Abstract
Background
MiR-301a and miR-301b are 2 oncomiRs involved in multiple types of cancer. In this study, we explored the expression change of miR-301a and miR-301b in prostate cancer cells in hypoxia and studied their regulation of autophagy and radiosensitivity of prostate cancer cells.
Material/Methods
QRT-PCR was performed to quantify the expression change of miR-301a and miR-301b in hypoxia. Their effects on autophagy were measured by Western blot analysis, and their effects on radiosensitivity were measured by clonogenic assay and flow cytometry. In addition, the regulation of miR-301a and miR-301b on NDRG2, a tumor-suppressor gene in prostate cancer, was also studied. The effect of miR-301a/b-NDRG2 axis on autophagy and radiosensitivity of prostate cancer cells was further investigated.
Results
MiR-301a and miR-301b are 2 hypoxia responsive miRNAs that are significantly upregulated in hypoxia in prostate cancer cells. Higher level of miR-301a and miR-301b expression results in elevated autophagy and increased radioresistance in LNCaP cells. MiR-301a and miR-301b simultaneously target NDRG2 and decrease its expression. Knockdown of NDRG2 leads to increased autophagy and radioresistance.
Conclusions
MiR-301a and miR-301b are 2 hypoxia-responsive miRNAs that decrease autophagy of prostate cancer cells in hypoxia by targeting NDRG2. Through downregulating NDRG2, miR-301a and miR-301b can promote radioresistance of prostate cancer cells.
MeSH Keywords: Autophagy, Cell Hypoxia, MicroRNAs, Prostatic Neoplasms
Background
Prostate cancer is the most common solid-tumor malignancy in men [1,2]. One of the key features associated with solid tumors is hypoxia [3]. Under hypoxia, the tumor cells usually make adaptive changes, including altered cellular metabolism and higher level of vascularization, leading to enhanced migratory potential and cell survival [3].
Currently, radiotherapy is used as an adjuvant therapy after physical resection for patients who can tolerate surgery and is also an optional therapeutic strategy for unresectable prostate tumors [4]. Successful radiotherapy largely depends on the radiosensitivity of cancer cells [5]. However, hypoxia may change the behavior of the tumor cells and results in decreased radiosensitivity [6,7] . Therefore, it is necessary to further investigate the association between hypoxia and radioresistance in prostate cancer cells.
Some previous studies found that hypoxia, autophagy, and radiosensitivity are closely related in solid tumors. For example, hypoxia-induced autophagy can promote radioresistance in colon cancer cells via the HIF-1alpha/miR-210/Bcl-2 pathway [8]. MiR-200c can inhibit autophagy and enhance radiosensitivity in breast cancer cells by targeting UBQLN1 [9]. miR-32 can induce radioresistance by elevating autophagy in prostate cancer cells via targeting DAB2IP [10]. MiR-301a and miR-301b are 2 miRNAs that cluster together. MiR-301a is viewed as an oncomiR in prostate cancer. It can lower the expression of androgen receptor (AR) and increase metastasis [11]. MiR-301a is also viewed as a predictor of prostate cancer metastasis in combination with miR-652/454/223/139 [12]. However, the function of miR-301b in prostate cancer is not completely understood.
In this study we investigated the changes in expression of miR-301a and miR-301b in hypoxia and studied their regulation of NDRG2, a tumor-suppressor in prostate cancer cells [13,14]. In addition, the role of miR-301a/b-NDRG2 axis in autophagy and radioresistance was studied.
Material and Methods
Cell culture
Prostate cancer cell lines PC-3, DU145, and LNCaP were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum in a cell incubator with a humidified atmosphere with 5% CO2 at 37°C. Oxygen supply for hypoxic culture was set at 1%.
