Abstract
Background
The objective of this study was to determine whether miR-24 can regulate malignant proliferation and chemotherapy sensitivity of EC cells by targeted silencing of the S100 Calcium Binding Protein A8 (S100A8) gene.
Material/Methods
The expression of miR-24 in EC tissues was detected by quantitative real-time PCR. The proliferation ability and chemotherapy sensitivity were analyzed by MTT assay. Bioinformatics software was used to predict some potential target genes of miR-24. Luciferase activity assay was used to verify the relationship between target genes and miR-24. S100A8 protein expression was detected by Western blot analysis.
Results
The low expression of miR-24 in EC tissues compared with normal control tissues suggests miR-24 might play a role in tumorigenesis of EC. EC HEC-1A cells were transfected with miR-24 agonist (agomiR-24) to up-regulate the expression of miR-24. Up-regulation of miR-24 inhibited the cell proliferation and advanced the chemotherapy sensitivity to paclitaxel in HEC-1A cells significantly. We used several types of bioinformatic software to predict that miR-24 could specifically combine with the 3′ untranslated region (3′UTR) of the S100A8 gene, and this prediction was verified by Western blot and luciferase activities assay. The regulation effects of miR-24 enhancement on cell proliferation and chemotherapy sensitivity were largely reversed by S100A8 up-regulation.
Conclusions
miR-24 acts as a tumor-suppressing gene to inhibit malignant proliferation and advance chemotherapy sensitivity to paclitaxel in EC by targeted silencing of the S100A8 gene.
MeSH Keywords: Antineoplastic Agents, Endometrial Neoplasms, MicroRNAs, Paclitaxel
Background
Endometrial carcinoma (EC) is the most common malignant tumor of the female genital tract world-wide [1]. The majority of patients with advanced stage or recurrence have poor prognosis due to limited effective treatment. The preferred therapy for EC is surgery, and chemotherapy following surgical excision is considered as an effective supplementary therapy to avoid recrudescence and metastasis of EC [2].
MicroRNAs are a kind of small noncoding RNA. MicroRNAs are well known for their aberrant expression and functions in various human diseases through their target genes [3,4]. Several previous studies reported miR-24 is under-expressed and plays a tumor-suppressing role in various tumors, including gastric cancer, bladder cancer, and breast cancer [5–7]. MiR-24 is involved in regulating critical cytobiological behaviors, such as proliferation, angiogenesis, and chemotherapy sensitivity, by silencing its target genes. A study by Liu showed miR-24 advances angiogenesis and cell growth in pancreatic carcinoma by suppressing the Bim gene [8]. Pan et al. found miR-24 down-regulation contributed to chemotherapy resistance in small-cell lung cancer to the combination of cisplatin (DDP) and etoposide (VP16) by targeting the autophagy-associated gene 4A (ATG4A) gene [9]. However, little is known about the expression and functions of miR-24 in EC, especially its influence on proliferation and chemotherapy sensitivity of EC cells.
In this study we detected the expression of miR-24 in EC tissues and the influence and mechanism of miR-24 on cell proliferation and chemotherapy sensitivity to paclitaxel of HEC-1A cells.
Material and Methods
Clinical specimens
All 46 EC tissues and normal endometrial tissues (NET) were obtained in Shengjing Hospital of China Medical University from November 2013 to December 2014. The age (mean ±SD) was 56.4±6.3 years (range 42–63 years). The clinical pathological data was confirmed by a pathologist according to WHO standards. This study was approved by the Ethics Committees of China Medical University, and patient consent was obtained before surgery.
Cell culture
EC cell lines HEC-1A and HEK293T cells were obtained from the China Academy of Chinese Medical Sciences. The cells were cultured in DMEM medium supplemented with 10% fetal bovine serum (Life Technologies Corporation, Carlsbad, CA), and culture condition was 5% CO2 at 37°C.
