Abstract
Background
It is still disputable whether negative effects of comorbid depression in diabetics can be diminished by successful treatment of depression. The primary aim of this study was to assess whether addition of antidepressants to existing insulin treatment would further improve glycemic control in these patients. A secondary objective was to assess whether such treatment impairs their lipid and inflammatory status.
Material/Methods
Total of 192 patients with poorly controlled diabetes (defined as HbA1c ≥8%) in the absence of any uncontrolled medical condition entered the 6-month run-in phase with optimization of diabetic therapy. Depression status was screened at the end of this phase by BDI-II depression testing. Patients with BDI-II ≥14 and psychiatric confirmation of depression (58 patients) entered the 6-month interventional phase with SSRI class antidepressants.
Results
Fifty patients completed the study. During the run-in phase, HbA1c dropped from 10.0±1.8% to 8.5±1.2% (p<0.001), and during the interventional phase it dropped from 8.5±1.2% to 7.7±0.7% (p<0.001). BDI-II scores improved significantly from 30.4±13.2 to 23.5±11.0 (p=0.02) during the interventional phase. A positive linear correlation between improvement in depression scale and improvement in glycemic control was observed (R2=0.139, p=0.008). Lipid profile and inflammatory status did not change significantly during the interventional phase.
Conclusions
Patients with poorly controlled diabetes and comorbid depression might benefit from screening and treatment of depression with SSRI antidepressants by achieving an incremental effect on glycoregulation. This therapy did not have any adverse effects on lipid profile or inflammatory status.
MeSH Keywords: Antidepressive Agents; Depression; Diabetes Mellitus, Type 2; Inflammation; Risk Factors; Serotonin Uptake Inhibitors
Background
Type 2 diabetes is currently reaching epidemic levels worldwide [1–3]. Depression, on the other hand, is a medical condition that burdens millions of people and is more than twice as likely to appear in diabetics [4–6], affecting as many as 1 in every 3 individuals with diabetes [4,7].
Over the past 30 years, findings from numerous studies have documented that coexistence of comorbid depression and diabetes is associated with worsened glycemic control [8,9], increased risk and severity of diabetes complications [1,10], worsened adherence to diabetes self-management behavior and medical treatment [11,12], increased functional disability and decreased quality of life [13], and increased serious psychiatric outcomes including attempted suicide [14], higher morbidity [15], higher early mortality [16,17], and higher medical costs [18]. Despite the great interest in the relationship between diabetes and depression and its directional nature, as well as for assessing the effects of depression treatment on glycemic control and on the long-term course of diabetes, these aspects have not been definitely resolved.
The primary aim of this study was to assess whether addition of antidepressants to the existing insulin treatment in patient with diabetes and comorbid depression would further improve their glycemic control. A Secondary objective was to assess whether treatment with antidepressants impairs lipid and inflammatory status in these patients, as they are responsible for developing diabetic complications and influencing its outcome.
To achieve these objectives, we conducted an interventional, prospective, single-center, multidisciplinary study with a self-controlled cohort including consecutive patients from our daily practice fulfilling study inclusion criteria.
Material and Methods
Patient selection
We targeted the population with poorly controlled diabetes, focusing on individuals with depressive symptoms in the absence of any uncontrolled or debilitating medical condition, being the potential confounder for objective assessment of comorbid depression.
The study was approved by the hospital Ethics Review Board, as well as by the Scientific and Educational Board at The Belgrade University School of Medicine. It was conducted according to the Declaration of Helsinki [19].
Patients with type 2 diabetes who were 18–65 years of age were screened for study participation. Patients were eligible to participate provided they had poorly controlled diabetes (defined as glycosylated hemoglobin ≥8%) and were able to give informed consent and fill out study research forms and questionnaires on their own. Using the study protocol, we excluded the following: patients with alcohol or substance dependence, diabetics in pregnancy or lactation, patients with the definite diagnose of coronary artery disease (angiographically proven, history of myocardial infarction or coronary interventions), patients with severe impairment of renal function, and patients with any uncontrolled medical condition other than type 2 diabetes.
Study protocol
Our study consisted of 2 phases. The first one was stabilization of diabetic treatment (conducted by an endocrinologist), being the run-in phase for the second, interventional phase with antidepressants (conducted by a psychiatrist). In the first phase, we either introduced insulin to the poorly controlled diabetic patients or optimized the dosing or the type of the existing insulin therapy. Our decisions were based either on highly elevated levels of HbA1c or following the failure of maximal dose of oral antidiabetic medications to achieve a proper diabetic control. Presence of diabetic complications and/or comorbidities was determined by the study investigator, an endocrinologist, paying particular attention to the presence of polyneuropathy, angina pectoris, nephropathy, retinopathy, hypertension, and hyperlipidemia. Anthropometric and relevant sociodemographic characteristics were recorded as well.
The run-in stabilization phase was introduced to provide glycoregulation as stable as possible before entering the interventional stage. Its purpose was to eliminate episodes of situational depression caused by improper management of diabetes, since a positive association with depressive symptoms has been demonstrated in patients with treated but not in untreated, type 2 diabetes, which was explained as secondary to psychological stress associated with diabetes management, and particularly with its mismanagement [20]. The run-in stabilization phase minimized stressful situations in general, since it is known that stress is one of the most common etiological factors for both depression and diabetes [21–23].
Starting from the stabilization phase, all patients were put on statin therapy according to the current guidelines [24–26]. Hypertensive patients received appropriate antihypertensive therapy in order to achieve recommended targets [27]. Patients with polyneuropathy received symptom-relieving therapy.
At the end of the stabilization phase, patients were screened for depression before entering the interventional stage with antidepressants. Screening for depression was performed by use of the Beck Depression Inventory-II (BDI-II), a patient self-assessment questionnaire. It requires self-rating on a scale of 0–3 on 21 items. This tool is shown to have high sensitivity (81%) and specificity (92%) and favorable correlation with more complex instruments and techniques for diagnosing depression, as well as respectable reproducibility [28,29]. A significant positive correlation with the Hamilton Depression Rating Scale (r=0.71) has been reported [28].
