Abstract
Background
The aim of this study was to investigate the use of transcranial Doppler (TCD) for diagnosis of brain death in patients with severe cerebral injury.
Material/Methods
This retrospective study enrolled 42 patients based on inclusion and exclusion criteria. All patients were divided into either the brain death group or the survival group according to prognosis. Blood flow of the brain was examined by TCD and analyzed for spectrum changes. The average blood flow velocity (Vm), pulse index (PI), and diastolic blood flow in reverse (RDF) were recorded and compared.
Results
The data demonstrated that the average speed of bilateral middle cerebral artery blood flow in the brain death group was significantly reduced (P<0.05). However, the PI of the brain death group increased significantly. Moreover, RDF spectrum and nail-like sharp peak spectrum of the brain death group was higher than in the survival group.
Conclusions
Due to its simplicity, high repeatability, and specificity, TCD combined with other methods is highly valuable for diagnosis of brain death in patients with severe brain injury.
MeSH Keywords: Brain Death; Craniocerebral Trauma; Ultrasonography, Doppler
Background
In clinical practice “brain death” refers mainly to the irreversible loss of all functions of the brain, including those of the brainstem [1]. Due to the unique characteristics of brain death, strict clinical criteria must be applied before a final conclusion can be made [1,2]. Generally, unexplained deep coma, absence of brainstem reflexes, and the end of independent respiration have been considered as the 3 major principles for diagnosing brain death [3,4]. A patient can only be considered as brain dead after meeting all 3 criteria. To date, more than 80 countries have defined the standard criteria for the diagnosis of brain death by legislative process. In China, the “Brain Death Assessment Criteria”, which was originally established in 2003 by the Ministry of Health, was further revised by the Health and Family Planning Commission in March 2013, indicating an improvement in the standardization of brain death diagnosis in China.
Assessment of brain death is a complex process with additional ethical concerns. A misdiagnosis of brain death not only harms patients but also affects their family members [4,5]. The prerequisites for brain death include coma with a clear cause and elimination of any possibility of reversible coma prior to a clinical diagnosis [6]. Generally, assessment of coma is based on the following 3 basic criteria: deep coma, absence of brainstem reflexes, and the end of independent respiration [7]. In recent years, several new methods for the clinical staging of brain death have emerged, including the atropine test [7]. Atropine accelerates the heart rate mainly via the excitation of the medulla oblongata and inhibition of vagal activity in the heart. It can be inferred that the brainstem is completely dead if the injection of atropine fails to alter the heart rate, indicating the death of the medulla oblongata [7–9]. Many countries have adopted the atropine test as part of the diagnostic criteria for brain death, including in China, due to its simplicity and convenience. However, since the primary effect of atropine is the production of an increase in heart rate, an assessment of the patient is required prior to using this method, especially for patients with heart disease, myocardial injuries, and those who have been administered cardiovascular medications [10].
It has been shown that cerebral circulatory arrest (CCA) demonstrated by angiography or radionuclear cerebral flow studies can be used to support the diagnosis of brain death because CCA will lead to brain death [11,12]. However, angiography is an invasive technique and contrast may cause vascular obstruction that is harmful to any remaining brain function [11]. Therefore, as a non-invasive and inexpensive technique, transcranial Doppler ultrasonography (TCD) could be a promising method to determine brain death because CCA can be visualized by TCD as well [11]. However, since many studies and reports suggested the value of TCD in confirming the CCA [11,13], some researchers still do not recommend this technique for confirming brain death [13–15]. In the present study, we investigated the significance of transcranial Doppler ultrasound (TCD) in the diagnosis of brain death, based on variations observed in TCD data.
Material and Methods
Ethics statement
This study was reviewed and approved by the Research Ethics Board of the Affiliated Hospital of Jining Medical College. This was a retrospective study. All legal guardians of participating patients were formally informed for the purpose of using patient data and a letter of consent was signed by the legal guardian of every subject involved.
Patients, inclusion criteria, and exclusion criteria
Clinical data were collected from 57 patients with severe brain injury admitted to the Neurosurgical Department of the Affiliated Hospital of Jining Medical College between January 2010 and December 2014.
