Summary
Polymer substances are commonly applied as surface coatings on endovascular catheters and vascular devices. Adverse effects related to their use have been reported, although the overall clinical significance and appropriate methods of detection of these complications have been unclear. In this analysis, we systematically reviewed clinical and diagnostic features in 32 patients (age, 36–87 years; mean, 59 years) in whom intracranial polymer reactions were documented following vascular interventions. Associated neuroradiologic and neuropathologic findings were variable and included cerebral vasculitis or vasculopathy (63%), abscess or granuloma formation (38%), ischemic infarcts (28%), parenchymal hematomas (28%), white matter change (25%), and/or chemical meningitis (22%). Location(s) of polymer reactions varied and included sites adjacent to and/or downstream from instrument insertion or implantation. Presenting clinical signs included focal neurologic deficits (41%), headache (22%), constitutional symptoms (19%), meningitis (16%), seizure and/or involuntary movements (9%), coma (6%), and syncope (3%). Adverse outcomes included stroke (31%), death (28%), delayed communicating hydrocephalus (9%), steroid dependency (9%), steroid complications (6%), and cerebral volume loss (3%). In some cases, these complications necessitated increased cost and length of medical care. In this review, we highlight the diverse features of polymer-induced reactions involving the central nervous system and summarize distinct diagnostic patterns that may enable earlier premortem detection of these lesions in the postprocedural clinical setting. Further work in this area is necessary to identify additional etiologic, preventative and therapeutic strategies. These data have potentially broad implications pertaining to the safety, efficacy, standards of use, storage, manufacturing, and regulation of new and emerging vascular devices and polymer nanotechnologies.
Keywords: Embolism, Endovascular procedure, HPE, Hydrophilic polymer, Iatrogenic complication, Inflammation, Vascular device
1. Introduction
Polymers are commonly used as surface coatings on endovascular catheters and vascular devices. Their use on sheaths, catheters, microcatheters, and guidewires, among other vascular devices, allows for less invasive therapeutic approaches and has facilitated novel endovascular techniques. These coating materials enhance device lubrication, maneuverability, and biocompatibility and facilitate access of small vessels while reducing vascular spasm [1]. Polymer-coated aneurysm coils have additionally been shown to improve volumetric occlusion and facilitate early inflammation and thrombus organization within cerebral aneurysms [2,3]. With evolving nanotechnologies and increased use of insertable and implantable vascular devices, applications of these coating materials are expected to increase.
Despite their advantages, polymer coatings have the potential to induce significant adverse reactions. Chemical injuries and unanticipated dissociation of polymer particles from vascular device surfaces have been increasingly recognized following routine use in patients [4–8]. These phenomena have been associated with diverse outcomes that depend on organ and site of involvement. In 2009, we reported to the US Food and Drug Administration the first fatal case of polymer embolism, which involved the brain [6]. In spite of limited recognition and reporting of this complication, multiple cases over recent years provide additional evidence that morbidity and mortality associated with these iatrogenic phenomena are underrecognized [5–14]. Further characterization of polymer reactions, including organ-specific effects, and identification of etiologic factors are therefore warranted and have potential to improve the safety of new and emerging vascular technologies.
Sensitive methods of earlier detection are needed to elucidate the true clinical incidence and significance of polymer phenomena. In this study, we review the spectrum of clinical, neuroradiologic, and neuropathologic findings and summarize premortem diagnostic features and outcomes of polymer complications involving the central nervous system. This review is performed with the intent to clarify the nature of clinical risks posed to patients; facilitate earlier diagnosis of polymer reactions by radiologists and pathologists; summarize available preventative and therapeutic strategies for treating physicians; and highlight areas of needed improvement to manufacturers, biomedical engineers, polymer chemists, and regulatory agencies.
