Abstract
The ability of Clostridium perfringens to form spores plays a key role during the transmission of this Gram-positive bacterium to cause disease. Of particular note, the spores produced by food poisoning strains are often exceptionally resistant to food environment stresses such as heat, cold and preservatives, which likely facilitates their survival in temperature-abused foods. The exceptional resistance properties of spores made by most type A food poisoning strains and some type C foodborne disease strains involves their production of a variant small acid soluble protein-4 that binds more tightly to spore DNA compared to the small acid soluble protein-4 made by most other C. perfringens strains. Sporulation and germination by C. perfringens and Bacillus spp. share both similarities and differences. Finally, sporulation is essential for production of C. perfringens enterotoxin, which is responsible for the symptoms of C. perfringens type A food poisoning, the second most common bacterial foodborne disease in the USA. During this foodborne disease, C. perfringens is ingested with food and then, using sporulation-specific alternate sigma factors, this bacterium sporulates and produces the enterotoxin in the intestines.
The ability of the Gram-positive, anaerobic rod Clostridium perfringens to form resistant spores contributes to its survival in many environmental niches, including soil, waste water, feces and foods (1). In addition, sporulation and germination play a significant role when this important pathogen causes disease (2, 3). As introduced in the next section of this chapter, spores often facilitate the transmission of C. perfringens to hosts and then germinate in vivo to cause disease.
Toxin production is well-appreciated as a critical factor for the pathogenicity of C. perfringens (1). At least 17 different C. perfringens toxins have been described in the literature; however, individual isolates produce only portions of this impressive toxin arsenal. Consequently, C. perfringens strains are commonly classified into one of five types (A-E) based upon their ability to produce four “typing” toxins, i.e., alpha, beta, epsilon and iota toxins. While all isolates produce alpha toxin, type B strains also express beta and epsilon toxin, type C isolates also make beta toxin, type D strains also produce epsilon toxin and type E isolates also express iota toxin. Besides producing one or more of the typing toxins, sporulating cells of some C. perfringens strains produce additional toxins such as C. perfringens enterotoxin (CPE), or a recently identified toxin named TpeL (1, 4). The connection between CPE production and sporulation has disease relevance, as introduced below.
The importance of spores for C. perfringens disease
In humans and several important livestock species, C. perfringens causes a spectrum of diseases that remain important medical and veterinary concerns. The most notable of those C. perfringens diseases are, i) histotoxic infections such as clostridial myonecrosis, also known as traumatic gas gangrene (5), and ii) diseases such as enteritis or enterotoxemias that originate in the intestinal tract (1, 2). As will now be described, spores can play an important role in the transmission of all these illnesses.
C. perfringens is the most common cause of traumatic human gas gangrene, which remains challenging to treat even using modern medical approaches (5). C. perfringens type A causes clostridial myonecrosis when spores or vegetative cells gain entry into muscle tissue via a wound. Spores can germinate if low oxidation-reduction (Redox) conditions are present in the muscle tissue; the resultant vegetative cells then grow rapidly to further reduce tissue Redox conditions, promoting additional bacterial growth. The growing C. perfringens vegetative cells produce alpha toxin and perfringolysin O, which cause local and regional necrosis in muscle, allowing rapid and progressive spread of the infection. In addition, these toxins can enter the systemic circulation to induce organ damage, circulatory problems and death (5).
Many cases of human or animal enteritis and enterotoxemia (i.e., absorption of toxins from the intestines into the circulation, from where they damage nonintestinal organs) are also caused by C. perfringens (2). Spores often play a critical role in transmission of the C. perfringens illnesses originating in the intestines, particularly during two human food-borne illnesses. The first of those diseases, i.e., C. perfringens type A food poisoning, is caused by CPE-producing type A strains and ranks as the second most prevalent bacterial food-borne illness in the USA at 1 million cases/year (6). While the enterotoxin (cpe) gene can be either chromosomal or plasmid-borne, ∼75% of all C. perfringens food poisoning cases are caused by type A strains carrying a chromosomal cpe gene (1). Food poisoning typically occurs when type A chromosomal cpe-positive strains are ingested with foods and then sporulate in the small intestine, where they produce CPE (further discussion later) (1). The second C. perfringens food-borne illness of humans is enteritis necroticans, which is caused by beta toxin-producing type C strains (7). Historically, enteritis necroticans was first observed in post-World-War II Germany, where it was known as Darmbrand (7). However, enteritis necroticans is most often associated with childhood infections in Papua New Guinea (where the disease is known locally as PigBel because it often follows ingestion of contaminated pork), although it occasionally occurs in developed countries (7). In both C. perfringens type A and type C food-borne diseases, improper cooking or holding of foods plays a critical role in transmission. This temperature abuse facilitates the survival of resistant C. perfringens spores present in foods; those spores later germinate and cause illness when the food is ingested (as discussed later in the chapter).
CPE-producing type A strains carrying a plasmid cpe gene are responsible for 2-15% of all cases of non-food-borne human gastrointestinal (GI) diseases, such as antibiotic-associated diarrhea (8). These illnesses are primarily acquired by ingesting spores that are present in the environment. Although less studied, spores could also contribute to C. perfringens enteritis and enterotoxemias in livestock, which can be caused by all types (A-E) of this bacterium.
After introducing C. perfringens spore ultrastructure and describing the basic processes of sporulation and germination in this bacterium, the remainder of this chapter will focus on recent insights into, i) the resistance properties that allow spores to contribute to C. perfringens disease transmission and ii) the molecular basis for the expression of CPE and TpeL by sporulating cells.
The ultrastructure of C. perfringens spores
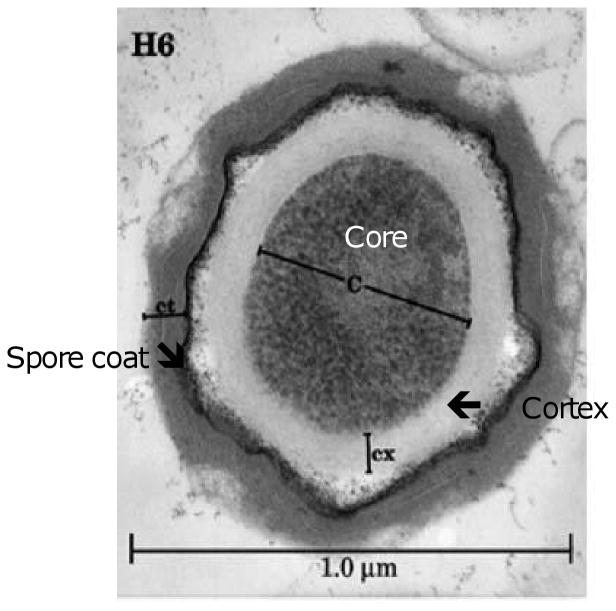
C. perfringens spores (Fig. 1) contain several different structural layers, all of which contribute to spore resistance properties (9, 10). Unlike some spore-forming species (11), C. perfringens does not possess an exosporium. Instead, the outermost layer of the C. perfringens spore is the spore coat. The composition and function of the spore coat in C. perfringens has not yet been carefully studied; however, in other Gram-positive spore formers, the spore coat is thought to be comprised of >50 spore-specific proteins and provides protection to the spore from reactive chemicals and lytic enzymes (12-14). In C. perfringens, a small fraction of the spore population (∼5%) is defective in spore coats (15). Those spores are permeable to lysozyme (15) so they can germinate inside the host under specific conditions.
Figure 1.
Ultrastructure of C. perfringens spores. Transmission electron micrograph of a spore from C. perfringens strain H-6, a food poisoning strain. Components of spore shown include: proteinaceous spore coat layers; cortex region; and the core with ribosomes giving a granular appearance. The bar represents 1.0 μM. Reproduced with permission from (9).
The layers that underlie the coat are common for all known Gram-positive spore formers, including C. perfringens. Beneath the coat is the outer membrane, which does not provide protection to dormant spores but is essential for spore formation and is presumably lost by shearing forces after short periods. The spore peptidoglycan cortex underlines the outer membrane, with a structure similar to that of peptidoglycan in a growing cell wall (16). The cortex plays an essential role in spore core dehydration and therefore directly contributes to spore resistance to environmental stress and chemicals. In studied spore-formers, and presumably also in C. perfringens, the spore peptidoglycan has three novel structural modifications that contribute to its resistance to cell wall hydrolases typically found in growing cells: (i) only one-quarter of cortex N-acetylmiramic acid (NAM) residues are substituted with short peptides, giving the cortex a lower degree of crosslinking than the germ cell wall; (ii) about one-quarter of the NAM residues carry a single L-alanine modification not present in the glycan strands of the germ cell wall; and (iii) nearly every second muramic acid residue in the cortex PG is converted to muramic-δ-lactam (MAL) (17, 18), which seems to be the recognition substrate element for the CLEs which uniquely hydrolyze the PG cortex, but not the germ cell wall during spore germination (19, 20). The germ cell wall underlines the spore PG cortex, has no role in spore resistance and is converted into the growing cell wall during spore outgrowth. The spore inner membrane underlies the germ cell wall and is the last layer of protection of the spore core. The spore inner membrane is significantly compressed resulting in highly immobile lipids (12), resulting in a low permeability to small molecules including water and DNA damaging chemicals (12, 21).
The core is the inner-most layer of the C. perfringens spore and contains the spore DNA, RNA and most enzymes. Three major factors, including the low water content of the core (20-50% of wet weight), its high levels of Ca-dipicolinic acid (Ca-DPA) (25% of core dry weight) and the saturation of DNA with small acid soluble proteins (SASPs, discussed further below) together contribute to the resistance properties of these spores (22, 23).
Sporulation of Clostridium perfringens
To survive unfavorable conditions, C. perfringens initiates the process of sporulation by undergoing an asymmetrical division of its cytoplasm membrane. This process gives rise to two compartments, i.e., a small compartment (termed the forespore), and a large compartment (termed the mother cell), each with a complete genome. As sporulation progresses through a series of morphological and biochemical changes, the forespore becomes the mature C. perfringens spore that is eventually released to the environment upon lysis of the mother cell (24).
