Abstract
Learned helplessness, the failure to escape shock induced by uncontrollable aversive events, was discovered half a century ago. Seligman and Maier (1967) theorized that animals learned that outcomes were independent of their responses—that nothing they did mattered – and that this learning undermined trying to escape. The mechanism of learned helplessness is now very well-charted biologically and the original theory got it backwards. Passivity in response to shock is not learned. It is the default, unlearned response to prolonged aversive events and it is mediated by the serotonergic activity of the dorsal raphe nucleus, which in turn inhibits escape. This passivity can be overcome by learning control, with the activity of the medial prefrontal cortex, which subserves the detection of control leading to the automatic inhibition of the dorsal raphe nucleus. So animals learn that they can control aversive events, but the passive failure to learn to escape is an unlearned reaction to prolonged aversive stimulation. In addition, alterations of the ventromedial prefrontal cortex-dorsal raphe pathway can come to subserve the expectation of control. We speculate that default passivity and the compensating detection and expectation of control may have substantial implications for how to treat depression.
Keywords: Learned Helplessness, Dorsal raphe nucleus, Ventromedial Prefrontal Cortex, Depression, Hope
In the early 1960s, Richard Solomon and his students at the University of Pennsylvania wanted to know how prior Pavlovian fear conditioning would influence later instrumental learning. To find out they restrained dogs in a hammock and the dogs got 64 mild-moderate electric shocks to their back paws, each shock heralded by a tone. Twenty-four hours later the dogs were placed in a shuttlebox and were supposed to learn to escape shock by jumping a short barrier between the two chambers. The two-factor theory of avoidance learning predicted that turning on the fear-inducing tone in the shuttlebox would generate fear and accelerate jumping. But to the experimenters' annoyance, they could not test this because the dogs often failed to escape altogether in the shuttlebox and passively waited the shock out (Leaf, 1964; Overmier & Leaf, 1965).
We arrived at Penn as first year graduate students in 1964. We thought that a profound failure to escape was the phenomenon and we began to try to understand it. After fifty years of research we believe we finally do understand it and this paper presents the evolution and destination of our theory.
From the beginning we thought the phenomenon looked like helplessness, as first suggested by Overmier and Seligman in 1967. But what, we puzzled, could helplessness consist of? How did it come about? How could we test for it?
Defining Helplessness
The intuitive notion of helplessness entails, we reasoned, the belief that nothing one does matters. This decomposes into objective and subjective helplessness. More formally, an animal is objectively helpless with respect to an important outcome (O) such as shock offset if the probability of (O), given a response (R) is not different from the probability of (O) given the absence of that response (notR). When this is true of all responses, objective helplessness exists.
But being subjectively helpless is another matter. We theorized that helplessness was cognitive and that it was learned. The animal must “detect” the lack of contingency as defined above and so must have “expected” that in the future shock would be independent of its responses. This was a radical suggestion for the 1960s. The learning theories of that era held that organisms could only learn associations or pairings, for example a response paired with shock strengthened this association (acquisition) or a response paired with no shock weakened this association (extinction). The rationale for the narrow associationistic view stemmed from behaviorists' shunning cognitions in animals and it seemed that the integration of two conditional probabilities—the probability of shock given a response integrated with the probability of shock in the absence of that response and then generalized across all responses—must be highly cognitive. Importantly we called the theory “learned” helplessness, rather than “conditioned” helplessness, because integrating these two conditional probabilities did not seem compatible with the associationistic premise that both Pavlovian conditioning and instrumental learning held dear. Emblematic of the tension between learning theory and cognitive theory was an encounter at the Princeton conference in which we first laid the theory out to the major learning theorists (Maier, Seligman & Solomon, 1969): Richard Herrnstein, a prominent Harvard Skinnerian, retorted, “You are proposing that animals learn that responding is ineffective. Animals learn responses; they don't learn that anything.”
Operationalizing Learned Helplessness
The triadic design to be described operationalizes this definition of objective and subjective learned helplessness. In order to know that it is the non-contingency between responding and shock and not the shock itself that produces later passivity, the non-contingency has to be isolated from the shock. So three groups are needed. One group gets escapable shock (ESC) where shock offset is contingent on the animal's response. So in the original stressor controllability experiment (Seligman & Maier, 1967), this group of dogs learned in the hammock to press a panel with their noses to turn off each shock. The second group is yoked to the ESC group. In this initial experiment, on each trial the yoked group subjects received the average duration of shock that the ESC group produced on each trial. However, in most subsequent experiments described below the yoking was done on each trial for each pair of subjects (ESC and yoked), so that the 2 groups receive exactly the same duration, intensity, and pattern of shocks, but for the yoked subjects their responses have no effect on the shock. In this inescapable shock group (INESC) shock offset and all of the animal's response are non-contingently related. A third group (0) gets no shock.
The next day the animals are tested in a very different environment – shuttlebox escape – and the well-replicated result was that two-thirds of the animals from the INESC group failed to learn to escape, whereas 90% of the animals in both the ESC and 0 groups easily learned to escape. Importantly the ESC and the 0 group learned to escape equally well. We concluded from this result that the animals in the INESC group had learned in the hammock that shock offset was independent of their responses and when shock occurred the next day in the shuttlebox they expected that its offset would once again be independent of responding. This expectation undermined their trying (“response initiation”) to escape. The fact that the escapable shock group did not do better than the zero group and that the main effect was that the inescapable group did worse than both other groups strongly influenced our belief that helplessness had been learned. It should be noted that at almost exactly the same time Weiss (1968) used a similar paradigm to study the effects of coping on ulcer formation.
In one variant Maier (1970) found that the passivity was not a superstitiously acquired response. The contiguity-minded learning theorists countered the cognitive account by claiming that in the hammock, shock offset occasionally was paired with not moving in the INESC group and this “superstitiously” reinforced the association of not moving with shock offset. Hence in the shuttlebox the animals engaged in not moving and eventually shock went off – further strengthening the superstitious no-movement—shock-off association. So Maier ran a special escapable shock group in the hammock: For this group shock went off when the animal held still, explicitly reinforcing not moving—one step better than superstition. The cognitive theory predicted that these animals would not sit still in the shuttlebox since they had learned that they could control shock; whereas the associationistic theory predicted that they would show the competing response of “helplessness.” This was a crucial test of contiguity versus cognition and Maier found that this escapable shock group easily learned to escape in the shuttlebox by jumping the barrier. Hence we discarded contiguity accounts of helplessness.
This work led us to define a dimension that we called control over outcomes, with control being present whenever the probability of (O), given a response (R) is different from the probability of (O) given the absence of that response (notR). Clearly, the escapable subjects have control over an aspect of the aversive event (when each shock terminates), and the inescapable subjects do not. This was exactly why we used the triadic design and included the escapable subjects as a control group because it isolated the element of uncontrollability—if failure to escape in the shuttlebox was caused by learning uncontrollability, then this failure should not occur if uncontrollability is removed but shock stays constant. That is, we assumed that uncontrollability is the active ingredient in producing passivity, and that escapable subjects were later normal because they lacked this critical learning ingredient. The escapable group was thus really included as a control group.
We must mention that running dog experiments was a harrowing experience for both of us. We are both dog lovers and as soon as we could we stopped experimenting with dogs and used rats, mice, and people in helplessness experiments, with exactly the same pattern of results. The next section provides a brief summary of this early work.
Animals
Research with animals quickly switched from dogs to rodents, but using the same triadic design that compares exactly equal ESC, yoked INESC, and no shock. Behavioral work focused on two issues. The first issue was whether the later effects of IS are limited to the induction of passivity in tasks such as the shuttlebox. To summarize briefly, a wide range of other behavioral changes followed INESC including reduced aggression, reduced social dominance, reduced food and water intake, exaggerated attention to external cues, reduced preference for sweet tastes, potentiated fear conditioning, slowed fear extinction, neophobia, anxious behavior on measures such as juvenile social exploration, potentiated opioid reward, exaggerated stereotypy to stimulants, and others. Importantly, none of these occurred if the shocks were escapable (see Maier & Watkins, 2005, for a review). Clearly, some of these outcomes can be related to passivity but others cannot. Many of these other behaviors seem indicative of anxiety, by which we mean fear out of proportion to an existing threat—potentiated fear conditioning, reduced fear extinction, neophobia, reduced juvenile social exploration, avoidance of open arms on an elevated maze, etc. In this paper for simplicity, we will refer to this suite of changes as “passivity/anxiety,” but we emphasize that we are interested in all of the changes separately—but not for the purpose of the present theorizing.
The second issue concerned testing the learned helplessness hypothesis against a variety of alternative ideas that were developed to explain why the experience of INESC leads to later failure to learn to escape in a different environment and whether control/lack of control is the critical underlying dimension (summarized in Maier & Seligman, 1976). Most of these investigations were focused on why INESC produces consequences such as failure to learn to escape, not why ESC did not do so. Indeed, the ESC group was typically omitted as this was not deemed of interest. In one important exception, Volpicelli, Ulm, Altenor, and Seligman (1983) using a triadic design found that the ESC group was much superior to the zero group and the INESC group. During later inescapable shock, the ESC group which had first learned to bar press to escape shock continued to run in a shuttlebox during long duration inescapable shock. This learned “mastery” effect foreshadows the main findings of the neuroscientific work below, in which first learning about escapable shock inhibits the default response of passivity. The work of R. L. Jackson and T. Minor was an exception. They argued that INESC produces later behavioral changes because it produces intense fear during the INESC session. With ESC however, the organism has a “safety signal” in that proprioceptive and other feedback from the escape response is followed by a shock-free interval of time. Indeed, these stimuli are as far away from the next shock as possible, and such stimuli do become conditioned inhibitors of fear (Maier, Rapaport, & Wheatley, 1976). This was argued to greatly reduce the total fear experienced during the session, and therefore no later behavioral “symptoms” (Jackson & Minor, 1988; Minor, Trauner, Lee, & Dess, 1990; Weiss, 1971). Note that by this view there is no learning about uncontrollability or controllability, just Pavlovian processes and fear. As support, the addition of external stimuli such as a light or a tone occurring immediately after each INESC prevented the occurrence of later failure to learn to escape (Minor et al., 1990; Weiss, 1971).
The early 1970s witnessed the first research directed at understanding the neural basis of these phenomena. Of course, these investigations could use only the neuroscience tools then available, and for the most part they involved either the systemic or intracerebroventicular administration of various receptor agonists and antagonists, and a role for a number of receptors and their endogenous ligands (e.g., acetylcholine, norepinephrine, dopamine, serotonin, and adenosine) was suggested (Anisman, Remington, & Sklar, 1979; Glazer, Weiss, Pohorecky, & Miller, 1975; Petty & Sherman, 1979). Given the nature of these studies no particular circuitry or structures could be implicated. However, the work of J. Weiss was an exception. He and his colleagues showed that INESC depletes norepinephrine (NE) in the region of the locus coeruleus (LC), a brainstem cell cluster that provides NE to most of the forebrain. Locus coeruleus NE neurons express alpha-2 receptors on their soma and dendrites, and these are inhibitory autoreceptors. Thus, NE within the locus coeruleus restrains the activity of locus coeruleus neurons, and so depletion of NE within this structure actually increases the activity of NE neurons, and Weiss had shown this to be important in the development of learned helplessness, a phenomenon he called behavioral depression (see Weiss & Simson, 1988, for review). However, it was never entirely clear how or why increased activity of these neurons would produce the passivity or anxiety. In sum, by the mid-1980s, there were nascent neurochemical views, but their detailed mechanism(s) of operation were necessarily murky given the tools that were then available, and their relationship to behavioral explanations unclear.
Humans
From this point on, we each went off to do other things. Seligman began to study humans exclusively. The human work went in three directions.
First, guided by the original theory, the learned helplessness procedures were replicated in apparently analogous human settings (e.g., Hiroto & Seligman, 1975). In the triadic design, for example, one group of college students received loud noise that could be escaped by button pressing, a second group was yoked, and a third group received nothing. Then they went to a human shuttlebox in which moving the hand from one side to the other turned off the noise. As with dogs and rats, most of the people from the yoked group failed to escape in the shuttlebox, whereas people from the escapable group and the zero group escaped well in the shuttlebox. Importantly the same pattern in the shuttlebox emerged when preceded by solvable and unsolvable anagrams (and no anagrams) instead of loud noise. In these studies, subjective unsystematic reports occasionally revealed that people from the inescapable group said that “nothing worked so why try?”
The second direction that Seligman took explored and manipulated the explanations people made for the causes of their failure to escape in the inescapable group (Abramson, Seligman, & Teasdale, 1978; Alloy, Peterson, Abramson, & Seligman, 1984). In the “attributional reformulation” of learned helplessness, Abramson et al. (1978) claimed that inescapability itself was not sufficient to produce anything more than momentary helplessness. Rather, the explanations that subjects made of the causes of their helplessness predicted the time course and the extent of helplessness. Subjects who attributed their helplessness to permanent causes (e.g., these problems will always be unsolvable) would show long-term helplessness in that situation. In contrast subjects who attributed their helplessness to temporary causes (e.g., only verbal puzzles are unsolvable) would not show helplessness later in that situation. Subjects who attributed their helplessness to pervasive factors (e.g., most problems are unsolvable) would show passivity across situations, whereas subjects who attributed helplessness to local factors (e.g., this problem is unsolvable) would only show helplessness in the original situation. These predictions were tested and largely borne out (Alloy et al., 1984).
