Abstract
Background
The aim of our study was to determine an influence of incarcerated inguinal hernia mesh repair on testicular circulation and to investigate consequent sperm autoimmunity as a possible reason for infertility.
Material/Methods
This prospective study was performed over a 3-year period, and 50 male patients were included; 25 of these patients underwent elective open mesh hernia repair (Group I). Group II consisted of 25 patients who had surgery for incarcerated inguinal hernia. Doppler ultrasound evaluation of the testicular blood flow and blood samplings for antisperm antibodies (ASA) was performed in all patients before the surgery, on the second day, and 5 months after. Main outcome ultrasound measures were resistive index (RI) and pulsative index (PI), as their values are inversely proportional to testicular blood flow.
Results
In Group I, RI, and PI temporarily increased after surgery and then returned to basal values in the late postoperative period. Friedman analysis showed a significant difference in RI and PI for all measurements in Group II (p<0.05), with a significant decrease between the preoperative, early, and late postoperative periods. All final values were within reference range, including ASA, despite significant increase of ASA in the late postoperative period.
Conclusions
Although statistically significant differences in values of testicular flow parameters and immunologic sensitization in observed time, final values remained within the reference ranges in all patients. Our results suggest that the polypropylene mesh probably does not cause any clinically significant effect on testicular flow and immunologic response in both groups of patients.
MeSH Keywords: Blood-Testis Barrier; Hernia, Inguinal; Infertility, Male; Surgical Mesh; Ultrasonography, Doppler
Background
According to European Hernia Society guidelines, patients with symptomatic inguinal hernias should be operated on electively to reduce complaints and to prevent complications such as incarceration or strangulation. It is believed that inguinal hernia occurs in 5% of adult men, and 5–15% of patients with inguinal hernia will need emergency surgery due to incarceration [1,2]. Current surgical treatment includes prosthetic mesh in both elective and urgent procedures [3]. Polypropylene meshes can be safely used in inguinal hernia surgery, even when intestine resection is needed [4–6]. Yavez et al. emphasized surgical treatment of inguinal hernia in 6.65% of infertile men as a main cause of infertility, and semen quality was markedly reduced in comparison with that of fertile men [7]. The connection between decreased testicular arterial flow and immunosensitization has been demonstrated in animal models. Unilateral testicular ischemia in the rat results in morphological damage in the contralateral testis due to increase in serum cytotoxic antisperm antibodies [8]. It has also been proven in animal models that mesh, besides direct compression on the spermatic cord, additionally narrows the lumen of the deferent duct by its contraction, and also reduces the arterial supply of the testis, consequently affecting spermatogenesis [9,10]. Clinical studies of testicular flow after inguinal hernia mesh repair showed different outcomes. Sucullu et al. support the hypothesis that mesh techniques in inguinal hernia repair significantly change the Doppler parameters in the early postoperative period, but do not have a significant effect on sperm concentration or rate of progressive motility [11]. In contrast, the study by Ramadan et al. showed that mesh placement does not adversely affect ipsilateral testicular flow [12]. The measurement of Doppler ultrasound parameters at the level of the testicular artery has been used to assess testicular flow in various studies, and Pinggera et al. reported that intratesticular level measurements are good spermatogenesis predictors [13]. Hillelsohn et al. confirmed those findings and supported intratesticular RI use as an independent indicator of testicular function [14]. They also proposed further investigations of Doppler changes occurring after surgical therapy and their association with spermatogenesis. Therefore, Štula et al. measured the flow in testicular, capsular, and intratesticular arteries following mesh hernia repair in elective patients; they found only transitory changes in testicular perfusion, without any long-lasting adverse effects on microcirculation [15].
Antisperm antibodies (ASA) in males cause the autoimmune disease ‘immune infertility’. The incidence of sperm autoimmunity in infertile couples is about 20%; ASA assessment is considered as an essential part of infertility management [16]. It is not clear how the mechanism of ASA causes male infertility, but recent studies showed significant negative effects of ASA on sperm concentration and sperm motility [17]. Testicles are paired organs; it has been proven that the unilateral damage to the deferent duct may affect fertility by production of ASA, which can be determined in serum before an evident histological unilateral or bilateral damage, as shown in a rat model [18]. Also, it can be affected by free radicals discharged after ischemia in gastrointestinal organs [19]. This is an autoimmune reaction which is responsible for damaging the function of the contralateral testis. It was hypothesized that increased testicular tissue ischemia and hypoxia damage to the basement membrane of endothelial and Sertoli cells in the seminiferous tubules may lead to the destruction of the blood-testis barrier, activating autoimmune reaction to produce ASA and result in infertility by direct interaction with sperm or indirect change to the local microenvironment. In their study, HaoXu et al. compared the effects of different types of hernia mesh on the reproductive function of adult male rats following open tension-free hernioplasty. In comparison with polytetrafluoroethylene groups, the concentration of ASA was significantly increased and the differences were statistically significant in the polypropylene groups [20]. The influence of elective inguinal hernia mesh repair on sperm autoimmunity and testicular flow was investigated by Štula et al., and there was no effect on testicular flow change on the blood-testicle barrier that could lead to a significant immune reaction [15].
The aim of this study was to determine the influence of incarcerated inguinal hernia mesh repair on testicular circulation and to investigate an autoimmunological reaction as a possible reason for infertility.