The sporulation process in spore-forming bacteria, including C. perfringens, is initiated by the integration of a wide range of environmental and physiological signals induced from changes in cell density, the Krebs cycle and nutrient starvation (25). In C. perfringens, initiation of sporulation requires the presence of inorganic phosphate (Pi) in the environment (26). In contrast, in sporulation medium containing Pi, C. perfringens was blocked at a very early stage of sporulation (i.e., the absence of polar septation and DNA partitioning) in cells reaching the stationary phase of growth (26). Importantly, Pi can neutralize the inhibitory effect of glucose at the onset of sporulation and induces spo0A expression, indicating that Pi acts as a key signal triggering spore-formation in C. perfringens (26). As introduced earlier, C. perfringens sporulation directly contributes to pathogenesis since sporulation leads to the synthesis of CPE and consequently to intestinal damage of epithelial cells (27). Coupling this finding with the fact that Pi is normally present in the GI tract of humans and animals, it appears that C. perfringens has efficiently adapted to sporulate in the GI tract. By extension, it can be speculated that Pi directly contributes to pathogenesis and survival of the progeny of C. perfringens type A food poisoning strains, i.e., it induces the production of CPE to cause diarrhea that disseminates metabolically dormant spores into the environment. These spores are able to withstand unfavorable conditions and remain viable for long periods of time.
Global regulation between the transition state and sporulation has not been studied in as great detail in C. perfringens as for the model sporulation system, B. subtilis. However, several studies have shown significant differences in the molecular regulation of sporulation between C. perfringens and B. subtilis (28, 29). As for B. subtilis, glucose has been found to act as a catabolic repressor of sporulation in C. perfringens (30). The transcriptional regulator carbon catabolite protein (CcpA) of the LacI/GalR family of repressor in C. perfringens (31) regulates many catabolite repressor effects from glucose. Interestingly, and in contrast to B. subtilis, CcpA is required for the efficient sporulation of C. perfringens (31). Initiation of sporulation in B. subtilis is mediated by the phosphorylation state of the master regulator of sporulation, i.e., the transcriptional factor Spo0A, which is present in all sequenced Clostridium species, including C. perfringens (32-35). Although the genome of CPE-negative C. perfringens strain 13 has a premature stop codon in spo0A (36), other C. perfringens isolates, including SM101 (a CPE-positive transformable derivative of a type A food poisoning strain), possess an intact spo0A gene (37). Evidence of a master regulatory role for Spo0A in C. perfringens sporulation was provided by a study showing that a spo0A knock out mutant of SM101 was unable to form spores. This spo0A phenotype was restored upon complementing the mutant with a recombinant plasmid carrying a wild-type spo0A copy (37). Interestingly, complementing the SM101 spo0A mutant with wild-type spo0A from other clostridial species revealed that Spo0A homologues can also induce the initiation of sporulation in enterotoxigenic C. perfringens (38).
The environmental signals that drive the initiation of sporulation are sensed by sporulation-specific orphan histidine kinases, which have not yet been identified in C. perfringens. Those histidine kinases integrate the sporulation signals and trigger a complex phosphorelay that increases the concentration of Spo0A in a phosphorylated state (Spo0A∼P) (39, 40). Once threshold levels of Spo0A∼P are reached, many genes (including those required for polar septum formation) become up- or down-regulated, leading to a series of biochemical and morphological events (41). For example, expression of small acid soluble protein-4 (SASP4, discussed later) and CPE by C. perfringens is dependent upon Spo0A (37, 42).
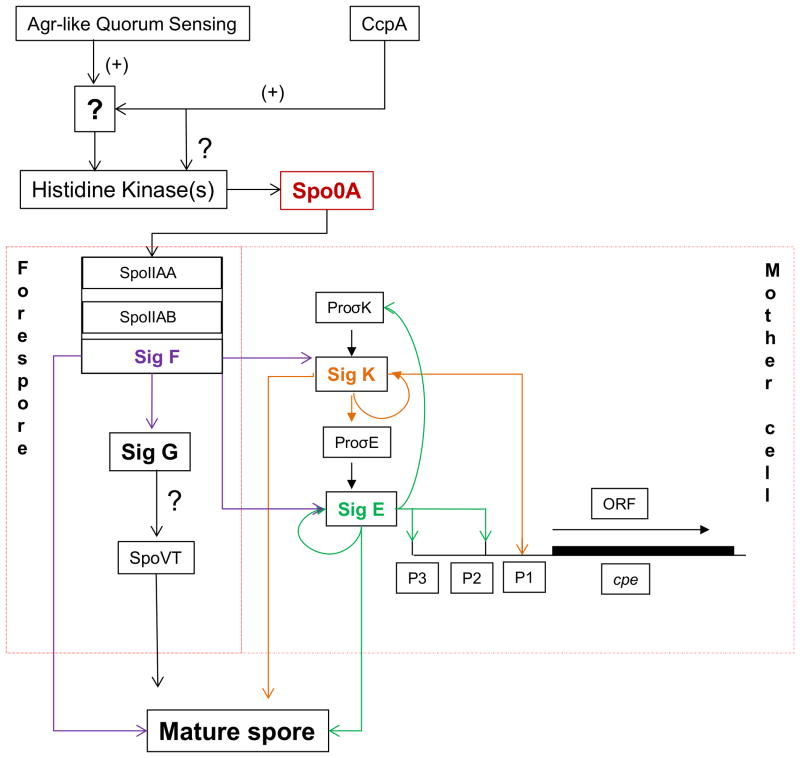
In C. perfringens, the morphological events during sporulation are divided into seven stages (I-VII), resembling those of sporulating B. subtilis cells. In B. subtilis, four major sporulation-specific sigma (σ) factors (σ F, σ E, σ G and σ K) regulate this sporulation process. The homologs of these genes are present in C. perfringens, where two recent studies (28, 29) demonstrated the expression and function of these sigma factors (Fig. 2). Those studies also presented evidence for both similarities and differences between the sporulation of C. perfringens and that of B. subtilis. Sporulation similarities between these bacteria include (i) Spo0A and SigF mediate control of expression of the other sporulation-associated sigma factors; (ii) SigG is expressed in the late sporulation stage; and (iii) sporulation requires production of all four sporulation-associated alternative sigma factors. Difference in sporulation include (i) C. perfringens lacks a B. subtilis-like phosphorelay and (ii) the expression of a key mother cell transcription factor (SpoIIID) depends on σE-associated RNA polymerase in B. subtlis, but not in C. perfringens.
Figure 2.
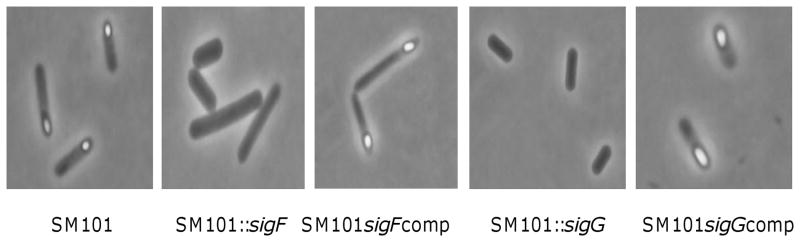
Sporulation-associated sigma factors are required for C. perfringens sporulation. Shown are photomicrographs of sporulating cultures of SM101, a transformable derivative of a food poisoning strain, after growth for 8 h in Duncan-Strong sporulation medium. Also shown is the absence of sporulating cells in similar Duncan-Strong cultures of a sigF or sigG null mutant of SM101 (SM101::sigF or SM101::sigG). This loss of sporulation was specifically due to inactivation of the sigF or sigG genes in those mutants since the effect was reversible by complementation, i.e., by adding back a wild-type sigF or sigG gene, respectively, to those mutants (SM101::sigFComp or SM101::sigGComp). Reproduced with permission from (28). Similar loss of sporulation was observed with sigE or sigK mutants of SM101 (29).
In detail (Fig. 3), C. perfringens transcribes sigF as part of a spoIIA tricistronic operon containing the sigF, spoIIAA and spoIIAB genes in the early sporulation stage (28). The bacterium then uses SigF to regulate the production of other sporulation-associated sigma factors (28). SigE and SigK, but not SigF or SigG, are initially made as inactive proproteins and then proteolytically-activated to their mature form (28, 29). Formation of C. perfringens mature spores requires expression of all four sigma factors.
Figure 3.
Sporulation in C. perfringens. Working through unidentified intermediates, the Agr quorum sensing system and CcpA affect Spo0A expression or, possibly, phosphorylation to initiate sporulation. This triggers a cascade of sigma factors where SigF controls production of the three other sporulation-associated sigma factors. Two of these sigma factors (SigE and SigK) then regulate CPE production during sporulation. Compiled from (28, 29, 31, 44). Not shown in this drawing, SigE (and possibly SigK) can also regulate production of TpeL toxin (97).
Most, if not all, C. perfringens strains possess (43-45) an Agr-like quorum-sensing (QS) system involving proteins homologous to, (i) AgrD, which is the putative precursor signaling peptide of this Agr system, and (ii) AgrB, a membrane protein that is thought to modify AgrD to the active form. A recent study (44) used an agrB mutant to demonstrate that the Agr-like QS system is also required for the initiation of sporulation in C. perfringens. Specifically, inactivating the agrB gene in C. perfringens strain F5603 decreased sporulation by ∼1,500 fold. Furthermore, inactivation of the agrB gene in F5603 results in reduced or lost expression of SigF and SigG, respectively, which are needed for sporulation. In addition, this agrB mutant produced less Spo0A, which is also necessary for C. perfringens sporulation; in fact, this reduced Spo0A production may explain why the agrB mutant produced reduced amounts of alternative sigma factors and sporulates poorly. Collectively, these results indicate that, in C. perfringens, the Agr-like QS system regulates sporulation at an early stage, i.e., by controlling Spo0A and SigF synthesis.
Recent studies identified two sporulation repressors, named virX mRNA and CodY protein (46, 47). The global regulator CodY repressed sporulation in a C. perfringens type D strain, and resulted in spores with lower germination ability (46). The virX gene encodes a regulatory RNA that significantly inhibits sporulation and CPE production in the type A SM101. Because transcription levels of sigE, sigF and sigK were higher in an isogenic virX-null mutant compared to wild-type SM101, it appears that virX RNA negatively regulates spore formation through the sporulation-specific sigma factors (47).