Quantitative real-time PCR (qRT-PCR)
Trizol reagent (Life Technologies Corporation, Carlsbad, CA) was used to extract total RNA, which was synthesized to cDNA by use of the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The expression of miR-24 and S100A8 gene was checked with the SYBR detection kit (Applied Biosystems, Foster City, CA). The primers of S100 Calcium Binding Protein A8 (S100A8) were 5′-TTGCTAGAGACCGAGTGTCC-3′ (sense) and 5′-CTTTGTGGCTTTCTTCATGG-3′ (antisense). U6 and GAPDH were used as endogenous controls. The relative expression was quantified with the 2−ΔΔCt method [9].
Transfection
Various vectors and miRNAs were transfected with Lipofectamine™ 3000 Reagent (Invitrogen, Foster City, CA) following manufacturer’s protocol. After 4 h, normal medium was applied to culture cells for 48 h.
Cell proliferation and chemotherapy sensitivity assay
The MTT Kit was used to detect the cell proliferation ability, as previously described [10]. Cells were treated with paclitaxel (Sigma, St. Louis, MO) at various concentrations (0.1, 1, 5, 10, 20, 50, and 100 μg/ml) 24 h later [11]. The dose-response curve at different concentrations was charted to calculated the IC50 using a Probit regression model.
MicroRNA targets prediction and Dual-luciferase reporter assay
Bioinformatic software, including miRanda and TargetScan, was used to predict that miR-24 could specifically combine with the 3′ untranslated region (3′UTR) of S100A8 gene. The wild-type S100A8 3′UTR binding site and mutant binding site were cloned into pmiR-RB-REPORT™ vector (RiboBio, Guangzhou, China) to construct wild-type luciferase reporter vector (pmiR-S100A8-wt) and mutant-type vector (pmiR-S100A8-mut) by Genescript (Nanjing, China). In this assay, the luciferase reporter vector, miR-24 agonist (agomiR-24), or negative control agonist (agomiR-NC) (Invitrogen, Foster City, CA) were cotransfected into HEK 293T cells. After that, the dual-luciferase reporter assay system (Promega, Madison, WI) was used to check the relative luciferase activity according to the manufacturer’s protocol.
Western blot analysis
Protein samples were handled with SDS-PAGE gels electrophoresis and transferred to PVDF membranes. PVDF membranes were incubated with primary S100A8 and GAPDH antibody (ab92331 and ab181602, Abcam, Cambridge, MA) and secondary antibody by turns, then treated with a chemiluminescence detection kit (Gene, Hong Kong, China). ImageJ software (BD, Franklin Lakes, NJ) was used to quantify the protein expression.
Vector construction
The open reading frame of S100A8 gene was cloned into pcDNA3.1 (Invitrogen, Foster City, CA) to construct the S100A8 expression vector pc-S100A8 by Sangon Company (Shanghai, China). The empty pcDNA3.1 vector was selected as the negative control (pc-NC).
Statistical analysis
SPSS 21.0 software (IBM, Somers, NY) was selected to complete the statistical analysis in this study. The paired t test was used to compare differences, with P<0.05 indicating a significant difference.
Results
MiR-24 was under-expressed in EC tissues
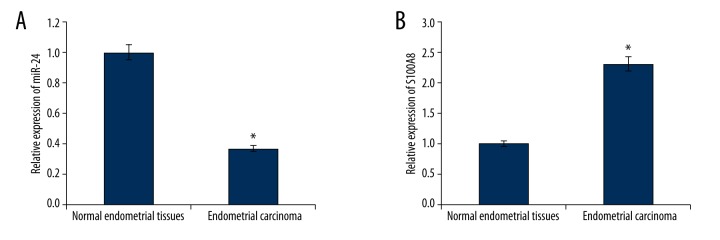
We found the expression level of miR-24 in EC tissue samples was much lower than that in NET samples (Figure 1A), which provided us initial evidence that miR-24 might play a role in tumorigenesis of EC.
Figure 1.