The third endeavor that Seligman pursued was the possibility that learned helplessness was a laboratory model of clinical depression (Seligman, 1974; Simson & Weiss, 1988). In the Diagnostic and Statistical Manual of the American Psychiatric Association Third Edition (DSM-III; American Psychiatric Association, 1980), and Fourth Edition (DSM-IV; American Psychiatric Association, 1994), major depressive disorder was diagnosed by the presence of at least 5 of the following 9 symptoms:
Sad mood
Loss of interest
Weight loss
Sleep problems
Psychomotor problems
Fatigue
Worthlessness
Indecisiveness or poor concentration
Thoughts of suicide
Learned helplessness in the laboratory – combining the animal and human experimental results – produced eight of the nine symptoms, with the only exception being suicide and suicidal thoughts—an unlikely symptom to be produced in the laboratory by mild aversive events. Not only did inescapable shock and noise produce the symptoms of depression, but the converse occurred as well: Depressed people, who had not received inescapable events, behaved in the laboratory as if they had—showing passivity in the shuttlebox and giving up on cognitive problems (Klein, Fencil-Morse, & Seligman, 1976; Klein & Seligman, 1976; Miller & Seligman, 1976). Overall learned helplessness by mapping into the symptoms of depression seemed like a plausible laboratory model.
After 2000, Seligman went in two further new directions: First he began to work on “Positive Psychology,” the study of the causes and consequences of positive events (Seligman & Csikszentmihalyi, 2000), among them having control as opposed to being helpless. Second, he began to work on “prospection,” the study of mental simulations and evaluations of possible futures, in contrast to the study of memory (the past) and perception (the present) (Seligman, Railton, Baumeister, & Sripada, 2013). As it turns out both these new directions are relevant to the unraveling of learned helplessness and we will return to positive psychology and prospection at the end of this paper.
Maier essentially switched fields, retrained, and went into neuroscience. As a neophyte neuroscientist he felt that issues of learned helplessness were too complicated to approach and he studied a variety of other phenomena. He eventually returned to learned helplessness and its neural basis. It is this body of work that illuminates uncontrollability much better than the original theory.
Here are the mechanisms that were assumed by the original theory:
First: DETECT: Animals DETECT the dimension of controllability and uncontrollability. (This was also called the dimension of contingency and non-contingency).
Second: EXPECT. Animals that DETECT uncontrollability EXPECT shock or other events to be once again uncontrollable in new situations and this undermines trying to escape in new situations.
This paper examines these two premises in the light of the neural evidence accumulated over the last two decades. We preview the new theory and its conclusions now to help the psychologically-minded reader go through the systematic neural evidence that follows.
First: PASSIVITY/ANXIETY. Aversive shock, among its other neural actions, activates serotonergic (5-HT) neurons in the dorsal raphe nucleus (DRN). The dorsal raphe nucleus sends 5-HT projecting neurons to numerous regions including the periaqueductal gray, striatum, and extended amygdala. 5-HT released in the periaqueductal gray and striatum acts at 5-HT receptors to inhibit active escape behavior, while 5-HT released in the amygdala acts at receptors to potentiate fear/anxiety. The intense activation of the dorsal raphe nucleus by shock sensitizes these neurons and this sensitization lasts for a few days and results in poor escape (passivity) and heightened anxiety. The excitation of the dorsal raphe nucleus is necessary and sufficient for passivity and heightened fear, these being mediated by 5-HT released in regions that proximately control their expression. The detection of uncontrollability is not necessary nor is it sufficient for passivity. This is caused by prolonged exposure to aversive stimulation per se.
Second: DETECT and ACT. When shock is initially escapable, the presence of control is DETECTed. This is accomplished by a circuit involving projections from the prelimbic region of the ventromedial prefrontal cortex (the PL) to the dorsal medial striatum (the DMS), and back. After detection of control, a separate and distinct population of prelimbic neurons are activated that here we call ACT. These neurons project to the dorsal raphe nucleus and inhibit the 5-HT cells that are activated by aversive stimulation, thus preventing dorsal raphe nucleus activation and thereby preventing sensitization of these cells, eliminating passivity and exaggerated fear. So it is the presence of control, not the absence of control, that is detected by prelimbic medial prefrontal circuits, leading to consequent prelimbic-mediated inhibition of stress-responsive brainstem structures such as the dorsal raphe nucleus. When these circuits are inactive the organism reacts passively and fearfully if the aversive event is prolonged.
Third: EXPECT. After the prelimbic-dorsal raphe nucleus ACT circuit is activated a set of changes that require several hours occurs in this pathway and involves the formation of new proteins related to plasticity. This is now a circuit that EXPECTS control. If the rat has previously had control, now even inescapable shock or other uncontrollable stressors activate this prelimbic- dorsal raphe nucleus pathway, which they would not otherwise do. Inescapable shock now activates the sensitized prelimbic- dorsal raphe nucleus pathway, which now operates as an EXPECT circuit. So, inescapable shock is not being detected as uncontrollable, but it is being responded to as if it were controllable. This bias to respond as if the shock was escapable we shall call an EXPECTATION of control. However, it should be clearly understood that this EXPECTATION may not be a cognitive process or entity as psychologists tend to view them. It is a circuit that provides an expectational function, in the sense that it changes or biases how organism's respond in the future as a consequence of the events that occur in the present.
The Neural Circuitry of Learned Helplessness
We now go through the neural circuitry dataset in detail using the PASSIVITY/ANXIETY, DETECT, ACT, and EXPECT terminology both to make the argument more easily understood and because we believe that it is a useful translation from the neural level of analysis to the psychological level of analysis. We are aware that any such translation is merely a hypothesis that can be tested and falsified.
By the mid-1990s it seemed that the neuroscience tools that had become available might allow a more detailed understanding of how the brain produces the behavioral consequences of uncontrollable aversive events. As noted above, a variety of neurotransmitters and receptors had already been implicated, but how the sequelae of inescapable shock are actually caused was obscure.
We state inclusion/exclusion criteria for any adequate neural learned helplessness study at the outset. A study must meet two criteria. First, control over the stressor must be manipulated to determine whether any neural change measured is indeed a consequence of the uncontrollability/controllability of the event. Otherwise, the measured change could be a simple consequence of the stressor per se. There are numerous consequences of exposure to an aversive event that are, in fact, not modulated by control (Helmreich et al., 1999; Maier, Ryan, Barksdale, & Kalin, 1986; McDevitt, et al., 2009; Woodmansee, Silbert, & Maier, 1993). Thus, it is not enough to compare only inescapable shock and non-shocked controls. In the research to be described below, rats are the subject and the response that the ESC subjects can perform to terminate each shock is the turning of a small wheel located on the front of the chamber. Of course, once having established that a particular outcome that follows a particular stressor is indeed a function of controllability, the triadic design may not then be needed in further studies designed to explore the mechanisms by which the incontrollable stressor produces behavioral outcomes. Second, the initial stressor must occur in an environment very different from the test environment since one of the hallmarks of learned helplessness is trans-situationality. When common cues are shared between the first environment and the test environment, processes such as fear conditioning could mediate the behavioral change. For example, there are a large number of reports under the label “biological mechanisms of learned helplessness” that have delivered inescapable gridshocks while the subjects are constrained to one side of a shuttlebox, and then escape learning is tested in that very same shuttlebox. Poor test shuttlebox escape learning could be mediated by fear conditioning to the shuttlebox environment, since freezing is a prominent fear response. Indeed, Greenwood, Strong, and Fleshner (2010) have shown this to be the case. In their studies, manipulations that reduce fear conditioning reduce the shuttle escape deficit when the prior inescapable shocks were administered in the shuttlebox, but not when they were administered outside the shuttlebox.
Passivity/Anxiety and the Dorsal raphe nucleus
It is, of course, difficult to know where to start in a search for the circuitry that mediates learned helplessness. Maier and his colleagues began by reasoning backwards from the behavioral sequelae of inescapable shock. As already noted, many of the behavioral consequences seemed to be captured as either inhibited fight/flight (poor escape, reduced aggression, reduced social dominance) or exaggerated fear/anxiety (decreased social investigation, potentiated fear conditioning, neophobia). By the mid-1990s there was quite a bit known about the neural circuitry that regulates fight/flight and fear/anxiety, and so this information could be used. Most behaviors and emotions are mediated not by a particular structure but rather by a circuit, so the idea was to identify structures that were the most proximal mediators of fight/flight and fear/anxiety, that is, the most efferent part of the circuit closest to the behaviors themselves. The most proximate mediator of fight/flight seemed to be the dorsal periaqueductal gray (dPAG), while the extended amygdala (bed nucleus of the stria terminalis, BNST, together with the amygdala proper) mediated fear/anxiety.
Serotonin (5-HT) and the Dorsal raphe nucleus
So it seemed as if the subjects that received inescapable shock later behaved as if they had inhibited dorsal periaqueductal gray function and exaggerated amygdala/BNST function. There is a structure—the dorsal raphe nucleus – that projects to both, inhibiting one and potentiating the other when it itself is activated. Activation of this structure might then recapitulate the behavioral pattern produced by inescapable shock. The dorsal raphe nucleus sends 5-HT projections to both the dorsal periaqueductal gray and to the amygdala, with 5-HT released in the dorsal periaqueductal gray inhibiting its function and 5-HT in the amygdala potentiating its function (see Graeff, Guimarães, De Andrade, & Deakin, 1996 for review).
Clearly, then, if inescapable shock were to produce a powerful activation of the dorsal raphe nucleus 5-HT neurons and lead to the release of 5-HT in structures such as the amygdala and dorsal periaqueductal gray, then this structure would hold the potential to be a crucial node in any learned helplessness circuit. It would also have to be true that escapable shock does not activate the dorsal raphe nucleus. Of course, it was necessary to investigate whether inescapable shock does not just activate it in some nonselective way, but rather that inescapable shock activates specifically 5-HT neurons. 5-HT containing cell bodies are largely localized to the raphe nuclei, with the dorsal raphe nucleus being the largest and providing much of the 5-HT innervation of forebrain and limbic structures. However, only roughly 1/3 of dorsal raphe nucleus neurons contain 5-HT, and so simply showing generalized activation is not enough. To approach this issue Grahn et al. (1999) labeled 5-HT cells in the dorsal raphe nucleus with an antibody directed at 5-HT. Then, subjects received escapable shock (ESC), yoked inescapable shock (INESC), or no shock, and the expression of markers for neural activation was examined (e.g., the expression of the protein product of the immediate-early gene c-fos) using immunohistochemistry specifically in the cells known to be 5-HT cells. Thus, she was able to show that inescapable shock activated the neurons in the dorsal raphe nucleus that contained 5-HT, and exactly equal escapable shock did not.
The technique of in vivo microdialysis allows the measurement of the levels of 5-HT in discrete brain regions in real-time in live, awake, behaving animals. The results were dramatic. Figure 1 shows the levels of 5-HT within the dorsal raphe nucleus during escapable and inescapable shock. The level of 5-HT within the dorsal raphe nucleus is a measure of dorsal raphe nucleus 5-HT neuronal activity since 5-HT is released within the dorsal raphe nucleus by axon collaterals when the neurons fire. First, baseline levels were measured before the stressors began. Both inescapable shock and escapable shock led to a rapid and large release of 5-HT. This elevated level of 5-HT within the dorsal raphe nucleus was maintained even after the session ended for the inescapable subjects. However, 5-HT dropped precipitously as the escapable subjects learned the instrumental wheel-turn escape response, even though the shocks continued. (We will ask below what made the 5-HT drop as the escapable subjects learned to escape.) Importantly, activation of dorsal raphe nucleus 5-HT neurons also occurs robustly during other essentially uncontrollable stressors such as social defeat (Amat, Aleksejev, Paul, Watkins, & Maier, 2010).
Figure 1.
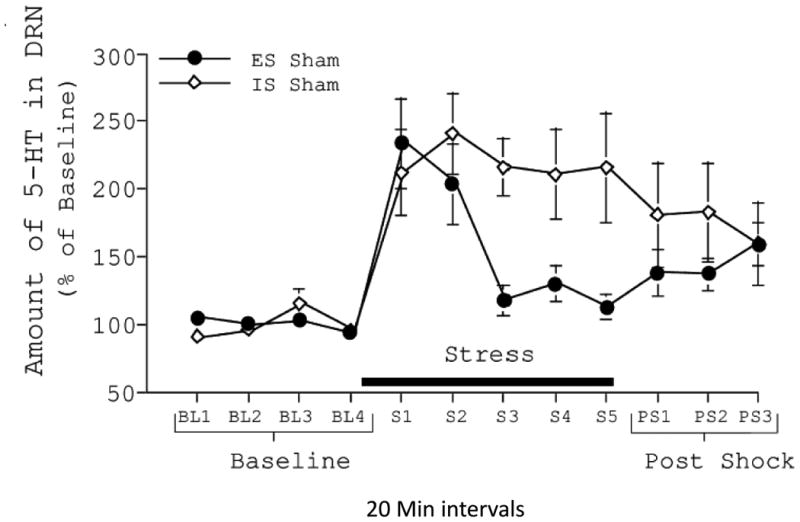
Levels of serotonin (5-HT) in the dorsal raphe nucleus (DRN) measured by in vivo microdialysis before, during, and after exposure to escapable (ESC) and yoked inescapable (IS) tailshocks. Level of 5-Ht is expressed as a percentage of baseline values, and the Baseline, during stress, and Post-Stress is measured in 20 min intervals. IN produced a sustained rise in levels of extracellular 5-HT, while levels during ESC dropped rapidly as the subjects learned the controlling response.