Reagents
MiR-301a and miR-301b mimics, miR-301a and miR-301b inhibitors (IH), NDRG2 siRNA, and the scrambled negative controls were purchased from RiBoBio (Shanghai, China). pDONR-NDRG2, a NDRG2 expression vector without 3′UTR, and a HIF1-α expression vector were purchased from Futai Bio (Taizhou, Jiangsu, China). pSELECT-GFP-LC3 plasmid was purchased from Invitrogen (Carlsbad, CA, USA). 3-methyladenine (3-MA) was purchased from Sigma-Aldrich (St Louis, MO, USA). For miR-301a and miR-301b overexpression, the cells were transfected with 100 nM miR-301a or miR-301b, respectively. For miR-301a and miR-301b knockdown, the cells were transfected with 75 nM miR-301a inhibitor and 75 nM miR-301a inhibitor simultaneously. To overexpress NDRG2 or HIF1-α, cells were transfected with pDONR-NDRG2 or pDONR-HIF1-α. To knock down endogenous NDRG2, cells were transfected with 100 nM NDRG2 siRNA. Lipofectamine 2000 (Invitrogen) was used for transfection according to the manufacturer’s instructions. In some studies, cells were treated with 3-MA (5 mM) 1 h before hypoxia for a duration of 36 h.
QRT-PCR analysis
Total miRNA and RNA isolation, reverse transcription of cDNA, and qRT-PCR analysis based on the prostate cancer cell samples followed the methods introduced in a previous study [15]. To quantify the change in miR-301a and miR-301b induced by hypoxia, the prostate cancer cells were subjected to hypoxia for up to 72 h. The level of miR-301a or miR-301b was quantified at indicated time points. The primers used for qRT-PCR analysis of NDRG2 were: forward, 5′-CAGGACAAACACCCGAGACT-3′; reverse, 5′-AGCCATAAGGTGTCTCCACAG-3′. qRT-PCR was performed using an ABI Prism 7500 device (Applied Biosystems, Foster City, CA, USA) and the results were calculated using 2−ΔΔct methods.
Clonogenic assay
After irradiation (dose rate: 5 Gy/min) using a 6-MV X-ray generated by a linear accelerator (Varian 2300EX, Varian, Palo Alto, CA) at the indicated dose, the plates were further incubated for 10–14 days, then the cells were fixed with 10% methanol and stained with 1% crystal violet in 70% ethanol. Colonies (>50 cells) were counted under a light microscope. The curve of survival fraction was derived from the multi-target single-hit model: SF=1-(1-exp(-x/D0))^N.
Flow cytometry analysis of cell apoptosis
Cell apoptosis was assessed using the Annexin V-FITC Apoptosis Detection Kit (V13241, Invitrogen) using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Transfection and selection of LNCaP cells with stable GFP-LC3 expression
LNCaP cells transfected with pSELECT-GFP-LC3 were seeded into 96-well plates and were selected using 250 μg/ml Zeocin (Sigma-Aldrich) in RPMI 1640. Stable clones were selected after 3-week screening. At 24 h after the indicated transfection, cells were subjected to hypoxia for another 36 h, and GFP-LC3 puncta were captured using an Olympus IX71 inverted microscope (Olympus, Tokyo, Japan).
Western blot analysis
Cells were lysed using a lysis buffer (Beyotime, Shanghai, China) with proteinase- and phosphatase-inhibitor cocktails (Sigma-Aldrich). Protein concentrations in the samples were measured by use of a BCA protein assay kit (Beyotime). Samples were then used for a conventional Western blot analysis according to the methods introduced in a previous study [16]. Primary anti-LC3B (ab48394), anti-p62 (ab91526), anti-NDRG2 (ab57429), anti-HIF1-α (ab16066), and anti-GAPDH (ab125247) and HRP-conjugated secondary antibodies were purchased from Abcam (Cambridge, MA, USA). The protein signals were detected using the BeyoECL Plus kit (Beyotime) according to the manufacturer’s instructions.
Dual luciferase assay
Two pieces of oligonucleotides containing wild-type predicted 301a and miR-301b binding site or the mutant binding site of 3′UTR of NDRG2 were chemically synthesized by RiBoBio (Shanghai, China). The sequences were inserted downstream of the luciferase gene using pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega, Madison, WI, USA). The reconstructed plasmids were named as pmirGLO-NDRG2-WT and pmirGLO-NDRG2-MT, respectively. The insertion was confirmed using sequencing. Then, the LNCaP cells were co-transfected with 200-ng plasmids, 40-ng control plasmids, and 100-nM miR-301a or miR-301b mimics using Lipofectamine 2000 (Invitrogen). Relative luciferase activity was analyzed 24 h after transfection by using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s protocol. Firefly luciferase activity was normalized to that of Renilla luciferase.