Inclusion criteria for patients were: 1) patients age 18–70 years; 2) admitted to the hospital for treatment within 24 h after the onset of symptoms; 3) initial Glasgow Coma Scale (GCS) of the patients less than or equal to, 8 without serious complications, injury, or shock; 4) no history of cardiovascular or cerebrovascular disease, including congenital vascular malformations; 5) temporal window sufficient to obtain a clear spectrum signal showing the flow of blood during the first TCD examination; and 6) no history of taking CNS antidepressants or neuromuscular-blocking agents.
Based on these criteria, 13 ineligible patients were excluded. The remaining 42 patients included in the study consisted of 27 males and 15 females, with an average age of 35 years. Twenty-nine patients suffered brain injuries due to traffic accidents, 10 patients had fall-related brain injuries, and the remaining 3 patients suffered brain injuries as a result of some other cause. Seven patients had GCS scores ranging from 3 to 5, while 35 patients had scores ranging from 5 to 8.
Research Methods
A total of 42 patients were divided into a clinical brain death group (15 patients) and a survival group (27 patients) based on final outcomes. Data were measured and recorded using transcranial Doppler ultrasound (EME, Germany). Anterior cerebral circulation was measured via the bilateral middle cerebral artery (MCA). A 2-MHz pulsed-wave Doppler ultrasound probe was inserted at a depth of 40–65 mm in the opposite direction of systolic blood flow over the temporal window. Measurements were taken at least once per day and increased to once every 6 h upon observation of oscillations or sharp peak waveforms. Each measurement was taken twice, with a 30-min interval. Average velocity of blood flow (Vm), pulsatility index (PI), and the frequency of oscillation and sharp peak waveforms were also recorded.
Statistical methods
The collected data (sex, age, date of admission, mean blood flow velocity, pulsatility index, and frequency of unusual waveforms) were compiled and sorted using the Excel program. Data were analyzed using SPSS19.0 statistical software, with the count data expressed as a percentage (%). The chi-square test was used to compare the frequency of oscillations/sharp peak waveforms between the brain death and survival groups. The t test was used to analyze differences in mean blood flow velocity (Vm) and pulsatility index (PI) observed between the 2 groups. Results are expressed as means ± standard deviations (x±s).
Results
Overall results
Between January 1, 2010 and December 30, 2014, a total of 57 patients with severe brain injury (various causes) were admitted to our Neurosurgery Department. We excluded 2 pediatric patients, 3 patients with an initial GCS >8, 5 patients with severe injuries to other organs due to traffic accidents and/or serious shock upon admission, and 3 patients with a history of heart disease. The included patients were then divided into the brain death group (n=15) and the survival group (n=27). It has been suggested that during the diagnosis of brain death by TCD, the specific intracranial flow patterns can be used as the indicator of cerebral circulatory arrest (CCA), such as oscillating waveform and sharp spikes or peaks waveforms [11]. In our study, oscillating waveforms were observed in 13 patients in the brain death group, while sharp peaks were observed for the remaining 2 patients. Based on real-time detection of changes in blood flow, we observed that characteristic changes in blood flow in patients with brain death preceded clinical diagnosis by 6–40 h.
However, it is notable that there were still 4 patients in the survival group who demonstrated the oscillation waveform. After carefully reviewing all medical records for these 4 patients, we noticed that the duration of oscillation waveform was less than 12 h. Moreover, after appearance of oscillation in these patients, emerging medical interventions, such as debridement of open intracranial injury, removal of hematoma, or reducing intracranial pressure by mannitol dehydration, were conducted, which may be a factor contributing to the survival of patients. However, based on our follow-up data, prognosis for these 4 surviving patients was poor. There were 2 patients who progressed into a vegetative state in first year of follow-up, suggesting brain function damage.
Results of statistical analysis
Hemodynamic differences between the brain death group and survival group are summarized in Table 1. The results presented in Table 1 indicate that MCA-Vm values in the brain death group were significantly lower than those of the survival group (p<0.05). PI values were significantly higher in the brain death group than in the survival group (p<0.05).