(A) Relative expression of miR-24 was under-expressed in endometrial cancer tissues (n=46). (B) Relative expression S100A8 was over-expressed in endometrial cancer tissues (n=46). * P<0.05.
MiR-24 inhibited cell proliferation and advanced chemotherapy sensitivity to paclitaxel in EC cells
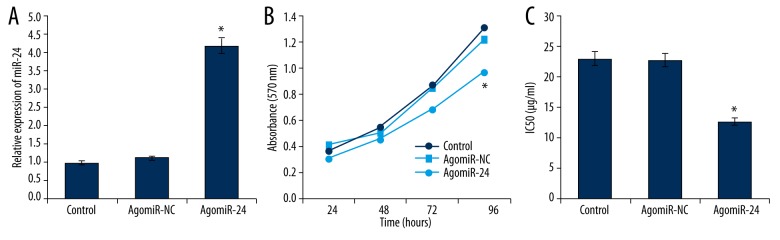
The influences of miR-24 on cell proliferation and chemotherapy sensitivity in HEC-1A cells were investigated. Agomir-24 was transfected into HEC-1A cells to up-regulate miR-24′s expression (Figure 2A). Compare with control cells, the cell proliferation was markedly inhibited by miR-24 enhancement (Figure 2B). Therefore, miR-24 acted as a tumor-suppressing gene in EC cells and suppressed malignant growth of EC cells.
Figure 2.
(A) Agomir-24 transfection up-regulated the expression of miR-24 in HEC-1A cells (n=5). (B) The cell viability of HEC-1A cells was inhibited by miR-24 enhancement (n=5). (C) Up-regulation of miR-24 decreased the IC50 of paclitaxel in HEC-1A cells (n=5). * P<0.05.
Paclitaxel is a common drug widely used in chemotherapy for EC patients. Figure 2C showed that up-regulation of miR-24 can decrease IC50 of paclitaxel from 23.08±1.82 μg/ml to 12.63±1.26μg/ml in HEC-1A cells (p<0.05), demonstrating that miR-24 enhancement advanced chemotherapy sensitivity to paclitaxel in EC cells.
MiR-24 functionally targets S100A8 in EC cell lines
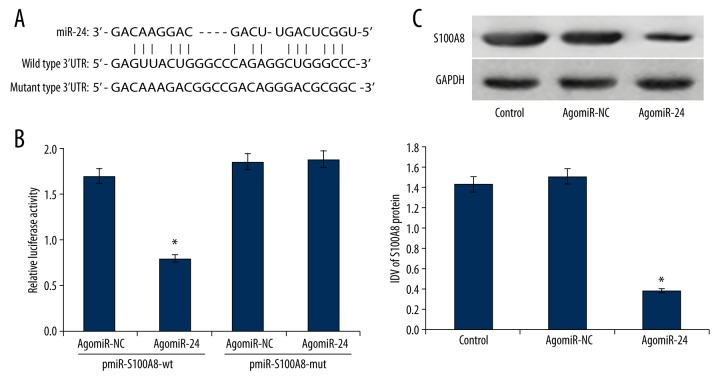
Because of the vital functions of miR-24 in EC, further mechanism analysis is becoming necessary. Bioinformatic software, including miRanda and TargetScan, was used to predict that miR-24 could specifically combine with the 3′ untranslated region (3′UTR) of S100A8 gene (5–29 bp) (Figure 3A).
Figure 3.
(A) 3′-UTR region of S100A8 mRNA is partially complementary to miR-24. (B) MiR-24 can bind to the seed zone of S100A8 3′UTR to inhibit the luciferase activity (n=5). (C) Up-regulation of miR-24 silenced the expression of S100A8 protein in HEC-1A cells (n=3). * P<0.05.
S100A8 expression was over-expression in EC tissues but nit in NET tissues (Figure 1B). Moreover, a negative correlation was identified between miR-24 and S100A8 gene by Pearson correlation analysis.