Statistical analysis
Data are presented as means ± standard deviation (SD) based on at least 3 repeats of 3 independent studies. Comparison between groups was performed using Student’s t-test with SPSS 18.0 software (SPSS). A p value of <0.05 was considered as statistically significant.
Results
MiR-301a and miR-301b are hypoxia-responsive miRNAs that enhance autophagy and radioresistance of prostate cancer cells
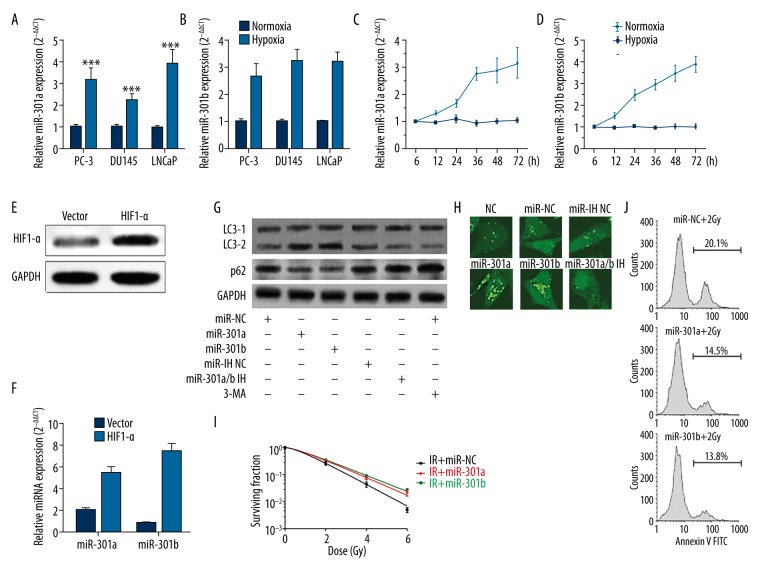
Tumor cells can make adaptive changes to stressful conditions, including hypoxia. MiR-301a and miR-301b are 2 oncogenes involved in multiple types of cancer [12,17]. However, how their expression changes in hypoxia has not been reported. By performing qRT-PCR analysis, we observed that both miRNAs increased significantly in hypoxia in PC-3, DU145, and LNCaP cells (Figure 1A, 1B). Notably, LNCaP cells had the largest increase in miR-301a and miR-301b expression (Figure 1A, 1B). Therefore, this cell line was used for the following analysis. The hypoxia-induced miR-301a and miR-301b increased in LNCaP cells was further confirmed by qRT-PCR analysis at indicated time points (Figure 1C, 1D). To preliminarily investigate how hypoxia induced higher miR-301a and miR-301b expression, LNCaP cells were transfected with HIF1-α expression vector (Figure 1E). The subsequent qRT-PCR analysis showed that HIF1-α overexpression resulted in significantly upregulated miR-301a and miR-301b expression (Figure 1F). Higher level of autophagy is usually a pro-survival mechanism of tumor cells in hypoxia. Then, we explored how these 2 miRNAs affect hypoxia-induced autophagy in prostate cancer cells. Both miR-301a and miR-301b increased LC3-II and facilitated p62 degradation (Figure 1G). By using LNCaP cells with stable LC3-GFP expression, we found miR-301a and miR-301b overexpression significantly enhanced LC3-GFP puncta accumulation in the cancer cells in hypoxia (Figure 1H). Some previous studies reported that elevated autophagy may contribute to increased radioresistance in some types of cancer [8,18] . Then, we investigated how these 2 miRNAs affect radioresistance of LNCaP cells. LNCaP cells with miR-301a or miR-301b overexpression had significantly higher survival fraction (Figure 1I) and lower rate of apoptotic cells (Figure 1J) after irradiation. These results suggest that miR-301a and miR-301b are 2 hypoxia-responsive miRNAs that enhance autophagy and radioresistance of prostate cancer cells.
Figure 1.