Table 1.
Comparison of differences in MAC-Vm and PI values between brain death and survival groups.
| Group | Number of patients | MCA-Vm (x±s; cm/s) | PI (x±s) |
|---|---|---|---|
| Survival group | 27 | 56.81±16.84 | 1.24±1.03 |
| Brain death group | 15 | 20.02±13.96 | 4.02±3.49 |
| t=7.189 | t=5.413 |
A comparison of the frequency of oscillation and sharp peak spectra between the brain death group and the survival group is presented in Table 2, which shows that the frequency of oscillation and sharp peak spectra in the brain death group was 93.33%, while the frequency of retrograde diastolic flow (RDF) in the survival group was 14.81%. Hence, the brain death group had a significantly higher frequency of RDF than the survival group (p<0.05).
Table 2.
The frequency of oscillation and sharp peak spectra in brain death and survival groups.
| Group | Number of patients | Number of patients with oscillation and sharp peak spectra | Frequency (%) |
|---|---|---|---|
| Brain death group | 15 | 13 (Oscillation); 2 (sharp peak) | 93.33 |
| Survival group | 27 | 4 (Oscillation) | 14.81 |
| χ2=28.71 |
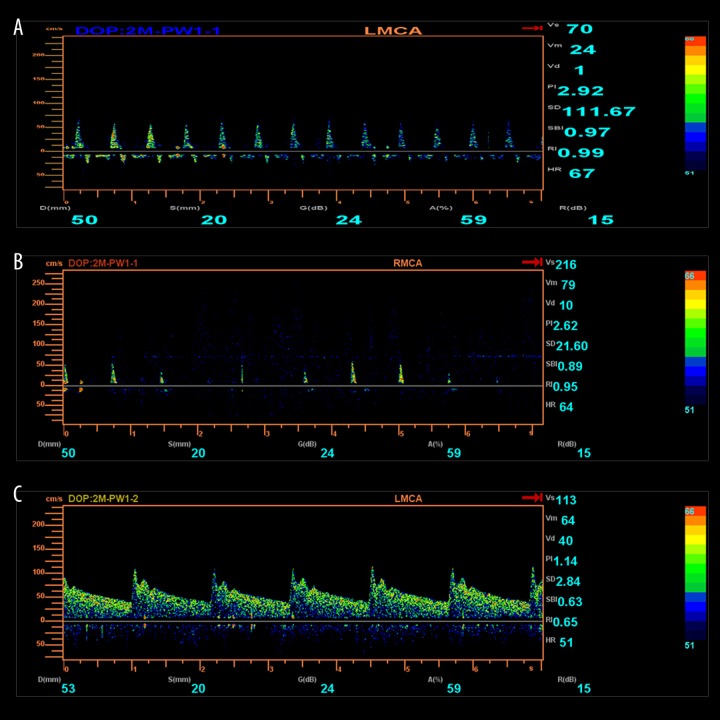
Figures 1 and 2 show the spectra of oscillation and sharp peaks in the brain death group and the survival group compared with the normal control spectrum.
Figure 1.
(A) Oscillation. (B) Sharp peaks. (C) Normal spectrum.
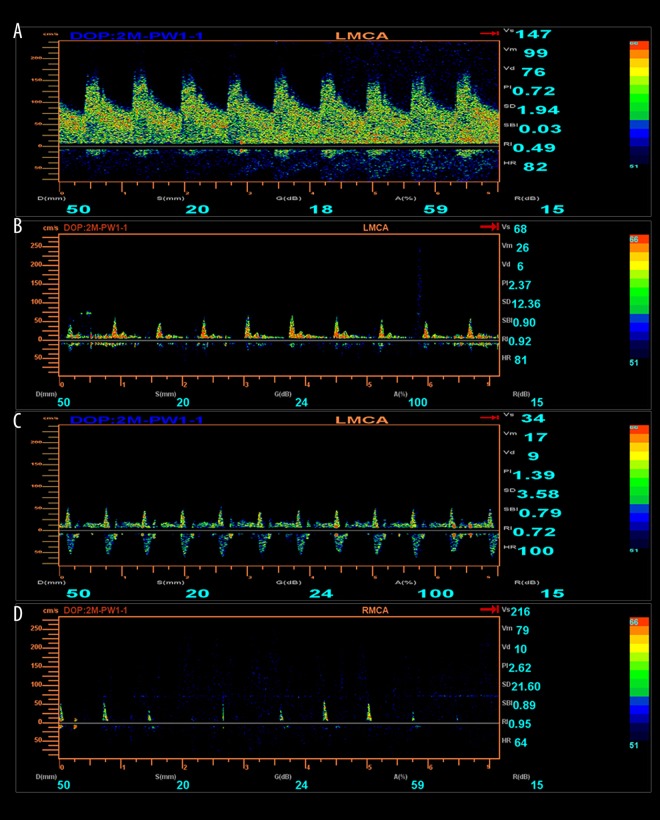
Figure 2.