Material and Methods
The prospective study was performed over a 3-year period (January 2012 to December 2014) in 25 male patients with incarcerated indirect inguinal hernia who underwent urgent surgical procedure at the Department of Surgery, University Hospital Split. Inclusion criteria were male sex and reducible hernia. Exclusion criteria were previous history of fertility problems, testicular trauma, operation or clinical detectable testicular disease, previous hernia repair, genitourinary infection, or immunodeficiency. Also, patients with direct inguinal hernias were not included in the study because of anatomical features of direct hernia and consequently less expected negative impact on testicular circulation. The control group consisted of 25 male patients hospitalized for a planned elective surgical treatment of indirect inguinal hernias who had given written consent to participate in the survey and have no exclusionary factors. The approval of the Ethics Committee of Split University Hospital was obtained for this study.
The preoperative preparation and postoperative care for patients who were treated electively was uniform. Also, preoperative preparation for patients who were treated from incarcerated inguinal hernia was also equable. Low-molecular weight heparin prophylaxis was administered to all patients before surgery, whereas a single dose of antibiotic prophylaxis (cefazolin 1 g) was administered to patients treated for incarcerated hernia. General anesthesia was administered to all patients, and all surgical procedures were performed by the same surgeon, using only anterior tension-free polypropylene mesh repair (Lichtenstein technique).
Operative technique
Mesh repairs of the inguinal hernia were performed via anterior tension-free approach. There were no signs of ischemic necrosis of content as a result of strangulation, and was no bowel resections were performed, so further procedures were identical in both groups. The posterior wall was covered by polypropylene mesh (Bard® Mesh – Monofilament, Davol a Bard Company, Providence, RI). The mesh was surgically shaped with a vertical cut cranial to the inguinal ligament and a round aperture was positioned around the spermatic cord and fixed with polyglactin 910 interrupted sutures (Vycril, Ethicon, Norderstedt, Germany).
Color Doppler ultrasound
Color Doppler ultrasound evaluation of the testicular blood flow was performed in all patients before the operation, in the early postoperative period (between 24 and 48 h after the operation), and in the late postoperative period (5 months after operation) (Figure 1). Testicular, capsular, and intratesticular arterial flow dynamics were measured by a single experienced radiologist.
Figure 1.
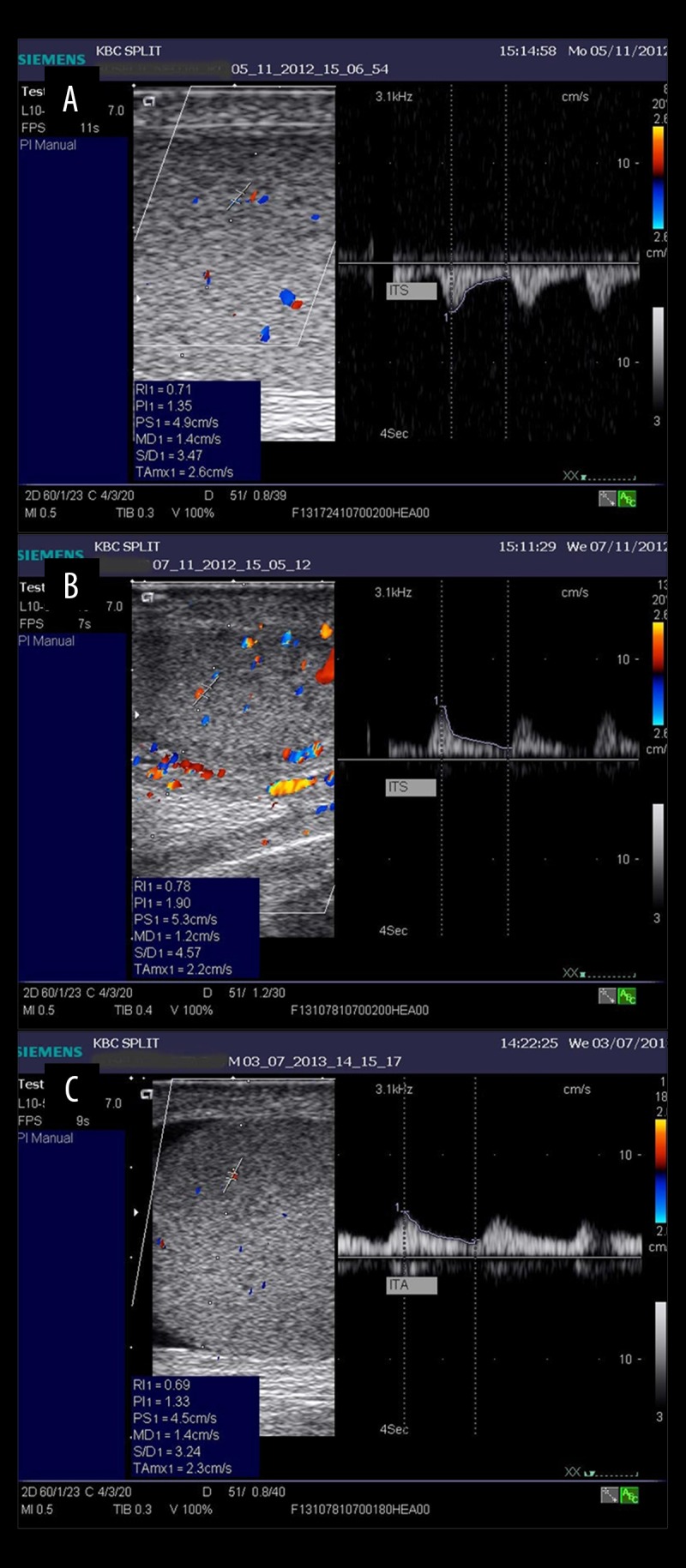
Intratesticular flow in a patient with incarcerated hernia: (A) preoperative, (B) early postoperative, (C) late postoperative measurement.