2. Materials and methods
This study was performed under exemption from the institutional review board and in accordance with the ethical standards of institutional and national research committees. Published reports, dating from 1997 to 2015, of intracranial polymer reactions due to routine catheterization and/or endovascular procedures were identified using PubMed. Cases previously reported by our group were systematically reanalyzed. Our archives were also searched for additional unreported consult cases. Patients in whom specific histopathologic or cerebrospinal fluid (CSF) laboratory data were unavailable were excluded. Demographics, procedure(s), imaging findings, laboratory and neuropathologic data, and outcomes on the remaining patients were reviewed.
3. Results
Thirty-two patients with documented intracranial polymer reactions and available pathology or CSF laboratory data were identified (age range, 36–87 years). Patients had undergone various procedures, including cerebral angiogram (94%), endovascular aneurysm coil embolization (50%), aneurysm flow diversion (25%), intraarterial thrombolysis (9%), mechanical thrombectomy (6%), peripherally inserted central catheter placement (3%), cardiac catheterization (3%), central venous catheterization (3%), and hemodialysis catheter placement (3%). Polymer phenomena were associated with diverse intracranial reactions that involved variable regions of the brain and spinal cord. Demographic, clinical, neuroradiologic, and neuropathologic findings and outcome on each patient are summarized in Tables 1 and 2. Representative neuroradiologic and neuropathologic patterns of tissue injury are summarized by location below.
Table 1.
Clinical, laboratory or histopathologic findings and outcome in 32 patients with intracranial polymeric reactions
| Source | Age (y), sex |
Procedure(s) | Time of sx onset a |
Presenting neurologic sign or sx a |
Documented laboratory or neuropathologic finding(s) a |
Treatment | Clinical outcome | |
|---|---|---|---|---|---|---|---|---|
| 1 | Barnwell (1997) [4] |
53 F | Cerebral angiogram, IA thrombolysis (R ICA) |
5 h | Coma | Polymer emboli with foreign body/giant cell rxn |
– | Stroke, death |
| 2 | " | 66 M | Cerebral angiogram, IA thrombolysis (L vertebral & basilar Aa), mechanical thrombectomy, angioplasty |
3 d | Neurological decline (NOS) |
Polymer emboli with evolving infarcts |
– | Stroke, death |
| 3 | " | 58 M | Cerebral angiogram, IA thrombolysis (basilar A) |
N/A | Coma | Polymer emboli with fibrin thrombi |
– | Stroke, death |
| 4 | " | 53 F | Cerebral angiogram and tumor embolization |
N/A | N/A | Polymer emboli | – | N/A |
| 5 | Meyers (2004) [9] |
52 F | Cerebral angiogram, coil embolization (balloon assist) of R carotid-ophthalmic A. aneurysm |
26 h | Fever, HA, nuchal rigidity, photophobia, meningismus |
CSF: ↑WBC, ↑protein; Serum: ↑WBC |
VP shunt, steroids |
Delayed hydrocephalus |
| 6 | " | 46 F | Cerebral angiogram, coil embolization (balloon and stent assist) of R PCA aneurysm |
3 wk, 7 wk | Fever, HA, n/v, meningismus, R CN palsies, L hemiparesis |
CSF: ↑ WBC, ↑protein | VP shunt, steroids |
Delayed hydrocephalus |
| 7 | Im (2007) [10] |
56 F | Cerebral angiogram, coil embolization of R ICA aneurysm |
28 h, 6 mo | Fever, HA, chills, malaise, meningismus, cognitive decline, gait abnormality |