The failure to escape produced by inescapable shock occurs for some number of days (see below for discussion of time course), but the elevation in 5-HT within regions such as the amygdala does not persist for this period of time. How could elevated 5-HT within the amygdala be responsible for behaviors such as passivity and increased anxiety when 5-HT elevations do not persist until testing? A little more information about the dorsal raphe nucleus helps. Receptors of the 5-HT1A subtype are expressed on the soma and dendrites of 5-HT cells within the dorsal raphe nucleus. These are inhibitory autoreceptors – 5-HT binding to these receptors inhibits 5-HT neuronal activity. This 5-HT comes from axon collaterals from neighboring 5-HT cells within the dorsal raphe nucleus. Thus, the activation of a dorsal raphe nucleus 5-HT neuron can lead to the inhibition of its neighbors, and so dorsal raphe nucleus 5-HT activity is under self-restraint. Interestingly, these receptors are desensitized or downregulated by high levels of 5-HT. Thus, 5-HT released within the dorsal raphe nucleus during the strong dorsal raphe nucleus 5-HT activation produced by inescapable shock could desensitize these receptors, leading to a loss of the normal inhibitory restraint on these cells, thereby sensitizing them. Indeed, this is precisely what happens (Rozeske et al., 2011). Inescapable shock, but not exactly equal escapable shock, desensitizes these receptors so that dorsal raphe nucleus5-HT neurons are sensitized for a number of days and to a remarkably large extent. For example, inescapable shock reduces later social investigation of a juvenile, a putative measure of anxiety (Christianson, Paul, et al., 2008)). Placing a juvenile into an adult's rat cage, as is done in this test, produces no increase in 5-HT activity at all in control subjects. However, the mere presence of a juvenile leads to a large increase in 5-HT within the amygdala in a subject that has experienced inescapable shock, but not escapable shock previously (Christianson et al., 2010). Of course, the desensitization of 5-HT1A receptors is not permanent, and recovers to normal within 3 days (Rozeske et al., 2011). Importantly, behavioral sequelae of IS such as escape deficits and anxiety also persist for just this period of time (Maier, 2001).
Dorsal raphe nucleus Activation is Necessary and Sufficient for Passivity/Anxiety
The fact that uncontrollable stressors differentially activate and sensitize dorsal raphe nucleus 5-HT neurons does not mean that this process is either necessary or sufficient to produce the passivity and anxiety that follows inescapable shock. Three strategies have been adopted to determine necessity.
Blockade of the dorsal raphe nucleus activation produced by inescapable shock. Here, activation of the dorsal raphe nucleus during inescapable shock was prevented by either lesion (Maier et al., 1993; Will et al., 2004) or microinjection of pharmacological agents that prevent dorsal raphe nucleus 5-HT activation (Maier, Grahn, & Watkins, 1995; Maier, Kalman, & Grahn, 1994). These treatments all prevented inescapable shock from producing its usual poor escape and heightened anxiety, and these subjects behaved as did non-shocked controls.
Prevention of the desensitization of 5-HT1A receptors on dorsal raphe nucleus5-HT neurons produced by inescapable shock. Here an antagonist to the 5-HT1A receptor was microinjected into the dorsal raphe nucleus during inescapable shock, and as above these subjects behaved later as if they had not received the inescapable shock.
Blockade of 5-HT receptors in the dorsal raphe nucleus target regions during later testing. The argument is that failure to escape and increased anxiety occur because excessive 5-HT is released in critical target structures such as the amygdala during behavioral testing. Thus, blocking the receptors to which the 5-HT binds should eliminate the passivity and increased fear that typically occurs after inescapable shock. Indeed, microinjection of 5-HT2C antagonists directly into these structures does block the passivity and increased anxiety (Christianson et al., 2010; Strong et al., 2011).
Sufficiency of Dorsal raphe nucleus Activity for Passivity/Anxiety
With regard to sufficiency, simply activating the dorsal raphe nucleus by microinjecting agents into the dorsal raphe nucleus that activate 5-HT neurons should produce the same passivity and anxiety as does inescapable shock. Although there is less work directed at this issue, this appears to be the case. Direct activation of the dorsal raphe nucleus by microinjection of the GABA antagonist picrotoxin or the benzodiazepine receptor antagonist beta-carboline both produce the typical behavioral outcomes that are produced by inescapable shock (Maier, Grahn, Maswood, & Watkins, 1995; Short & Maier, 1993).
Learning: How and What does the Dorsal raphe nucleus Know?
The work above indicates that dorsal raphe nucleus 5-HT neurons are selectively activated if the shock is inescapable, and that this activation is necessary and sufficient to produce passivity and anxiety. But the key question is why the dorsal raphe nucleus responds only if the shock is inescapable. The most obvious option is that the dorsal raphe nucleus DETECTS the uncontrollability of the shock. To do so the dorsal raphe nucleus would have to extract the conditional probability of the shock offset given that the wheel turn or some other escape response occurs, and the conditional probability of the shock offset occurring in the absence of those responses, and compare these two probabilities. When the probabilities are equal the shock is uncontrollable. However, to do this, the dorsal raphe nucleus would require inputs informing it whether the motor responses have occurred and whether the shock is present or not, but the dorsal raphe nucleus does not receive these types of somatomotor and somatosensory inputs.
The next possibility is that the dorsal raphe nucleus receives greater excitatory inputs during inescapable than during escapable shock, thereby leading to more activation with inescapable shock. Indeed, a number of inputs to the dorsal raphe nucleus during stress have been discovered, but none provide more excitatory input during inescapable shock than during escapable shock. For example, recall that Weiss and his colleagues (Weiss & Simson, 1988) found that inescapable shock activates locus coeruleus norepinephrine (NE)-containing neurons. These project to the dorsal raphe nucleus, and consistent with the Weiss work, blockade of NEreceptors in the dorsal raphe nucleus with a microinjected antagonist during inescapable shock eliminated the passivity and anxiety (Grahn et al., 2002). However, both escapable and inescapable shock produced exactly equal levels of locus coeruleus NE activation (McDevitt et al., 2009). That is, although locus coeruleus input to the dorsal raphe nucleus was required for learned helplessness, both inescapable and escapable shock led to equivalent inputs to the dorsal raphe nucleus . Moreover, a similar pattern was found for several other inputs to the dorsal raphe nucleus occurring during the shock. So, the conclusion is that the dorsal raphe nucleus does not receive any heightened excitation from inescapable shock relative to escapable shock.
Learning: The Ventromedial Prefrontal Cortex does DETECT and EXPECT
In sum, a number of inputs to the dorsal raphe nucleus, using a number of different transmitters, was necessary to produce learned helplessness behaviors, but these inputs did not discriminate inescapable from escapable shock. If inescapable shock produces a much greater activation of dorsal raphe nucleus 5-HT neurons than does escapable shock, but both provide equivalent excitatory input, then there is only one obvious possibility left—the presence of control must somehow inhibit dorsal raphe nucleus 5-HT neurons that would otherwise be activated by shock per se without regard to controllability. The computational complexity of detecting the presence of control suggests a cortical process, and the dorsal raphe nucleus receives virtually all of its cortical input from the prelimbic region (PL) of the ventromedial prefrontal cortex (vmPFC) (Peyron, Petit, Rampon, Jouvet, & Luppi, 1997; Vertes, 2004). Importantly, electrical stimulation of the neurons that descend from the prelimbic area to the dorsal raphe nucleus inhibits dorsal raphe nucleus neuronal activity. Although these descending neurons are glutamatergic and so excitatory, they synapse preferentially on GABAergic interneurons in the dorsal raphe nucleus that inhibit the 5-HT cells (see Figure 2 for a cartoon). This arrangement leads to the hypothesis that escapability (control) is DETECTed by the ventromedial prefrontal cortex, and that the ventromedial prefrontal cortex then ACTs to inhibit shock-induced dorsal raphe nucleus activation. The dorsal raphe nucleus is a site of convergence that sums inputs from a number of structures themselves activated by shock (Figure 3). One idea is that these different inputs encode different aspects of aversive events, and so the more that are activated the more serious the threat. The dorsal raphe nucleus is important because it has this integrative function, and in turn projects to structures that are the proximate mediators of passivity/anxiety, our shorthand for the various behavioral and mood changes that follow inescapable shock. Thus, the dorsal raphe nucleus plays a role with respect to passivity somewhat analogous to that of the central nucleus of the amygdala in mediating fear. However, the dorsal raphe nucleus 5-HT neurons are under the inhibitory control of the prelimbic region of the ventromedial prefrontal cortex, and the detection of escapable shock activates this top-down inhibition of the dorsal raphe nucleus. We will return to a discussion of how this detection is accomplished.
Figure 2.
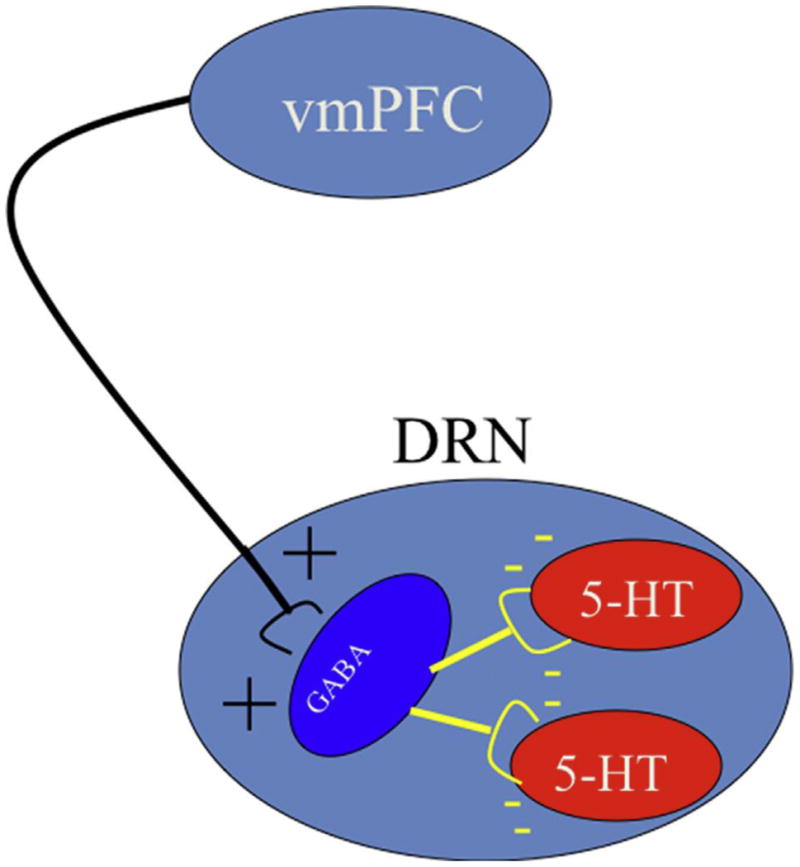
Schematic depiction of ventromedial medial prefrontal cortex (vmPFC) dorsal raphe nucleus (DRN) interactions. Excitatory glutamatergic projections from the vmPFC synapse onto inhibitory GABAergic interneurons within the DRN that inhibit the serotonin (5-HT) neurons.
Figure 3.
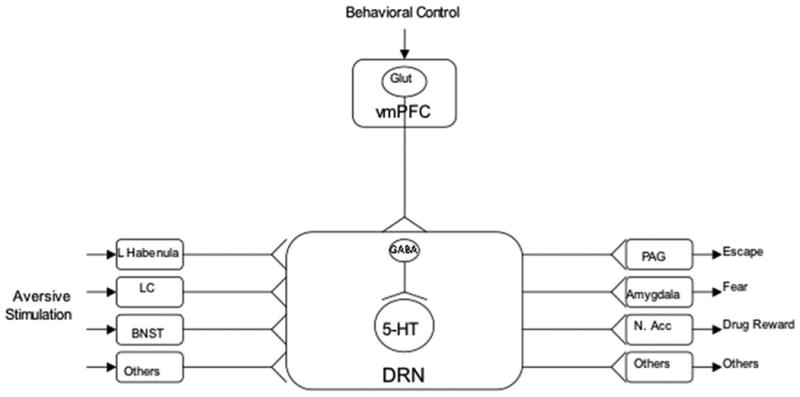
Schematic depiction of the proposed model. Serotonin (5-HT) neurons in the dorsal raphe nucleus (DRN) integrate stress-responsive inputs that encode different aspects of a stressor and then activate brain regions that are the proximate mediators of the behavioral effects of uncontrollable stress. Glut=glutamate; vmPFC=ventral medial prefrontal cortex; GABA=gamma aminobutyric acid; 5-HT=serotonin; DRN=dorsal raphe nucleus; habenula=habenula; LC=locus coeruleus; BNST=bed nucleus of the stria terminalis; PAG=periaqueductal gray; amygdala=amygdala; N. Acc.=nucleus accumbens.
Does the ventromedial prefrontal cortex actually regulate dorsal raphe nucleus activity and passivity as specified by this model (Figure 3)?