The patients were scanned in supine position using an ultrasound unit (Acuson X500, Siemens Medical Systems, Erlangen, Germany) with a 10 MHz linear array probe placed directly on the scrotal skin. Realtime scans were obtained in standard longitudinal and transverse plans to identify testicular blood flow on the hernia side. After that, point spectral analysis was performed over the testicular artery 1 cm superior to the pole of the testicle, over the capsular artery, and over the largest intratesticular vessel identified, in the middle and on both poles of the testicle. After testicular blood flow had been identified, the following parameters were evaluated: peak systolic velocity (PSV), end-diastolic velocity (EDV), pulsative index (PI), and resistive index (RI).
Antisperm antibodies (ASA)
Blood samples were drawn in all patients before hernia repair and 5 months after. The serum samples were immediately frozen and kept at a temperature of −70°C until they were analyzed. The antisperm antibodies were analyzed by enzyme-linked immunoadsorbent assay (ELISA, Iason, Germany).
Statistical analysis
The data were analyzed using nonparametric tests (Friedman, Wilcoxon, Mann-Whitney, χ2 test; Statistica for Windows Release 7.0, StatSoft, Tulsa, OK). All p values less than 0.05 were considered to indicate statistical significance.
Results
Fifty male patients underwent indirect inguinal hernia open repair using tension-free polypropylene mesh technique between January 2012 and December 2014. Twenty-five of them underwent elective open repair (Group I), and the other twenty-five had incarcerated hernia and underwent urgent open repair (Group II). The median age of patients in Group I was 57 years (range 40–81 years) and in Group II 64 years (range 28–80 years). No significant differences between the 2 groups were found regarding age (p=0.123) (Table 1).
Table 1.
Patient characteristics comparison: non-complicated hernia (Group I) and incarcerated hernia (Group II).
| All patients | ||||
|---|---|---|---|---|
| Group I | Group II | |||
| Patients (n, %) | 25 (50%) | 25 (50%) | ||
| Hernia side (n, %) | Right | 9 (36%) | 11 (44%) | |
| Left | 16 (64%) | 14 (56%) | ||
| Age (years) median (min.–max.) | 57 (40–81) | 64 (28–80) | p=0.123* | |
Mann-Whitney U test.
Testicular blood flow measurements
Color Doppler ultrasound evaluation of the testicular blood flow (resistive index-RI, pulsative index-PI, peak systolic velocity-PSV, and end-diastolic velocity-EDV) was performed in all patients preoperatively, 24–48 hours after the operation (early postoperative), and in 49 patients 5 months after the operation (late postoperative) at testicular, capsular, and intratesticular arteries levels.
No significant differences between the 2 groups were found in terms of Doppler ultrasound parameters for the preoperative period (Mann-Whitney test; p>0.05).
Friedman analysis showed significant difference of RI only in Group II at testicular artery level (χ2=32.4; p<0.001), capsular artery level (χ2=23.6; p<0.001), and intratesticular arteries level (χ2=20.9; p <0.001) over observed time. In the late postoperative period median value of RI decreased by 0.17 at testicular artery level, 0.14 at capsular artery level, and 0.09 at intratesticular arteries level in comparison to the preoperative values (Table 2).
Table 2.
Resistive index (RI) according to level and time of measurement in elective surgery patients (Group I) and in patients who underwent emergency surgery (Group II).
| Level of measurement | Time of measurement | RI Group I Median (min.–max.) |
p* | RI Group II Median (min.–max.) |
p* |
|---|---|---|---|---|---|
| Testicular artery | Preoperative | 0.81 (0.56–0.92) | 0.550 | 0.86 (0.57–1.0) | <0.001 |
| Early postoperative | 0.85 (0.5–0.96) | 0.74 (0.41–0.93) | |||
| Late postoperative | 0.83 (0.51–0.94) | 0.69 (0.51–0.95) | |||
| Capsular artery | Preoperative | 0.74 (0.52–0.86) | 0.228 | 0.75 (0.54–1) | <0.001 |
| Early postoperative | 0.74 (0.35–0.9) | 0.63 (0.54–0.94) | |||
| Late postoperative | 0.74 (0.56–0.88) | 0.61 (0.39–0.83) | |||
| Intratesticular artery | Preoperative | 0.65 (0.5–0.74) | 0.399 | 0.67 (0.52–1) | <0.001 |
| Early postoperative | 0.66 (0.38–0.8) | 0.62 (0.42–0.94) | |||
| Late postoperative | 0.65 (0.52–0.79) | 0.58 (0.41–0.74) |
Friedman analysis.