Subsequently, the direct relationship between miR-24 and S100A8 3′UTR was identified by luciferase reporter assay. Co-transfection with pmiR-S100A8-wt and agomiR-24 significantly inhibited the relative luciferase activity in HEK 293T cells compared with the other 3 control groups (P<0.05) (Figure 3B), which verified that miR-24 can specifically combine with the seed zone in 3′UTR of S100A8 gene to suppress relative luciferase activity. S100A8 gene is a target of miR-24.
The endogenous modulation of miR-24 to S100A8 protein was assessed by Western blot analysis. MiR-24 up-regulation silenced the expression of S100A8 protein remarkably compared with control groups (Figure 3C). Therefore, we concluded that miR-24 silenced the expression of S100A8 protein in HEC-1A cells.
Over-expression of S100A8 largely reversed miR-24-induced regulative effects on EC cells
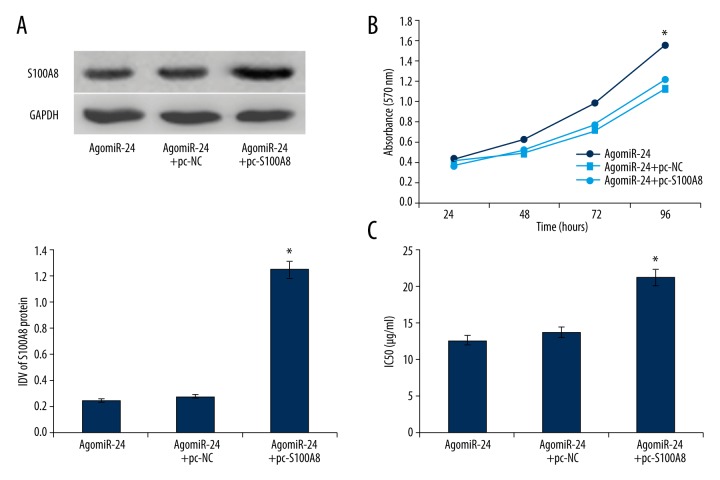
Because S100A8 gene is a target of miR-24 and miR-24 modulates cell proliferation and chemotherapy sensitivity of HEC-1A cells, we therefore suspected that S100A8 is involved in the tumor-suppressive effects of miR-24. Transfection of pc-S100A8 up-regulated the expression of S100A8 protein silenced by miR-24 enhancement (Figure 4A). Over-expression of S100A8 reversed the regulation of miR-24 to cell proliferation and chemotherapy sensitivity to paclitaxel in HEC-1A cells, and the IC50 of paclitaxel increased from 12.63±1.26 μg/ml to 21.31±1.37 μg/ml (Figure 4B, 4C). In view of the above results, we determined that miR-24 controls cell proliferation and chemotherapy sensitivity of EC cells largely through targeted silencing of the S100A8 gene.
Figure 4.
(A) Transfection of pc-S100A8 up-regulated the S100A8 expression silence induced by miR-24 enhancement (n=3). (B) MiR-24 enhancement advanced the cell viability of HEC-1A cells (n=5). (C) Up-regulation of miR-24 increased the IC50 of paclitaxel in HEC-1A cells (n=5). * P<0.05.
Discussion
EC possesses vigorous proliferative ability, and has powerful resistance to chemotherapy and radiotherapy [12,13]. Those characteristics contribute to malignant growth and might be an important reason for the inevitable recurrence of EC.
MiRNAs are well known to be involved in regulating some key cytobiological characteristics, including cell proliferation and chemotherapy sensitivity, which advance EC cells development and result in poor prognosis [14,15]. In this study, we discovered that miR-24 is under-expressed in EC tissues. Previous studies reported miR-24 is under-expressed and plays a tumor-suppressing role in modulating cell proliferation and chemotherapy sensitivity in some kinds of malignant tumors [6–9]. Those results suggested that miR-24 depletion might be involved in tumorigenesis and chemotherapy resistance of EC. Our subsequent experiments confirmed that miR-24 over-expression significantly inhibits cell proliferation and advances chemotherapy sensitivity to paclitaxel in HEC-1A cells. Paclitaxel is a cytoskeletal drug targeted to tubulin, and is widely used in chemotherapy for EC patients. During cell division, paclitaxel is involved in interference with the normal breakdown of microtubules.