First, does the presence of escapable shock, but not inescapable shock, activate ventromedial prefrontal cortex neurons that project to the dorsal raphe nucleus? It would be easy to administer escapable shock, yoked inescapable shock, or no shock treatment and then determine whether the ventromedial prefrontal cortex is selectively activated by the escapable shock. However, most of the cells in the ventromedial prefrontal cortex have nothing to do with projections to the dorsal raphe nucleus, and so more is needed to indicate that the specific ventromedial prefrontal cortex pathways that project to the dorsal raphe nucleus are activated by escapable shock. To answer this question Baratta et al. (2009) microinjected a retrograde tracer into the dorsal raphe nucleus. Retrograde tracers are taken up by axon terminals within the dorsal raphe nucleus and transported back to the neuronal cell bodies. This labels all cell bodies in the ventromedial prefrontal cortex that project to the dorsal raphe nucleus. Baratta et al. (2009) then later administered escapable shock, inescapable shock, or no shock. It was then only necessary to determine whether the cells that were labeled as projecting from the ventromedial prefrontal cortex to the dorsal raphe nucleus were activated, which was done by examining within these labeled neurons the expression of markers of neuronal activation such as the immediate-early gene c-fos. Indeed, escapable shock but not exactly equal inescapable shock, increased c-fos protein in the labeled projecting neurons.
Second, is activation of this pathway necessary for escapable shock to reduce dorsal raphe nucleus activation and block the passivity and anxiety usually produced by inescapable shock? To answer this question Amat et al. (2005) inactivated the ventromedial prefrontal cortex-to-dorsal raphe nucleus pathway during the experience of escapable shock, inescapable shock, or no shock. This was done by microinjecting a pharmacological agent into the prelimbic area that inhibits the glutamatergic pyramidal neurons that project to the dorsal raphe nucleus (see Figure 4). The results were dramatic. Although the subjects with control learned the escape response perfectly, this learning was no longer protective—the dorsal raphe nucleus was activated as if the tailshocks were actually inescapable, and the subjects showed the passivity and heightened anxiety typical of exposure to inescapable shock. That is, inactivating the ventromedial prefrontal cortex-to-dorsal raphe nucleus pathway turned an animal with control into an animal without control.
Figure 4.
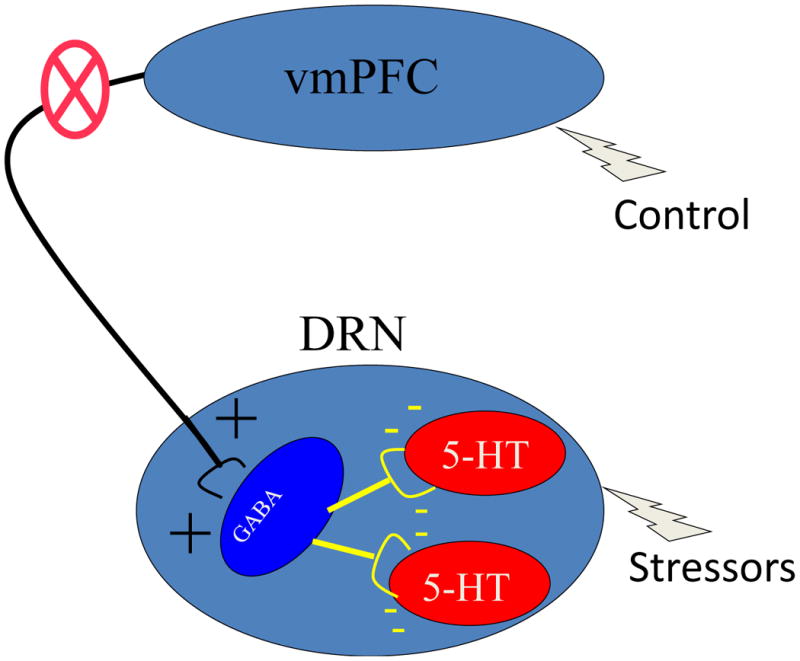
Schematic depiction of experimental strategy to determine whether activation of the ventromedial prefrontal cortex (vmPFC) to dorsal raphe nucleus (DRN) pathway is necessary for the presence of behavioral control to be protective. Blockade of the vmPFC to DRN pathway would prevent behavioral control from activating the inhibitory GABAergic cells that control the 5-HT neurons.
Third, is activation of this pathway sufficient for control to reduce dorsal raphe nucleus activation and block the passivity typically produced by inescapable shock? That is, does the organism actually need to escape at all, or is the mere activation of this pathway during the shock enough? To answer this question Amat, Paul, Watkins, and Maier (2008) activated this pathway directly with a microinjected pharmacological agent during the experience of inescapable shock. Now the dorsal raphe nucleus was inhibited as if the stressor was escapable, which it was not, and passivity was prevented. That is, activating the ventromedial prefrontal cortex-to-dorsal raphe nucleus pathway turned an animal without control into an animal with control.
Detection of control
So the presence of control activates descending inhibition of shock-induced dorsal raphe nucleus activation and thereby blocks passivity and anxiety. However, this does not mean that control is necessarily detected by the ventromedial prefrontal cortex in isolation, or by the ventromedial prefrontal cortex at all. Control could be detected by a different circuit, with this information then conveyed over to the ventromedial prefrontal cortex.
The clue to how Maier and his students proceeded with this issue came from the literature on the neural mechanisms underlying appetitive instrumental learning—for example a rat learning to press a lever for food. The history of psychology witnessed a debate as to whether instrumental learning involves the formation of a Stimulus-Response habit or instead a Response-Reinforcer expectancy. Neuroscience research indicates that each can take place and each involves different neural systems (Balleine & O'Doherty, 2010). One system, called the “act/outcome” system, is sensitive to the contingency between response and reinforcer. Contingency is “the difference between the probability of obtaining a target reward (r) given that a specific action (a) is performed and the probability of gaining the reward in the absence of the action” (Liljeholm, Tricomi, O'Doherty, & Balleine, 2011, p. 2474). The act/outcome system leads to “flexible” learning, and it is sensitive to contingency changes in the reward. A second system, the “habit” system, is a mere habit that is not sensitive to contingency but only to the temporal pairing between response and reward, and it produces inflexible learning that is not sensitive to changes in the contingency of the reward (Balleine & Dickinson, 1998). Importantly, the act/outcome system involves a corticostriatal circuit consisting of the prelimbic area within the ventromedial prefrontal cortex and the posterior dorsal medial striatum (DMS), while the habit system has no prefrontal cortical involvement, but instead involves the sensorimotor cortex and the dorsal lateral striatum (DLS). Thus, lesion, NMDA receptor blockade, and inactivation of either the prelimbic area or the dorsal medial striatum prevents contingency sensitive act/outcome learning. Responses can be learned, but only the habit system is then used, and so the learning is insensitive to contingency (Shiflett & Balleine, 2011).
Interestingly, this definition of instrumental contingency is identical to the definition of control that Maier and Seligman (1976) provided, although Maier and Seligman were referring to shock rather than food. Maier and Seligman defined control as being present whenever the conditional probability of the outcome (shock termination) after some response is different from the conditional probability of the outcome in the absence of that response, an identical formalism that provided for contingency in the appetitive instrumental learning literature 40 years later.
All this suggested that perhaps DETECTion of control over shock is done by a circuit involving the prelimbic area and the dorsal medial striatum, just as is instrumental appetitive contingency learning. As would be predicted, Amat et al. (2014) found that escapable shock, but not inescapable shock activates the contingency-sensitive dorsal medial striatum, but not the habit-oriented contingency-insensitive dorsal lateral striatum. The impact of inactivating the dorsal medial striatum during the experience of escapable and inescapable shock was even more intriguing. First, inactivating the dorsal medial striatum did not interfere with the learning and performance of the escape response. This suggests that the rats could use the “dumber” habit system to acquire and perform the escape response. Dramatically, even though the escapable subjects performed the controlling escape response perfectly, this was not protective against passivity/anxiety later. So when the habit system and not the contingency-sensitive system is used, detecting control seems to be absent. That is, the dorsal raphe nucleus was activated as if the shocks were uncontrollable and passivity/anxiety followed. Thus, it is not turning the wheel and actually escaping the shock by wheel turning that is necessary to prevent later passivity, but rather the detection of escapability by the prelimbic-dorsal medial striatum act/outcome system. As this conclusion also predicts, inactivating the dorsolateral striatum and the habit system did not reduce the protective effects of escapability. It might be noted that the precise mechanism by which the prelimbic-dorsomedial striatum circuit does the “detecting” is not known.
In sum, the prelimbic region of the ventromedial prefrontal cortex is involved in two separable functions-the DETECTion of control in a circuit with the dorsal medial striatum and then ACTing to inhibit the dorsal raphe nucleus (see Figure 5). Is it the same prelimbic neurons that are involved in both detection of contingency and ACTing on this information by transmitting it to the dorsal raphe nucleus and inhibiting it? To answer this question M. Barratta (unpublished) in Maier's group microinjected one color retrograde tracer in the dorsal medial striatum and a differently colored retrograde tracer into the dorsal raphe nucleus. These tracers were transported backwards along the neurons to the cell bodies of the neurons in the prelimbic area that project to these structures. If the same prelimbic neurons project to both regions then both colors should be present in the same cell bodies in the prelimbic area. There was no colocalization at all, indicating that these are separate populations of prelimbic neurons. Thus, DETECT escapability and ACT to pass this information that then inhibits the dorsal raphe nucleus are subserved by different populations of ventromedial prefrontal cortex cells and so are truly different functions.
Figure 5.
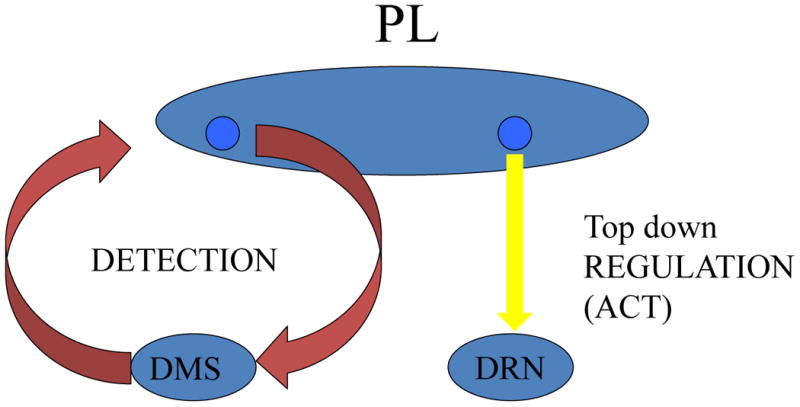
Schematic depiction of the role of the prelimbic cortex (PL) in mediating the impact of behavioral control. Separate systems are involved in the detection of control, and then acting on this detection. The detection circuit involves bidirectional flow between the dorsomedial striatum (DMS) and the prelimbic cortex while the action circuit consists of neurons that project from the PL to the dorsal raphe nucleus (DRN).
Immunization and EXPECTation
Initial exposure to escapable shock prevents or reduces the passivity/anxiety induced by later inescapable shock, a phenomenon we called immunization (Maier et al., 1969). Two features of immunization are essential. First, it is quite enduring, but perhaps not permanent. Second, it is trans-situational and so inescapable shock in one situation blocks the passivity/anxiety caused by even stressors that do not involve shock in different situations. For example, Amat et al. (2010) reported that experience with escapable shock blocked the passivity in shuttle escape and also blocked the reduced social investigation (anxiety) produced by social defeat occurring seven days later. Social defeat involves placing the experimental subject together with a larger and aggressive dominant subject. The experimental subject inevitably loses and adopts defeat postures, and so there is a strong element of uncontrollability. Here there is no shock at all, no restraint, the defeat is conducted on a different floor of the building by different experimenters, and yet escapable shock immunized against the effects of defeat. As would be expected, social defeat also increased dorsal raphe nucleus 5-HT activity, and this increase was prevented by the prior escapable shock (Amat et al., 2010).
Why does the inescapable shock (or defeat) fail to activate dorsal raphe nucleus 5-HT neurons and thus produce the typical passivity/anxiety if the organism has first experienced control over shock? Given the circuitry above, perhaps the uncontrollable shock (or defeat) now activates the prelimbic-to dorsal raphe nucleus inhibitory pathway, even though without the prior immunizing experience of escapable shock it would not do so. To determine whether this is the case, Baratta et al. (2009) microinjected a retrograde tracer in the dorsal raphe nucleus in order to label prelimbic neurons that project to the dorsal raphe nucleus. Recall that escapable shock but not inescapable shock activates these labeled cells, as assessed by examining activation markers such as c-fos in these labeled neurons. Dramatically, inescapable shock now does activate these cells as if the inescapable shock was escapable shock – if the organism had experienced immunizing control seven days earlier! Furthermore, when these prelimbic neurons were inactivated during the inescapable shock via the microinjection of inhibitory pharmacological agents, immunization no longer occurred (Amat, Paul, Zarza, Watkins, & Maier, 2006).
Thus, the experience of escapable shock (control) produced a specific and persistent change in the prelimbic-dorsal raphe nucleus circuit that led to the inhibition of the dorsal raphe nucleus and prevented passivity in response to even uncontrollable stress. The obvious possibility is that the activation of this pathway that occurs during escapable shock is sufficient to produce the persistent change. To test this idea Amat et al. (2006) activated this pathway directly by microinjecting a pharmacological agent with no actual escapable shock present, but this did not produce the persistent pathway change or produce immunization. They then reasoned that perhaps it is the joint activation of this pathway and the occurrence of the shock that is critical. To test this, the prelimbic-dorsal raphe nucleus pathway was activated pharmacologically during the occurrence of inescapable shock, the uncontrollable form of the stressor. Remarkably, now immunization occurred. That is, inescapable shock produces immunization as long as the prelimbic pathway is activated during shock.