Friedman analysis showed significant difference of PI only in Group II at testicular artery level (χ2=11.2; p=0.004), capsular artery level (χ2=7.9; p=0.019), and intratesticular arteries level (χ2=10.3; p=0.006) over observed time. In the late postoperative period the median value of PI decreased by 0.41 at testicular artery level, by 0.1 at capsular artery level, and by 0.1 at intratesticular arteries level in comparison to the preoperative values (Table 3).
Table 3.
Pulsative index (PI) preview according to level and time of measurement in elective surgery patients (Group I) in comparison with patients who underwent emergency surgery (Group II).
| Level of measurement | Time of measurement | PI Group I Median (min.–max.) |
p* | PI Group II Median (min.–max.) |
p* |
|---|---|---|---|---|---|
| Testicular artery | Preoperative | 2.23 (0.96–3.67) | 0.549 | 1.79 (0–4.05) | 0.004 |
| Early postoperative | 2.27 (0.68–4.63) | 1.54 (0.81–4.51) | |||
| Late postoperative | 2.22 (1.04–3.49) | 1.38 (0.98–3.06) | |||
| Capsular artery | Preoperative | 1.54 (0.81–2.95) | 0.909 | 1.12 (0–2.19) | 0.019 |
| Early postoperative | 1.67 (0.61–3.16) | 1.11 (0.71–2.59) | |||
| Late postoperative | 1.59 (0.61–2.74) | 1.02 (0.51–2.15) | |||
| Intratesticular artery | Preoperative | 1.13 (0.73–1.67) | 0.392 | 1.07 (0–2.48) | 0.006 |
| Early postoperative | 1.27 (0.49–2.06) | 1.02 (0.57–3.39) | |||
| Late postoperative | 1.18 (0.8–1.92) | 0.97 (0.59–1.55) |
Friedman analysis.
In the Group I median PSV value rose for 0.7 cm/s at testicular artery level (χ2=10.2; p=0.006) and for 0.6 cm/s at intratesticular arteries level (χ2=6.2; p=0.044) in the early postoperative period in comparison to the preoperative value and returned to its preoperative value in the late postoperative measuring (Table 4).
Table 4.
Peak systolic velocity (PSV) preview according to level and time of measurement in elective surgery patients (Group I) in comparison with patients who underwent emergency surgery (Group II).
| Level of measurement | Time of measurement | PSV cm/s Group I Median (min.–max.) |
p* | PSV cm/s Group II Median (min.–max.) |
p* |
|---|---|---|---|---|---|
| Testicular artery | Preoperative | 9.75 (4.7–16.3) | 0.006 | 9.1 (0–20.6) | 0.453 |
| Early postoperative | 10.45 (7.7–30.5) | 9.9 (4.9–16.2) | |||
| Late postoperative | 9.35 (3.7–16.2) | 7.95 (5.1–18.3) | |||
| Capsular artery | Preoperative | 6.85 (4–11.2) | 0.947 | 7 (0–9.9) | 0.582 |
| Early postoperative | 6.6 (4–13.2) | 6.8 (4.6–21.7) | |||
| Late postoperative | 7 (3.8–10.7) | 6.1 (2.7–9.1) | |||
| Intratesticular artery | Preoperative | 4.85 (2.6–6.2) | 0.044 | 4.5 (0–8.1) | 0.002 |
| Early postoperative | 5.45 (3.3–9.3) | 5.3 (3.2–8.2) | |||
| Late postoperative | 4.8 (3.3–6) | 4.7 (2.6–6.8) |
Friedman analysis.
PSV also changed in Group II during observed time but only at the intratesticular arteries level (χ2=12.2; p=0.002). Median PSV value rose by 0.8 cm/s in the early postoperative period in comparison to the preoperative value and returned to its preoperative value in the late postoperative measuring.
In Group I, median EDV value rose from 0.25 cm/s at the intratesticular artery level (χ2=6.33; p=0.042) in the early postoperative period in comparison to the preoperative value, and returned to its preoperative value in the late postoperative measuring.
In Group II, median EDV value rose from 0.8 cm/s at testicular arteries level (χ2=10; P=0.007) and from 0.4 cm/s at intratesticular arteries level (χ2=12.4; P=0.002) in the early postoperative period in comparison to the preoperative value and remained at that level in the late postoperative measurement (Table 5).
Table 5.
End diastolic velocity (EDV) preview according to level and time of measurement in elective surgery patients (Group I) in comparison with patients who underwent emergency surgery (Group II).
| Level of measurement | Time of measurement | EDV cm/s Group I Median (min.–max.) |
p* | EDV cm/s Group II Median (min.–max.) |
p* |
|---|---|---|---|---|---|
| Testicular artery | Preoperative | 1.6 (0.8–3.5) | 0.297 | 1.7 (0–4.2) | 0.007 |
| Early postoperative | 1.8 (0.9–7.9) | 2.5 (1.1–3.6) | |||
| Late postoperative | 1.75 (0.7–4) | 2.7 (0.4–3.3) | |||
| Capsular artery | Preoperative | 1.7 (0.9–3.8) | 0.685 | 1.8 (0–3.5) | 0.502 |
| Early postoperative | 1.8 (0.9–3.9) | 2.5 (0.2–5.3) | |||
| Late postoperative | 1.7 (0.7–2.8) | 2.3 (1–3.7) | |||
| Intratesticular artery | Preoperative | 1.6 (0.8–2.8) | 0.042 | 1.5 (0–2.7) | 0.002 |
| Early postoperative | 1.85 (1.2–3.3) | 1.9 (0.5–2.8) | |||
| Late postoperative | 1.65 (0.8–2.3) | 1.85 (1.1–2.8) |
Friedman analysis.