Given that miRNAs play their roles by silencing their target genes, we hypothesized that miR-24 can regulate some target genes to modify the regulative network in EC. We used several different types of bioinformatics software to predict the potential target genes of miR-24, of which S100A8 gene is the most intriguing. We previously reported proof of targeted silencing of S100A8 expression by miR-24 in laryngeal carcinoma [16], but whether a similar regulative mechanism exists in EC is not clear. The following expression detection and luciferase activity assay affirmed that S100A8 is over-expressed in EC and is a specific target of miR-24.
S100A8 gene is a member of the S100 family, and has been reported to be over-expressed in various kinds of cancer [17,18]. Previous studies suggested that S100A8 plays key roles in modulation of inflammation and immune response, which is conducive to genesis of almost all tumors [19,20]. The abnormal expression and/or functions of S100A8 gene exhibit a key step in genesis and development of cancer. There has been no previous conclusive report about the expression and functions of S100A8 gene in EC.
Because S100A8 gene is a target of miR-24, and up-regulation of miR-24 suppresses cell proliferation and advances chemotherapy resistance in EC cells, miR-24 was suggested to exert its regulative effects through targeted silencing of S100A8. To test this hypothesis, S100A8 expression vector was used to raise S100A8 expression silenced by miR-24 enhancement in EC cells. Subsequent experiments found that up-regulation of S100A8 could reverse the regulation of miR-24 to cell proliferation and chemotherapy resistance to paclitaxel in EC cells. Consequently, the validation of S100A8 gene as a target of miR-24 provides a possible explanation of how the under-expressed of miR-24 can function as a tumor suppressor gene in EC.
Conclusions
In summary, miR-24, which is under-expressed in EC, functions as a tumor-suppressing gene to inhibit malignant proliferation and advance chemotherapy sensitivity to paclitaxel in EC by targeted silencing of S100A8. Our results elucidate the process of tumorigenesis in EC, and may offer a novel potential therapeutic target for EC.
Footnotes
Source of support: This work was supported by a grant from the Science and Technology Development Fund of Macao Special Administrative Region (028/2011/A2) and Macau Polytechnic Institute (RP/ESS-05/2013)
Disclosure statement
The authors have no conflicts of interest.
References
- 1.Sorbe B. Prognostic importance of DNA ploidy in non-endometrioid, high-risk endometrial carcinomas. Oncol Lett. 2016;11(3):2283–89. doi: 10.3892/ol.2016.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Saadeh F, Langhe R, Galvin DM, et al. Procoagulant activity in gynaecological cancer patients; the effect of surgery and chemotherapy. Thromb Res. 2016;139:135–41. doi: 10.1016/j.thromres.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Shang C, Hong Y, Guo Y, Xue Y. Mir-338-3p inhibits malignant biological behaviors of glioma cells by targeting MACC1 gene. Med Sci Monit. 2016;22:710–16. doi: 10.12659/MSM.897055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang C, Hong Y, Guo Y, et al. miR-128 regulates the apoptosis and proliferation of glioma cells by targeting RhoE. Oncol Lett. 2016;11(1):904–8. doi: 10.3892/ol.2015.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Duan J, Qu Y, et al. Onco-miR-24 regulates cell growth and apoptosis by targeting BCL2L11 in gastric cancer. Protein Cell. 2016;7(2):141–51. doi: 10.1007/s13238-015-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S, Zhang C, Liu W, et al. MicroRNA-24 upregulation inhibits proliferation, metastasis and induces apoptosis in bladder cancer cells by targeting CARMA3. Int J Oncol. 2015;47(4):1351–60. doi: 10.3892/ijo.2015.3117. [DOI] [PubMed] [Google Scholar]