CSF: ↑WBC | VP shunt, steroids |
Delayed hydrocephalus |
| 8 | " | 66 F | Cerebral angiogram, coil embolization of L ICA aneurysm |
22 h | Fever, HA, weakness, nuchal rigidity |
CSF: ↑WBC, ↑protein | Steroids | N/A |
| 9 | " | 68 M | Cerebral angiogram, coil embolization of R MCA aneurysm |
20 h, 2 wk | Fever, HA, chills, agitation, meningismus, LUE weakness |
CSF: ↑ WBC, ↑protein | Steroids | Stroke |
| 10 | Fealey (2008) [11] |
58 F | Cerebral angiogram, coil embolization of R ICA aneurysm |
9 mo | L arm and leg weakness and numbness, tonic-clonic seizure |
Polymer emboli with granulomata and abscess |
Surgical biopsy/ excision |
Progressive inflammation with abscess |
| 11 | Mehta (2009) [6] |
87 F | Cerebral angiogram, coil embolization of L ICA aneurysm |
1 d | Right hemiparesis | Polymer emboli with scattered infarcts, foreign body/giant cell rxn, granulomas and vasculitis, fibrinoid vascular necrosis, fibrin thrombi, IELd, micro thrombus formation, luminal fibrosis, Hemo, perivascular rarefaction, neointimal proliferation, focal meningitis |
Supportive measures |
Stroke, death |
| 12 | Mehta (2010) [7] |
36 F | PICC, cerebral angiogram | 10 d | Neurological decline (NOS) |
Polymer emboli with foreign body/giant cell rxn, fibrinoid vascular necrosis, microthrombus formation, IELd, luminal fibrosis, perivascular rarefaction, Hemo, focal neuronal ischemia |
Surgical biopsy/ excision |
N/A |
| 13 | " | 54 M | Cerebral angiogram, mechanical thrombectomy of L MCA thromboembolus |
3 d | R hemiplegia, aphasia | Polymer emboli with infarcts and parenchymal hematoma, foreign body rxn, Hemo, microthrombus formation, IELd, white matter astrogliosis, focal neuronal ischemia |
Surgical biopsy/ excision |
Stroke |
| 14 | Skolarus (2010) [12] |
46 F | Cerebral angiogram, coil embolization of basilar artery tip aneurysm |
4 wk, 10 wk |
Scintillating scotomas RUE dysmetria |
CSF: ↑ IgG, ↑myelin basic protein |
– | N/A |
| 15 | " | 56 F | Cerebral angiogram, coil embolization of basilar artery tip aneurysm, subsequent aneurysm retreatment |
9 mo | LUE and LLE paresthesias, difficulty ambulating |
CSF: ↑protein | – | Stroke |
| 16 | Hu (2014) [13] |
73 M | Cerebral angiogram, flow diversion L ICA aneurysm |
3 d | Syncope | Polymer emboli with parenchymal hematoma |
– | Stroke, death |
| 17 | " | 63 F | Cerebral angiogram, flow diversion L ICA aneurysm |
6 d | Neurological decline (NOS) |
Polymer emboli with parenchymal hematoma |
– | Stroke, death |
| 18 | " | 66 F | Cerebral angiogram, flow diversion L ICA aneurysm |
2 wk | Death | Polymer emboli with parenchymal hematoma |
– | Stroke, death |
| 19 | Mehta (2015) [14] |
73 F | Dialysis catheter, CVC, cardiac catheterization |
2 d | Fever, altered mental status |
↑PT/PTT/Ddimer/p-ANCA/ ESR/CRP/FSP, ↓Fib, polymer emboli with foreign body/giant cell rxn, neutrophils, fibrinoid vascular necrosis, vasculitis, fibrin thrombi, IELd, Hemo and perivascular rarefaction |
– | SIRS bdeath |
| 20 | Grewal (2015) [15] |
65 F | Cerebral angiogram, coil embolization R PCom aneurysm |
4 d | L sided weakness, aseptic inflammation groin access site |
CSF: ↑WBC, ↑protein; serum: ↑ESR |
Chronic steroids/ IS |
Cerebral volume loss, steroid/IS dependency |
| 21 | Shapiro (2015) [16] |
N/A | Cerebral angiogram, stent–assisted flow diversion of R trigeminal aneurysm |