Displays a series of TCD changes in a patient with severe brain injury, from coma to vegetative state, and finally to brain death. (A) The spasticity of L-MCA during coma on the first day of admission. (B) Low perfusion in L-MCA during the vegetative state on the 20th day of admission. (C) Oscillation of L-MCA on the 25th day of admission. (D) Sharp peaks on the 26th day of admission.
Discussion
Severe brain injury with GCS ≤8 is the most serious type of traumatic brain injury, accounting for about 13–21% of traumatic brain injury, with a mortality rate of 30–50% [16]. The main causes of death are brain edema, cerebral contusion, and primary or secondary injury to the brainstem [5,13]. Severe brain injury is generally accompanied by numerous complications such as intracranial hypertension and is the leading cause of brain death [6].
In 2013, the updated diagnostic criteria and technical specification of brain death in China clearly defined the diagnostic criteria for brain death and includes well-defined prerequisites for clinical diagnosis, as well as auxiliary tests for confirmation. The auxiliary diagnostic tests for the confirmation of brain death include median nerve short-latency somatosensory evoked potentials (SLSEP), electroencephalogram (EEG), and transcranial Doppler ultrasound (TCD) [17].
According to these updated diagnostic criteria, TCD is the only method used to assess cerebral blood flow. TCD examination has advantage over EEG for patients treated with sedative drugs because TCD is not affected by them. Moreover, TCD is relatively resistant to environmental factor compared with SLSEP examination. When using TCD for the diagnosis of brain death, the occurrence of RDF, early sharp systolic peaks of forward flow (sharp peaks), and disappearance of blood flow may suggest an onset of brain death in the patient.
In this study, we examined a total of 15 patients with proposed brain death, including 13 patients with oscillating spectra and 2 patients with sharp peaks prior to clinical diagnosis of brain death. Hence, it is reasonable to believe that clinical treatment is useless after the appearance of oscillation and sharp peak waveforms. Therefore, our data suggest that physicians should consider use of TCD, EEG, and brainstem auditory evoked potentials to confirm the occurrence of brain death prior to making a final diagnosis, which may facilitate organ donation and transplantation. Moreover, it is surprising for us that there were still 4 patients in survival group who demonstrated the oscillation waveform. Based on the records for these patients, the duration of oscillation waveform was less than 12 h, and these patients received medical interventions. This suggests that to determine the brain death via TCD, long-term observation is needed to reach a final conclusion.
It is still unclear if disappearance of the oscillation waveform was due to medical interventions or self-recovery of patients. Moreover, even after these patients recovered from brain death, the poor prognosis suggested functional damage to the brain. However, it is interesting that our data suggest there was still a chance for the patient to recover, even with the appearance of oscillation waveform, which needs further investigation.
Conclusions
Transcranial Doppler ultrasound (TCD) is highly valuable for diagnosis of brain death in patients with severe brain injury due to its simplicity, high repeatability, and high specificity. Therefore, it can be used in combination with other methods to confirm brain death in patient with severe brain injury.
Footnotes
Source of support: Departmental sources
Disclosure of conflict of interest
None.