Antisperm antibodies measurements
Both preoperative and late postoperative antisperm antibodies (ASA) values were measured in 46 patients (Table 6). There were no statistically significant differences in basal values (z=0.736, p=0.220, Mann-Whitney U test) amongst the elective and urgent patient groups.
Table 6.
Antisperm antibodies in basal and postoperative measurements [median (min.–max.)].
| Median (min.–max.) | P* | ||
|---|---|---|---|
| Group I | Basal | 30.5 (4.5–53.1) | 0.001 |
| After operation | 35.0 (15–56) | ||
| Group II | Basal | 24.4 (8.3–56.0) | <0.001 |
| After operation | 32.7 (19.2–63.0) |
Wilcoxon test.
With the elective patient group, median ASA rose for 4.5 U/ml after surgery in comparison to the basal value (z=3.36; p=0.001). With the urgent patient group, median ASA rose from 8.3 U/ml after the surgery in comparison to the preoperative value (z=3.7; p<0.001).
Discussion
The impact of inguinal hernia mesh repair on fertility of men remains a subject of scientific debate. The results of recent prospective studies in men do not support the hypothesis that inguinal hernia repair with alloplastic mesh prosthesis causes male infertility at a significantly greater rate than those operated on without mesh [21]. A number of clinical studies indicate that potential adverse effects of mesh repairs do not seem to have a clinical impact on male fertility [22]. The numbers of included patients were mainly limited, especially with bilateral hernias, and new observational cohort studies are being designed and in progress to assess the clinical relevance of the effects of inguinal hernia and hernia surgery on male fertility [23,24]. Despite the results stated in these studies, there is still a major ineradicable stance amongst surgeons that precautions are necessary when applying mesh on younger men who are planning to have children. Besides the direct damage to testis circulation, a cause of infertility can also be damage to the vas deferens as well as an autoimmune reaction with patients treated with mesh. Animal models show substantial effects of mesh hernia repair on structures in the spermatic cord. Histological examination revealed a foreign body reaction causing decreased spermatogenesis [10,25]. Inguinal vessel obstruction related to inguinal herniorrhaphy in humans is an uncommon and frequently unrecognized cause of male infertility. Infertility due to vas deferens dysfunction can be related to direct iatrogenic injury or delayed obstruction caused by scar tissue created as a result of inflammatory tissue response to a foreign body. Shin et al. evaluated 14 men with infertility who underwent hernia repairs with polypropylene mesh. Surgical exploration in the inguinal region at the previous hernia site revealed a dense fibro-plastic response encompassing the polypropylene mesh, with either trapped or obliterated vas deferens, and obstruction was confirmed by intraoperative vasograms [26].
Sucullu et al. measured postoperative spermiogram after mesh hernia repair and did not find statistically significant differences to preoperative values [11]. Also, Lee et al. reported that the presence of serum ASA was highly accurate in predicting obstructive azoospermia [16].
Vas deferens injury, testicle ischemia, and inflammatory reaction are considered to be the main causes of intolerance to sperm antigens and ASA production [27].
ASA can be detected prior to obvious testicle damage, and an antispermal immunological reaction can lead to dysfunction of the contralateral testis in infertile men who underwent unilateral mesh hernia repair [18].
There are only a few studies that have investigated ASA serum levels after hernia repair. Matsuda et al. and Friberg et al. found a significant increase of ASA in infertile patients treated by non-mesh repair [27,28]. On the other hand, Kapral et al. found insignificant ASA level increase after hernia repair [29]. Štula et al. investigated ASA levels in 2 groups of patients and found higher ASA values in the group treated by the anterior open free-tension method compared with the group treated by TAPP, but the difference in ASA value was not clinically important. A possible reason was greater tissue damage and consecutive inflammatory response, which is expected with the anterior approach. Also, there was no significant correlation between the change in testicular flow and the increased ASA value [15].
The present study assessed ASA values in patients treated for incarcerated hernia with mesh; we expected to find a more pronounced sensitization and therefore higher ASA values due to changes in testicular flow and consequently greater impact on blood-testicle barrier, in comparison to patients treated electively. Time of postoperative ASA measurement was based on 2 facts: ASA production is not evident until 15–30 days after the blood-testicle barrier is broken, and the possibility of extrinsic compression leaving a scar ends within 3 months when the wound healing process after repair is over [18]. Obviously, the short interval between incarceration and surgery was the explanation of immune response absence and preoperatively we did not found significant differences in ASA values between the 2 groups (p=0.220). We found a statistically significant increase of antisperm antibodies (ASA) in the late postoperative period compared to preoperative values in both groups of patients (Table 6). The functional blood-testicle barrier is the result of CD8+ suppressor T lymphocytes domination. A possible reason for an increase in postoperative ASA values is inflammatory reaction with increased prevalence of B-cells on immunosuppressor T cells after the mesh application and the consequent lack of autoimmune response inhibition. The ASA values were within the reference range (0–60 IU/ml), except in 3 patients with incarcerated hernia who had postoperative values slightly above the upper limit (60–63 IU/ml). Extensive manipulation with spermatic cord structures during incarcerated hernia surgery may be the cause of some higher postoperative ASA values in those patients. Nevertheless, the 2 groups of patients did not show statistically significant differences in postoperatively measured ASA values (p=0.826).