Diabetics with major depression (having BDI-II score ≥14) were eligible to enter the interventional phase of the study. In each of these patients, the presence of major depression was further confirmed by a psychiatrist. At that point, patients were excluded from the study if there was a suicidal tendency, history of bipolar or any other psychiatric disorder, alcohol/drug abuse, or if they were currently taking psychoactive medications. All patients were put on selective serotonin reuptake inhibitors (SSRI) antidepressants. The choice of a particular antidepressant from this group in each individual patient was at the psychiatrist’s discretion, taking in account the patient’s profile, liver and renal function, drug pharmacokinetics, and local availability. The prescribed drugs were titrated to the optimal dose range by the psychiatrist during the treatment course. All diabetic medications were kept at the same dosage and regimen as already established during the stabilization phase. If these medications needed substantial change or readjustment, patients were excluded from the study. Patients were also excluded if they needed hospitalization longer than one day for any newly-arisen medical condition. Patients were vigorously instructed and supported to comply all dietary and activity measures established over the stabilization phase throughout the study.
All patients had at least one control examination in both study phases at 3 months, as well as the additional ones if deemed needed in particular cases. Patient compliance to both diabetic medications and antidepressants was monitored at the scheduled examinations, as well as at drug prescription and renewal checkpoints, necessitating presentation of emptied containers of dispensed medications.
Laboratory measurements
Glycemic control was evaluated by HbA1c measurement, being accepted as the best indicator of recent integrated glycemic control and used in guiding clinical management [30]. HbA1c concentrations were determined by the Siemens Dimension® RxL Max® Integrated Chemistry System. The range of HbA1c levels for normal glucose tolerance in our population is 4–6%.
Lipid profile was assessed by measuring total cholesterol and triglycerides levels. Measurements were also done by the Siemens Dimension® RxL Max® System. Lipid status evaluation was performed at the study entry and repeated at the end of both phases, as presented in Figure 1.
Figure 1.
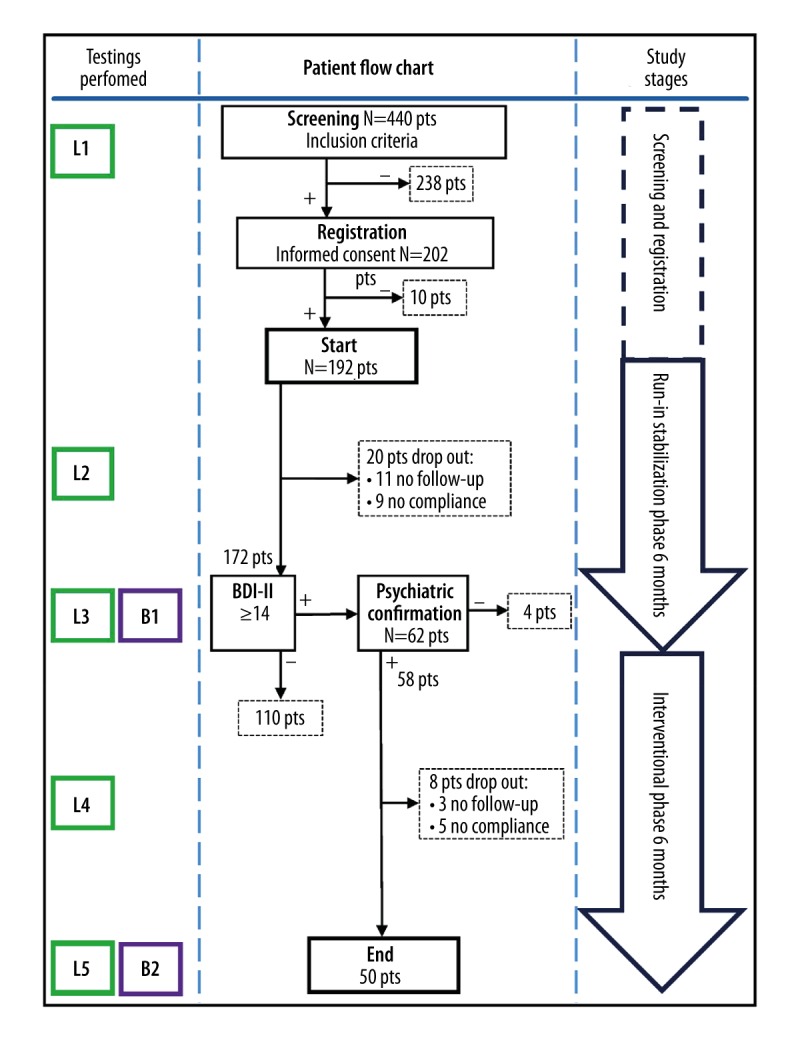
Study protocol flowchart. L1, L3, L5 – full laboratory testing including HbA1c, CRP, and lipid profile at the study milestones (L1 beginning of the study, L3 end of run-in stabilization, and L5 end of interventional phase). L2, L4 – laboratory testing points at the mid-phase visits, including blood glucose, urine, and basic biochemical screening. B1 and B2 – BDI-II questioning points at the end of each study phase.
Bearing in mind that inflammation has been proposed as a common link between insulin resistance, obesity, diabetes and depression, we assessed inflammatory status in studied patients by CRP measurements, being a practicable and universally used global marker. For that purpose, CRP measurements were performed at the same checkpoints as lipid profile. CRP was measured using the Siemens Dimension® RxL Max® System. The cutoff for normal values in our population is <5 mg/L.
Statistical analysis
Patient data were extracted from the hospital information system (Heliant Health®, Heliant LLC., Belgrade, SRB) to a Microsoft Excel database. Final data analysis was performed using SPSS software (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.).
For descriptive statistics, continuous variables are presented as means ± standard deviation (SD), or as median with corresponding range, depending on the data distribution. Normality of sample distribution was analyzed by Kolmogorov-Smirnoff test. Nonparametric variables were presented as frequency distributions. For testing the hypothesis on the treatment effect Student’s t-test for serial measurements, or Wilcoxon signed-rank test were used, depending on normality of data distribution. Two-tailed probability level of p<0.05 was considered to be statistically significant. To investigate the relationship, its directional nature and the strength of association between improvement in diabetes status and improvement in glycoregulation, Pearson’s correlation was applied. Based on calculated Pearson correlation coefficient r, significance was expressed as two-tailed probability value with the same p<0.05 threshold.