MiR-301a and miR-301b are hypoxia-responsive miRNAs that enhance autophagy and radioresistance of prostate cancer cells. (A, B) PC-3, DU145, and LNCaP cells were cultured in hypoxia for 36 h. The expression of miR-301a (A) and miR-301b (B) were determined by qRT-PCR (n=3). (C, D) LNCaP cells were subjected to hypoxia for up to 72 h and the expression of miR-301a (C) and miR-301b (D) at indicated time points were determined by qRT-PCR. (E, F) LNCaP cells were transfected with HIF1-α expression vector or the negative control. (E) Western blot analysis was performed 72 h after the transfection to detect the expression of HIF1-α. (F) QRT-PCR analysis was performed to detect the expression of miR-301a and miR-301b. (G) LNCaP cells with miR-301a or miR-301b overexpression or with knockdown of both miR-301a and miR-301b were exposed to hypoxia (1%) for 36 h. LNCaP cells without any transfection were treated with 3-MA treatment (5 mM) 1 h before hypoxia and then exposed to hypoxia (1%) for 36 h. Autophagy was visualized by detecting LC3B and p62 via Western blot. (H) LNCaP cells with stable GFP-LC3 expression were transfected with miR-301a mimics, miR-301b mimics, or co-transfected with miR-301a and miR-301b inhibitors, then the cells were subjected to hypoxia for 36 h. GFP-LC3 puncta accumulation was then observed under confocal microscopy. (I) Survival fraction of LNCaP cells with or without miR-301a or miR-301b overexpression after irradiation. (J) Flow cytometry analysis of apoptotic LNCaP cells with or without miR-301a or miR-301b overexpression 24 h after irradiation. * p<0.05, ** p<0.01, *** p<0.001.
MiR-301a and miR-301b simultaneously target NDRG2 and decrease its expression
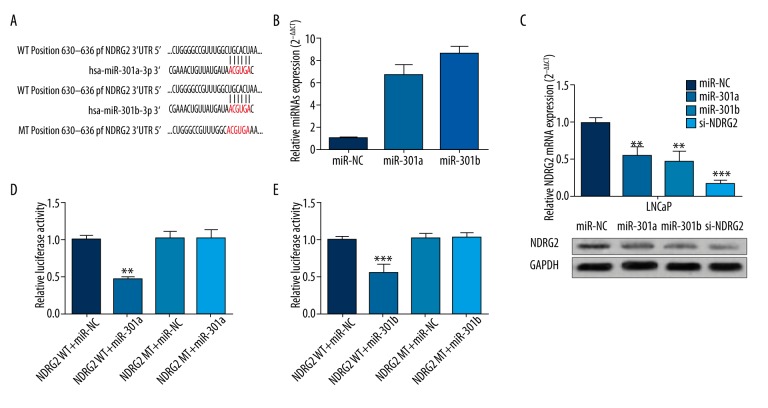
By performing target prediction using online databases, we observed that the 3′UTR of NDRG2 has a sequence targeted by both miR-301a and miR-301b (Figure 2A). Previous studies confirmed that NDRG2 is a tumor-suppressor gene in prostate cancer [13,14]. LNCaP cells were first transfected with miR-301a or miR-301b mimics for overexpression (Figure 2B). Both miR-301a and miR-301b overexpression could substantially inhibit NDRG2 expression, and the effect was similar to that of NDRG2 siRNA (Figure 2C). To further verify the direct binding between miR-301a/miR-301b and 3′UTR of NDRG2, we produced dual luciferase reporter plasmids inserted with wild-type or mutant predicted binding site. Both miR-301a and miR-301b substantially suppressed the relative luciferase activity of wild-type reporter plasmids, but had little effect on the mutant reporter plasmids (Figure 2D, 2E).
Figure 2.
MiR-301a and miR-301b simultaneously targets NDRG2 and decrease its expression. (A) The predicted binding sequence between miR-301a, miR301b and 3′UTR of NDRG2. (B–D) LNCaP cells were transfected with 100 nM miR-301a mimics, miR-301b mimics, or NDRG2 siRNA. QRT-PCR analysis was performed to detect miR-301a, miR-301b (B), and NDRG2 mRNA (C) expression. NDRG2 protein expression (D) was measured by Western blot. (D, E) LNCaP cells were co-transfected with 100-nM miR-301a mimics (D) or miR-301b mimics (E) and pmirGLO-NDRG2-WT or pmirGLO-NDRG2-MT. The relative luciferase activity was measured 24 h after transfection. * p<0.05, ** p<0.01, *** p<0.001.