A wide variety of genes are expressed during sporulation in C. perfringens. Whole-genome expression profiling of the sporulation process in type A food poisoning strain SM101 was recently performed using DNA microarrays (48). This analysis revealed that a large number of genes showing sporulation-associated upregulated expression are homologs of known Bacillus genes involved in sporulation or germination. Expression levels of 106 SM101 genes exceeded 5 log2-fold increases during sporulation. Similarly, 294 SM101 genes showed up-regulation between 3 and 5 log2-fold increases and 451 genes were up-regulated at a lower level (2 to 3 log2-fold increases) in SM101 sporulation cultures.
Germination of C. perfringens spores
C. perfringens spores can remain in dormancy for extended periods of time and survive extreme environmental conditions (see next section). However, in the presence of favorable conditions they can return to life, with outgrowth in less than 20 min (19). Spore germination is also an important factor for C. perfringens food-borne disease transmission. From a practical food safety perspective, the process of germination is of considerable interest because, (i) germination of C. perfringens spores in food stuffs can lead to food poisoning; and (ii) upon germination, these spores lose their resistance and become susceptible to mild decontamination treatments. Therefore, understanding the molecular mechanism of C. perfringens spore germination might allow modulation of the germination process in foods by either inhibitors or artificial germinants that could allow the control of spore contamination loads with milder treatment conditions.
Germination is also important for transmission of other C. perfringens diseases. As already mentioned, spore germination in wounds can lead to clostridial myonecrosis. Furthermore, germination of C. perfringens spores in the intestines is presumably important during CPE-associated non-food-borne human GI diseases, since these illnesses are thought to be transmitted by ingestion of environmental spores. Those spores would need to germinate in the human intestinal tract before they could colonize and then cause disease.
Germination does not require metabolism and is initiated by small molecules called germinants, which can include amino acids, sugars, purines, nucleosides and salts (19, 49, 50). Germinant specificity can vary significantly between bacterial species and strains, and this selectivity is likely to be influenced by adaptation to specific environmental niches. Indeed, significant differences in specificity of germinants are observed between spores of C. perfringens food poisoning strains versus non-food-borne GI disease isolates. Spores of C. perfringens food poisoning isolates are able to germinate in the presence of KCl, NaPi (pH 6.0), L-asparagine or exogenous 1:1 chelate of Ca2+ and Ca-DPA, while spores of non-food-borne GI disease isolates initiate germination in the presence L-alanine, L-valine and with the mixture of KCl and L-asparagine (51, 52). Also, spores of a non-food-borne GI isolate germinated to a greater extent than spores of a food poisoning isolate in the presence of cultured intestinal epithelial cells (53). These results support the hypothesis that spores of food poisoning isolates have adapted to food niches (i.e., processed meat products) where nutrients like KCl and NaPi are highly abundant, while spores of non-food-borne GI disease isolate are better adapted to germinate in the host's intestinal epithelium environment.
In addition to recognizing the aforementioned germinants, bacterial spores in the host encounter several host-derived components that are capable of inducing germination. Among these are lysozyme, which is released by Peyer patches in the small intestine (54), present in the serum (55), and comprises part of the antibacterial arsenal of phagocytic cells. Lysozyme can trigger germination of spores of C. perfringens strain SM101 by directly degrading the spore peptidoglycan cortex (15). This germination pathway might have implications for the pathogenesis of C. perfringens (15), especially for superdormant spores that are extremely slow to germinate (56).
The germinant receptors (GRs) that recognize these nutrient germinants are relatively low abundance proteins that localize to the spore inner membrane and belong to the GerA family of GRs (19, 21, 49). In B. subtilis spores, three tricistronic operons (gerA, gerB and gerK) encode the three major GRs, with different receptors responding to different germinants (57). In contrast, C. perfringens has no tricistronic gerA-like operon and only a monocistronic gerAA that is far from the gerK locus. This gerK locus contains a bicistronic gerKA-KC operon and a monocistronic gerKB upstream of, and in the opposite orientation from, gerKA-KC (58, 59). Interestingly, the tricistronic gerA operons found in B. subtilis accounts for ∼ 50% of GRs, while the gerK locus found in C. perfringens accounts for nearly 5% of the GRs present in sequenced genomes of endospore-forming members of Bacillales and Clostridiales (49). In C. perfringens strain SM101, gene knock-out studies have identified the main receptors for L-asparagine, KCl, AK, and NaPi as the products of the bicistronic operon gerKA-KC, while the products of gerAA and gerKB play auxiliary roles in germination (51, 52, 60). Further gene knock-out and protein localization studies demonstrated that GerKC is the essential GR for germination of C. perfringens spores and also that GerKC is located in spore inner membrane (58).
GerKA-KC and GerKB receptors are also required for viability and outgrowth of C. perfringens spores (51, 60). Binding of nutrient germinants to GRs located in the spore's inner membrane triggers the release of monovalent ions (Na+ and K+) and the spore core's depot of dipicolinic acid as a 1:1 chelate with Ca2+ (Ca-DPA) is replaced by water (49). Although the precise mechanism of monovalent ion release remains unknown, GrmA-like antiporter homologues (named GerO and GerQ, which are not to be confused with the coat protein GerQ in B. subtilis and the germination receptor GerQ in B. anthracis) in C. perfringens strain SM101 were shown to be involved in transport of K+ and/or Na+, and GerO was also required for normal germination (61). However, because both GerO and GerQ are expressed in the mother cell compartment during sporulation, it is likely that their effect on spore germination is primarily during spore formation (61).
A major event after germinant nutrient binding to GRs is the release of the large deposit of Ca-DPA in the spore core (19). The precise mechanism of Ca-DPA release remains to be fully understood, although proteins encoded by the spoVA operon are thought to be involved in Ca-DPA movement (49). Instead of a hexacistronic spoVA operon as in B. subtilis, C. perfringens carries a tricistronic (spoVAC, spoVAD and spoVAE) spoVA operon (49). Interestingly, C. perfringens SM101 spoVA null mutant spores lacking DPA are stable and germinate well, suggesting that Ca-DPA is not required for either C. perfringens spore stability or signal transduction from GRs to downstream effectors as is the case in B. subtilis (49). This release of ions allows a slight hydration of the spore core that does not restore enzymatic activity but does lead to a decrease in spore wet heat resistance (19). Taken together, the aforementioned events constitute an initial stage of germination known as Stage I (19).
Once Ca-DPA is released from the spore core (signaling the start of Stage II of germination), a series of biochemical events take place, with the hallmark being the hydrolysis of the peptidoglycan cortex of the spore (19). The cortex, which acts as a strait jacket restricting spore core hydration and therefore expansion, is hydrolyzed by cortex lytic enzymes (CLEs) that will specifically degrade the cortex (19). Two CLEs are present in the C. perfringens spore; one of these, SleC, is synthesized as an inactive zymogen and is the sole essential CLE for cortex hydrolysis of C. perfringens food poisoning isolates. SleC is a bifunctional enzyme with lytic transglycosylase and N acetylmuramoyl-L-alanine amidase activity on cross-linked peptide moieties in the cortex (62). In contrast to the case of B. subtilis CLE CwlJ, which is activated by Ca-DPA released from the spore core (63), C. perfringens SleC is controlled by the Csp proteins (64) that belong to the subtilisin family of serine proteases. Csp proteins are localized in the cortex and activate cortex hydrolysis by converting pro-SleC to active SleC (64).
In contrast to SleC, the second CLE, SleM, is synthesized in a mature form with N-acetylmuramidase activity (65) and has little role in cortex hydrolysis of spores made by chromosomal cpe food poisoning isolates (66). Interestingly, complementation of a sleC mutant of a food poisoning isolate with wild-type sleC from a non-food-borne human GI disease isolate only partially restored the germination phenotype, suggesting that the precise role of both CLEs (i.e., SleC and SleM) in non-food-borne GI disease isolates might be different from that in chromosomal cpe food poisoning isolates (67). Significant differences between spores of both food poisoning and non-food-borne GI disease isolates also exist in regard to Csp proteins. While spores of chromosomal cpe food poisoning isolates possess only one Csp protein, CspB (64), spores of non-food-borne isolates have three Csp proteins (i.e., CspA, CspB, and CspC) encoded by a tricistronic operon (68). Studies with C. perfringens chromosomal cpe strain SM101 demonstrated that CspB alone is localized to the spore coat and alone is sufficient for converting pro-SleC to active SleC and activate cortex hydrolysis (58, 64). Degradation of the spore peptidoglycan cortex allows full core hydration, remodeling of the germ cell wall, resumption of metabolism, degradation of SASPs and complete loss of spore resistance properties (19).
Resistance properties of C. perfringens spores
One reason why C. perfringens is such a successful food-borne pathogen is because it can form resistant spores that allow survival in improperly held or incompletely cooked foods. Specifically, spores provide C. perfringens with resistance against such common food environment stresses as low or high temperatures, osmotic pressure, chemical preservatives and pH. For example, while vegetative cells of this bacterium cannot survive even brief exposure to 55°C, spores of some C. perfringens strains can survive boiling for an hour or longer (7, 69).
Importantly, C. perfringens spores exhibit significant strain-to-strain differences in their food environment stress resistance properties (7, 69-71). As mentioned earlier in this chapter, type A strains with a chromosomal cpe gene are strongly associated with food poisoning; it is those isolates that also typically form the most resistant spores, regardless of their geographic origin, date of isolation, or isolation source (72). For example, in terms of decimal reduction values (D100 value or the time that a culture must be kept at 100°C to obtain a one log reduction in viable spore numbers), the spores of type A chromosomal cpe food poisoning isolates are, on average, ∼60-fold-higher than the D100 values for spores of type A isolates carrying a plasmid cpe gene or cpe-negative type A strains. Notably, spores produced by some chromosomal cpe food poisoning isolates have D100 values exceeding 2 h (69).
Of epidemiologic significance, a survey detected both spores and vegetative cells of cpe-positive type A isolates in non-outbreak raw meats and seafood sold retail in the USA (73). Importantly, those cpe-positive type A retail food isolates all carried a chromosomal cpe gene. Furthermore, the spores made by each of these raw food isolates exhibited exceptionally strong heat resistance, indicating that the spore heat-resistant phenotype is an intrinsic trait of most type A chromosomal cpe isolates, rather than a survivor trait selected by cooking. This spore resistance phenotype should be an important virulence determinant since it likely favors survival of type A chromosomal cpe isolates in improperly warmed or incompletely cooked foods.