Results
Demographic and clinical characteristics
Following patient screening and registration, total of 192 patients entered the run-in stabilization phase of the study (Figure 1). Excluding dropouts during this phase, 172 patients reached BDI-II testing at its end. Out of them, 62 patients had BDI-II scoring result ≥14. Four patients were excluded further on by the psychiatrist, so a total of 58 patients entered the interventional phase of the study. During this 6-month phase, 3 patients were excluded since they did not show up at their scheduled control. An additional 5 patients were excluded because they were not adherent to therapy (either antidepressants or insulin). Antidepressant therapy prescribed by psychiatrist was well tolerated in all remaining patients, without serious adverse events necessitating the need for its interruption, or switch to other forms of treatment.
Therefore, all results are based on the remaining 50 patients. Basic demographic and relevant clinical characteristics of these patients at the beginning of the study are presented in the Table 1. There were 31 (62%) female and 19 (38%) male patients. All participants were white and none of them belonged to populations with increased risk for familiar hemoglobinopathies that would alter the erythrocyte lifespan and consecutive glycosylated hemoglobin readings. Exclusion of patients with severe renal or hepatic failure also contributed to the unaltered erythrocyte lifespan in the studied population.
Table 1.
Baseline demographic and clinical characteristics.
| Variable | Study population (n=50) |
|---|---|
| Age(years) ±SD | 57.8±7.3 |
| Gender | |
| Male | 19 (38%) |
| Female | 31 (62%) |
| Body weight (kg) ±SD | 81.3±13.3 |
| BMI (kg/m2) ±SD | 27.1±3.7 |
| 18.5–24.9 | 14 (28%) |
| 25–29.9 | 23 (46%) |
| ≥30 | 13 (26%) |
| Physical activity | |
| No activity | 7 (14.0%) |
| 1×weekly | 12 (24.0%) |
| 2×weekly | 21 (42.0%) |
| ≥3×weekly | 10 (20.0%) |
| Educational status | |
| Elementary school | 7 (14%) |
| Secondary school | 32 (64%) |
| High school | 9 (18%) |
| University education | 2 (4%) |
| Marital status | |
| Married | 24 (48%) |
| Not married | 26 (52%) |
| Household income (dinars, national currency) | |
| No income | 3 (6%) |
| <20.000 | 9 (18%) |
| 20.000–40.000 | 26 (52%) |
| 40.000–60.000 | 11 (22) |
| >60.000 | 1 (2%) |
| Alcohol intake | 9 (18%) |
| Smoking | 25 (50%) |
| Hypertension | 35 (70%) |
| Hypercholesterolemia | 39 (78%) |
| Angina pectoris | 8 (16%) |
| Nephropathy | 6 (12%) |
| Retinopathy | 11 (22%) |
| Neuropathy | 10 (20%) |
| Antidepressants used | |
| Escitalopram | 24 (48%) |
| Sertraline | 20 (40%) |
| Paroxetine | 3 (6%) |
| Citalopram | 2 (4%) |
| Fluoxetine | 1 (2%) |
Serial changes in glycemic and metabolic-related parameters, as well as results of psychological testing, are shown in the Table 2. All laboratory measurements were done at 3 control points: 1) beginning of the study, 2) end of run-in stabilization phase, and 3) end of interventional phase with antidepressants, as presented at the study protocol flowchart (Figure 1).
Table 2.
Glycemic, metabolic, and psychiatric parameters across the study.
| Variable | Beginning of the study (n=50)* | End of run-in stabilization phase (n=50)# | End of interventional phase (n=50)§ | P |
|---|---|---|---|---|
| HbA1c (mean ±SD) | 10.0±1.8 | 8.5±1.2 | 7.7±0.7 | */# p<0.001 |
| #/§ p<0.001 | ||||
| Total cholesterol (mean ±SD) | 6.1±1.3 | 5.6±1.0 | 5.7±1.1 | */# p=0.003 |
| #/§ p=0.316 | ||||
| Triglycerides (median (range)) | 2.2 (0.4–16.4) | 1.8 (0.3–4.4) | 1.8 (0.4–9.3) | */# p=0.004 |
| #/§ p=0.481 | ||||
| CRP (median (range)) | 5.8 (1.1–22.3) | 3.8 (0.8–12.0) | 3.2 (0.5–12.9) | */# p= 0.002 |
| #/§ p=0.132 | ||||
| BDI-II score (mean ±SD) | / | 30.4±13.2 | 23.5±11.0 | #/§ p=0.002 |
– Significance of difference between the beginning of the study and the end of the run-in stabilization phase;
– Significance of difference between the end of the run-in stabilization phase and the end of the interventional phase with antidepressants. Units of measure: HbA1c (%), cholesterol (mmol/L), triglycerides (mmol/L), CRP (mg/L).
Glycosylated hemoglobin dropped significantly from 10.0±1.8% at the study entry to 8.5±1.2% (p<0.001) at the end of stabilization phase (stabilization effect), and from 8.5±1.2% at the end of stabilization phase to 7.7±0.7% (p<0.001) at the end of interventional phase (interventional effect). The observed HbA1c stabilization improvement of 1.5%, as well as the interventional improvement of 0.8%, were within the clinically meaningful range according to the standards of practice and the study proposed goals. The stabilization improvement was a direct result of optimization of diabetes therapy and stringent dietary and activity modifications. The interventional improvement was attributed to the effects of introduced antidepressants per se since all other influential factors remained constant throughout the interventional phase. The achieved significant effect of antidepressants on improvement of glycoregulation, as the primary endpoint of the study, is presented in Figure 2.
Figure 2.
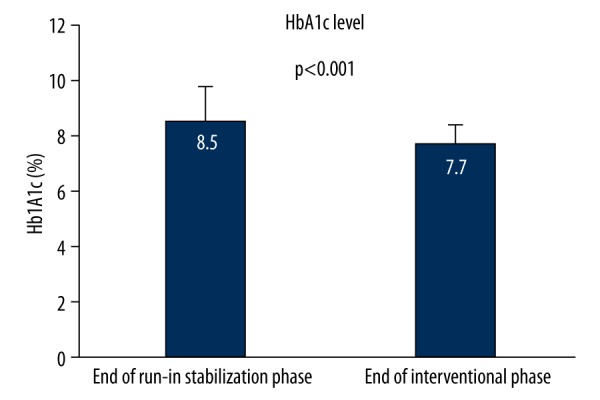
Interventional effect of antidepressants on glycemic control.