MiR-301a and miR-301b promote radioresistance of prostate cancer cells via downregulating NDRG2
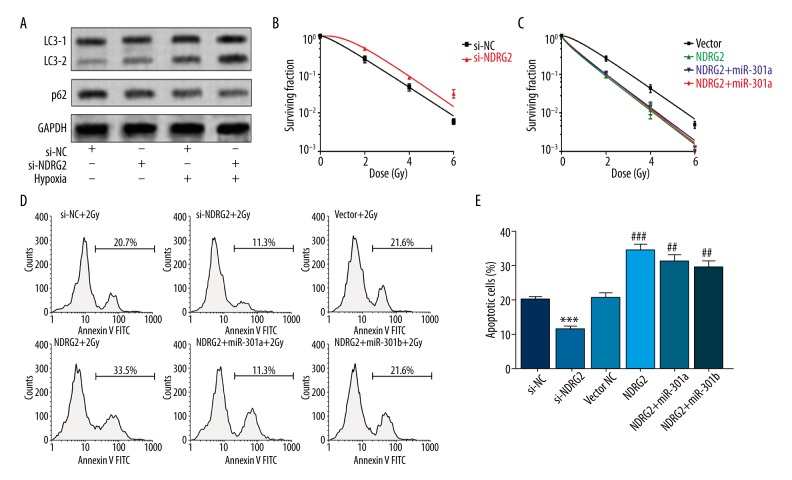
We further studied how the miR-301a/b-NDRG2 axis influences autophagy and radioresistance. LNCaP cells with NDRG2 knockdown had a significantly higher level of autophagy than the control in both normoxic and hypoxic culture (Figure 3A). NDRG2 knockdown promoted radioresistance of LNCaP cells (Figure 3B), while NDRG2 overexpression reduced radioresistance (Figure 3C). In addition, cells with NDRG2 knockdown had a significantly reduced rate of apoptotic cells after irradiation (Figure 3D, 3E). However, NDRG2 overexpression enhanced the apoptosis after irradiation (Figure 3D, 3E). Since the NDRG2 expression vector lacks the 3′UTR, miR-301a or miR-301b had little effect on NDRG2-induced lower radioresistance (Figure 3C, 3E).
Figure 3.
MiR-301a and miR-301b promote radioresistance of prostate cancer cells via downregulating NDRG2. (A) LNCaP cells were first transfected with 100-nM NDRG2 siRNA and then were exposed to normoxia or hypoxia for 36 h. Then, the level of autophagy was detected by measuring LC3B and p62 via Western blot. (B, C) Survival fraction of LNCaP cells with NDRG2 knockdown (B) or with NDRG2 overexpression in combination with miR-301a or miR-301b (C) after irradiation. (D, E) Representative images (D) and quantification (E) of flow cytometry analysis of apoptotic LNCaP cells with indicated treatment in Figure B, C 24 h after irradiation. * Comparison with si-NC, # comparison with vector NC. # p<0.05, ## p<0.01, *** and ### p<0.001.
Discussion
MiR-301a has been reported to be an oncomiR in prostate cancer. MiR-301a can directly bind to AR and lower its expression, leading to a higher level of metastasis via the miR-301a/AR/TGF-beta1/Smad/MMP9 signaling pathway [11]. It is also viewed as a predictor of prostate cancer metastasis in combination with miR-652/454/223/139 [12]. Although the function of miR-301b in prostate cancer has not been reported, it usually acts as a tumor promoter, contributing to progression of hepatocellular carcinoma [19] and triple-negative breast cancer [17].
Hypoxia-induced dysregulated miRNAs and their involvement in pathological process of tumor development have been widely reported [5,7,16] . In this study, we found hypoxia induced significantly higher expression of miR-301a and miR-301b in prostate cancer cells. Autophagy is an evolutionarily conserved process that involves degradation and recycling of defective organelles and proteins to maintain cellular homeostasis [20]. Autophagy is a natural response of cancer cells to stressful environments, including hypoxia. Some recent studies also reported that autophagy acts as an important survival mechanism of prostate cancer cells to androgen deprivation and hypoxia [20,22]. Inhibition of autophagy in prostate cancer cells induces a higher level of apoptosis in hypoxia [19]. In some types of cancer, elevated autophagy in hypoxia is a mechanism of reduced radiosensitivity. This mechanism is also observed in prostate cancer. For example, a recent study observed that PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through reducing autophagy [23]. Some recent studies suggested that miRNAs may also be involved in this process of regulation. For example, miR-200c can suppress autophagy and decrease radioresistance of breast cancer cells via targeting UBQLN1 [9]. MiR-32 can enhance radioresistance by downregulating DAB2IP and promoting autophagy in prostate cancer cells [10].