8 wk | L homonymous quadrantanop-sia |
CSF: ↑protein | Steroids | N/A |
| 22 | " | N/A | Cerebral angiogram, stent assisted flow diversion R ICA aneurysm |
8 wk | HA, neck pain, L inf. homonymous quadrantanopsia |
CSF: ↑protein | Steroids | N/A |
| 23 | " | N/A | Cerebral angiogram, coil–supported flow diversion of b/l ICA aneurysms |
2 wk | Persistent HA | CSF: ↑protein; polymer emboli with giant cells, granulomas, reactive gliosis |
Steroids; surgical biopsy/excision |
N/A |
| 24 | " | N/A | Cerebral angiogram, flow diversion supported coiling of R ICA aneurysm |
1 d 3 mo |
L hemiparesis, involuntary movements LUE and L eyelid |
CSF: ↑protein Polymer emboli with granulomatous angiitis |
Antiepileptics, chronic steroids/IS; surgical biopsy/ excision |
Steroid/IS dependency with related effects |
| 25 | " | N/A | Cerebral angiogram, stent–assisted coiling of L MCA aneurysm |
2 wk | RUE paresthesia, spasms | CSF: ↑protein | Steroids | N/A |
| 26 | Lorentzen (2015) [17] |
52 F | Cerebral angiogram, flow diversion L ICA aneurysm |
3 mo | Global aphasia, R hemiparesis, ataxia |
CSF: ↑protein Polymer emboli within meningeal vessel, giant cell rxn, cerebral microabscess with neutrophilic rxn |
Chronic steroids/ IS |
Steroid/IS dependency with related effects, chronic HA, fatigue, long-term disability |
| 27 | Mehta (consult) |
N/A | Cerebral angiogram | 24 h | Neurological decline (NOS) |
Polymer emboli with intravascular macrophages; hemorrhage |
– | N/A |
| 28 | " | N/A | Cerebral angiogram | 2 mo | Neurological decline (NOS) |
Polymer emboli with intravascular macrophage, giant cell, neutrophilic and granulomatous rxn; vasculitis, fibrin thrombi, IELd, Hemo, hemorrhage |
– | N/A |
| 29 | " | N/A | Catheterization, NOS | N/A | Neurological decline (NOS) |
Polymer emboli with pituitary necrosis |
– | N/A |
| 30 | " | N/A | Cerebral angiogram, coil embolization |
N/A | Neurological decline (NOS) |
Polymer emboli with hemorrhage | – | Death |
| 31 | " | N/A | Cerebral angiogram, coil embolization |
N/A | Neurological decline (NOS) |
Polymer emboli with intravascular macrophages, luminal fibrosis, ischemic infarct |
– | N/A |
| 32 | " | N/A | Cerebral angiogram, coil embolization |
N/A | Neurological decline (NOS) |
Polymer emboli with perivascular macrophages, ischemic infarct |
– | N/A |
Abbreviations: A, artery; Aa, arteries; b/l, bilateral; CN, cranial nerves; CRP, C-reactive protein; CSF, cerebrospinal fluid; CVC, central venous catheter; ESR, erythrocyte sedimentation rate; F, female; Fib, fibrinogen; FSP, fibrin split products; HA, headache; Hemo, perivascular hemosiderin; IA, intraarterial; ICA, internal carotid artery; IELd, internal elastic lamina disruption; IgG, immunoglobulin G; inf, inferior; IS, immunosuppressive; L, left; LLE, left lower extremity; LUE, left upper extremity; M, male; MCA, middle cerebral artery; N/A, not applicable or not available; NOS, not otherwise specified; n/v, nausea and vomiting; p-ANCA, perinuclear anti-neutrophil cytoplasmic antibodies; PCA, posterior cerebral artery; PCom, posterior communicating artery; PICC, peripherally inserted central catheter; PT, prothrombin time; PTT, partial thromboplastin time; R, right; RUE, right upper extremity; rxn, reaction; SIRS, systemic inflammatory response syndrome; sx, symptoms; VP, ventriculoperitoneal; WBC, white blood cells.