How can this be understood? It is known that without prior escapable shock activation of the prelimbic-dorsal raphe nucleus ACT pathway requires the detection of control by the prelimbic-dorsal medial striatum DETECT circuit. We suspect that it also requires the presence of a potent aversive event, as there would be no reason to inhibit the dorsal raphe nucleus if there were not an aversive event present. This assertion could be tested, although the appropriate experiment has not yet been done. Plasticity, or increased connectivity between neurons at a synapse, typically occurs when both are activated together, as they say, “neurons that fire together wire together”. Thus, the joint occurrence of shock and control might induce increased connectivity so that later just the presence of the shock, without control, is sufficient to activate the pathway. Recent evidence supports this idea: 1) The development of persistent increases in connectivity requires the production of new proteins in the cells in question, and blockade of new protein synthesis in the prelimbic area after the escapable shock experience prevents immunization (Amat et al., 2006). That is, even though the subjects exert control and the prelimbic area is activated, immunization only happens if new proteins can be formed. Importantly, control still blunts the impact of the stressor being experienced, but longer-term immunization is eliminated; 2) The production of particular proteins, called plasticity proteins, is required for long term-increases in connectivity. Increases in these particular proteins (e.g., phosphorylated extracellular signal-regulated kinase) are indeed induced in the prelimbic region of theventromedial prefrontal cortex by escapable shock (Christianson et al., 2014); 3) Inhibitors of just these plasticity proteins prevent immunization when microinjected in the prelimbic area (Christianson et al., 2014); and 4) Direct electrophysiological measurement of projecting prelimbic neurons indicates that escapable shock, but not inescapable shock increases their excitability (Varela, Wang, Christianson, Maier, & Cooper, 2012). We conclude from this that the prelimbic-dorsal raphe nucleus ACT pathway can be modified over several hours after the joint experience of control and aversive stimulation, to respond to stressors in general as if they were controllable, and this is compatible with the idea that this altered pathway subserves the EXPECTation that shock will be controllable in new aversive situations. Thus, the same prelimbic-dorsal raphe nucleus pathway that operates as ACT can later operate as EXPECT.
Amygdala
The ventromedial prefrontal cortex projects to many structures other than the dorsal raphe nucleus. The amygdala is especially interesting in this regard. The role of the amygdala in fear and conditioned fear is well known. Briefly, the association between a stimulus predicting shock and the shock forms in basolateral regions of the amygdala. From there the information passes to the central nucleus of the amygdala, which in turns projects to the regions that control the behaviors and physiological responses that are the symptoms of fear (Davis, Rainnie, & Cassell, 1994; LeDoux, 2003; Maren & Quirk, 2004). For example, the central nucleus of the amygdala projects to regions of the periaqueductal gray that produce freezing, a behavioral component of conditioned fear. Both the prelimbic and the infralimbic region of the ventromedial prefrontal cortex project to parts of the amygdala. Of special note, the infralimbic region sends excitatory glutamatergic projections to a region of the amygdala known as the intercalated cell region. The cells in this region are GABAergic and project to and inhibit the central nucleus of the amygdala (Berretta, Pantazopoulos, Caldera, Pantazopoulos, & Pare, 2005). Thus, stimulation of the infralimbic region should inhibit fear expression, and it does (Sierra-Mercado, Padilla-Coreano, & Quirk, 2011). Since the prelimbic-dorsal medial striatum circuit DETECTS control, and since the prelimbic communicates with the infralimbic region of the ventromedial prefrontal cortex, Baratta et al. (2007) wondered whether the experience of control over an aversive event might reduce later fear in a different situation. Thus, subjects were exposed to escapable shock or yoked inescapable shock in the wheel-turn apparatus and given fear conditioning in standard conditioning chambers seven days later. Inescapable shock potentiated later fear conditioning, a well-known phenomenon (Rau, DeCola, & Fenselow, 2005). It would not have been surprising if initial control merely prevented this potentiating effect, but it did more than that: Instead prior escapable shock actually retarded fear conditioning and facilitated fear extinction. This indicated an EXPECTation of control over shock. Moreover, these effects of prior control depended on the ventromedial prefrontal cortex (Baratta, Lucero, Amat, Watkins, & Maier, 2008), showing that this structure exerts top-down inhibition of more than just the dorsal raphe nucleus, and the limits of this arrangement await further exploration.
Neurobiology of Human Control
Although there is a long history of research investigating the controllability dimension in humans, studies using methods that allow the measurement of neural activity are quite recent and few in number. A number of studies employing painful stimuli have found that providing control, or inducing perceived control, reduces the experienced intensity of the painful stimulus. Moreover, perceived control in these pain studies increases ventromedial prefrontal cortex activity (Salomons, Johnstone, Backonja, & Davidson, 2004). In the only relevant triadic design of which we are aware, Kerr, McLaren, Mathy, and Nitschke (2012) used exposure to snake videos to subjects with snake phobias. Each trial began with an anticipation period of variable duration in which a cue signaled that a snake video or a neutral fish video might follow. A second cue indicated whether the subject would or would not have control over whether the video would occur on that trial. After a variable period of time a target then occurred and the subject was instructed to press it as rapidly as possible. The video or a fixation point then appeared. On a controllable trial subjects were told that if they responded fast enough the fixation point rather than the video would appear, but if they were too slow they would see the video. On uncontrollable trials the subjects were told that no matter of how quickly they pressed, the video and the fixation point would each occur half the time, but subjects were asked to press as fast as possible anyway. However, the speed required on controllable trials was adjusted so that the subjects succeeded about half the time in avoiding the video, and so the actual frequencies on the uncontrollable trials was equated to this frequency. Thus, the controllable and uncontrollable trails were exactly yoked, as in animal studies. As expected, perceived control over the snake presentation reduced anticipatory anxiety on snake trials. Importantly, there was one condition that selectively excited ventromedial prefrontal cortex activity – snake controllable trials. Control did not increase ventromedial prefrontal cortex activity on neutral fish trials, even though the subjects pressed. Ventromedial prefrontal cortex activity was higher on controllable snake trials than in any of the other conditions. Finally, there was a negative relationship between ventromedial prefrontal cortex and amygdala activity on snake trials. These findings provide some support for generalizing the animal data reviewed above to humans.
Contrasting Psychological and Neural Explanations of Learned Helplessness
We believe that the neural explanations strongly inform the psychological explanations. We suspect that the learned helplessness work now provides a good, generalizable example of the complementarity between neural and psychological explanations. In the present case, the detailed knowledge concerning neural processes enabled the testing and major revision of the original psychological theory of learned helplessness—refinements that could not have happened without knowing the neural circuitry (examples below). On the other hand, the neural work would likely never have been done without the original behavioral work and psychological theorizing. Recall that the phenomenon that began this line of work was that exposure to aversive Pavlovian fear conditioning leads to later failure to learn instrumental escape/avoidance responses (Leaf, 1964). It was behavioral work and psychological theorizing that led to the isolation of behavioral control/lack of control as being the key feature of the Pavlovian conditioning that led to the failure to learn, and without this work there would not have been neuroscientific research directed at understanding the mechanisms that underlie controllability effects. The neuroscience circuitry work then clarified numerous issues (see below), but then translation back to psychological concepts also seems useful. As will be discussed below, the translation back to the psychological level enables the neuroscience work to potentially inform clinical practice.
Hypothesis Testing
First, Maier's group was able to test hypotheses that did not seem testable at the psychological level. The psychological theorizing concerning learned helplessness flowed from the triadic design that compared subjects with no control and those with control. The basic result was that the subjects without control later revealed passivity and a number of other behavioral changes, while those with control did not and appeared to be similar to non-shocked controls. Given this pattern we inferred that detecting and expecting a lack of control was the active ingredient. The non-difference between the zero group and the escapable shock group led us to believe that organisms expected controllability as the basic “default option”. Alternatively, it has been argued (e.g., Minor, Dess, & Overmier, 1991) that the reverse could be true, that stressors per se have deleterious effects, and that these effects could then be blocked when control was added as the active ingredient. However, it was difficult to separate these two possibilities with behavioral experiments, and the idea that uncontrollability was learned remained the dominant view.
The neural work allowed the testing of whether control is the active ingredient and lack of control is the default option, rather than the other way around as the psychological theory claimed. There are several key points. The neural evidence strongly suggests that activation and sensitization of the dorsal raphe nucleus leads to the passivity and anxiety characteristic of learned helplessness. Of course, inescapable shock produces a greater activation of the dorsal raphe nucleus than does controllable shock. But there were two obvious possibilities as to why: Is this differential activation because inescapable shock provides more excitatory input to the dorsal raphe nucleus than does escapable shock, or is it because escapable shock provides more inhibitory input to the dorsal raphe nucleus? Either would produce differential activation of the dorsal raphe nucleus by inescapable versus escapable shock and of course, both could be true. But the neural data are clear. Inescapable shock does not provide more excitatory input—both forms of shock produce equal excitation of the dorsal raphe nucleus. However, when shock is escapable this is DETECTed by the ventromedial prefrontal cortex-dorsal medial striatum circuit, and then the ventromedial prefrontal cortex ACTs, sending inputs to the dorsal raphe nucleus that inhibit it, thereby turning off the activation produced by shock per se. That is, there is nothing in the brain that is selectively turned on by a lack of control, only something that turns things off when there is the presence of control. So, aversive events per se (either controllable or uncontrollable) excite the dorsal raphe nucleus, but control over stress actively turns this off.
The reader may wonder why then in all the initial helplessness experiments the previously nonshocked group and the previously escapably shocked group performed equally well in shuttlebox escape. Recall that passivity/anxiety is explained by 5-HT accumulation during the testing in projection regions of the dorsal raphe nucleus that mediate these behaviors. If control leads to sensitization of the prelimbic-dorsal raphe nucleus pathway (EXPECT), then 5-HT activity should be inhibited from the start during shuttlebox escape testing in this group, but not in the non-shocked controls. It is easy to see why the inescapably shocked group should perform more poorly than controls—the dorsal raphe nucleus 5-HT neurons are sensitized in these subjects at the start of testing and so the aversive stimulus in the shuttlebox test (the gridshock) would lead to large and rapid 5-HT activation and consequent passivity/anxiety. But, if control leads to EXPECT (sensitized prelimbic-dorsal raphe nucleus inhibition), why should the escapably shocked subjects not perform better than controls that had not been previously stressed and so do not have EXPECT? The answer likely lies in an accidental feature of shuttlebox escape learning—it is learned very rapidly. Indeed, rodents escape with almost asymptotically fast latencies by the second or third trial (e.g., Grahn, Watkins, & Maier, 2000). This is likely because running is elicited as a species specific defense response (Bolles & Fanselow, 1980) on the very first trials. It is important to understand that 5-HT in response to aversive stimulation accumulates gradually across trials, and so the non-shocked controls learn control before 5-HT levels that could induce passivity have accumulated in regions such as the dPAG and striatum. This DETECTion of control, would, of course, inhibit the dorsal raphe nucleus. Even if there were a slight difference in 5-HT, the shuttle response is learned so rapidly that there is a ceiling effect. This argument would suggest that in tasks in which the non-shocked control is not at ceiling a difference between the previously escapably shocked and non-shocked controls might emerge, and this appears to be the case (Baratta et al., 2007).
A second theoretical advance came from the neural circuitry: We know that the part of the brain that DETECTs control is a circuit formed by the prelimbic area of the ventromedial prefrontal cortex and the dorsal medial striatum. When this system was inactivated so that control/lack of control information could not be detected and subjects were exposed to escapable shock or inescapable shock, the rats reacted to the shock as if it were inescapable – both in terms of passivity/anxiety and of neurochemistry. The data showed that all the animals, regardless of whether they had an escape response that they learned perfectly, acted later as if the stressor had been inescapable. That is, if the control detecting circuit was taken off line, all animals acted as if the shock was inescapable whether the animal actually was able to escape the shock or not. This suggests that if the DETECT control circuit is absent the animal invariably reverts to the default of helplessness following exposure to any prolonged stressor.
The neural circuitry also allowed the test of a competing theory of learned helplessness. It had been argued that the feedback from the escape response becomes a Pavlovian inhibitor of fear, a safety signal that reduces the total fear experienced and that it is this excess fear—if unreduced – that produces passivity (see above). There is no question that the presence of safety signals that predict a period of time free from shock can reduce the behavioral impact of aversive events. With knowledge of the underlying neural circuitry it became simple to ask whether safety signals blunt the impact of stressors via the same or a different mechanism. As discussed above, the escape response exerts its behavioral effects by activating ventromedial prefrontal cortex top-down inhibition of brainstem and limbic stress-responsive structures. It is straightforward to ask whether the protective effects of safety signals also requires the ventromedial prefrontal cortex, and the answer is no. For example, ventromedial prefrontal cortex lesions eliminated the ability of behavioral control to blunt the passivity and fear caused by inescapable shock, but these lesions did not even reduce the passivity and fear blunting impact of safety signals (Christianson, Benison, et al., 2008). Instead, safety signals had their impact via the insular cortex and insular cortex lesions eliminated the protective effects of safety signals. However, insular lesions did not reduce the passivity blunting effects of having an escape response, thereby demonstrating a double dissociation (Christianson, Benison, et al., 2008). Thus, control cannot be reduced to safety. This does not mean that safety signals are not stress-blunting, nor that safety signals do not have clinical uses, but only that stressor control and safety signals exert their effects via different neural mechanisms.
Another theoretical advance provided by the neural circuitry concerns understanding how experiences of control alter how organisms respond to future events. If the rats first experience is with escape the organism is immunized and reacts to subsequent stressors in new situations as if they are escapable. This suggests that the rat EXPECTs that shock will be escapable in the new situation and that plasticity in the prelimbic-dorsal raphe nucleus subserves this expectation and inhibits the dorsal raphe nucleus, thus blocking learned helplessness.