Therefore, we further investigated the association between miR-301a/miR-301b, autophagy, and radioresistance. The results showed that miR-301a and miR-301b significantly promoted autophagy in hypoxia and that miR-301a and miR-301b can also enhance radioresistance. Then, we decided to study the regulators in between. NDRG2 is a tumor-suppressive gene in prostate cancer [13,24]. In fact, it is also a gene that may be involved in regulation of autophagy. The loss of NDRG2 results in enhanced phosphorylation of PTEN [25,26], which is viewed as a mechniam of enhanced autophagy in response to DNA-damaging agents in cancer cells [27]. Through performing dual luciferase assay, Western blot, and qRT-PCR analysis, we confirmed that both miR-301a and miR-301b can bind to 3′UTR of NDRG2 and downregulate its expression. In addition, we also observed that NDRG2 knockdown resulted in enhanced radioresistance, while its overexpression promoted radiosensitivity. These results suggest that the miR-301a/miR-301b-NDRG2 axis is an important signaling pathway modulating radiosensitivity of prostate cancer cells.
Conclusions
miR-301a and miR-301b are 2 hypoxia-responsive miRNAs that decrease autophagy of prostate cancer cells in hypoxia by targeting NDRG2. Through downregulating NDRG2, miR-301a and miR-301b can promote radioresistance of prostate cancer cells.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Carson CC., III Carcinoma of the prostate: Overview of the most common malignancy in men. N C Med J. 2006;67:122–27. [PubMed] [Google Scholar]
- 2.Tao ZQ, Shi AM, Wang KX, Zhang WD. Epidemiology of prostate cancer: current status. Eur Rev Med Pharmacol Sci. 2015;19:805–12. [PubMed] [Google Scholar]
- 3.Marignol L, Rivera-Figueroa K, Lynch T, Hollywood D. Hypoxia, notch signalling, and prostate cancer. Nat Rev Urol. 2013;10:405–13. doi: 10.1038/nrurol.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberti C. Prostate cancer: Radioresistance molecular target-related markers and foreseeable modalities of radiosensitization. Eur Rev Med Pharmacol Sci. 2014;18:2275–82. [PubMed] [Google Scholar]
- 5.Chang L, Graham PH, Hao J, et al. Emerging roles of radioresistance in prostate cancer metastasis and radiation therapy. Cancer Metastasis Rev. 2014;33:469–96. doi: 10.1007/s10555-014-9493-5. [DOI] [PubMed] [Google Scholar]
- 6.Yang W, Wei J, Guo T, et al. Knockdown of miR-210 decreases hypoxic glioma stem cells stemness and radioresistance. Exp Cell Res. 2014;326:22–35. doi: 10.1016/j.yexcr.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Zhu H, Yang X, et al. MicroRNA-21 is a novel promising target in cancer radiation therapy. Tumour Biol. 2014;35:3975–79. doi: 10.1007/s13277-014-1623-8. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Xing X, Liu Q, et al. Hypoxia-induced autophagy reduces radiosensitivity by the HIF-1alpha/miR-210/Bcl-2 pathway in colon cancer cells. Int J Oncol. 2015;46:750–56. doi: 10.3892/ijo.2014.2745. [DOI] [PubMed] [Google Scholar]
- 9.Sun Q, Liu T, Yuan Y, et al. MiR-200c inhibits autophagy and enhances radiosensitivity in breast cancer cells by targeting UBQLN1. Int J Cancer. 2015;136:1003–12. doi: 10.1002/ijc.29065. [DOI] [PubMed] [Google Scholar]
- 10.Liao H, Xiao Y, Hu Y, et al. microRNA-32 induces radioresistance by targeting DAB2IP and regulating autophagy in prostate cancer cells. Oncol Lett. 2015;10:2055–62. doi: 10.3892/ol.2015.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie H, Li L, Zhu G, et al. Infiltrated pre-adipocytes increase prostate cancer metastasis via modulation of the miR-301a/androgen receptor (AR)/TGF-beta1/Smad/MMP9 signals. Oncotarget. 2015;6:12326–39. doi: 10.18632/oncotarget.