Storage of foods at low temperatures (in refrigerators or freezers) is another important food safety approach. The spores of most chromosomal cpe strains also show exceptional cold resistance compared with the spores of type A plasmid cpe strains or cpe-negative strains. For example, after a 6 month storage at 4°C or -20°C, the average log reduction in viability for spores of plasmid cpe or cpe-negative strains was about three to four-fold greater, respectively, compared against the average log reduction in viability of spores made by chromosomal cpe strains (71). These results suggest that the chromosomal cpe strains are strongly associated with food poisoning not only because of their exceptional spore heat resistance properties, but also because their spores are unusually tolerant of storage at low temperature (71).
Other factors besides temperature are also used to control the presence of pathogens in foods. For example, commercial curing of meats often involves use of sodium nitrite, which can inhibit outgrowth of clostridial spores or, at high concentrations, kill bacterial spores. One study (70) showed that the spores of type A chromosomal cpe isolates exhibit significantly better tolerance of, and survival against, nitrite-induced stress compared against the spores of other type A isolates. This nitrite resistance should further facilitate the ability of the chromosomal cpe, type A strains to cause foodborne illness.
Mechanisms of C. perfringens spore resistance
C. perfringens spore resistance depends upon a synergistic interplay of multiple factors, which include sporulation temperature, mineralization of the core with DPA and its cations, binding of α/β-type small soluble proteins (SASPs) to spore DNA, and core water content. Spore core water content is directly affected by sporulation temperature; a higher sporulating temperature produces C. perfringens spores with higher heat resistance. Spores of an spmA/B null mutant have more core water content, which directly reduces heat resistance by 50% (74). A spoVA null mutant makes spores with two-fold more core water than wild-type spores and those mutant spores also exhibit lower resistance to moist heat, UV radiation and chemical treatment (75). A possible explanation for this decreased resistance is that increased hydration decreases SASP binding to spore DNA (75). The degree of cross-linking of the spore PG cortex also plays a major role in C. perfringens spore resistance since dacF/B null mutant strains, which have significantly increased cross-linking of muropeptides, make spores with decreased heat resistance (74). However, the degree of cross-linking of muropeptides does not affect the core water content of C. perfringens spores (74).
SASPs bind to and saturate spore DNA, providing protection from various environmental stresses. The C. perfringens genome encodes four major α/β-type SASPs (i.e., SASP1, SASP2, SASP3 and SASP4). When SASP1, 2 and 3 levels in sporulating cells were reduced by >90% using antisense-RNA-mediated down regulation approaches, a 5-fold reduction in spore heat-resistance was observed (76).(73, 74) Those spores also showed greater sensitivity to chemicals (i.e., nitrous acid, hydrogen peroxide, formaldehyde and HCL) and UV radiation. However, SASP1, 2 and 3 levels could not explain the resistance differences observed between spores of type A chromosomal cpe isolates vs. spores of other C. perfringens since all strains produce similar amounts of SASP1, 2 and 3 and no consistent sequence variation in these proteins occurs amongst C. perfringens strains (76, 77). (73, 74)
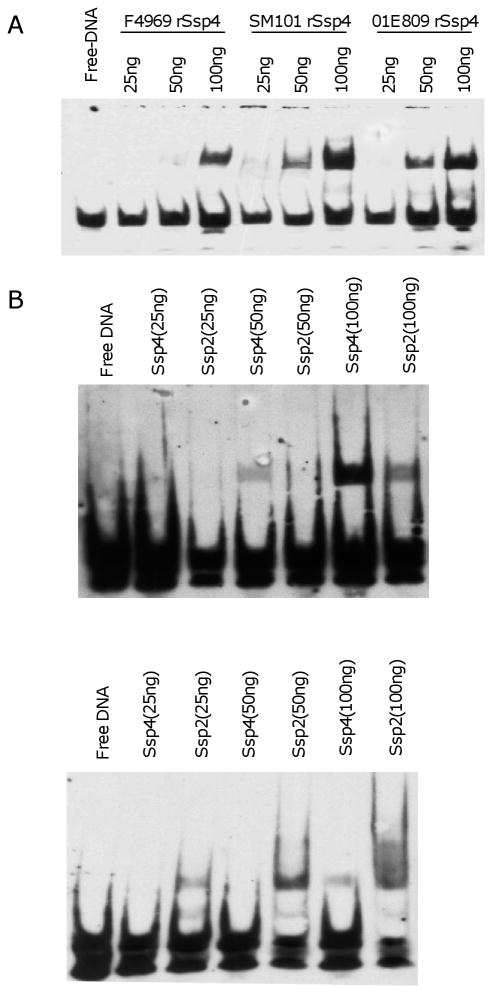
However, an Asp is found at residue 36 of the SASP4 made by most, if not all, of the type A chromosomal cpe isolates forming highly resistant spores (78). In contrast, Gly is consistently present at this SASP4 residue in those C. perfringens strains producing more sensitive spores. An important contribution of the Asp 36 SASP4 variant to the exceptional heat, sodium nitrite and cold resistance properties of spores made by most chromosomal cpe food poisoning strains has been directly demonstrated using ssp4 null mutants (78). Furthermore, electrophoretic mobility shift assays (EMSA) and DNA binding studies showed that SASP4 variants with an Asp at residue 36 binds DNA more efficiently and tightly than do SASP4 variants with a Gly at residue 36 (Fig. 4A). Results from saturation mutagenesis experiments (42) indicated that both amino acid size and charge at SASP4 residue 36 are important for tight DNA binding and for spore resistance properties. It was also shown that C. perfringens SASP4 binds preferentially to AT-rich DNA sequences, while SASP2 binds better to GC-rich DNA sequences (Fig. 4B). Since the C. perfringens genome is more than 70% AT rich, these binding preferences may help to explain why SASP4 plays such an important role in providing cold, heat and nitrite resistance by protecting spore DNA. However, maximal spore resistance requires production of all four C. perfringens SASPs.
Figure 4.
DNA binding properties of recombinant His6-tagged SASP4. A) Electromobility shift assays (EMSA) showing binding to biotin-labelled C. perfringens DNA by purified rSASP4 from F4969 (a CPE-positive nonfood-borne human GI disease strain that forms sensitive spores and produces an SASP4 variant with a Gly at residue 36), SM101 or 01E809 (two CPE-positive food poisoning isolates that form resistant spores and produce an SASP4 variant with Asp at residue 36). B) EMSA showing binding by purified SM101 rSASP4 or rSASP2 to (left) C. perfringens AT-rich biotin-labelled DNA or (right) biotin-labeled C. perfringens GC-rich DNA. Reproduced with permission from (42, 78).
While SASP4 variations are clearly a major determinant of relative stress resistance for C. perfringens spores, the ssp4 null mutant of a type A chromosomal cpe strain still showed somewhat more resistance than did wild-type spores of other type A isolates, indicating that other factors also contribute to the exceptional spore resistance associated with chromosomal cpe strains. Several studies (9, 10) have attempted to correlate structural features of C. perfringens spores with the striking heat resistance of chromosomal cpe positive isolates. Factors analyzed included the core, cortex, coat and total spore size (9, 10), but the most significant correlation noted between C. perfringens spore structure and heat resistance was for the ratio of core volume and core plus peptidoglycan layer, with a lower ratio giving higher heat resistance (10). However, in general, the ultra-structural features of C. perfringens spores are similar to those of other spore former species, so it is likely that the main differences involved in spore resistance differences between C. perfringens spores action at the molecular, rather than structural, level.
Multilocus Sequence Typing (MLST) analyses (79) of 8 housekeeping genes determined that chromosomal cpe isolates represent a distinct genetic cluster within the global C. perfringens population, i.e., these studies (72) identified many genetic differences in typical type A chromosomal cpe isolates besides their carriage of a chromosomal cpe gene, a variant ssp4 gene, and ability to produce highly resistant spores. These type A chromosomal cpe isolates have apparently now evolved to excel at causing food-borne disease.
Since those initial MLST studies, molecular analyses have also been performed on type C strains (7), the only non-type-A isolates that cause human enteritis necroticans. In post-World War II Germany, this disease was named Darmbrand, and recent molecular analyses (7) of these Darmbrand isolates showed they carry both beta (cpb) and cpe genes on large plasmids. Even though these Darmbrand isolates carry a plasmid-borne cpe, they produce highly heat-resistant spores. Interestingly, these type C Darmbrand strains produce the same variant Ssp4 made by chromosomal cpe strains. MLST analysis indicated that these type C Darmbrand strains and type A chromosomal cpe strains also share a similar genetic background. This helps to explain why both Darmbrand strains and type A chromosomal cpe strains are so well-suited to cause human foodborne illness, i.e., besides producing enterically-active toxins, they both produce spores that are highly resistant to food environment stresses so that those surviving spores can later germinate into vegetative cells that are ingested in foods. In terms of evolution, it is likely that these strains emerged by entry of different mobile genetic elements (conjugative plasmids and/or transposons) into C. perfringens strains with a similar background, including production of the Asp36 SASP variant.
Sporulation-associated toxins
Clostridium perfringens enterotoxin (CPE)
As already introduced, CPE-producing type A strains of C. perfringens cause the second most common bacterial food-borne illness in the USA, along with many cases of non-food-borne human GI diseases such as antibiotic-associated diarrhea. During this food poisoning (1), spores often germinate in temperature-abused foods, followed by rapid multiplication of the resultant vegetative cells in those contaminated foods (note that C. perfringens has a doubling time of only ∼10 minutes). After the food is ingested, many vegetative cells are killed by exposure to the low pH of the stomach. However, when a food was sufficiently contaminated, some ingested bacteria survive and pass into the small intestines. Initially these bacteria multiply, but they soon commit to in vivo sporulation, possibly when they encounter Pi in the intestinal tract. It is during this sporulation in the small intestines that CPE is produced during C. perfringens type A food poisoning.
Considerable evidence implicates CPE as the toxin responsible for the GI symptoms that characterize both C. perfringens type A food poisoning and CPE-associated non-food-borne human GI diseases (1). For example, ingestion of purified CPE plus bicarbonate was shown to be sufficient to induce diarrhea and cramping in human volunteers (1). Additionally, studies fulfilling molecular Koch's postulates demonstrated that CPE production is essential for the GI pathogenicity of CPE-positive type A human food poisoning and non-food-borne human GI disease isolates in animal models (27).