- 7.Lu K, Wang J, Song Y, et al. miRNA-24-3p promotes cell proliferation and inhibits apoptosis in human breast cancer by targeting p27Kip1. Oncol Rep. 2015;34(2):995–1002. doi: 10.3892/or.2015.4025. [DOI] [PubMed] [Google Scholar]
- 8.Liu R, Zhang H, Wang X, et al. The miR-24-Bim pathway promotes tumor growth and angiogenesis in pancreatic carcinoma. Oncotarget. 2015;6(41):43831–42. doi: 10.18632/oncotarget.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan B, Chen Y, Song H, et al. Mir-24-3p downregulation contributes to VP16-DDP resistance in small-cell lung cancer by targeting ATG4A. Oncotarget. 2015;6(1):317–31. doi: 10.18632/oncotarget.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang C, Guo Y, Zhang H, Xue YX. Long noncoding RNA HOTAIR is a prognostic biomarker and inhibits chemosensitivity to doxorubicin in bladder transitional cell carcinoma. Cancer Chemother Pharmacol. 2016;77(3):507–13. doi: 10.1007/s00280-016-2964-3. [DOI] [PubMed] [Google Scholar]
- 11.Xu C, Li X, Li T, et al. Combination effects of paclitaxel with signaling inhibitors in endometrial cancer cells. Asian Pac J Cancer Prev. 2011;12(11):2951–57. [PubMed] [Google Scholar]
- 12.Fukuda T, Oda K, Wada-Hiraike O, et al. The anti-malarial chloroquine suppresses proliferation and overcomes cisplatin resistance of endometrial cancer cells via autophagy inhibition. Gynecol Oncol. 2015;137(3):538–45. doi: 10.1016/j.ygyno.2015.03.053. [DOI] [PubMed] [Google Scholar]
- 13.Bougen NM, Steiner M, Pertziger M, et al. Autocrine human GH promotes radioresistance in mammary andendometrial carcinoma cells. Endocr Relat Cancer. 2012;19(5):625–44. doi: 10.1530/ERC-12-0042. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Sun KX, Liu BL, et al. MicroRNA-505 functions as a tumor suppressor in endometrial cancer by targeting TGF-α. Mol Cancer. 2016;15(1):11. doi: 10.1186/s12943-016-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Qiu XM, Li QH, et al. MicroRNA-424 may function as a tumor suppressor in endometrial carcinoma cells by targeting E2F7. Oncol Rep. 2015;33(5):2354–60. doi: 10.3892/or.2015.3812. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y, Fu W, Chen H, et al. miR-24 functions as a tumor suppressor in Hep2 laryngeal carcinoma cells partly through down-regulation of the S100A8 protein. Oncol Rep. 2012;27(4):1097–103. doi: 10.3892/or.2011.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao YI, Wang A, Mo J. S100A8/A9 is associated with estrogen receptor loss in breast cancer. Oncol Lett. 2016;11(3):1936–42. doi: 10.3892/ol.2016.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Ponti A, Wiechert L, Schneller D, et al. A pro-tumorigenic function of S100A8/A9 in carcinogen-induced hepatocellular carcinoma. Cancer Lett. 2015;369(2):396–404. doi: 10.1016/j.canlet.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Ai F, Li X, et al. Inflammation-induced S100A8 activates Id3 and promotes colorectal tumorigenesis. Int J Cancer. 2015;137(12):2803–14. doi: 10.1002/ijc.29671. [DOI] [PubMed] [Google Scholar]
- 20.Narumi K, Miyakawa R, Ueda R, et al. Proinflammatory Proteins S100A8/S100A9 Activate NK Cells via Interaction with RAGE. J Immunol. 2015;194(11):5539–48. doi: 10.4049/jimmunol.1402301. [DOI] [PubMed] [Google Scholar]