In addition to theory testing, knowledge of the underlying circuitry explained a number of learned helplessness phenomena that were simply mysteries at a psychological level. Here are two examples.
Time course of learned helplessness
The passivity produced by inescapable shock is transient, lasting for only a few days after the inescapable shock. If the behavioral effects of inescapable shock are mediated by the learned expectation that active responding will not produce relief, the original idea, then why this time course? No satisfactory explanation could be conjured at the psychological level. The neuroscience work predicts the time course. The passivity occurs because excessive 5-HT is released in projection regions of the dorsal raphe nucleus, and this occurs because dorsal raphe nucleus 5-HT neurons have become sensitized due to the desensitization of 5-HT1A receptors on the soma and dendrites of these cells. Thus, these behavioral changes should exist only as long as the receptors remain desensitized, which proved to be for only a few days (Rozeske et al., 2011).
The hypothalamo-pituitary-adrenal (HPA) response
The hypothalamo-pituitary-adrenal response begins with the production of corticotropin releasing hormone (CRH) in the paraventricular nucleus of the hypothalamus. Corticotropin releasing hormone travels to the anterior pituitary where it stimulates the production and release of adrenocorticotrophic hormone (ACTH) into the bloodstream. Adrenocorticotrophic hormone in turn stimulates the production and release of glucocorticoids (corticosterone in the rat, cortisol in humans) from the adrenal cortex into the blood. The hypothalamo-pituitary-adrenal response is often considered to be the hallmark of the bodily reaction to stressors, so it would be natural to assume that inescapable shock would produce a larger hypothalamo-pituitary-adrenal response than equated escapable shock. But, at least in the rodent, it does not (see Dess, Linwick, Patterson, Overmier, & Levine, 1983 for different results in dogs). In the rodent, the corticosterone rise is not greater or more prolonged (Helmreich et al., 2012; Maier et al., 1986), the adrenocorticotrophic hormone rise is not greater or more prolonged (Maier et al., 1986), nor is the increase in corticotropin releasing hormone in the hypothalamus larger (Helmreich et al., 1999). This unexpected finding has been inexplicable by psychological theory or behavioral considerations. Why should control modulate passivity, fear, and the neurochemical impact of a stressor but not the hypothalamo-pituitary-adrenal response? It follows from the circuitry. Retrograde and anterograde tracing studies indicate that the dorsal raphe nucleus does not send a major projection to the paraventricular nucleus of the hypothalamus, and perhaps none at all (Larsen, Hay-Schmidt, Vrang, & Mikkelsen, 1996). Thus, the dorsal raphe nucleus is not a major source of the hypothalamo-pituitary-adrenal activation during stress (Herman & Cullinan, 1997). The presence of control could reduce or inhibit paraventricular activation only if the structures that DETECT control project to the paraventricular nucleus. There is a pathway from the ventromedial prefrontal cortex to the paraventricular nucleus, but it goes through a relay in the bed nucleus of the stria terminalis, rather than directly (Radley & Sawchenko, 2011). We (Baratta (2015) thus utilized retrograde tracing techniques combined with the assessment of activation markers to determine whether the projections from the ventromedial prefrontal cortex to the bed nucleus of the stria terminalis are controllability-sensitive, and they are not. Thus, we would expect that control would not modulate the HPA axis response to stress because the paraventricular nucleus is not informed about controllability by the ACT circuit.
What Did Original Learned Helplessness Theory Get Right and What Did It Get Wrong?
On the positive side, we found that as the original theory claimed, organisms are sensitive to the dimension of control, and this dimension is critical. However, the part of the dimension that is detected or expected seems now to be the presence of control, not the absence of control. It also appears that the passivity and increased anxiety that follows uncontrollable stressors for several days is not produced by any expectancy at all, but rather is an unlearned reaction to prolonged aversive stimulation that sensitizes a specific set of neurons. Importantly, the presence of control aborts this process. However, expectancy does play a role, but it does in the immunization process. Here, an expectancy of control does blunt the impact of subsequent stressors. Clearly, the neurobiological data are at odds with the theory we held fifty years ago. When we first found that dogs given inescapable shock later failed to learn to escape in a shuttlebox, but that dogs given exactly equated escapable shock later escaped normally, the ideas that we developed were shaped by thinking of what might be most adaptive for dogs, rats and people. We reasoned that active coping is generally best because this would minimize exposure, and so we assumed that organisms would initially expect control to be possible. If the stressor proved to be uncontrollable, organisms would then learn this and expect it to be true in related situations in the future, with this expectation of uncontrollability undermining trying active coping. However, the neural basis of inescapable and escapable shock effects does not support this general schema. Instead, the presence of control seems to be the active ingredient, leading to the inhibition of threat-induced changes in limbic and brainstem structures.
Perhaps this counterintuitive arrangement becomes more intelligible if one considers our phylogenetic ancestors. In primitive organisms threats engage defensive reflexes (Walters & Erickson, 1986). However, these reflexes are energy intensive, and so if unsuccessful it might be adaptive to inhibit them and conserve energy for use in physiological adjustments that promote survival, such as altering the responsivity of the immune system to be better able to fight any infection or wound that might occur after an attack (Frank, Watkins, & Maier, 2013). Primitive organisms do not have the sensory apparatus to detect threats at a distance, nor do they have a complex behavioral repertoire that can be used for what we are calling behavioral control. That is, adverse events for primitive organisms are generally uncontrollable by their voluntary behavior, and such organisms do not need mechanisms to detect controllability. Thus, the “successfulness” of defensive reflexes is likely related to the duration of the existing threat—if it is prolonged then conservation/withdrawal would be adaptive with energy being shifted to physiological adjustments to threat. It is important to note that 5-HT is phylogenetically very old (Hen, 1993). Moreover, 5-HT has, from the beginning, been involved in controlling and shifting the balance and flow of energy (see Andrews, Bharwani, Lee, Fox, & Thomson, 2015, for review).
As organisms became more complex, they could detect and identify threats at a distance. And so they developed rich behavioral and cognitive skills that could be used to cope with threats. Control became possible even against threats that persist over time. Clearly, if such control will work this is the best course because it will minimize injury and harm. For complex organisms behavioral control can be possible over threats that are repeated, intermittent, and so persist across time. Thus, conservation/withdrawal and other energy adjustments set in motion by the continuation of threat should be inhibited.
Thus, when encountering a threat we envisage the following scenario. First, defensive behavior will be elicited. The aversive event would activate structures such as the dorsal raphe nucleus. This is a cumulative process with 5-HT building over time, and if the transmitter reaches some threshold in target structures, defensive behaviors become inhibited and energy flow is shifted. However, if control is possible this is detected and leads to the inhibition of this process so that active responding can continue. In addition, plasticity is induced in the prelimbic-to dorsal raphe nucleus circuit so that the system is biased to initially react to aversive events as if they are controllable, thereby prolonging the duration of active responding.
Unresolved Issues
At the level of basic neural circuits, there are several important unresolved issues. The data suggest that there are two important circuits within the ventromedial prefrontal cortex engaged by control that mediate the protective effects of control—a prelimbic-dorsomedial striatum pathway and a prelimbic-dorsal raphe nucleus pathway. The prelimbic-dorsomedial striatum circuit detects control (DETECT) when control is present, and then activates the prelimbic-dorsal raphe nucleus pathway (ACT) to then blunt the behavioral effects of stress. Since Baratta (2015) has shown that the prelimbic neurons that participate in these two circuits are quite discrete, there has to be a pathway from somewhere in the prelimbic-dorsomedial striatum circuit that projects to and activates the prelimbic neurons in the prelimbic- dorsal raphe nucleus pathway, and this pathway is as yet unknown. The “gold standard” discovery of this pathway will require measuring the activity of the prelimbic neurons in each pathway separately, with the critical result being that activity in the prelimbic-dorsomedial striatum pathway precedes activity in the prelimbic- dorsal raphe nucleus pathway. This requires a method that allows the experimenter to know that a prelimbic neuron that is being recorded is in one of these two pathways (most prelimbic neurons are in neither), and which one. This further requires the experimenter to be able to activate or inhibit each pathway selectively. The experiments described earlier in this paper that activated or inhibited prelimbic neurons did so non-selectively, as they involved microinjecting excitatory or inhibitory drugs that would act on all prelimbic neurons.
Thus, the existing neural evidence although strong, it is not conclusive. However genetic/molecular tools are now available that allow these gold standard experiments, and they are underway in the Maier laboratory.
At the psychological level, there are several other loose ends.
As a general statement, neural processes in the prefrontal cortex become narrowed by stress (Arnsten, 2015). Thus, the fact that in an aversive situation the brain seems to detect control as the active ingredient rather than a lack of control, does not mean that the brain cannot detect lack of control in other types of circumstances, such as uncontrollable food or unsolvable cognitive problems, or even loud noise. That is, the findings that we have reviewed do not imply that the brain does not have circuitry to detect non-contingency between events that include actions and outcomes. Rather, it may be that this processing can occur, but is not deployed in situations that are highly aversive such as the original helplessness experiments. So it is important to distinguish between what the brain does under a given set of conditions, and what the brain is capable of under different conditions. This possibility is in need of further research.
Speculations
The neural circuitry explains and predicts phenomena that are not explained or predicted at the psychological level. There are three main takeaways from the neural circuitry that might inform thinking about therapy and psychopathology. The first is that the default response of higher organisms to prolonged bad events seems to be passivity and heightened anxiety and that this is caused by the activation of the dorsal raphe nucleus. The second is that top-down higher cortical processes from the ventromedial prefrontal cortex inhibit this default response. The passivity and heightened anxiety symptoms of learned helplessness map quite well into symptoms of depression (Seligman 1975; Weiss, Simson, Ambrose, Webster, & Hoffman, 1985) and perhaps those of posttraumatic stress disorder (LoLordo & Overmier, 2011). The third has to do with the well-established enduring effects of cognitive interventions (Cuijpers et al., 2013). Here we have reviewed research that indicates that the experience of behavioral control over a stressor also has an enduring impact, and have suggested that this occurs because that experience induces plasticity in ventromedial prefrontal cortex neurons that project to midbrain and brainstem stress-responsive structures, leading to their later inhibition. It is tempting to suggest that cognitive therapies operate via the same mechanism. Assuming that learned helplessness models these phenomena, we now speculate on the implications of the neural circuitry particularly for the treatment of depression.
The first blush reaction is that we should measure these structures in humans, and then excite the ventromedial prefrontal cortex and inhibit the dorsal raphe nucleus, pharmacologically, electrically, trans-magnetically or psychologically in therapy. So for example one might ask if the dorsal raphe nucleus is highly excited during deep depression and if it becomes less excited as depression wanes either in time or in therapy. One might ask if medial prefrontal cortical activity inhibits the dorsal raphe nucleus when therapy or medication is successful. One might even look at the effect of medications and of trans-magnetic stimulation of dorsal raphe and medial prefrontal cortical structure during the course of depression. But a set of cautions should temper these speculations. A variety of psychological processes lead to increased ventromedial prefrontal cortex activation as measured by fMRI and also blunt the impact of stressors. However, this does not mean that these processes do so by activating the crucial PL-dorsal raphe nucleus pathway. The ventromedial prefrontal cortex is a large and complex structure encompassing cell types releasing a variety of transmitters and neuropeptides. Moreover, neurons in the ventromedial prefrontal cortex participate in numerous circuits with neurons in other brain regions, and the functions served by these circuits are likely unrelated, except that they share cells in a large piece of heterogeneous geography. The prelimbic neurons that are involved in the prelimbic-dorsomedial striatum and Prelimbic- dorsal raphe nucleus pathways represent an extremely small percentage of the cells in the ventromedial prefrontal cortex and would not contribute measurably to a BOLD signal in the ventromedial prefrontal cortex. Thus, measuring a global ventromedial prefrontal cortex fMRI signal will not remotely imply activation of the control-related critical prelimbic neurons. It might seem that the dorsal raphe nucleus might be usefully imaged, but the dorsal raphe nucleus is a small structure, with roughly 25,000 5-HT cells in the rat and 150,000 in humans. Furthermore, the critical dorsal raphe nucleus5-HT neurons that are involved in mediating the effects of uncontrollable stress and that are inhibited from the prelimbic by control are restricted to the caudal dorsal raphe nucleus (Grahn et al., 1999), maybe 8,000 neurons in the rat and 50,000 in the human. This is too small a number of cells by at least an order of magnitude to be imaged currently.
With regard to therapy, ventromedial prefrontal cortex dysregulation and impaired top-down inhibition of stress responsive limbic and brainstem structures have often been noted (e.g., DeRubeis, Siegle, & Hollon, 2008; Hartley & Phelps, 2010; Koenigs & Grafman, 2009; Rive et al., 2013; Shin & Liberzon, 2010). There is a growing and complex literature concerning the impact of therapies such as cognitive-behavioral therapy (CBT) on neural function that cannot be reviewed here. However, there are reports that CBT alters ventromedial prefrontal cortex activity and reduces negative emotion relative to wait-listed controls (e.g., Goldin et al., 2013).