CPE can induce substantial small intestinal histologic damage, which includes villus blunting along with epithelial necrosis and desquamation (1). This damage is thought to cause intestinal fluid and ion loss, effects that manifest clinically as diarrhea (1). Evidence with experimental animals suggests that CPE can sometimes be absorbed into the systemic circulation, where it can bind to and damage the liver and other organs. These effects can lead to a lethal increase in serum potassium levels, which could explain some deaths associated with C. perfringens type A food poisoning (80).
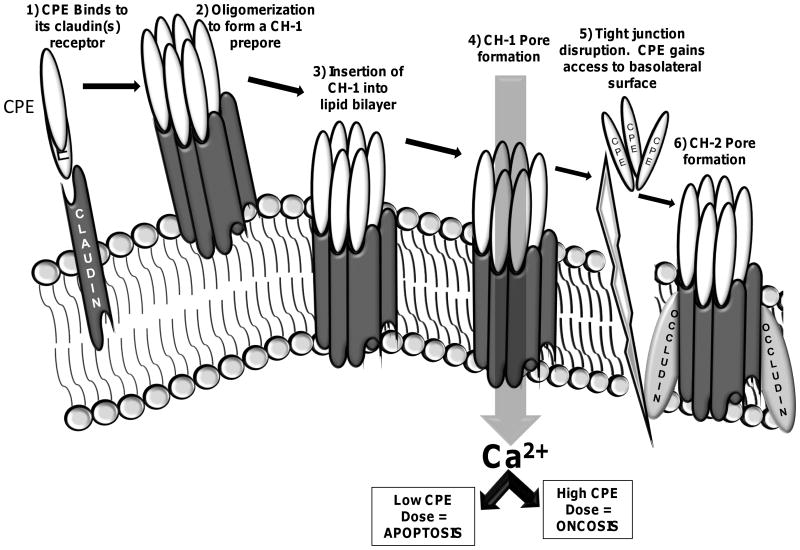
The histologic damage that occurs in the CPE-treated small intestine is a consequence of the cellular action of this toxin (Fig. 5). This action starts with CPE binding to receptors that include certain members of the claudin family of tight junction proteins (1). There are ∼ 27 members of the claudin family, but only some claudins can serve as CPE receptors (1). An Asn residue located in the middle of the second extracellular loop of receptor claudins in necessary for a claudin to bind CPE (81) and amino acid residues near this Asn residue modulate the affinity of CPE binding properties (82). Once bound to a claudin receptor, the toxin becomes localized in a small complex of ∼90 kDa that can contain, at minimum, a CPE, claudin receptor and a claudin non-receptor (1).
Figure 5.
Current model for the mechanism of action of CPE. CPE binds to claudin receptors to form small complexes. Those small complexes then oligomerize on the host cell surface to form an ∼450 kDa prepore known as CH-1. The prepore inserts into the membrane to form an active pore that alters host plasma membrane permeability for small molecules. As a result, calcium enters the cytoplasm and triggers either apoptosis (caused by low CPE doses, where there is a modest calcium influx) or oncosis (caused by high CPE doses, where there is a strong calcium influx). Reproduced with permission from (1).
At 37°C, several small complexes rapidly oligomerize to form a larger CPE complex named CH-1 (for CPE hexamer-1). CH-1 contains, at minimum, six CPE molecules as well as both receptor claudins and claudin nonreceptors (83). Formation of CH-1, which is ∼450 kDa in size, occurs on the host cell surface; however, once formed, this CH-1 prepore soon inserts into the plasma membrane to form a pore.
CPE pore formation increases the permeability of mammalian cells for the second messenger calcium, amongst other ions. Calcium influx plays a critical role in CPE-induced cell death (84). At low CPE doses, where small amounts of the CH-1 pore form, there is a modest calcium influx and host cells die by a classical caspase-3 mediated apoptosis. At high CPE doses, where more CH-1 pores form, a massive calcium influx triggers mammalian cell death via oncosis.
Substantial morphologic damage, such as cell rounding, develops in CPE-treated host cells. This effect damages the tight junction and exposes the basolateral surface of the cell, which allows formation of a second large CPE complex named CH-2. CH-2 is ∼600 kDa in size; like CH-1 it contains six CPE molecules and both receptor and nonreceptor claudins. However, CH-2 uniquely contains another tight junction protein named occludin (83). The consequences of CH-2 formation are less clear than for CH-1 formation but may include formation of additional pores, further damage to the tight junction by sequestering even more tight junction proteins in CPE complexes, and inducing the internalization tight junction proteins into the host cell cytoplasm.
The CPE protein is a single polypeptide consisting of 319 amino acids, with a molecular weight of ∼35 kDa. The structure of the toxin was recently solved and shown to consist of two major domains with a resemblance to the aerolysin-like pore forming toxin family (85, 86). When this structural information is collated with results from structure/function mutagenesis studies (87), it revealed that claudin binding activity is mediated by the C-terminal domain of CPE. This binding involves several tyrosine residues located near the extreme C-terminus of the toxin that interact with the ECL-2 loop of receptor claudin. In contrast, the N-terminal domain of the toxin, which consists of two halves, mediates both CPE oligomerization and pore formation. The extreme N-terminal sequences of the toxin are susceptible to cleavage by intestinal proteases such as trypsin or chymotrypsin and thus may be removed in the intestines during disease. Proteolytic removal of these sequences increases cytotoxicity by about 2 to 3-fold, as it exposes the CPE residues (notably residue D48) to promote toxin oligomerization (2).
During sporulation, some C. perfringens strains produce very large amounts of CPE, which can comprise up to 20% of the total protein present in a sporulating cell (2). The cpe gene is transcribed as a message of ∼1.2 kb, beginning ∼3 h after inoculation into Duncan-Strong sporulation medium (2). The CPE protein becomes detectable by Western blotting at 4-5 h post-inoculation into Duncan-Strong medium. CPE is not secreted from sporulating cells, but instead accumulates in the cytoplasm until the mother cell lyses to release the mature spore (2).
Regardless of whether an isolate carries a chromosomal cpe gene or a plasmid-borne cpe gene, CPE production is strictly sporulation-associated. Therefore, it is not surprising that a spo0A null mutant of SM101, which cannot sporulate, is unable to produce CPE (37). Similarly, the Agr-like quorum sensing system, which regulates sporulation in C. perfringens by reducing production of Spo0A, is also required for wild-type production levels of CPE (44). In contrast, virX RNA inhibits CPE production by repressing sporulation (47).
One reason for the strong, sporulation-associated expression of CPE by cpe-positive type A strains is the presence of three promoters upstream of the cpe ORF (Fig. 3). Two of those cpe promoters (named P2 and P3) are similar to consensus SigE dependent promoters that drive mother cell gene expression, while the other cpe promoter (named P1) resembles a consensus SigK-dependent promoter. Consistent with cpe transcription being dependent on these two sporulation-associated sigma factors, sigK- and sigE-null mutants of strain SM101 failed to drive beta-glucuronidase production when transformed with a plasmid carrying the cpe promoter region fused to the Escherichia coli reporter gene gusA (29). Another study (28) investigated the role of SigF and SigG in CPE production and reported that cpe transcription is also blocked in an SM101 sigF null mutant, but not in a SM101 sigG null mutant. The role of SigF in controlling CPE production can be explained by the dependence of SigE and SigK upon SigF expression. The ability of a sigG-null mutant to produce CPE indicates that, while all four sporulation-associated sigma factors are needed for C. perfringens sporulation, those sigma factors are not all necessary for cpe transcription and CPE production (28, 29).
TpeL toxin
Many C. perfringens isolates encode a novel toxin named TpeL. TpeL was initially identified in the supernatant of C. perfringens strain CP4 and shown to be cytotoxic to Vero cells by causing cell rounding (4). TpeL has a molecular mass of ∼205 kDa (88) and belongs to the family of large clostridial toxins (LCTs) that encompass Clostridium difficile toxin A (TcdA) and B (TcdB), along with similar toxins such as Clostridium sordellii lethal toxin (TcsL) made by other clostridial species (89). TpeL has no signal peptide region within the open reading frame (4).
Classically, large clostridial toxins were thought to contain four domains: i) A-domain: involved in N-terminal biological activity; ii) B-domain: C-terminal carbohydrate-binding repeats, often assumed to be involved in receptor binding; iii) C-domain: autoproteolytic cleavage during toxin-processing; and iv) D-domain: delivery of the A-domain into cytosol (89). LCTs enter host cells via endocytosis and insert via the D-domain into the endosome membrane. The protease C-domain is activated by intracellular inositolhexaphosphate (InsP6), resulting in toxin-cleavage and release of the A domain to the cytosol, where it glycosylates small GTPases inactivating their cellular functions.
Notably, TpeL has a shorter amino acid sequence than other LCTs, with homology encompassing to the N-terminal domain, including the DXD motif (essential for glycosyltransferase activity) and a conserved W102 (essential for enzymatic activity) (4, 89-91). Interestingly, despite the dogma that LCTs use their B-domain to bind to host cell membrane receptor(s) (89, 92), the carbohydrate binding repeats present in the C-terminal domain of other LCTs are absent from TpeL (88). Recently, this puzzle was resolved when a receptor binding domain was identified in the C-terminal half of the TpeL D domain (93). This region allows TpeL to bind to LDL receptor-related protein 1 (LRP1) as a receptor (93).
The tpeL gene is present ∼3 kb downstream of the beta toxin-encoding gene (cpb) on large plasmids in many C. perfringens type B and C strains (94, 95). The tpeL gene is also present in some type A isolates (96), although its location (plasmid vs. chromosomal) has not been determined in those strains. Studies have shown that tpeL is present, i) in ∼18% of type A necrotic enteritic outbreak isolates (96); ii) in ∼2% of C. perfringens isolates from retail chicken samples (96); and iii) in 100% and 75%, respectively, of type B and type C isolates (94, 95). The contribution of TpeL, when produced, to C. perfringens pathogenesis remains unclear.