Until now there have been only a few studies of testicular flow and immunological response to sperm antigens that could be related to male infertility in patients treated by mesh hernia repair. Koksal et al. compared testicular perfusion in 2 groups of patients: those treated by totally extraperitoneal mesh repair, and those treated by Lichtenstein hernia repair. Resistive index (RI) results in both groups were statistically insignificant in all preoperative, early, and late postoperative periods [30]. In their ultrasound study, Brisinda et al. measured testicular blood flow and testicular volume after tension-free inguinal hernia repair, and no statistically significant differences were found between preoperative and postoperative measurements [31].
Štula et al. investigated testicular circulation using a linear array probe of 10 MHz which had a better resolution than a linear array probe of lower frequency used in other studies. In their study, measurements were made at 3 levels: testicular, capsular, and intratesticular. The study showed only transitory change in testicular perfusion without long-term adverse effects on microcirculation [15]. Hillelsohn et al. suggested that intratesticular RI can be a reliable indicator in routine clinical use to establish diagnostic criteria for normal and pathological sperm counts and to identify sub-fertile men [14]. They noticed that RI values show greater testicular vascular resistance in patients with pathological sperm counts than in those with normal sperm counts.
Until now there has been no Doppler study published which researched testicular flows in patients treated for hernia incarceration. Our hypothesis was that patients treated for hernia incarceration by urgent surgical procedures with inguinal mesh placement would have significant changes in testicular flow in the late postoperative period in comparison to the control group.
Therefore, in our study, flow was measured on 3 levels: the testicular, capsular, and intratesticular artery level. This was mandatory due to the fact that increased vascular resistance inside testicular tissue is related to spermatogenesis disturbance. Although resistance index (RI) and pulsative index (PI) values were of most importance, peak-systolic velocity (PSV) and end-diastolic velocity (EDV) values were also measured in our study as they are strongly correlated with testicular blood flow.
In our study, preoperative RI was higher in patients with incarcerated hernia (Group II) compared to the control group at all levels, but we did not prove a significant difference. It was to be expected that incarcerated hernia caused greater testicular flow changes before surgical intervention (Table 2).
With patients who underwent elective surgery, RI values did not change significantly in the early and late postoperative periods, so we can conclude that the surgical procedure, with all its consequential tissue changes, does not lead to temporary or permanent changes which could reflect in changes of RI values, regardless of the level of measurement.
The decompression effect of the surgical procedure and reactive hyperemia as a part of higher inflammatory response is a possible reason for the significant decrease of RI in the early postoperative period in Group II. It is known that testicular or funicular inflammatory processes can lead to significant RI decline [32]. A surprising finding was the significant decline in RI measurements at all levels in the late postoperative period in Group II, compared to Group I which had ultimately higher RI values in the late postoperative period. The development of collateral circulation as an adjustment due to chronic pressure on the narrow hernial neck is a possible explanation for lower RI values in the late postoperative period in Group II.
Preoperative values of PI were not different in the 2 groups of patients, which suggests that incarceration does not necessarily reduce testicular blood flow.
In the electively operated group, PI increased in the early postoperative period and returned to baseline levels in the late postoperative period, and value differences were not statistically significant (Table 3). On the other hand, measurements in emergency patients showed decline of PI values at all levels in the early postoperative period, with a statistically significant decrease in the late postoperative period. We found significantly lower values of pulsatile index at all levels in the late postoperative period in Group II. The dynamics of PI changes could be compared to RI changes in all patients, and both parameters show a good correlation with the testicular blood flow.
Research on blood velocity is important to develop a better understanding of flow change. End-diastolic velocity (EDV) increased with all patients in the early postoperative period (Table 5). The increase in value with Group I was temporary and the values returned to their basal values in the late postoperative period, whereas with urgent patients (Group II) EDV value remained at the same level as at previous measurements. Although the dynamics of changes was similar at all levels of measuring, the difference was statistically significant at the testicular artery level in Group I, and at intratesticular arteries level in patients from both groups. The dynamics of these transitory changes of EDV can be explained by postoperative tissue changes which are reversible in elective patients, and possibly permanent in patients with incarcerated hernia. However, it should be noted that there were no statistically significant differences in the measured values of EDV in the late postoperative period at the level of intratesticular arteries in both groups (z=1.47; p=0.141).
Peak systolic velocity (PSV) showed a significant increase in the early postoperative period in both groups at the intratesticular artery level and in Group I at the testicular artery level (Table 4). The reason for the transient increase of PSV may be an increase in vascular resistance or inflammatory reaction [33].