Reappraisal of situations seen as catastrophic is central to CBT and there is now a large literature that explores the brain regions that might be involved. In reappraisal research subjects make some stimulus or event seem less negative or anxiety arousing (e.g., “imagine that the snake is not poisonous and cannot get at you”). Subjects that are able to successfully reduce negative reactions show reduced amygdala activity and increased activity in lateral and dorsal regions of the prefrontal cortex (Beauregard, Lévesque, & Bourgouin, 2001). Importantly Urry et al. (2006) noted that these regions of the prefrontal cortex do not project to the amygdala, but they do project to the ventromedial prefrontal cortex. She found that when subjects reduced negative emotional reactions successfully, there was a strong negative correlation between amygdala and ventromedial prefrontal cortex activity. This and a variety of other evidence led Ray and Zald (2012) to conclude,
These investigators either implicitly or explicitly describe emotion regulation as the deployment of top-down “cold” cognitive control region of the prefrontal cortex to down regulate bottom-up “hot” reactive processes involving the subcortical limbic regions like the amygdala. Failures in the successful deployment of prefrontal cortex top-down cognitive control mechanisms or overactive bottom-up amygdala processes have been proposed to contribute to several forms of psychopathology (p. 487).
Reappraisal as a tool of therapy is behavioral control. It blunts the impact of a negative event, and likely involves top-down inhibition from the ventro to lower structures. Whether the ventromedial prefrontal cortex activation produced by reappraisal involves the specific pathways that are critical to mediating the impact of behavioral control awaits future research. This encourages speculation that CBT engages the same top-down protective circuitry that has been isolated in the study of behavioral control. After all, CBT teaches cognitive tools that can be used to reduce destructive negative thoughts and emotions. That is, they are taught that there are things that they can do—control.
Why Versus Whither
Given our caution about the multifarious functions and structures of the ventromedial prefrontal cortex, it has not escaped our notice that one such function is prospection, the representation of possible futures (Seligman et al., 2013). In their review of prospection research Gilbert and Wilson (2007) conclude “An extensive body shows that prefeeling depends critically on the ventromedial prefrontal cortex and that people with damage to this area find it difficult to predict the hedonic consequences of future events” (p. 1352).
Notice that the top-down process from the prelimbic to the dorsal raphe nucleus captures the notion of EXPECTing that future bad events will be controllable. This circuit is about the future and it buffers against, but does not annihilate, the default reaction of the dorsal raphe nucleus. A default of helplessness eventually overcome by the experience of mastery over aversive events is compatible with the ontogeny of the human species: beginning life in a state of almost utter helplessness and only gradually learning to control bad events.
The possibility that the prelimbic- dorsal raphe nucleus circuitry involves prospection points to a class of psychological interventions that should be useful and to another class of psychological interventions that should be less useful. This is at the heart of our most speculative thoughts.
The default reaction to past and present bad events may be concurrent passivity and heightened anxiety. These cannot be undone directly or annihilated. They can, however, be inhibited by top-down cortical, control. Treatment can only buffer against past and present events with moves that produce control over bad events in the future: These are therapy's end-runs around bad events. We speculate that it is expectations of a better future that most matter in treatment. Psychotherapy might usefully spend less time on what is likely default and spend more time on what are likely the “end-runs” of DETECTing and then EXPECTing control.
“Why?” is a question that psychotherapy often asks. Perhaps a better question is “whither?” An exhaustive discussion of therapeutic moves that focus on understanding and undoing past and coping with present events as opposed to building buffers for the future is beyond the scope of this paper. So we will only give a few examples. Consider the class of therapy moves in which the patient reviews a past trauma in order to gain insight into its causes or to have catharsis about it. The dorsal raphe nucleus default reaction to trauma suggests that this is an uphill battle that will likely fail. The circuitry suggests that there is not much that can be achieved merely by confronting, understanding and reliving the trauma.
This uphill battle about confronting the past is not the province only of psychodynamic therapy, but it is also common in CBT. Re-appraisal, in general, re-interprets a past or present bad event. All of the following are other examples of a prima facie past focus (see Dobson, 2010 for details): discussion of post-event processing and attendant rumination; discussion of memory biases like selective filtering (where the patient only attended to a negative part of something that happened and ignored the positive parts); behavior chain analysis (where the patient looks at all the steps that led up to a bad outcome, such as an eating or drinking binge, and considers how those steps set him up to ‘fail’); and functional analyses (determining antecedents, behaviors, & consequences).
Similarly many moves in CBT (see Dobson, 2010 for details) focus prima facie on coping with the present. So therapists discuss “mind reading” and “catastrophization,” to facilitate reappraisal of the attributions and meaning a person is making for an ongoing event. Attentional control and mindfulness similarly emphasize the present. Exposure therapies (and emotion-focused therapies) emphasize the present emotional experience and the patient observes how emotion changes over time when he stays in the situation.
We are quick to note, of course, that in the hands of a skilled and experienced therapist these exercises about the past and the present are typically done with the purpose of changing future behavior, such as better recognizing triggers for past maladaptive responses in order to avoid those triggers in the future or gaining insight into catastrophizing in order to learn how to be more optimistic in the future. Nevertheless, from our circuitry speculation, it is the preparation for the future that is likely to be the most effective ingredient and so it is worthwhile to be explicit about the locus of its effectiveness.
Indeed much of cognitive behavioral therapy is actually future–oriented, even if it is not taught in this way. Problem solving, activity scheduling, crisis response plans, role play in assertiveness training, and what doors open when one door closes, all involve simulating future situations and trying to prepare for those effectively. We note that there actually exists a variant, as yet insufficiently validated, of CBT, called “Future Directed Therapy” (Vilhauer, 2014).
Perhaps one CBT “whither” vignette may help the clinician: A young man was distressed about the upcoming one-year anniversary of his psychiatric hospitalization. He was afraid he would be distraught on this anniversary and engage in self-harm.
The therapist could tell that he was anxious and depressed about having been hospitalized, and what this meant for his future. He was probably having some distorted thoughts about it (e.g., “this means I'll always be a fuck-up,” etc.). The therapist considered using classic CBT moves – inquiring about these automatic thoughts and helping him to reappraise them.
Instead, she played dumb: “Wow, the one-year anniversary. How are you going to celebrate it?” He was initially confused. Celebrate? “Well,” she replied, “you've obviously come a really long way since then: You're working again, you're in a great relationship. How would you commemorate that progress, if you wanted to?”
This totally refocused the conversation on future mastery. They also planned ways to prevent self-harm, and they planned the ways that he would capitalize on his progress in the coming year and they explored how he could continue to be in a good place one year from now.
In conclusion, the neural circuitry underlying the phenomenon of learned helplessness strongly suggests that helplessness was not learned in the original experiments. Rather passivity and heightened anxiety are the default mammalian reaction to prolonged bad events. What can be learned is cortical—that bad events will be controllable in the future. The top-down circuitry that descends from the ventromedial prefrontal cortex down to the dorsal raphe nucleus and other structures acts to inhibit this default. We are mindful that in the theory of explanatory style, “hope” consists largely in the habit of expecting that future bad events will not be permanent, global, and uncontrollable, rather they will be temporary, local and controllable (Seligman, 1991, pp. 48-49). Such expectations are likely the best natural defense against helplessness and we speculate that the ventromedial prefrontal cortex-dorsal raphe nucleus circuit may be usefully thought of as the “hope circuit.”
Footnotes
Steven Maier, Department of Psychology and Neuroscience, Campus Box 345, University of Colorado at Boulder, Boulder, CO. 80309-0345
Many colleagues and students have contributed to these ideas and experiments over the years, and without them there would be none. Special thanks go to J. Amat, S. Bland, M. Baratta, J. Christianson, A. Der-Avakian, R. Drugan, J. Elstein, R. Grahn, J. Hammack, R.L. Jackson, K. Kubala, S. Maswood, T. Minor, K. Short, P. Sparks, B. Teachman, L.R. Watkins, M. Will, W. Woodmansee, D. Yaden.
Contributor Information
Steven F. Maier, Email: smaier@psych.colorado.edu, University of Colorado.
Martin E. P. Seligman, University of Pennsylvania
References
- Abramson LY, Seligman MEP, Teasdale I. Learned helplessness in humans: Critique and reformulation. Journal of Abnormal Psychology. 1978;87:49–59. http://dx.doi.org/10.1037/0021-843X.87.1.49. [PubMed] [Google Scholar]
- Alloy LB, Peterson C, Abramson LY, Seligman MEP. Attributional style and the generality of learned helplessness. Journal of Personality and Social Psychology. 1984;46:681–687. doi: 10.1037//0022-3514.46.3.681. http://dx.doi.org/10.1037/0022-3514.46.3.681. [DOI] [PubMed] [Google Scholar]
- Amat J, Aleksejev RM, Paul E, Watkins LR, Maier SF. Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience. 2010;165:1031–1038. doi: 10.1016/j.neuroscience.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neuroscience. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Christianson JP, Aleksejev RM, Kim J, Richeson KR, Watkins LR, Maier SF. Control over a stressor involves the posterior dorsal striatum and the act/outcome circuit. European Journal of Neuroscience. 2014;40:2352–2358. doi: 10.1111/ejn.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral effects of later uncontrollable stress: Role of the ventral medial prefrontal cortex. Journal of Neuroscience. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd. Washington, DC; 1980. Author. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC; 1994. Author. [Google Scholar]
- Andrews PW, Bharwani A, Lee KR, Fox M, Thomson JA. Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response. Neuroscience & Biobehavioral Reviews. 2015;51:164–188. doi: 10.1016/j.neubiorev.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Anisman H, Remington G, Sklar LS. Effect of inescapable shock on subsequent escape performance: Catecholaminergic and cholinergic mediation of response initiation and maintenance. Psychopharmacology. 1979;61:107–124. doi: 10.1007/BF00426724. http://dx.doi.org/10.1007/BF00426724. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress weakens prefrontal networks: Molecular insults to higher cognition. Nature Neuroscience. 2015;18:1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: Contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/S0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV.October2015Prefrontal control of resilience to adverse events Society for Neuroscience Chicago, IL [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Maier SF. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Lucero TR, Amat J, Watkins LR, Maier SF. Role of the ventral medial prefrontal cortex in mediating behavioral control-induced reduction of later conditioned fear. Learning & Memory. 2008;15:84–87. doi: 10.1101/lm.800308. [DOI] [PubMed] [Google Scholar]
- Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. European Journal of Neuroscience. 2009;30:1111–1116. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Lévesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:6993–7000. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. Retrieved from http://www.jneurosci.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC, Fanselow MS. A perceptual-defensive-recuperative model of fear and pain. Behavioral and Brain Sciences. 1980;3:291–301. http://dx.doi.org/10.1017/S0140525X0000491X. [Google Scholar]
- Christianson JP, Benison AM, Jennings J, Sandsmark EK, Amat J, Kaufman RD, Maier SF. The sensory insular cortex mediates the stress-buffering effects of safety signals but not behavioral control. The Journal of Neuroscience. 2008;28:13703–13711. doi: 10.1523/JNEUROSCI.4270-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Flyer-Adams JG, Drugan RC, Amat J, Daut RA, Foilb AR, Maier SF. Learned stressor resistance requires extracellular signal-regulated kinase in the prefrontal cortex. Frontiers in Behavioral Neuroscience. 2014;8(348) doi: 10.3389/fnbeh.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Maier SF. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behavioural Brain Research. 2008;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Maier SF. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biological Psychiatry. 2010;67:339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, Hollon SD, van Straten A, Bockting C, Berking M, Andersson G. Does cognitive behavior therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. British Medical Journal Open. 2013;26:e002542. doi: 10.1136/bmjopen-2012-002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends in Neuroscience. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: Treatment outcomes and neural mechanisms. Nature Reviews Neuroscience. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dess NK, Linwick D, Patterson J, Overmier JB, Levine S. Immediate and proactive effects of controllability and predictability on plasma cortisol responses to shocks in dogs. Behavioral Neuroscience. 1983;97:1005–1016. doi: 10.1037//0735-7044.97.6.1005. [DOI] [PubMed] [Google Scholar]
- Dobson K. Handbook of cognitive-behavioral therapies. 3rd. New York, NY: Guilford; 2010. [Google Scholar]