Improvement in depression status by antidepressants therapy was confirmed by the significant decrease of BDI-II scores from 30.4±13.2 to 23.5±11.0 (p=0.02) over the interventional phase. This direct and sustained effect of antidepressants on depression is presented in Figure 3. The observed reduction in DBI-II score of 6.9 represents a clinically meaningful improvement of depression status in study patients [28].
Figure 3.
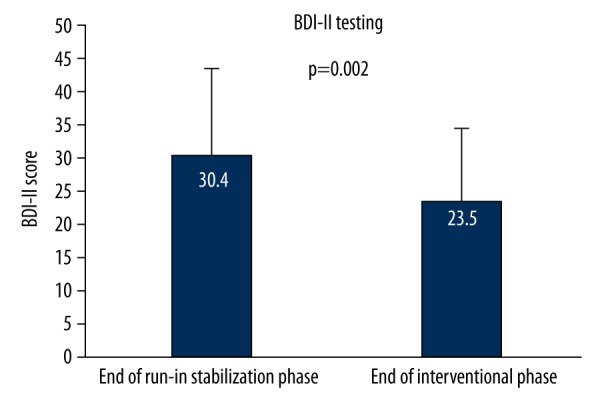
Interventional effect of antidepressants on depression scale.
Concerning cholesterol trends, there was a significant decrease of 7.7% from 6.1±1.3 to 5.6±1.0, (p=0.03) over the stabilization phase in the overall studied population, as a direct and predictable effect of statin therapy and complementary dietary interventions. Atorvastatin was chosen as a statin of choice and prescribed to all patients because of its evidence-based benefit in treating diabetics [31], favorable safety profile in general populations [32–34], as well as in diabetics [35], and potent effects on the lipid profile [36,37]. It was used with the median dose of 10 mg, and the dose range of 10–20 mg. The reduction of cholesterol was more pronounced (20.5%) among statin-naïve patients (n=21) at the study entry, from 6.8±1.3 to 5.4±0.9 over the stabilization phase (p<0.001). Over the subsequent interventional phase with antidepressants, cholesterol values were not altered significantly, changing from 5.6±1.0 to 5.7±1.1 (p=0.32).
Triglycerides dropped significantly (18.2%) over the stabilization phase, from 2.2 (0.4–16.4) at the beginning to 1.8 (0.3–4.4) at the end, presented as median (range), (p=0.004) as result of improved glycemic control and partially secondary to atorvastatin. Triglycerides remained unchanged afterward, from 1.8 (0.3–4.4) at the beginning to 1.8 (0.4–9.3) at the end of the interventional phase with antidepressants (p=0.48).
The observed nonsignificant alterations of both cholesterol and triglycerides over the interventional phase with antidepressants confirm the secondary objective of the study, the lipid neutrality of SSRI antidepressants in patients with diabetes and depression.
Finally, CRP values dropped significantly (31.2%) during the stabilization phase, from 5.8 (1.1–22.3) at the beginning to 3.8 (0.8–12.0) at its end, presented as mean (range) (p=0.002). CRP values did not change significantly thereafter, from 3.8 (0.8–12) at the end of the stabilization phase to 3.2 (0.5–12.9) at the end of the interventional phase (p=0.132), confirming their neutral effect on inflammation, which was the secondary objective of the study.
Association and directional nature between changes in depression and diabetes status were addressed by checking the relation between the change of BDI-II score (ΔBDI-II) and the change in glycated hemoglobin levels (ΔHbA1c) during the interventional phase by means of Pearson’s correlation testing. A strong positive linear correlation between improvement in depression scale (ΔBDI-II) and improvement in glycemic control (ΔHbA1c) was observed (R2=0.139), with corresponding p=0.008, as shown in Figure 4.
Figure 4.
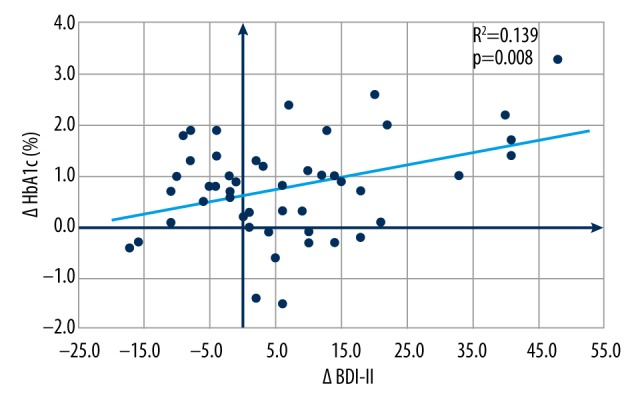
Interventional effect of antidepressants: Association between improvement in depression status and improvement in metabolic control.
Discussion
Improvement of glycemic control during the treatment of depression
Our study showed a beneficial effect of antidepressants on glycemic control beyond standard diabetic treatment practice, even though some other studies may have not. In point of fact, despite the great interest in this area, this issue has not been resolved definitely. Several large size studies in this field were cross-sectional and methodologically unable to establish directional effect, and longitudinal ones included limited numbers of participants, being underpowered and using different methodologies, limiting suitability of their inclusion in subsequent meta-analyses. Therefore, it remains disputable whether, and to what extent, the negative effects of comorbid depression in diabetic patients can be diminished by successful treatment of depression.
There are several non-interventional publications dealing with the effect of depression per se on glycemic control. Richardson and colleagues assessed the long-term effects of depression on glycemic control. They reported that over 4 years of follow-up, there was a significant longitudinal relationship between depression and glycemic control and that depression was associated with persistently higher HbA1c levels over the entire period [38].
Furthermore, numerous studies have been published on the effects of treating depression in diabetes, encompassing case reports, uncontrolled studies, randomized controlled studies, systematic reviews, and meta-analysis. Treatment options included a vast range of modalities, including patient education and counseling, various forms of behavioral psychotherapy, and pharmacological therapy with antidepressant medications, either alone or in combination.