In the late postoperative period, PSV values returned to their preoperative values in both groups of patients. Analyses of blood flow velocity results reveal that both groups of patients had similar changes in the early postoperative period, especially at intratesticular arteries level. The increased PSV and EDV values in both groups may be due to tissue reaction to surgical procedures. As postoperative hyperemia and edemas recede, PSV returned to its initial values in the late postoperative period in both groups of patients, whereas EDV had a similar dynamic only in elective patients. A possible explanation of retention of higher EDV values in the late postoperative period with patients who underwent surgery due to hernia incarceration is the existence of a prominent testicular collateral irrigation, which explains the decrease in RI value, and a fall in peripheral resistance accelerates the diastolic flow.
Finally, RI values in the late postoperative period were within the reference ranges in both groups and the clinical significance of these changes is questionable. In our patients, there was no significant correlation between change in testicular flow and in the increase in ASA value. Although we measured an increase in ASA in both groups of patients, we conclude that mesh hernia repair does not cause clinically significant immunological reactions, even in patients with incarcerated hernia.
To the best of our knowledge, this is the first study to investigate both testicular circulation and antisperm antibodies in incarcerated hernia mesh repair.
Conclusions
Transitory changes in testicular flow and autoimmune reaction due to changes on the blood-testicle barrier were comparable patients treated for incarcerated and those treated for non-complicated indirect inguinal hernia. Further investigations are needed to make firm conclusions regarding the effect of incarcerated inguinal hernia mesh repair on immunologic sensitization and, perhaps, male fertility.
Footnotes
Source of support: Departmental sources
Conflict of interest
No conflicts of interest or financial ties to disclose.
References
- 1.Rai S, Chandra SS, Smile SR. A study of the risk of strangulation and obstruction in groin hernias. Aust NZ J Surg. 1998;68(9):650–54. doi: 10.1111/j.1445-2197.1998.tb04837.x. [DOI] [PubMed] [Google Scholar]
- 2.Kulah B, Kulacoglu IH, Oruc MT, et al. Presentation and outcome of incarcerated external hernias in adults. Am J Surg. 2001;181(2):101–4. doi: 10.1016/s0002-9610(00)00563-8. [DOI] [PubMed] [Google Scholar]
- 3.Hentati H, Dougaz W, Dziri C. Mesh repair versus non-mesh repair for strangulated inguinal hernia: Systematic review with meta-analysis. World J Surg. 2014;38(11):2784–90. doi: 10.1007/s00268-014-2710-0. [DOI] [PubMed] [Google Scholar]
- 4.D’Ambrosio R, Capasso L, Sgueglia S, et al. The meshes of polypropylene in emergency surgery for strangulated hernias and incisional hernias. Ann Ital Chir. 2004;75:569–73. [PubMed] [Google Scholar]
- 5.Kamtoh G, Pach R, Kibil W, et al. Effectiveness of mesh hernioplasty in incarcerated inguinal hernias. Wideochir Inne Tech Malo Inwazyjne. 2014;9(3):415–19. doi: 10.5114/wiitm.2014.43080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohsiriwat V, Sridermma W, Akaraviputh T, et al. Surgical outcomes of Lichtenstein tension-free hernioplasty for acutely incarcerated inguinal hernia. Surg Today. 2007;37(3):212–14. doi: 10.1007/s00595-006-3380-9. [DOI] [PubMed] [Google Scholar]
- 7.Yavetz H, Harash B, Yogev L, et al. Fertility of men following inguinal hernia repair. Andrologia. 1991;23(6):443–46. doi: 10.1111/j.1439-0272.1991.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 8.Kearney SE, Lewis-Jones DI. Effect of ACTH on contralateral testicular damage and cytotoxic antisperm antibodies after unilateral testicular ischaemia in the rat. J Reprod Fertil. 1985;75(2):531–35. doi: 10.1530/jrf.0.0750531. [DOI] [PubMed] [Google Scholar]
- 9.Uzzo RG, Lemack GE, Morrissey KP, Goldstein M. The effects of mesh bioprosthesis on the spermatic cord structures: A preliminary report in a canine model. J Urol. 1999;161(4):1344–49. [PubMed] [Google Scholar]
- 10.Peiper C, Junge K, Klinge U, et al. Is there a risk of infertility after inguinal mesh repair? Experimental studies in the pig and the rabbit. Hernia. 2006;10(1):7–12. doi: 10.1007/s10029-005-0055-1. [DOI] [PubMed] [Google Scholar]
- 11.Sucullu I, Filiz AI, Sen B, et al. The effects of inguinal hernia repair on testicular function in young adults: A prospective randomized study. Hernia. 2010;14(2):165–69. doi: 10.1007/s10029-009-0589-8. [DOI] [PubMed] [Google Scholar]
- 12.Ramadan SU, Gokharman D, Tuncbilek I, et al. Does the presence of a mesh have an effect on the testicular blood flow after surgical repair of indirect inguinal hernia? J Clin Ultrasound. 2009;37(2):78–81. doi: 10.1002/jcu.20516. [DOI] [PubMed] [Google Scholar]
