [Google Scholar]
- 70.Abdul-Hay SO, Kang D, McBride M, Li L, Zhao J, Leissring MA. Deletion of insulin-degrading enzyme elicits antipodal, age-dependent effects on glucose and insulin tolerance. PLoS One. 2011;6:e20818. doi: 10.1371/journal.pone.0020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- 72.Cadoret A, Ovejero C, Terris B, Souil E, Levy L, Lamers WH, Kitajewski J, Kahn A, Perret C. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 2002;21:8293–8301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
- 73.Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci. 2008;31:151–173. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hone E, Martins IJ, Fonte J, Martins RN. Apolipoprotein E influences amyloid-beta clearance from the murine periphery. J Alzheimers Dis. 2003;5:1–8. doi: 10.3233/jad-2003-5101. [DOI] [PubMed] [Google Scholar]
- 75.Tamaki C, Ohtsuki S, Iwatsubo T, Hashimoto T, Yamada K, Yabuki C, Terasaki T. Major involvement of low-density lipoprotein receptor-related protein 1 in the clearance of plasma free amyloid beta-peptide by the liver. Pharm Res. 2006;23:1407–1416. doi: 10.1007/s11095-006-0208-7. [DOI] [PubMed] [Google Scholar]