Postprocedure sign or symptom suspected from polymer reaction.
Patient was found to have multifocal intracranial and extracranial polymer emboli and developed clinical vasculitis, coagulopathy, lymphadenopathy, and systemic inflammatory response syndrome.
Table 2.
Imaging findings in 32 patients with intracranial polymeric reactions
| Source | Age (y), sex |
Radiologic study | Location of abnormality | |
|---|---|---|---|---|
| 1 | Barnwell (1997) [4] | 53 F | CT | Basal ganglia/R cerebellar hemisphere |
| 2 | " | 66 M | MRI | B/l thalami and pons |
| 3 | " | 58 M | N/A | Pons |
| 4 | " | 53 F | N/A | Meningioma |
| 5 | Meyers (2004) [9] | 52 F | CT, MRI | Perianeurysmal region, ventricles |
| 6 | " | 46 F | CT, MRI | Perianeurysmal region, ventricles |
| 7 | Im (2007) [10] | 56 F | MRA | Ventricles |
| 8 | " | 66 F | N/A | CSF/ventricle |
| 9 | " | 68 M | MRI, Cath Ang | R parietal lobe, MCA at site of aneurysm |
| 10 | Fealey (2008) [11] | 58 F | MRI | R parietal lobe |
| 11 | Mehta (2009) [6] | 87 F | MRI, MRA | B/l cerebrum, cerebellum, pons |
| 12 | Mehta (2010) [7] | 36 F | MRI | B/l cerebral white matter and cortex |
| 13 | " | 54 M | CT, CTA, MRI, MRA | L cerebral hemisphere (MCA, PCA territories) |
| 14 | Skolarus (2010) [12] | 46 F | MRI | Occipital and parietal lobes |
| 15 | " | 56 F | MRI | Pons, midbrain, cerebellum, cerebral white matter |
| 16 | Hu (2014) [13] | 73 M | N/A | L cerebral hemisphere |
| 17 | " | 63 F | CT | L cerebral hemisphere |
| 18 | " | 66 F | N/A | L frontal lobe |
| 19 | Mehta (2015) [14] | 73 F | CT | Cerebral white matter, spinal cord |
| 20 | Grewal (2015) [15] | 65 F | MRI | R frontal, parietal white matter |
| 21 | Shapiro (2015) [16] | N/A | CT, MRI | R cerebral hemisphere |
| 22 | " | N/A | CT, MRI | R parieto-occipital lobe, R cerebral white matter |
| 23 | " | N/A | MRI | B/l cerebral hemispheres |
| 24 | " | N/A | CT, MRI, Cath Ang | R cerebral white matter |
| 25 | " | N/A | MRI | L MCA territory |
| 26 | Lorentzen (2015) [17] | 52F | CT, CTA, MRI, MRA | L cerebral hemisphere |
| 27 | Mehta (consult) | N/A | N/A | Cortex, NOS |
| 28 | " | N/A | N/A | Cortex, NOS |
| 29 | " | N/A | N/A | Sellar/parasellar |
| 30 | " | N/A | N/A | Cerebellar cortex |
| 31 | " | N/A | N/A | Cortex, NOS |
| 32 | " | N/A | N/A | Cortex, NOS |
Abbreviations: Cath Ang, cerebral catheter angiography; CT, computed tomography; CTA, computed tomography angiogram; MRA, magnetic resonance angiogram; MRI, magnetic resonance imaging.
3.1. Polymer injuries and deposition local to device implantation
Imaging studies revealed evidence of perianeurysmal inflammation, manifested as aneurysm wall thickening and enhancement, in 2 of 16 patients (13%) who underwent endovascular aneurysm treatment using polymer-coated coils [9]. Perianeurysmal parenchymal edema was present and involved the midbrain in 1 of these patients (6%) (Fig. 1). Additional arterial abnormalities identified on imaging studies included clot within the parent artery adjacent to an occluded aneurysm that was treated with polymer-coated coils (6%) [10]. Postmortem examination revealed abundant polymer materials within the cavernous internal carotid arteries, at the site of stent placement, in 2 of 8 patients (25%)who underwent flow diversion for aneurysm therapy [13].