A recent systematic review [39] showed that psychological and pharmacological interventions have a moderate but clinically significant effect on depression outcomes in diabetics. However, concerning their impact of diabetes control, it was shown that in pharmacological trials improved glycemic control moderately, while for psychological interventions the evidence was inconclusive [39]. In general, the evidence was scattered and conflicting due to several trials with inappropriate design, including substantial risk of bias, and the vast diversity of interventional regimes and populations.
The effect of antidepressants on diabetes in numerous individual studies published in this field was positive regarding improvement in depression scale points, but HbA1c remained unchanged. One of the first prospective studies was published by Lustman in 1997, applying the tricyclic antidepressant nortriptyline in 68 diabetic patients. Results showed significant reduction in depression symptoms; however, no such effects were observed on glycemic control. The treatment drug was not statistically superior to placebo in reducing glycated hemoglobin in the depressed subjects, but the treatment period was only 8 weeks, which was not long enough for the HbA1c to fully drop [40].
In 2013 Nicolau and colleagues published results on 48 patients (38 patients on antidepressants and 10 patients as control group) treated with citalopram, determining the effects on depressive symptoms and metabolic control in patients with type 2 diabetes. Patients were followed for 6 months. Significant improvement in depression score and in almost all areas of quality of life was achieved; however, no differences were found in glycemic control assessed by HbA1c [41]. Lipid profile did not change significantly after 6 months of antidepressant therapy. Whether the explanation for the negative result is the relatively small sample size or more stringent definition of depression remains unresolved.
Paile-Hyvärinen and colleagues reported in 2007 the results from a randomized, double-blind, placebo-controlled trial dealing with the quality of life and metabolic status of patients with sub-threshold depression and type 2 diabetes. They demonstrated that at the end of 6-month follow-up, no statistically significant difference between treatment (paroxetine) and placebo groups was observed, neither for glycemic control nor for depression score, despite favorable effects observed at 3 months.
Several studies of antidepressants showed improvement of glycoregulation, assessed by lowering of HbA1c or related parameters of diabetic control. Okamura, published results from a 2000 study including 20 nondiabetic patients with depression, evaluating insulin sensitivity and its changes during the clinical course of depression. Therefore, tricyclic or tetracyclic antidepressants (maprotiline, amitriptyline, dosulepin, or amoxapine) were used. Patients demonstrated lowering of the depression score, as well as the improvement of the parameters of glycemic control, aside from HbA1c, such as hyperinsulinemia and insulin response during the OGTT. The authors concluded that patients with depression had impaired insulin sensitivity and hyperinsulinemia and that those could be resolved after successful medical treatment of depression. However, none of the patients in the study had diabetes, so its findings are not directly comparable to ours [42].
In 2000, Lustman and colleagues published results on 60 patients treated with fluoxetine. The treatment period was only 8 weeks and results showed that HbA1c levels fell, but not enough to reach a level of statistical significance. Fluoxetine, as a medication of choice, had a negative effect on weight gain, but it positively influenced insulin resistance and insulin availability [43].
Dhavale and colleagues investigated the effect of escitalopram in a series of 100 consecutive patients with type 2 diabetes mellitus with increased blood glucose levels. Depressed patients were then started on escitalopram while their diabetes treatment remained unchanged. They were reviewed 6 weeks later and blood glucose levels were repeated. At the follow-up, 47% of patients started on escitalopram showed clinically and statistically significant lower fasting and postprandial blood glucose levels [22].
Lustman and colleagues published a 2006 study of 152 patients treated with sertraline for 16 weeks. During the 52-week follow-up, HbA1c was lowered. The study design was not standard; it was a randomized, double-blind, and placebo-controlled trial. Participants who recovered from depression after treatment with sertraline continued to receive sertraline (n=79) or placebo (n=73) and were followed up for up to 52 weeks or until depression recurred. The study concluded that maintenance therapy with sertraline prolongs the depression-free interval following recovery from major depression. Depression recovery with sertraline was associated with improvements in glycosylated hemoglobin levels for at least 1 year [44].
Echeverry and colleagues reported in 2009 results of a randomized, double-blind, placebo-controlled trial on 89 patients with depression and diabetes. After 6 months of treatment with sertraline, significant decreases in HbA1c and systolic blood pressure levels were found compared to a placebo [5].
Concerning the most recent studies in this field, Petrak and colleagues published in 2015 noteworthy results on the Diabetes and Depression (DAD) study. It was a prospective multicenter study from 70 secondary care centers across Germany, comparing the long-term efficacy of cognitive behavioral group therapy (CBT) with sertraline in patients with diabetes and poor glycemic control (initial mean HbA1c value at the entry was 9.3%) [45]. Depression improved both under CBT and sertraline (with a significant advantage for sertraline) in patients with diabetes and depression; however, glycemic control remained unchanged [45]. The lack of a positive effect on glycemic control might be discussed in the light of study design and related patient characteristics. Concerning the baseline characteristics, the recruited group consisted of very “difficult-to-treat” patients, at least with respect to their glycemic control, since the mean glycosylated hemoglobin values were higher than reported in other studies in this field.
In regard to this aspect, our patients were recruited at the Endocrinology Department of our hospital, representing the tertiary level of diabetes care. Concerning patient inclusion, our study, as a majority of others exploring effects of antidepressants, was confined to type 2 diabetes, but in the DAD study patients with type 1 diabetes represented 51% of the group. The important difference in study design between our study and the DAD study was that we excluded patients with diagnosed coronary artery disease, while in the DAD study they represented 12.7% of the population. These 2 studies share some common approaches: definition of poorly controlled diabetes, introduction of run-in phase, and the definition of the primary outcome. Also, despite the thorough methodology, adherence to diabetes treatment in the DAD study has not been directly assessed.
Finally, bearing in mind the results of our study, as well the data from other publications that we have reviewed, we believe that further investigations in this field are needed to produce definitive results on the significance and range of psychiatric treatment in patients with diabetes and comorbid depression, including the development of more comprehensive and collaborative models for treating both depression and diabetes.
Association between changes in depression status and metabolic control
To the best of our knowledge, our study is the first one reporting a linear association between improvement in BDI-II score (delta BDI-II) and improvement in glycated hemoglobin values (ΔHbA1c) in diabetic patients with comorbid depression treated with SSRI antidepressants, indicating the reciprocal interaction between depression and glycoregulation, suggesting that treatment of depression may be beneficial to both mood and glycemic control.