References
- 1.Ghoshal S, Greer DM. Why is diagnosing brain death so confusing? Curr Opin Crit Care. 2015;21:107–12. doi: 10.1097/MCC.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 2.Redelmeier DA, Scales DC. Missing the diagnosis of brain death as a self-erasing error. Am J Respir Crit Care Med. 2015;192:280–82. doi: 10.1164/rccm.201503-0499OE. [DOI] [PubMed] [Google Scholar]
- 3.Brasil S, Bor-Seng-Shu E, de-Lima-Oliveira M, et al. Role of computed tomography angiography and perfusion tomography in diagnosing brain death: A systematic review. J Neuroradiol. 2016;43(2):133–40. doi: 10.1016/j.neurad.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Shah SK, Kasper K, Miller FG. A narrative review of the empirical evidence on public attitudes on brain death and vital organ transplantation: The need for better data to inform policy. J Med Ethics. 2015;41:291–96. doi: 10.1136/medethics-2013-101930. [DOI] [PubMed] [Google Scholar]
- 5.Wahlster S, Wijdicks EF, Patel PV, et al. Brain death declaration: Practices and perceptions worldwide. Neurology. 2015;84:1870–79. doi: 10.1212/WNL.0000000000001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wijdicks EF. Determining brain death. Continuum (Minneap Minn) 2015;21:1411–24. doi: 10.1212/CON.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 7.Szurhaj W, Lamblin MD, Kaminska A, Sediri H. EEG guidelines in the diagnosis of brain death. Neurophysiol Clin. 2015;45:97–104. doi: 10.1016/j.neucli.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Anastasian ZH, Khan N, Heyer EJ, et al. Effect of atropine dose on heart rate during electroconvulsive therapy. J ECT. 2014;30:298–302. doi: 10.1097/YCT.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 9.Gill H, Thoresen M, Smit E, et al. Sedation management during therapeutic hypothermia for neonatal encephalopathy: Atropine premedication for endotracheal intubation causes a prolonged increase in heart rate. Resuscitation. 2014;85:1394–98. doi: 10.1016/j.resuscitation.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Arbour RB. Brain death: Assessment, controversy, and confounding factors. Crit Care Nurse. 2013;33:27–46. doi: 10.4037/ccn2013215. [DOI] [PubMed] [Google Scholar]
- 11.Monteiro LM, Bollen CW, van Huffelen AC, et al. Transcranial Doppler ultrasonography to confirm brain death: A meta-analysis. Intensive Care Med. 2006;32:1937–44. doi: 10.1007/s00134-006-0353-9. [DOI] [PubMed] [Google Scholar]
- 12.Sawicki M, Bohatyrewicz R, Walecka A, et al. CT angiography in the diagnosis of brain death. Pol J Radiol. 2014;79:417–21. doi: 10.12659/PJR.891114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang JJ, Tsivgoulis G, Katsanos AH, et al. Diagnostic accuracy of transcranial Doppler for brain death confirmation: Systematic review and meta-analysis. Am J Neuroradiol. 2016;37:408–14. doi: 10.3174/ajnr.A4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escudero D, Otero J, Quindos B, Vina L. Transcranial Doppler ultrasound in the diagnosis of brain death. Is it useful or does it delay the diagnosis? Med Intensiva. 2015;39:244–50. doi: 10.1016/j.medin.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Al-Jehani HM, Sheikh BY. Transcranial Doppler in brain death assessment. Perspective and implications in the Saudi Arabian health system. Neurosciences (Riyadh) 2013;18:122–25. [PubMed] [Google Scholar]
- 16.Aleksandrova EV, Iusupova MM, Tenedieva VD, et al. [Clinical and prognostic significance of genetic markers in craniocerebral injury (Part III)]. Zh Vopr Neirokhir Im N N Burdenko. 2014;78:53–61. [in Russian] [PubMed] [Google Scholar]
- 17.Naqvi J, Yap KH, Ahmad G, Ghosh J. Transcranial Doppler ultrasound: A review of the physical principles and major applications in critical care. Int J Vasc Med. 2013;2013:629378. doi: 10.1155/2013/629378. [DOI] [PMC free article] [PubMed] [Google Scholar]