Fig. 1.
Arteritis, with aseptic meningitis and communicating hydrocephalus. A 46-year-old woman (case 6) underwent endovascular coil embolization of an unruptured PCA aneurysm and subsequently developed thickening and enhancement of the aneurysm wall, perianeurysmal edema, and aseptic meningitis due to presumed polymer reaction (A and B, FLAIR; C, postcontrast T1-weighted MRI images; reprinted with permission from Wolters Kluwer Health, Inc. [9]). Postmortem histology (case 11) revealed marked meningeal vascular inflammation downstream from polymer emboli (D, H&E).
3.2. Polymer embolization with parenchymal inflammation
Imaging studies revealed inflammatory changes in brain parenchyma downstream from sites of intervention in 10 patients (31%); granulomas were found in 9 patients (28%), whereas sterile abscesses were found in 1 patient (3%) [11,12,15–17] (Fig. 2). Three additional patients (9%) showed evidence of microabscess formation on pathologic examination. In all patients with available histology, analysis confirmed inflammation centered around polymer emboli. Additional inflammatory responses identified on histopathologic examination included intravascular or perivascular histiocytic or giant cell reaction (25%) and granulomatous vasculitis (9%) [6,17]. Vasogenic edema in each case was variable in extent and progression.
Fig. 2.
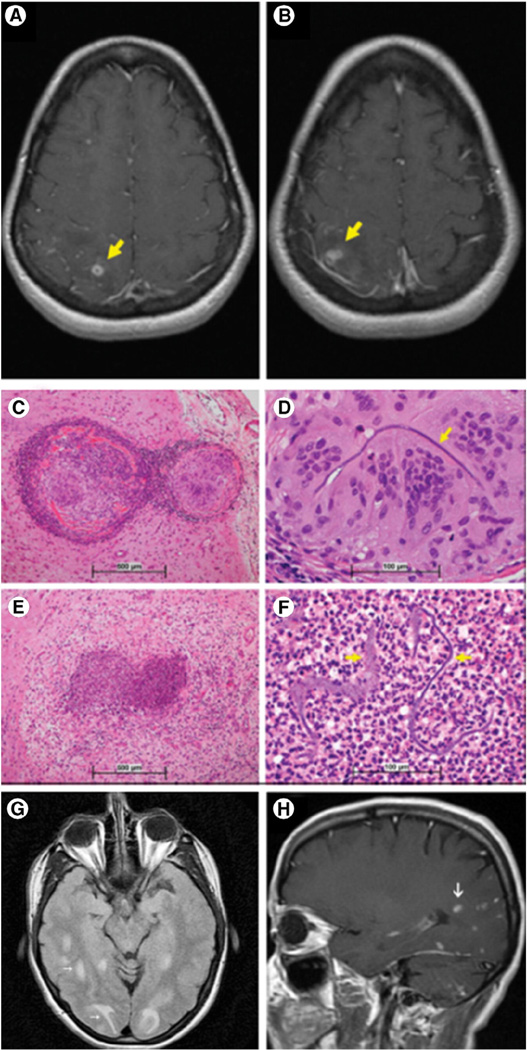
Sterile microabscess and granuloma formation, with foreign body inflammation. A 58-year-old woman (case 10) was treated via endovascular coil embolization for a supraclinoid right ICA aneurysm and subsequently developed parietal lobe ring-enhancing lesions with surrounding vasogenic edema. Biopsy confirmed necrotizing granulomatous inflammation and abscess, with foreign polymer material. (A and B, postcontrast T1-weighted MRI images; C–F, H&E; reprinted with permission from Wolters Kluwer Health, Inc. [11]). Another 46-year-old woman (case 14) developed symptomatic nodular enhancing lesions with associated vasogenic edema in the parietal and occipital lobes following basilar artery aneurysm coiling (G, T2-weighted and H, postcontrast T1-weighted MRI images; reprinted with permission from Elsevier [12]).