Bioinformatic analysis of the tpeL promoter region on the 65-kb plasmid of C. perfringens type B strain ATCC 3626 revealed the presence of σE-and σK-dependent promoter sequences (97). In contrast, no sequence with similarity to the consensus OA box was found (97). Evidence that tpeL can be expressed during sporulation came from fusion of the tpeL promoter region with the Escherichia coli gusA reporter gene; when that construct was introduced into C. perfringens strain SM101, no significant beta-glucuronidase (GUS) activity was observed in the vegetative growth of SM101 transformants. However, GUS activity became significant in sporulating cultures of those SM101 transformants within as little as 4 h after initiation of sporulation (97). Sporulation-specific expression of tpeL was confirmed by introducing plasmids carrying the tpeL-gusA fusions into a SM101 spo0A mutant (37); no GUS specific activity was observed when this spo0A mutant carrying the tpeL-gusA fusion construct was grown under sporulation conditions, indicating that sporulation-regulated tpeL expression is dependent upon spo0A expression.
Evidence of σE-dependent expression of tpeL came from experiments that measured GUS specific activity in vegetative and sporulation cultures of a SM101 sigE mutant carrying the tpeL-gusA fusion construct (97). No significant GUS specific activity was observed during vegetative or sporulation growth of sigE mutant carrying the tpeL-gusA fusion construct (97). The expression of tpeL was only detectable in the mother cell compartment and at ∼80 to 150 fold lower levels than cpe expression (97). Given the presence of the putative σK-dependent promoter upstream of tpeL, it is possible that other σ factors such as σK might also be involved in regulating tpeL expression (97). Further detailed studies on tpeL promoter binding with Spo0A, σE and σK should clarify the mechanism of sporulation-regulated tpeL expression (97).
While the above evidence supports tpeL expression during sporulation, TpeL production by C. perfringens isolates during vegetative growth has also been reported (98, 99). Furthermore, those studies found repression of TpeL production mediated by glucose in a similar manner to LCT production by C. difficile. During vegetative growth, the regulator TpeR is critical for TpeL production, similar to the cases of C. difficile TcdR and C. sordellii TcsR.
Summary
The important pathogen C. perfringens utilizes its spores to survive in harsh environments. Spores are also important for transmission of this food-borne disease pathogen, particularly where type A chromosomal cpe food poisoning isolates often produce highly resistant spores that can survive in temperature-abused foods. Another linkage between sporulation and virulence for C. perfringens is the expression of two C. perfringens toxins from promoters recognized by sporulation-associated sigma factors. The production of one of those toxins, CPE, is essential for the pathogenesis of the second most common bacterial food-borne disease in the USA. Interestingly, another Gram-positive spore-former, B. thuringiensis, also produces sporulation-associated, pore-forming toxins that affect the gastrointestinal tract, albeit in insects rather than humans. The similarities between the pathogenesis of C. perfringens and B. thuringiensis suggest that these bacteria show a common strategy of producing toxins when sporulating in the GI tract of their host in order to induce diarrhea, which may then facilitate transmission of their spores back into the environment so they can be picked up by additional hosts. The prevalence of C. perfringens type A food poisoning suggests this strategy has been highly successful.
Acknowledgments
Preparation of this chapter was supported in part by AI019844-32 from the National Institute of Allergy and Infection Disease (To BMc) and by Department of Defense Multi-disciplinary University Research Initiative (MURI) award through the U.S. Army Research Laboratory and the U. S. Army Research Office under contract number W911NF-09-1-0286 (to MRS); and by grants from MECESUP UAB0802, the Fondo Nacional de Ciencia y Tecnología de Chile (FONDECYT Grant 1110569) and from the Research Office of Universidad Andres Bello (DI-35-11/R) (to D.P.-S)
Contributor Information
Jihong Li, Email: jihongli@pitt.edu, Department of Microbiology and Molecular Genetics, University of Pittsburgh School of Medicine, Pittsburgh, PA 15219, Phone: 412-648-9021, Fax: 412-624-1401.
Daniel Paredes-Sabja, Email: Daniel.paredes.sabja@unab.cl, Departamento de Ciencias Biológicas, Universidad Andrés Bello, Santiago, Chile, Phone: 56(02)7703225, Fax: 56(02)661 8421.
Mahfuzur R. Sarker, Email: sarkerm@oregonstate.edu, Department of Biomedical Sciences, College of Veterinary Medicine, Department of Microbiology, College of Science, Oregon State University, Corvallis, OR 15219, Phone: 541-737-6918, Fax: 541-737-2730.
Bruce A. McClane, Email: bamcc@pitt.edu, Department of Microbiology and Molecular Genetics, University of Pittsburgh School of Medicine, Pittsburgh, PA 15219, Phone: 412-648-9022, Fax: 412-624-1401.
References
- 1.McClane BA, Robertson SL, Li J. Clostridium perfringens. In: Doyle MP, Buchanan RL, editors. Food Microbiology: Fundamentals and Frontiers. 4th. ASM press; Washington, D.C.: 2013. pp. 465–489. [Google Scholar]
- 2.McClane BA, Uzal FA, Miyakawa MF, Lyerly D, Wilkins TD. The Enterotoxic Clostridia. In: Dworkin M, Falkow S, Rosenburg E, Schleifer H, Stackebrandt E, editors. The Prokaryotes. 3rd. Springer NY press; New York: 2006. pp. 688–752. [Google Scholar]
- 3.Mallozzi M, Viswanathan VK, Vedantam G. Spore-forming Bacilli and Clostridia in human disease. Future Microbiol. 2010;5:1109–1123. doi: 10.2217/fmb.10.60. [DOI] [PubMed] [Google Scholar]
- 4.Amimoto K, Noro T, Oishi E, Shimizu M. A novel toxin homologous to large clostridial cytotoxins found in culture supernatant of Clostridium perfringens type C. Microbiology. 2007;153:1198–1206. doi: 10.1099/mic.0.2006/002287-0. [DOI] [PubMed] [Google Scholar]
- 5.Stevens DL, Rood JI. Histotoxic Clostridia. In: Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI, editors. Gram-positive pathogens. 2nd. ASM press; Washington, DC: 2006. [Google Scholar]
- 6.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma M, Li J, McClane BA. Genotypic and phenotypic characterization of Clostridium perfringens isolates from Darmbrand cases in post-World War II Germany. Infect Immun. 2012;80:4354–4363. doi: 10.1128/IAI.00818-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carman RJ. Clostridium perfringens in spontaneous and antibiotic-associated diarrhoea of man and other animals. Rev Med Microbiol. 1997;8(supplement 1):S43–S5. [Google Scholar]
- 9.Novak JS, Juneja VK, McClane BA. An ultrastructural comparison of spores from various strains of Clostridium perfringens and correlations with heat resistance parameters. Int J Food Microbiol. 2003;86:239–247. doi: 10.1016/s0168-1605(02)00550-0. [DOI] [PubMed] [Google Scholar]
- 10.Orsburn B, Melville SB, Popham DL. Factors contributing to heat resistance of Clostridium perfringens endospores. Appl Environ Microbiol. 2008;74:3328–3335. doi: 10.1128/AEM.02629-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriques AO, Moran CP., Jr Structure, assembly, and function of the spore surface layers. Annu Rev Microbiol. 2007;61:555–588. doi: 10.1146/annurev.micro.61.080706.093224. [DOI] [PubMed] [Google Scholar]
- 12.Setlow P. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol. 2006;101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 13.Klobutcher LA, Ragkousi K, Setlow P. The Bacillus subtilis spore coat provides “eat resistance” during phagocytic predation by the protozoan Tetrahymena thermophila. Proc Natl Acad Sci USA. 2006;103:165–170. doi: 10.1073/pnas.0507121102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laaberki MH, Dworkin J. Role of spore coat proteins in the resistance of Bacillus subtilis spores to Caenorhabditis elegans predation. J Bacteriol. 2008;190:6197–6203. doi: 10.1128/JB.00623-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paredes-Sabja D, Sarker MR. Host serum factor triggers germination of Clostridium perfringens spores lacking the cortex hydrolysis machinery. J Med Microbiol. 2011;60:1734–1741. doi: 10.1099/jmm.0.031575-0. [DOI] [PubMed] [Google Scholar]
- 16.Popham DB, Bernhards CB. Spore peptidoglycan. In: Eichenberger P, Driks A, editors. The Bacterial Spore. ASM Press; Washington, DC: in press. [Google Scholar]
- 17.Warth AD, Strominger JL. Structure of the peptidoglycan of bacterial spores: occurrence of the lactam of muramic acid. Proc Natl Acad Sci USA. 1969;64:528–535. doi: 10.1073/pnas.64.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warth AD, Strominger JL. Structure of the peptidoglycan from spores of Bacillus subtilis. Biochemistry. 1972;11:1389–1396. doi: 10.1021/bi00758a010. [DOI] [PubMed] [Google Scholar]
- 19.Setlow P. Spore germination. Curr Opin Microbiol. 2003;6:550–556. doi: 10.1016/j.mib.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Makino S, Moriyama R. Hydrolysis of cortex peptidoglycan during bacterial spore germination. Med Sci Monit. 2002;8:RA119–RA127. [PubMed] [Google Scholar]
- 21.Moir A, Corfe BM, Behravan J. Spore germination. Cell Mol Life Sci. 2002;59:403–409. doi: 10.1007/s00018-002-8432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Setlow P. I will survive: DNA protection in bacterial spores. Trends Microbiol. 2007;15:172–180. doi: 10.1016/j.tim.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Setlow P. Spore resistance properties. Microbiol Spectrum. 2014;2(4):TBS-0003-2012. doi: 10.1128/microbiolspec.TBS-0003-2012. [DOI] [PubMed] [Google Scholar]
- 24.Labbe RG. Sporulation (Morphology) of Clostridium. In: Durre P, editor. Handbook on Clostridia. CRC Press; Boca Raton: 2005. pp. 647–658. [Google Scholar]
- 25.Stragier P. A gene odyssey: exploring the genomes of endospore-forming bacteria. In: Sonenshein AL, Hoch JA, Losick R, editors. Bacillus subtilis and its closest relatives: from genes to cells. ASM Press; Washington, D.C.: 2002. pp. 519–525. [Google Scholar]