Wiltink and colleagues reported in 2014 on results from the Gutenberg Heath Study, showing a linear and consistent association of the intensity of depression with the presence of diabetes, increasing from 6.9% in no or minimal depression to 7.6% in mild, 9% in moderate, and 10.5% in severe depression; i.e., the prevalence of diabetes was elevated substantially (1.5 fold) in severe vs. no depression [46]. However, this was a community study with the cross-sectional design.
Metabolic and lipid effects of antidepressants
The choice of a particular SSRI drug in the study was made by the psychiatrist. Overall, 5 drugs from this class were used in the study, with dominant usage of escitalopram and sertraline (88% together). This practice has disabled us to statistically assess the individual power of certain medications in modulating glycemic and metabolic effects so that the existence of the class effect was presumed.
Concerning the published data on SSRI antidepressants, it has been shown that fluoxetine has a favorable effect onto glucose tolerance and insulin sensitivity. Some other medications have negative impacts on insulin resistance (duloxetine, desvenlafaxine, and mirtazapine) [47]; however, these drugs were not used in this study. Concerning lipid profile, it has been established that mirtazapine and paroxetine (used in 6% of our study population) are associated with clinically significant increases in serum triglyceride level and LDL levels [48,49]. Fluoxetine (used in only 2% of our study population) had a favorable effect on both of the fractions of lipids, including the non-depressed diabetic population [50,51].
Effects on inflammation
CRP is one of the positive acute-phase proteins, secreted in the liver. Depression has been shown to be associated with the increased inflammatory response [52]. Also, it has been suggested that the link between depression, obesity, and diabetes may be mediated through inflammation.
We consider the significant drop in CRP values observed during the stabilization phase in our patients as a direct effect of statin therapy and improved metabolic control. Reduction of CRP is a well-known class effect of statins. Particularly for atorvastatin, used in our patients, this observation has well been broadly documented in the general population [53–55] as well as in diabetics [56,57].
In our study, during the interventional phase, we have demonstrated the neutral effect of SSRI antidepressants on inflammation assessed by serial CRP measurements. This observation certainly precludes concerns about their eventual negative consequences on underlying inflammatory mechanisms been the common link between diabetes and depression.
Concerning the published data on the effects of antidepressants on inflammation, accumulated evidence from ex-vivo studies have been published demonstrating that these medications modulate cytokine production, proliferative activity of T cells, and the cytotoxic activity of natural killer cells, being particularly true for tricyclic antidepressants and SSRI antidepressants [58]. Clinical data on the correlation between CRP levels and depression are not consistent across studies. Some authors showed a positive correlation and normalization of these values after therapy with antidepressants [59,60]. However, these finding were not consistent with negative findings from other studies in this field [52].
Statins and depression
When designing the present study, we were particularly aware of the potential relationship between cholesterol lowering and suicidality, proposed by some circumstantial evidence at that time, as well as by some early epidemiological studies [61]. Although this concept has recently been disputed on the basis of studies with more refined accuracy that have not found any correlation [62–65], we decided to, unless clinically necessary, apply a conservative but proven atorvastatin dose of 10 mg [31] in all patients. Atorvastatin was well tolerated, and the prescribed dose was maintained during the study. No dropouts from the study occurred because of its adverse effects or intolerance. Besides its proven effect on lipid and inflammatory status, this therapy does not adversely affect the efficacy of SSRI antidepressants in treating depression.
Potential long-term benefits of treating comorbid depression
Due to the number of treated patients and the treatment duration, our study was underpowered to assess the impact of such combined treatment on the course of diabetes-related complications.
However, concerning the latest data on the J-shaped link between diabetes duration and depression, reporting almost tripled risk after the third decade of living with diabetes [6], we believe that timely initiation of treatment of depression in diabetic patients might disentangle such late catch-up of depression.
Study limitations and strengths
Patients in our country had to be started on human insulin therapy (regulatory affairs) rather than on analogs, being harder for them regarding flexibility and with greater incidence of hypoglycemia. Anabolic characteristics of human insulins resulting in a more pronounced weight gain potentially might have led to worsening of depression per se.
Also, at the time and due to the circumstances of study implementation, we were not able to obtain the complete lipid status in all participating patients, so we ended up presenting only the total cholesterol and triglycerides that were common to all participants. The same limitation applies to the methods for assessing inflammation.
Our prospective study did not include a control group because the institutional position of the local ethics committee prohibited us from deterring psychiatric consultation and consecutive treatment in patients with a high BDI-II score corresponding to moderate or severe depression.
The anticipated strength of the study directly arises from its design with introduction of run-in stabilization phase, exclusion of diagnosed coronary disease and other uncontrolled medical conditions as influential confounders, length of interventional phase, and good compliance to medications with its inherent control.
Conclusions
We found that patients with poorly controlled diabetes and comorbid depression might benefit from screening and treatment of depression with SSRI antidepressants by achieving an incremental effect on glycoregulation over one accomplished by more stringent control of diabetic therapy alone. This therapy did not have adverse effects on lipid profile or inflammatory status, which are held responsible for cardiovascular and other diabetes-related complications.
Our findings suggest that clinicians should be aware of the increased risk of depression in patients with poorly controlled type 2 diabetes. Comorbid depression in such patients can be identified by proper and routine screening, opening avenues for their effective treatment and follow-up. By building bridges with mental health providers, endocrinologists can implement therapy with antidepressants, facilitating treatment of both conditions.
Footnotes
Source of support: Departmental sources
Conflicts of interest
No potential conflicts of interest relevant to this article were reported.