- 13.Pinggera GM, Mitterberger M, Bartsch G, et al. Assessment of the intratesticular resistive index by colour Doppler ultrasonography measurements as a predictor of spermatogenesis. BJU Int. 2008;101(6):722–26. doi: 10.1111/j.1464-410X.2007.07343.x. [DOI] [PubMed] [Google Scholar]
- 14.Hillelsohn JH, Chuang KW, Goldenberg E, Gilbert BR. Spectral Doppler sonography: A noninvasive method for predicting dyspermia. J Ultrasound Med. 2013;32(8):1427–32. doi: 10.7863/ultra.32.8.1427. [DOI] [PubMed] [Google Scholar]
- 15.Štula I, Družijanić N, Sršen D, et al. Influence of inguinal hernia mesh repair on testicular flow and sperm autoimmunity. Hernia. 2012;16(4):417–24. doi: 10.1007/s10029-012-0918-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee R, Goldstein M, Ullery BW, et al. Value of serum antisperm antibodies in diagnosing obstructive azoospermia. J Urol. 2009;181(1):264–69. doi: 10.1016/j.juro.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Cui D, Han G, Shang Y, et al. Antisperm antibodies in infertile men and their effect on semen parameters: A systematic review and meta-analysis. Clin Chim Acta. 2015;444:29–36. doi: 10.1016/j.cca.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Chehval MJ, Doshi R, Kidd CF, et al. Antisperm autoantibody response after unilateral vas deferens ligation in rats: when does it develop? J Androl. 2002;23(5):669–73. [PubMed] [Google Scholar]
- 19.Isik A, Peker K, Gursul C, et al. The effect of ozone and naringin on intestinal ischemia/reperfusion injury in an experimental model. Int J Surg. 2015;21:38–44. doi: 10.1016/j.ijsu.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Chen M, Xu Q, et al. Application of tension-free hernioplasty with hernia meshes of different materials and the postoperative effects on the reproductive function of male rats. Mol Med Rep. 2014;9(5):1968–74. doi: 10.3892/mmr.2014.2014. [DOI] [PubMed] [Google Scholar]
- 21.Hallen M, Sandblom G, Nordin P, et al. Male infertility after mesh hernia repair: A prospective study. Surgery. 2011;149:179–84. doi: 10.1016/j.surg.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 22.Tekatli H, Schouten N, van Dalen T, et al. Mechanism, assessment, and incidence of male infertility after inguinal hernia surgery: A review of the preclinical and clinical literature. Am J Surg. 2012;204(4):503–9. doi: 10.1016/j.amjsurg.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Peeters E, Spiessens C, Oyen R, et al. Sperm motility after laparoscopic inguinal hernia repair with lightweight meshes: 3-year follow-up of a randomised clinical trial. Hernia. 2014;18(3):361–67. doi: 10.1007/s10029-012-1028-9. [DOI] [PubMed] [Google Scholar]
- 24.Schouten N, van Dalen T, Smakman N, et al. Male infertility after endoscopic Totally Extraperitoneal (Tep) hernia repair (Main): Rationale and design of a prospective observational cohort study. BMC Surg. 2012;12:7. doi: 10.1186/1471-2482-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maciel LC, Glina S, Palma P, et al. Histopathological alterations of the vas deferens in rats exposed to polypropylene mesh. BJU Int. 2007;100:87–190. doi: 10.1111/j.1464-410X.2007.06782.x. [DOI] [PubMed] [Google Scholar]
- 26.Shin D, Lipshultz LI, Goldstein M, et al. Herniorrhaphy with polypropylene mesh causing inguinal vasal obstruction. Ann Surg. 2005;241:553–58. doi: 10.1097/01.sla.0000157318.13975.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda T, Ogura K, Muguruma K, et al. Serum antisperm antibodies in men with vas deferens obstruction caused by chilhood inguinal herniorrhaphy. Fertil Steril. 1993;59:1095–97. doi: 10.1016/s0015-0282(16)55934-2. [DOI] [PubMed] [Google Scholar]
- 28.Friberg J, Fritjoffson A. Inguinal herniorhaphy and sperm-agglutinating antibodies in infertile men. Arch Androl. 1979;2:317–22. doi: 10.3109/01485017908987332. [DOI] [PubMed] [Google Scholar]
- 29.Kapral W, Kollaritsch H, Stemberger H. Correlation of inguinal hernia and agglutinating sperm antibodies. Entralbl Chir. 1990;115:369–77. [PubMed] [Google Scholar]
- 30.Koksal N, Altinli E, Sumer A, et al. Impact of herniorraphy technique on testicular perfusion: Results of a prospective study. Surg Laparosc Endosc Percutan Tech. 2010;20(3):186–89. doi: 10.1097/SLE.0b013e3181e19f0b. [DOI] [PubMed] [Google Scholar]
- 31.Brisinda G, Cina A, Nigro C, et al. Duplex ultrasound evaluation of testicular perfusion after tension-free inguinal hernia repair: Results of a prospective study. Hepatogastroenterology. 2008;55(84):974–78. [PubMed] [Google Scholar]
- 32.Jee WH, Choe BY, Byun JY, et al. Resistive index of the intrascrotal artery in scrotal inflammatory disease. Acta Radiol. 1997;38:1026–30. doi: 10.1080/02841859709172124. [DOI] [PubMed] [Google Scholar]
- 33.Brown JM, Hammers LW, Barton JW, et al. Quantitative Doppler assessment of acute scrotal inflammation. Radiology. 1995;197:427–31. doi: 10.1148/radiology.197.2.7480687. [DOI] [PubMed] [Google Scholar]