- 26.Philippe VA, Méndez MB, Huang IH, Orsaria LM, Sarker MR, Grau RR. Inorganic phosphate induces spore morphogenesis and enterotoxin production in the intestinal pathogen Clostridium perfringens. Infect Immun. 2006;74:3651–3656. doi: 10.1128/IAI.02090-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarker MR, Carman RJ, McClane BA. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol Microbiol. 1999;33:946–958. doi: 10.1046/j.1365-2958.1999.01534.x. [DOI] [PubMed] [Google Scholar]
- 28.Li J, McClane BA. Evaluating the involvement of alternative sigma factors SigF and SigG in Clostridium perfringens sporulation and enterotoxin synthesis. Infect Immun. 2010;78:4286–4293. doi: 10.1128/IAI.00528-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harry KH, Zhou R, Kroos L, Melville SB. Sporulation and enterotoxin (CPE) synthesis are controlled by the sporulation-specific sigma factors SigE and SigK in Clostridium perfringens. J Bacteriol. 2009;191:2728–2742. doi: 10.1128/JB.01839-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shih NJ, Labbe RG. Effect of glucose on sporulation and extracellular amylase productio by Clostridium perfringens type A in defined medium. Curr Microbiol. 1994;29:163–169. [Google Scholar]
- 31.Varga J, Stirewalt VL, Melville SB. The CcpA protein is necessary for efficient sporulation and enterotoxin gene (cpe) regulation in Clostridium perfringens. J Bacteriol. 2004;186:5221–5229. doi: 10.1128/JB.186.16.5221-5229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bettegowda C, Huang X, Lin J, Cheong I, Kohli M, Szabo SA, Zhang X, Diaz LA, Jr, Velculescu VE, Parmigiani G, Kinzler KW, Vogelstein B, Zhou S. The genome and transcriptomes of the anti-tumor agent Clostridium novyi-NT. Nat Biotechnol. 2006;24:1573–1580. doi: 10.1038/nbt1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruggemann H, Baumer S, Fricke WF, Wiezer A, Liesegang H, Decker I, Herzberg C, Martinez-Arias R, Merkl R, Henne A, Gottschalk G. The genome sequence of Clostridium tetani, the causative agent of tetanus disease. Proc Natl Acad Sci USA. 2003;100:1316–1321. doi: 10.1073/pnas.0335853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers GS, Rasko DA, Cheung JK, Ravel J, Seshadri R, DeBoy RT, Ren Q, Varga J, Awad MM, Brinkac LM, Daugherty SC, Haft DH, Dodson RJ, Madupu R, Nelson WC, Rosovitz MJ, Sullivan SA, Khouri H, Dimitrov GI, Watkins KL, Mulligan S, Benton J, Radune D, Fisher DJ, Atkins HS, Hiscox T, Jost BH, Billington SJ, Songer JG, McClane BA, Titball RW, Rood JI, Melville SB, Paulsen IT. Skewed genomic variability in strains of the toxigenic bacterial pathogen, Clostridium perfringens. Genome Res. 2006;16:1031–1040. doi: 10.1101/gr.5238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nölling J, Breton G, Omelchenko MV, Makarova KS, Zeng Q, Gibson R, Lee HM, Dubois J, Qiu D, Hitti J, Wolf YI, Tatusov RL, Sabathe F, Doucette-Stamm L, Soucaille P, Daly MJ, Bennett GN, Koonin EV, Smith DR. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J Bacteriol. 2001;183:4823–4838. doi: 10.1128/JB.183.16.4823-4838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu T, Ohtani K, Hirakawa H, Ohshima K, Yamashita A, Shiba T, Ogasawara N, Hattori M, Kuhara S, Hayashi H. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc Natl Acad Sci USA. 2002;99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang IH, Waters M, Grau RR, Sarker MR. Disruption of the gene (spo0A) encoding sporulation transcription factor blocks endospore formation and enterotoxin production in enterotoxigenic Clostridium perfringens type A. FEMS Microbiol Lett. 2004;233:233–240. doi: 10.1016/j.femsle.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Huang IH, Sarker MR. Complementation of a Clostridium perfringens spo0A mutant with wild-type spo0A from other Clostridium species. Appl Environ Microbiol. 2006;72:6388–6393. doi: 10.1128/AEM.02218-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonenshein AL. Control of sporulation initiation in Bacillus subtilis. Curr Opin Microbiol. 2000;3:561–566. doi: 10.1016/s1369-5274(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 40.Kroos L. The Bacillus and Myxococcus developmental networks and their transcriptional regulators. Annu Rev Genet. 2007;41:13–39. doi: 10.1146/annurev.genet.41.110306.130400. [DOI] [PubMed] [Google Scholar]
- 41.Molle V, Fujita M, Jensen ST, Eichenberger P, González-Pastor JE, Liu JS, Losick R. The Spo0A regulon of Bacillus subtilis. Mol Microbiol. 2003;50:1683–1701. doi: 10.1046/j.1365-2958.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Paredes-Sabja D, Sarker MR, McClane BA. Further characterization of Clostridium perfringens small acid soluble protein-4 (Ssp4) properties and expression. PLoS One. 2009;4:e6249. doi: 10.1371/journal.pone.0006249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidal JE, Chen J, Li J, McClane BA. Use of an EZ-Tn5-based random mutagenesis system to identify a novel toxin regulatory locus in Clostridium perfringens strain 13. PLoS One. 2009;4:e6232. doi: 10.1371/journal.pone.0006232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Chen J, Vidal JE, McClane BA. The Agr-like quorum-sensing system regulates sporulation and production of enterotoxin and beta2 toxin by Clostridium perfringens type A non-food-borne human gastrointestinal disease strain F5603. Infect Immun. 2011;79:2451–2459. doi: 10.1128/IAI.00169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohtani K, Yuan Y, Hassan S, Wang R, Wang Y, Shimizu T. Virulence gene regulation by the agr system in Clostridium perfringens. J Bacteriol. 2009;191:3919–3927. doi: 10.1128/JB.01455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Ma M, Sarker MR, McClane BA. CodY is a global regulator of virulence-associated properties for Clostridium perfringens type D strain CN3718. MBio. 2013;4:e00770–e13. doi: 10.1128/mBio.00770-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohtani K, Hirakawa H, Paredes-Sabja D, Tashiro K, Kuhara S, Sarker MR, Shimizu T. Unique regulatory mechanism of sporulation and enterotoxin production in Clostridium perfringens. J Bacteriol. 2013;195:2931–2936. doi: 10.1128/JB.02152-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao Y, van Hijum SA, Abee T, Wells-Bennik MH. Genome-wide transcriptional profiling of Clostridium perfringens SM101 during sporulation extends the core of putative sporulation genes and genes determining spore properties and germination characteristics. PLoS One. 2015;10:e0127036. doi: 10.1371/journal.pone.0127036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paredes-Sabja D, Setlow P, Sarker MR. Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol. 2011;19:85–94. doi: 10.1016/j.tim.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Moir A, Cooper G. Spore germination. Microbiol Spectrum. 2014;3(5):TBS-0014-2012. doi: 10.1128/microbiolspec.TBS-0014-2012. [DOI] [PubMed] [Google Scholar]
- 51.Paredes-Sabja D, Torres JA, Setlow P, Sarker MR. Clostridium perfringens spore germination: characterization of germinants and their receptors. J Bacteriol. 2008;190:1190–1201. doi: 10.1128/JB.01748-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paredes-Sabja D, Udompijitkul P, Sarker MR. Inorganic phosphate and sodium ions are cogerminants for spores of Clostridium perfringens type A food poisoning-related isolates. Appl Environ Microbiol. 2009;75:6299–6305. doi: 10.1128/AEM.00822-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paredes-Sabja D, Sarker MR. Germination response of spores of the pathogenic bacterium Clostridium perfringens and Clostridium difficile to cultured human epithelial cells. Anaerobe. 2011;17:78–84. doi: 10.1016/j.anaerobe.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Mercado-Lubo R, McCormick BA. A unique subset of Peyer's patches express lysozyme. Gastroenterology. 2010;138:36–39. doi: 10.1053/j.gastro.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reitamo S, Klockars M, Adinolfi M, Osserman EF. Human lysozyme (origin and distribution in health and disease) Ric Clin Lab. 1978;8:211–231. [PubMed] [Google Scholar]
- 56.Ghosh S, Zhang P, Li YQ, Setlow P. Superdormant spores of Bacillus species have elevated wet-heat resistance and temperature requirements for heat activation. J Bacteriol. 2009;191:5584–5591. doi: 10.1128/JB.00736-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moir A, Smith DA. The genetics of bacterial spore germination. Annu Rev Microbiol. 1990;44:531–553. doi: 10.1146/annurev.mi.44.100190.002531. [DOI] [PubMed] [Google Scholar]
- 58.Banawas S, Paredes-Sabja D, Korza G, Li Y, Hao B, Setlow P, Sarker MR. The Clostridium perfringens germinant receptor protein GerKC is located in the spore inner membrane and is crucial for spore germination. J Bacteriol. 2013;195:5084–5091. doi: 10.1128/JB.00901-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Udompijitkul P, Alnoman M, Banawas S, Paredes-Sabja D, Sarker MR. New amino acid germinants for spores of the enterotoxigenic Clostridium perfringens type A isolates. Food Microbiol. 2014;44:24–33. doi: 10.1016/j.fm.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 60.Paredes-Sabja D, Setlow P, Sarker MR. Role of GerKB in germination and outgrowth of Clostridium perfringens spores. Appl Environ Microbiol. 2009;75:3813–3817. doi: 10.1128/AEM.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paredes-Sabja D, Setlow P, Sarker MR. GerO, a putative Na+/H+-K+ antiporter, is essential for normal germination of spores of the pathogenic bacterium Clostridium perfringens. J Bacteriol. 2009;191:3822–3831. doi: 10.1128/JB.00158-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumazawa T, Masayama A, Fukuoka S, Makino S, Yoshimura T, Moriyama R. Mode of action of a germination-specific cortex-lytic enzyme, SleC, of Clostridium perfringens S40. Biosci Biotechnol Biochem. 2007;71:884–892. doi: 10.1271/bbb.60511. [DOI] [PubMed] [Google Scholar]