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nam RK, Amemiya Y, Benatar T, et al. Identification and validation of a five MicroRNA signature predictive of prostate cancer recurrence and metastasis: A cohort study. J Cancer. 2015;6:1160–71. doi: 10.7150/jca.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao L, Wu GJ, Liu XW, et al. Suppression of invasion and metastasis of prostate cancer cells by overexpression of NDRG2 gene. Cancer Lett. 2011;310:94–100. doi: 10.1016/j.canlet.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Yu C, Wu G, Li R, et al. NDRG2 acts as a negative regulator downstream of androgen receptor and inhibits the growth of androgen-dependent and castration-resistant prostate cancer. Cancer Biol Ther. 2015;16:287–96. doi: 10.1080/15384047.2014.1002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuo YL, Li XM, Luo J. Long noncoding RNA UCA1 modulates breast cancer cell growth and apoptosis through decreasing tumor suppressive miR-143. Eur Rev Med Pharmacol Sci. 2015;19:3403–11. [PubMed] [Google Scholar]
- 16.Ma Y, Yang HZ, Dong BJ, et al. Biphasic regulation of autophagy by miR-96 in prostate cancer cells under hypoxia. Oncotarget. 2014;5:9169–82. doi: 10.18632/oncotarget.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YY, Kuo WH, Hung JH, et al. Deregulated microRNAs in triple-negative breast cancer revealed by deep sequencing. Mol Cancer. 2015;14:36. doi: 10.1186/s12943-015-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He WS, Dai XF, Jin M, et al. Hypoxia-induced autophagy confers resistance of breast cancer cells to ionizing radiation. Oncol Res. 2012;20:251–58. doi: 10.3727/096504013x13589503483012. [DOI] [PubMed] [Google Scholar]
- 19.Mitani T, Minami M, Harada N, et al. Autophagic degradation of the androgen receptor mediated by increased phosphorylation of p62 suppresses apoptosis in hypoxia. Cell Signal. 2015;27:1994–2001. doi: 10.1016/j.cellsig.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Carew JS, Kelly KR, Nawrocki ST. Autophagy as a target for cancer therapy: new developments. Cancer Manag Res. 2012;4:357–65. doi: 10.2147/CMAR.S26133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chhipa RR, Wu Y, Ip C. AMPK-mediated autophagy is a survival mechanism in androgen-dependent prostate cancer cells subjected to androgen deprivation and hypoxia. Cell Signal. 2011;23:1466–72. doi: 10.1016/j.cellsig.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang N, Ji N, Jiang WM, et al. Hypoxia-induced autophagy promotes human prostate stromal cells survival and ER-stress. Biochem Biophys Res Commun. 2015;464:1107–12. doi: 10.1016/j.bbrc.2015.07.086. [DOI] [PubMed] [Google Scholar]
- 23.Chang L, Graham PH, Hao J, et al. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 2014;5:e1437. doi: 10.1038/cddis.2014.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren GF, Tang L, Yang AQ, et al. Prognostic impact of NDRG2 and NDRG3 in prostate cancer patients undergoing radical prostatectomy. Histol Histopathol. 2014;29:535–42. doi: 10.14670/HH-29.10.535. [DOI] [PubMed] [Google Scholar]
- 25.Nakahata S, Ichikawa T, Maneesaay P, et al. Loss of NDRG2 expression activates PI3K-AKT signalling via PTEN phosphorylation in ATLL and other cancers. Nat Commun. 2014;5:3393. doi: 10.1038/ncomms4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichikawa T, Nakahata S, Fujii M, et al. Loss of NDRG2 enhanced activation of the NF-kappaB pathway by PTEN and NIK phosphorylation for ATL and other cancer development. Sci Rep. 2015;5:12841. doi: 10.1038/srep12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JH, Zhang P, Chen WD, et al. ATM-mediated PTEN phosphorylation promotes PTEN nuclear translocation and autophagy in response to DNA-damaging agents in cancer cells. Autophagy. 2015;11:239–52. doi: 10.1080/15548627.2015.1009767. [DOI] [PMC free article] [PubMed] [Google Scholar]