References
- 1.Egede LE, Ellis C. Diabetes and depression: Global perspectives. Diabetes Res Clin Pract. 2010;87(3):302–12. doi: 10.1016/j.diabres.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–29. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 3.Chen G-Y, Li L, Dai F, et al. Prevalence of and risk factors for type 2 diabetes mellitus in hyperlipidemia in China. Med Sci Monit. 2015;21:2476–84. doi: 10.12659/MSM.894246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression. Diabetes Care. 2001;24(6):1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 5.Echeverry D, Duran P, Bonds C, et al. Effect of pharmacological treatment of depression on A1C and quality of life in low-income hispanics and African Americans with diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Care. 2009;32:2156–60. doi: 10.2337/dc09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida OP, McCaul K, Hankey GJ, et al. Duration of diabetes and its association with depression in later life: The Health In Men Study (HIMS) Maturitas. 2016;86:3–9. doi: 10.1016/j.maturitas.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Mikaliukstiene A, Zagminas K, Juozulynas A, et al. Prevalence and determinants of anxiety and depression symptoms in patients with type 2 diabetes in Lithuania. Med Sci Monit. 2014;20:182–90. doi: 10.12659/MSM.890019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lustman PJ, Anderson RJ, Freedland KE, et al. Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–42. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 9.Papelbaum M, Moreira RO, Coutinho W, et al. Depression, glycemic control and type 2 diabetes. Diabetol Metab Syndr. 2011;3(1):26. doi: 10.1186/1758-5996-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Groot M, Anderson R, Freedland KE, et al. Association of depression and diabetes complications: A meta-analysis. Psychosom Med. 2001;63(4):619–30. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: A meta-analysis. Diabetes Care. 2008;31(12):2398–403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egede LE, Ellis C. The effects of depression on diabetes knowledge, diabetes self-management, and perceived control in indigent patients with type 2 diabetes. Diabetes Technol Ther. 2008;10(3):213–19. doi: 10.1089/dia.2007.0278. [DOI] [PubMed] [Google Scholar]
- 13.Clouse RE, Lustman PJ, Freedland KE, et al. Depression and coronary heart disease in women with diabetes. Psychosom Med. 2003;65(3):376–83. doi: 10.1097/01.psy.0000041624.96580.1f. [DOI] [PubMed] [Google Scholar]
- 14.Kim G-M, Woo J-M, Jung S-Y, et al. Positive association between serious psychiatric outcomes and complications of diabetes mellitus in patients with depressive disorders. Int J Psychiatry Med. 2015;50(2):131–46. doi: 10.1177/0091217415605024. [DOI] [PubMed] [Google Scholar]
- 15.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26(10):2822–28. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Norris SL, Gregg EW, et al. Depressive symptoms and mortality among persons with and without diabetes. Am J Epidemiol. 2005;161(7):652–60. doi: 10.1093/aje/kwi089. [DOI] [PubMed] [Google Scholar]
- 17.Egede LE, Nietert PJ, Zheng D. Heart disease mortality among adults. Diabetes Care. 2005;28(6):1339–45. doi: 10.2337/diacare.28.6.1339. [DOI] [PubMed] [Google Scholar]
- 18.Simon GE, Katon WJ, Lin EHB, et al. Diabetes complications and depression as predictors of health service costs. Gen Hosp Psychiatry. 2005;27(5):344–51. doi: 10.1016/j.genhosppsych.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Association WM. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–94. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 20.Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751–59. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surwit RS, Schneider MS. Role of stress in the etiology and treatment of diabetes mellitus. Psychosom Med. 1993;55(4):380–93. doi: 10.1097/00006842-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Dhavale HS, Panikkar V, Jadhav BS, et al. Depression and diabetes: Impact of antidepressant medications on glycaemic control. J Assoc Physicians India. 2013;61(12):896–99. [PubMed] [Google Scholar]
- 23.Roberts AL, Agnew-Blais JC, Spiegelman D, et al. Posttraumatic stress disorder and incidence of type 2 diabetes mellitus in a sample of women. JAMA Psychiatry. 2015;72(3):203–10. doi: 10.1001/jamapsychiatry.2014.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32(14):1769–818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 26.Grant PJ, Chairperson E, Germany SDA, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2013;34(39):3035–87. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 27.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Steer RA. Manual for the beck depression inventory-2. Psychol Corp. 1996 [Google Scholar]
- 29.Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionna. Arthritis Care Res (Hoboken) 2011;63(S11):S454–66. doi: 10.1002/acr.20556. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761–73. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 31.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet (London, England) 2004;364(9435):685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 32.Newman CB, Palmer G, Silbershatz H, Szarek M. Safety of atorvastatin derived from analysis of 44 completed trials in 9,416 patients. Am J Cardiol. 2003;92(6):670–76. doi: 10.1016/s0002-9149(03)00820-8. [DOI] [PubMed] [Google Scholar]
- 33.Newman C, Tsai J, Szarek M, et al. Comparative safety of atorvastatin 80 mg versus 10 mg derived from analysis of 49 completed trials in 14,236 patients. Am J Cardiol. 2006;97(1):61–67. doi: 10.1016/j.amjcard.2005.07.108. [DOI] [PubMed] [Google Scholar]
- 34.Alsheikh-Ali AA, Ambrose MS, Kuvin JT, Karas RH. The safety of rosuvastatin as used in common clinical practice: A postmarketing analysis. Circulation. 2005;111(23):3051–57. doi: 10.1161/CIRCULATIONAHA.105.555482. [DOI] [PubMed] [Google Scholar]
- 35.Barakat L, Jayyousi A, Bener A, et al. Comparison of efficacy and safety of rosuvastatin, atorvastatin and pravastatin among dyslipidemic diabetic patients. ISRN Pharmacol. 2013;2013:146579. doi: 10.1155/2013/146579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study) Am J Cardiol. 1998;81(5):582–87. doi: 10.1016/s0002-9149(97)00965-x. [DOI] [PubMed] [Google Scholar]