- 63.Paidhungat M, Ragkousi K, Setlow P. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca(2+)-dipicolinate. J Bacteriol. 2001;183:4886–4893. doi: 10.1128/JB.183.16.4886-4893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paredes-Sabja D, Setlow P, Sarker MR. The protease CspB is essential for initiation of cortex hydrolysis and dipicolinic acid (DPA) release during germination of spores of Clostridium perfringens type A food poisoning isolates. Microbiology. 2009;155:3464–3472. doi: 10.1099/mic.0.030965-0. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y, Miyata S, Makino S, Moriyama R. Molecular characterization of a germination-specific muramidase from Clostridium perfringens S40 spores and nucleotide sequence of the corresponding gene. J Bacteriol. 1997;179:3181–3187. doi: 10.1128/jb.179.10.3181-3187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paredes-Sabja D, Setlow P, Sarker MR. SleC is essential for cortex peptidoglycan hydrolysis during germination of spores of the pathogenic bacterium Clostridium perfringens. J Bacteriol. 2009;191:2711–2720. doi: 10.1128/JB.01832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paredes-Sabja D, Sarker MR. Effect of the cortex-lytic enzyme SleC from non-food-borne Clostridium perfringens on the germination properties of SleC-lacking spores of a food poisoning isolate. Can J Microbiol. 2010;56:952–958. doi: 10.1139/w10-083. [DOI] [PubMed] [Google Scholar]
- 68.Shimamoto S, Moriyama R, Sugimoto K, Miyata S, Makino S. Partial characterization of an enzyme fraction with protease activity which converts the spore peptidoglycan hydrolase (SleC) precursor to an active enzyme during germination of Clostridium perfringens S40 spores and analysis of a gene cluster involved in the activity. J Bacteriol. 2001;183:3742–3751. doi: 10.1128/JB.183.12.3742-3751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarker MR, Shivers RP, Sparks SG, Juneja VK, McClane BA. Comparative experiments to examine the effects of heating on vegetative cells and spores of Clostridium perfringens isolates carrying plasmid genes versus chromosomal enterotoxin genes. Appl Environ Microbiol. 2000;66:3234–3240. doi: 10.1128/aem.66.8.3234-3240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Li J, McClane BA. Comparative effects of osmotic, sodium nitrite-induced, and pH-induced stress on growth and survival of Clostridium perfringens type A isolates carrying chromosomal or plasmid-borne enterotoxin genes. Appl Environ Microbiol. 2006;72:7620–7625. doi: 10.1128/AEM.01911-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li J, McClane BA. Further comparison of temperature effects on growth and survival of Clostridium perfringens type A isolates carrying a chromosomal or plasmid-borne enterotoxin gene. Appl Environ Microbiol. 2006;72:4561–4568. doi: 10.1128/AEM.00177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deguchi A, Miyamoto K, Kuwahara T, Miki Y, Kaneko I, Li J, McClane BA, Akimoto S. Genetic characterization of type A enterotoxigenic Clostridium perfringens strains. PLoS One. 2009;4:e5598. doi: 10.1371/journal.pone.0005598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wen Q, McClane BA. Detection of enterotoxigenic Clostridium perfringens type A isolates in American retail foods. Appl Environ Microbiol. 2004;70:2685–2691. doi: 10.1128/AEM.70.5.2685-2691.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paredes-Sabja D, Sarker N, Setlow B, Setlow P, Sarker MR. Roles of DacB and Spm proteins in Clostridium perfringens spore resistance to moist heat, chemicals, and UV radiation. Appl Environ Microbiol. 2008;74:3730–3738. doi: 10.1128/AEM.00169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paredes-Sabja D, Setlow B, Setlow P, Sarker MR. Characterization of Clostridium perfringens spores that lack SpoVA proteins and dipicolinic acid. J Bacteriol. 2008;190:4648–4659. doi: 10.1128/JB.00325-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raju D, Setlow P, Sarker MR. Antisense-RNA-mediated decreased synthesis of small, acid-soluble spore proteins leads to decreased resistance of Clostridium perfringens spores to moist heat and UV radiation. Appl Environ Microbiol. 2007;73:2048–2053. doi: 10.1128/AEM.02500-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raju D, Waters M, Setlow P, Sarker MR. Investigating the role of small, acid-soluble spore proteins (SASPs) in the resistance of Clostridium perfringens spores to heat. BMC Microbiol. 2006;6:50. doi: 10.1186/1471-2180-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J, McClane BA. A novel small acid soluble protein variant is important for spore resistance of most Clostridium perfringens food poisoning isolates. PLoS Pathog. 2008;4:e1000056. doi: 10.1371/journal.ppat.1000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miyamoto K, Li J, Akimoto S, McClane BA. Molecular approaches for detecting enterotoxigenic. Clostridium perfringens Res Adv Appl Environ Microbiol. 2009;2:1–7. [Google Scholar]
- 80.Caserta JA, Robertson SL, Saputo J, Shrestha A, McClane BA, Uzal FA. Development and application of a mouse intestinal loop model to study the in vivo action of Clostridium perfringens enterotoxin. Infect Immun. 2011;79:3020–3027. doi: 10.1128/IAI.01342-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robertson SL, Smedley JG, III, McClane BA. Identification of a claudin-4 residue important for mediating the host cell binding and action of Clostridium perfringens enterotoxin. Infect Immun. 2010;78:505–517. doi: 10.1128/IAI.00778-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shrestha A, McClane BA. Human claudin-8 and -14 are receptors capable of conveying the cytotoxic effects of Clostridium perfringens enterotoxin. MBio. 2013;4:e00594–e12. doi: 10.1128/mBio.00594-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robertson SL, Smedley JG, III, Singh U, Chakrabarti G, Van Itallie CM, Anderson JM, McClane BA. Compositional and stoichiometric analysis of Clostridium perfringens enterotoxin complexes in Caco-2 cells and claudin 4 fibroblast transfectants. Cell Microbiol. 2007;9:2734–2755. doi: 10.1111/j.1462-5822.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- 84.Chakrabarti G, McClane BA. The importance of calcium influx, calpain and calmodulin for the activation of CaCo-2 cell death pathways by Clostridium perfringens enterotoxin. Cell Microbiol. 2005;7:129–146. doi: 10.1111/j.1462-5822.2004.00442.x. [DOI] [PubMed] [Google Scholar]
- 85.Kitadokoro K, Nishimura K, Kamitani S, Fukui-Miyazaki A, Toshima H, Abe H, Kamata Y, Sugita-Konishi Y, Yamamoto S, Karatani H, Horiguchi Y. Crystal structure of Clostridium perfringens enterotoxin displays features of beta-pore-forming toxins. J Biol Chem. 2011;286:19549–19555. doi: 10.1074/jbc.M111.228478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Briggs DC, Naylor CE, Smedley JG, III, Lukoyanova N, Robertson S, Moss DS, McClane BA, Basak AK. Structure of the food-poisoning Clostridium perfringens enterotoxin reveals similarity to the aerolysin-like pore-forming toxins. J Mol Biol. 2011;413:138–149. doi: 10.1016/j.jmb.2011.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao Z, McClane BA. Use of Clostridium perfringens enterotoxin and the enterotoxin receptor-binding domain (C-CPE) for cancer treatment: opportunities and challenges. J Toxicol. 2012;2012:981626. doi: 10.1155/2012/981626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guttenberg G, Hornei S, Jank T, Schwan C, Lü W, Einsle O, Papatheodorou P, Aktories K. Molecular characteristics of Clostridium perfringens TpeL toxin and consequences of mono-O-GlcNAcylation of Ras in living cells. J Biol Chem. 2012;287:24929–24940. doi: 10.1074/jbc.M112.347773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Belyi Y, Aktories K. Bacterial toxin and effector glycosyltransferases. Biochim Biophys Acta. 2010;1800:134–143. doi: 10.1016/j.bbagen.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 90.Busch C, Hofmann F, Gerhard R, Aktories K. Involvement of a conserved tryptophan residue in the UDP-glucose binding of large clostridial cytotoxin glycosyltransferases. J Biol Chem. 2000;275:13228–13234. doi: 10.1074/jbc.275.18.13228. [DOI] [PubMed] [Google Scholar]
- 91.Busch C, Hofmann F, Selzer J, Munro S, Jeckel D, Aktories K. A common motif of eukaryotic glycosyltransferases is essential for the enzyme activity of large clostridial cytotoxins. J Biol Chem. 1998;273:19566–19572. doi: 10.1074/jbc.273.31.19566. [DOI] [PubMed] [Google Scholar]
- 92.Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schorch B, Song S, van Diemen FR, Bock HH, May P, Herz J, Brummelkamp TR, Papatheodorou P, Aktories K. LRP1 is a receptor for Clostridium perfringens TpeL toxin indicating a two-receptor model of clostridial glycosylating toxins. Proc Natl Acad Sci USA. 2014;111:6431–6436. doi: 10.1073/pnas.1323790111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gurjar A, Li J, McClane BA. Characterization of toxin plasmids in Clostridium perfringens type C isolates. Infect Immun. 2010;78:4860–4869. doi: 10.1128/IAI.00715-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sayeed S, Li J, McClane BA. Characterization of virulence plasmid diversity among Clostridium perfringens type B isolates. Infect Immun. 2010;78:495–504. doi: 10.1128/IAI.00838-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chalmers G, Bruce HL, Hunter DB, Parreira VR, Kulkarni RR, Jiang YF, Prescott JF, Boerlin P. Multilocus sequence typing analysis of Clostridium perfringens isolates from necrotic enteritis outbreaks in broiler chicken populations. J Clin Microbiol. 2008;46:3957–3964. doi: 10.1128/JCM.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paredes-Sabja D, Sarker N, Sarker MR. Clostridium perfringens tpeL is expressed during sporulation. Microbial pathogen. 2011;51:384–8. doi: 10.1016/j.micpath.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 98.Chen J, McClane BA. Characterization of Clostridium perfringens TpeL toxin gene carriage, production, cytotoxic contributions, and trypsin sensitivity. Infect Immun. 2015;83:2369–2381. doi: 10.1128/IAI.03136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carter GP, Larcombe S, Li L, Jayawardena D, Awad MM, Songer JG, Lyras D. Expression of the large clostridial toxins is controlled by conserved regulatory mechanisms. Int J Med Microbiol. 2014;304:1147–1159. doi: 10.1016/j.ijmm.2014.08.008. [DOI] [PubMed] [Google Scholar]