- Frank MG, Watkins LR, Maier SF. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain, Behavior, and Immunity. 2013;33:1–6. doi: 10.1016/j.bbi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DT, Wilson TD. Prospection: Experiencing the future. Science. 2007;317:1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- Glazer HI, Weiss JM, Pohorecky LA, Miller NE. Monamines as mediators of avoidance-escape behavior*. Psychosomatic Medicine. 1975;37:535–543. doi: 10.1097/00006842-197511000-00007. Retrieved from http://journals.lww.com/psychosomaticmedicine/pages/default.aspx. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: Randomized clinical trial. JAMA Psychiatry. 2013;70:1048–1056. doi: 10.1001/jamapsychiatry.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff FG, Guimarães FS, De Andrade TG, Deakin JFW. Role of 5-HT in stress, anxiety, and depression. Pharmacology Biochemistry and Behavior. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Hammack SE, Will MJ, O'Connor KA, Deak T, Sparks PD, Maier SF. Blockade of alpha1 adrenoreceptors in the dorsal raphe nucleus prevents enhanced conditioned fear and impaired escape performance following uncontrollable stressor exposure in rats. Behavioural Brain Research. 2002;134:387–392. doi: 10.1016/S0166-4328(02)00061-X. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Watkins LR, Maier SF. Impaired escape performance and enhanced conditioned fear in rats following exposure to an uncontrollable stressor are mediated by glutamate and nitric oxide in the dorsal raphe nucleus. Behavioural Brain Research. 2000;112:33–41. doi: 10.1016/S0166-4328(00)00161-3. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Research. 1999;826:35–43. doi: 10.1016/S0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Fleshner M. Lesions of the basolateral amygdala reverse the long-lasting interference with shuttle box escape produced by uncontrollable stress. Behavioural Brain Research. 2010;211:71–76. doi: 10.1016/j.bbr.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: The neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmreich DL, Tylee D, Christianson JP, Kubala KH, Govindarajan ST, O'Neill WE, Maier SF. Active behavioral coping alters the behavioral but not the endocrine response to stress. Psychoneuroendocrinology. 2012;37:1941–1948. doi: 10.1016/j.psyneuen.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmreich DL, Watson LR, Deak T, Maier SF, Akil H, Watson SJ. The effect of stressor controllability on stress-induced neuropeptide mRNA expression within the paraventricular nucleus of the hypothalamus. Journal of Neuroendocrinology. 1999;11:121–128. doi: 10.1046/j.1365-2826.1999.00300.x. [DOI] [PubMed] [Google Scholar]
- Hen R. Structural and functional conservation of serotonin receptors throughout evolution. EXS. 1993;63:266–278. doi: 10.1007/978-3-0348-7265-2_14. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo–pituitary–adrenocortical axis. Trends in Neurosciences. 1997;20:78–84. doi: 10.1016/S0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hiroto DS, Seligman MEP. Generality of learned helplessness in man. Journal of Personality and Social Psychology. 1975;31:311–327. doi: 10.1037//0022-3514.46.3.681. http://dx.doi.org/10.1037/h0076270. [DOI] [PubMed] [Google Scholar]
- Jackson RL, Minor TR. Effects of signaling inescapable shock on subsequent escape learning: Implications for theories of coping and “learned helplessness. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:390–400. http://dx.doi.org/10.1037/0097-7403.14.4.390. [PubMed] [Google Scholar]
- Kerr DL, McLaren DG, Mathy RM, Nitschke JB. Controllability modulates the anticipatory response in the human ventromedial prefrontal cortex. Frontiers in Psychology. 2012;3(557) doi: 10.3389/fpsyg.2012.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC, Fencil-Morse E, Seligman MEP. Learned helplessness, depression, and the attribution of failure. Journal of Personality and Social Psychology. 1976;33:508–516. doi: 10.1037//0022-3514.33.5.508. http://dx.doi.org/10.1037/0022-3514.33.5.508. [DOI] [PubMed] [Google Scholar]
- Klein DC, Seligman MEP. Reversal of performance deficits and perceptual deficits in learned helplessness and depression. Journal of Abnormal Psychology. 1976;85:11–26. doi: 10.1037//0021-843x.85.1.11. http://dx.doi.org/10.1037/0021-843X.85.1.11. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. Posttraumatic stress disorder: The role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Hay-Schmidt A, Vrang N, Mikkelsen JD. Origin of projections from the midbrain raphe nuclei to the hypothalamic paraventricular nucleus in the rat: A combined retrograde and anterograde tracing study. Neuroscience. 1996;70:963–988. doi: 10.1016/0306-4522(95)00415-7. [DOI] [PubMed] [Google Scholar]
- Leaf RC. Avoidance response evocation as a function of prior discriminative fear conditioning under curare. Journal of Comparative and Physiological Psychology. 1964;58:446–449. doi: 10.1037/h0045975. http://dx.doi.org/10.1037/h0045975. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeholm M, Tricomi E, O'Doherty JP, Balleine BW. Neural correlates of instrumental contingency learning: Differential effects of action–reward conjunction and disjunction. The Journal of Neuroscience. 2011;31:2474–2480. doi: 10.1523/JNEUROSCI.3354-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoLordo VM, Overmier B. Trauma, learned helplessness, its neuroscience, and implications for posttraumatic stress disorder. In: Schactman TR, Reilly SS, editors. Associative learning and conditioning theory: Human and non-human applications. New York, NY: Oxford University Press; 2011. pp. 121–151. [Google Scholar]
- Maier SF. Failure to escape traumatic shock: Incompatible skeletal-motor responses or learned helplessness? Learning and Motivation. 1970;1:157–169. doi: 10.1016/0023-9690(70)90082-2. [DOI] [Google Scholar]
- Maier SF. Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biological Psychiatry. 2001;49:763–773. doi: 10.1016/S0006-3223(00)01095-7. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Kalman BA, Sutton LC, Wiertelak EP, Watkins LR. The role of the amygdala and dorsal raphe nucleus in mediating the behavioral consequences of inescapable shock. Behavioral Neurosciences. 1993;107:377–389. doi: 10.1037//0735-7044.107.2.377. http://dx.doi.org/10.1037/0735-7044.107.2.377. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Maswood S, Watkins LR. The benzodiazepine receptor antagonists flumazenil and CGS8216 block the enhancement of fear conditioning and interference with escape behavior produced by inescapable shock. Psychopharmacology. 1995;121:250–258. doi: 10.1007/BF02245636. http://dx.doi.org/10.1007/BF02245636. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and the interference with escape produced by exposure to inescapable shock. Behavioral Neuroscience. 1995;109:404–413. doi: 10.1037//0735-7044.109.3.404. http://dx.doi.org/10.1037/0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Maier SF, Kalman BA, Grahn RE. Chlordiazepoxide microinjected in the region of the dorsal raphe nucleus eliminates the interference with escape responding produced by inescapable shock whether administered before inescapable shock or escape testing. Behavioral Neuroscience. 1994;108:121–130. doi: 10.1037//0735-7044.108.1.121. http://dx.doi.org/10.1037/0735-7044.108.1.121. [DOI] [PubMed] [Google Scholar]
- Maier SF, Rapaport PM, Wheatley K. Conditioned inhibition and the UCS-CS interval. Animal Learning and Behavior. 1976;4:217–222. doi: 10.3758/bf03214039. Retrieved from http://www.psychonomic.org/learning-behavior. [DOI] [PubMed] [Google Scholar]
- Maier SF, Ryan SM, Barksdale CM, Kalin NH. Stressor controllability and the pituitary-adrenal system. Behavioral Neuroscience. 1986;100:669–674. doi: 10.1037//0735-7044.100.5.669. http://dx.doi.org/10.1037/0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman MEP. Learned helplessness: Theory and evidence. Journal of Experimental Psychology: General. 1976;105:3–46. http://dx.doi.org/10.1037/0096-3445.105.1.3. [Google Scholar]
- Maier SF, Seligman MEP, Solomon RL. Pavlovian fear conditioning and learned helplessness: Effects on escape and avoidance behavior of (a) the CS-US contingency, and (b) the independence of the US and voluntary responding. In: Campbell BA, Church RM, editors. Punishment. New York, NY: Appleton-Century-Crofts; 1969. pp. 299–342. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin releasing hormone. Neuroscience and Biobehavioral Reviews. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Szot P, Baratta MV, Bland ST, White SS, Maier SF, Neumaier JF. Stress-induced activity in the locus coeruleus is not sensitive to stressor controllability. Brain Research. 2009;1285:109–118. doi: 10.1016/j.brainres.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Seligman MEP. Learned helplessness, depression, and the perception of reinforcement. Behaviour Research and Therapy. 1976;14:7–17. doi: 10.1016/0005-7967(76)90039-5. [DOI] [PubMed] [Google Scholar]
- Minor TR, Dess NK, Overmier J. Inverting the traditional view of “learned helplessness.”. In: Denny MR, editor. Fear, avoidance, and phobias. Hillsdale, NJ: Erlbaum; 1991. pp. 87–133. [Google Scholar]
- Minor TR, Trauner MA, Lee CY, Dess NK. Modeling signal features of escape response: Effects of cessation conditioning in “learned helplessness” paradigm. Journal of Experimental Psychology: Animal Behavior Processes. 1990;16:123–126. http://dx.doi.org/10.1037/0097-7403.16.2.123. [PubMed] [Google Scholar]
- Overmier JB, Leaf RC. Effects of discriminative Pavlovian fear conditioning upon previously or subsequently acquired avoidance responding. Journal of Comparative and Physiological Psychology. 1965;60:213–217. doi: 10.1037/h0022340. [DOI] [PubMed] [Google Scholar]
- Overmier JB, Seligman ME. Effects of inescapable shock upon subsequent escape and avoidance responding. Journal of Comparative and Physiological Psychology. 1967;63:28–33. doi: 10.1037/h0024166. http://dx.doi.org/10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- Petty F, Sherman AD. Reversal of learned helplessness by imipramine. Communications in Psychopharmacology. 1979;3:371–373. [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1997;82:443–468. doi: 10.1016/S0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. The Journal of Neuroscience. 2011;31:9683–9695. doi: 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: An animal model of posttraumatic stress disorder. Neuroscience & Biobehavioral Reviews. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Ray RD, Zald DH. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience & Biobehavioral Reviews. 2012;36:479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Evans AK, Frank MG, Watkins LR, Lowry CA, Maier SF. Uncontrollable, but not controllable, stress desensitizes 5-HT1A receptors in the dorsal raphe nucleus. The Journal of Neuroscience. 2011;31:14107–14115. doi: 10.1523/JNEUROSCI.3095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Davidson RJ. Perceived controllability modulates the neural response to pain. The Journal of Neuroscience. 2004;24:7199–7203. doi: 10.1523/JNEUROSCI.1315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman MEP. Depression and learned helplessness. In: Friedman RJ, Katz MM, editors. The psychology of depression: Contemporary theory and research. New York, NY: Winston-Wiley; 1974. pp. 83–113. [Google Scholar]
- Seligman MEP. Helplessness: On depression, development, and death. San Francisco, CA: Freeman; 1975. [Google Scholar]
- Seligman MEP. Learned optimism: How to change your mind and your life. New York, NY: Pocket Books; 1991. [Google Scholar]
- Seligman MEP, Csikszentmihalyi M. Positive psychology: An introduction. American Psychologist. 2000;55:5–14. doi: 10.1037//0003-066x.55.1.5. http://dx.doi.org/10.1037/0003-066X.55.1.5. [DOI] [PubMed] [Google Scholar]
- Seligman MEP, Maier SF. Failure to escape traumatic shock. Journal of Experimental Psychology. 1967;74:1–9. doi: 10.1037/h0024514. http://dx.doi.org/10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Seligman MEP, Railton P, Baumeister RF, Sripada C. Navigating into the future or driven by the past. Perspectives on Psychological Science. 2013;8:119–141. doi: 10.1177/1745691612474317. [DOI] [PubMed] [Google Scholar]
- Shiflett MW, Balleine BW. Contributions of ERK signaling in the striatum to instrumental learning and performance. Behavioural Brain Research. 2011;218:240–247. doi: 10.1016/j.bbr.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Maier SF. Stressor controllability, social interaction, and benzodiazepine systems. Pharmacology Biochemistry and Behavior. 1993;45:827–835. doi: 10.1016/0091-3057(93)90128-G. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simson P, Weiss J. Altered activity of the locus coeruleus in an animal model of depression. Neuropsychopharmacology. 1988;1:287–295. [PubMed] [Google Scholar]
- Strong PV, Christianson JP, Loughridge AB, Amat J, Maier SF, Fleshner M, Greenwood BN. 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience. 2011;197:132–144. doi: 10.1016/j.neuroscience.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela JA, Wang J, Christianson JP, Maier SF, Cooper DC. Control over stress, but not stress per se increases prefrontal cortical pyramidal neuron excitability. The Journal of Neuroscience. 2012;32:12848–12853. doi: 10.1523/JNEUROSCI.2669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vilhauer J. Think forward to thrive: How to use the mind's power of anticipation to transcend your past and transform your life. Novato, CA: New World Library; 2014. [Google Scholar]
- Volpicelli JR, Ulm RR, Altenor A, Seligman MEP. Learned mastery in the rat. Learning and Motivation. 1983;14:204–222. [Google Scholar]
- Walters ET, Erickson MT. Directional control and the functional organization of defensive responses in Aplysia. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1986;159:339–351. doi: 10.1007/BF00603980. [DOI] [PubMed] [Google Scholar]
- Weiss JM. Effects of coping responses on stress. Journal of Comparative and Physiological Psychology. 1968;65:251–260. doi: 10.1037/h0025562. [DOI] [PubMed] [Google Scholar]
- Weiss JM. Effects of coping behavior with and without a feedback signal on stress pathology in rats. Journal of Comparative and Physiological Psychology. 1971;77:22–30. doi: 10.1037/h0031581. http://dx.doi.org/10.1037/h0031581. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Simson PE. Neurochemical and electrophysiological events underlying stress-induced depression in an animal model. Advances in Experimental Medicine and Biology. 1988;245:425–440. doi: 10.1007/978-1-4899-2064-5_33. Retrieved from http://www.springer.com/series/5584. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Simson PG, Ambrose MJ, Webster A, Hoffman LJ. Neurochemical basis of behavioral depression. In: Katkin E, Manuck S, editors. Advances in Behavioral Medicine. Vol. 1. Greenwich, CT: JAI Press; 1985. pp. 233–275. [Google Scholar]
- Will MJ, Der-Avakian A, Bland ST, Grahn RE, Hammack SE, Sparks PD, Maier SF. Electrolytic lesions and pharmacological inhibition of the dorsal raphe nucleus prevent stressor potentiation of morphine conditioned place preference in rats. Psychopharmacology. 2004;171:191–198. doi: 10.1007/s00213-003-1572-1. [DOI] [PubMed] [Google Scholar]
- Woodmansee WW, Silbert LH, Maier SF. Factors that modulate inescapable shock-induced reductions in daily activity in the rat. Pharmacology Biochemistry and Behavior. 1993;4(5):553–559. doi: 10.1016/0091-3057(93)90505-N. [DOI] [PubMed] [Google Scholar]