- 37.Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial) Am J Cardiol. 2003;92(2):152–60. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 38.Richardson LK, Egede LE, Mueller M, et al. Longitudinal effects of depression on glycemic control in veterans with Type 2 diabetes. Gen Hosp Psychiatry. 2008;30(6):509–14. doi: 10.1016/j.genhosppsych.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database Syst Rev. 2012;12(12):CD008381. doi: 10.1002/14651858.CD008381.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lustman PJ, Griffith LS, Clouse RE, et al. Effects of nortriptyline on depression and glycemic control in diabetes: Results of a double-blind, placebo-controlled trial. Psychosom Med. 1997;59(3):241–50. doi: 10.1097/00006842-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Nicolau J, Rivera R, Frances C, et al. Treatment of depression in type 2 diabetic patients: Effects on depressive symptoms, quality of life and metabolic control. Diabetes Res Clin Pract. 2013;101(2):148–52. doi: 10.1016/j.diabres.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Okamura F, Tashiro A, Utumi A, et al. Insulin resistance in patients with depression and its changes during the clinical course of depression: Minimal model analysis. Metabolism. 2000;49(10):1255–60. doi: 10.1053/meta.2000.9515. [DOI] [PubMed] [Google Scholar]
- 43.Lustman PJ, Freedland KE, Griffith LS, Clouse RE. Fluoxetine for depression in diabetes: A randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23(5):618–23. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- 44.Lustman PJ, Clouse RE, Nix BD, et al. Sertraline for prevention of depression recurrence in diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2006;63:521–29. doi: 10.1001/archpsyc.63.5.521. [DOI] [PubMed] [Google Scholar]
- 45.Petrak F, Herpertz S, Albus C, et al. Cognitive behavioral therapy versus sertraline in patients with depression and poorly controlled diabetes: The Diabetes and Depression (DAD) study: A Randomized Controlled Multicenter Trial. Diabetes Care. 2015;38:767–75. doi: 10.2337/dc14-1599. [DOI] [PubMed] [Google Scholar]
- 46.Wiltink J, Michal M, Wild PS, et al. Associations between depression and diabetes in the community: Do symptom dimensions matter? Results from the Gutenberg Health Study. PLoS One. 2014;9(8):e105499. doi: 10.1371/journal.pone.0105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McIntyre RS, Park KY, Law CWY, et al. The association between conventional antidepressants and the metabolic syndrome: A review of the evidence and clinical implications. CNS Drugs. 2010;24(9):741–53. doi: 10.2165/11533280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Nicholas LM, Ford AL, Esposito SM, et al. The effects of mirtazapine on plasma lipid profiles in healthy subjects. J Clin Psychiatry. 2003;64(8):883–89. doi: 10.4088/jcp.v64n0805. [DOI] [PubMed] [Google Scholar]
- 49.Laimer M, Kramer-Reinstadler K, Rauchenzauner M, et al. Effect of mirtazapine treatment on body composition and metabolism. J Clin Psychiatry. 2006;67(3):421–24. doi: 10.4088/jcp.v67n0313. [DOI] [PubMed] [Google Scholar]
- 50.Fisar Z, Anders M, Tvrzicka E, Stankova B. Effect of long-term administration of antidepressants on the lipid composition of brain plasma membranes. Gen Physiol Biophys. 2005;24(2):221–36. [PubMed] [Google Scholar]
- 51.Masuda Y, Ohnuma S, Sugiyama T. Relationship between antidepressants and glycolipids in the forced swimming test in mice. Methods Find Exp Clin Pharmacol. 2000;22(9):667–70. doi: 10.1358/mf.2000.22.9.802281. [DOI] [PubMed] [Google Scholar]
- 52.Stanojević A, Popović I, Nenadović M, et al. Metabolic syndrome and C-reactive protein in patients with depressive disorder on antidepressive medication. Srp Arh Celok Lek. 2013;141:511–15. doi: 10.2298/sarh1308511s. [DOI] [PubMed] [Google Scholar]
- 53.Kent SM, Flaherty PJ, Coyle LC, et al. Effect of atorvastatin and pravastatin on serum C-reactive protein. Am Heart J. 2003;145(2):e8. doi: 10.1067/mhj.2003.34. [DOI] [PubMed] [Google Scholar]
- 54.van Wissen S, Trip MD, Smilde TJ, et al. Differential hs-CRP reduction in patients with familial hypercholesterolemia treated with aggressive or conventional statin therapy. Atherosclerosis. 2002;165(2):361–66. doi: 10.1016/s0021-9150(02)00280-0. [DOI] [PubMed] [Google Scholar]
- 55.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: A randomized controlled trial. JAMA. 2004;291(9):1071–80. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 56.van de Ree MA, Huisman MV, Princen HMG, et al. Strong decrease of high sensitivity C-reactive protein with high-dose atorvastatin in patients with type 2 diabetes mellitus. Atherosclerosis. 2003;166(1):129–35. doi: 10.1016/s0021-9150(02)00316-7. [DOI] [PubMed] [Google Scholar]
- 57.Ridker PM, Morrow DA, Rose LM, et al. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dl and C-reactive protein <2 mg/l: An analysis of the PROVE-IT TIMI-22 trial. J Am Coll Cardiol. 2005;45(10):1644–48. doi: 10.1016/j.jacc.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 58.De Berardis D, Conti CMV, Serroni N, et al. The effect of newer serotonin-noradrenalin antidepressants on cytokine production: A review of the current literature. Int J Immunopathol Pharmacol. 2010;23(2):417–22. doi: 10.1177/039463201002300204. [DOI] [PubMed] [Google Scholar]
- 59.Suarez EC. C-reactive protein is associated with psychological risk factors of cardiovascular disease in apparently healthy adults. Psychosom Med. 2004;66(5):684–91. doi: 10.1097/01.psy.0000138281.73634.67. [DOI] [PubMed] [Google Scholar]
- 60.O’Brien SM, Scott LV, Dinan TG. Antidepressant therapy and C-reactive protein levels. Br J Psychiatry. 2006;188:449–52. doi: 10.1192/bjp.bp.105.011015. [DOI] [PubMed] [Google Scholar]
- 61.Colin A, Reggers J, Castronovo V, Ansseau M. [Lipids, depression and suicide]. Encephale. 2003;29(1):49–58. [in French] [PubMed] [Google Scholar]
- 62.Manfredini R, Caracciolo S, Salmi R, et al. The association of low serum cholesterol with depression and suicidal behaviours: New hypotheses for the missing link. J Int Med Res. 2000;28:247–57. doi: 10.1177/147323000002800601. [DOI] [PubMed] [Google Scholar]
- 63.Yang C, Jick SS, Jick H. LIpid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926–32. doi: 10.1001/archinte.163.16.1926. [DOI] [PubMed] [Google Scholar]
- 64.De Berardis D, Marini S, Piersanti M, et al. The relationships between cholesterol and suicide: An update. ISRN Psychiatry. 2012;2012:387901. doi: 10.5402/2012/387901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koponen H, Kautiainen H, Leppänen E, et al. Association between suicidal behaviour and impaired glucose metabolism in depressive disorders. BMC Psychiatry. 2015;15:163. doi: 10.1186/s12888-015-0567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