3.3. Polymer embolization with parenchymal infarct
Embolic polymer phenomena involved the brain in 21 patients (66%) and were associated with small artery occlusion in all cases [4,6,7,10–17]. Among other microvascular changes, there were associated internal elastic lamina disruption in 5 patients (16%), perivascular hemosiderin deposition in 5 patients (16%), fibrin thrombi in 4 patients (13%), intravascular fibrosis in 4 patients (13%), microthrombus formation in 3 patients (9%), and fibrinoid vascular necrosis in 3 patients (9%) (Table 1). Embolic polymer phenomena led to parenchymal hemorrhages in 9 patients (28%) [4,7,13,16] (Fig. 3), acute ischemic infarcts in 5 patients (16%) [4,6,7,10,12] (Fig. 4), and other white matter ischemic lesions in 4 patients (13%) [6,7,14]. Involved vessels measured 13 to 600 µm in diameter and varied in number from 2 to more than 100 per case. Large artery (middle cerebral artery or internal cerebral artery) narrowing due to suspected polymer emboli was present in 3 patients (9%) [6,7,16].
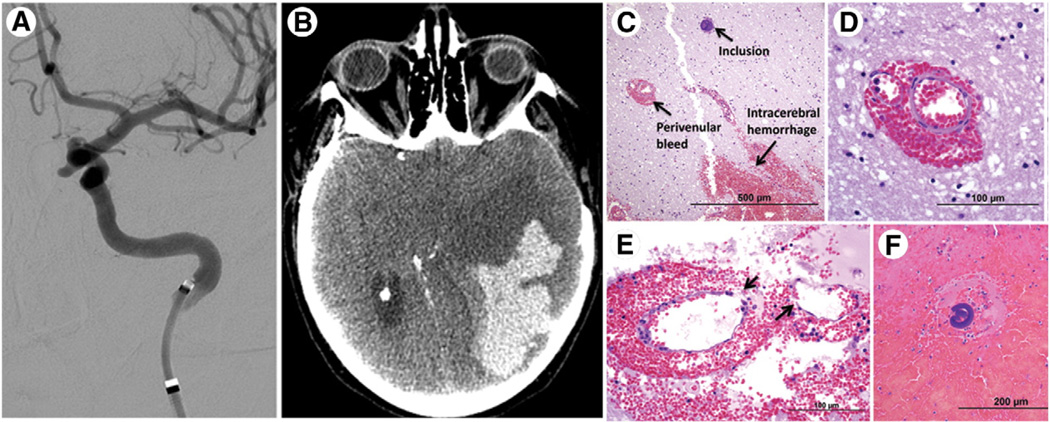
Fig. 3.
Parenchymal hematoma. A 63-year-old woman (case 17) underwent endovascular aneurysm flow diversion for a left paraophthalmic ICA aneurysm and developed a large ipsilateral parenchymal hematoma with associated brain herniation that led to her demise; autopsy revealed hemorrhagic cerebral infarcts due to occlusive polymer emboli (A, selective ICA catheter angiogram; B, noncontrast CT image; C–F, H&E; reprinted with permission from Rockwater, Inc. [13]).
Fig. 4.
Multifocal embolic infarcts. An 87-year-old woman (case 11) with a left ICA aneurysm was treated with coil embolization and subsequently died from multiple ischemic infarcts, which were most extensive within the left MCA/PCA territories. At autopsy, polymer emboli were present throughout the brain and were associated with intravascular inflammation, granulomas, fibrosis, and areas of infarcts, which led to her demise (A, CT angiogram image; B, selective ICA catheter angiogram; C and D, DWI MRI images; reprinted with permission from Elsevier [6]) (E–H, H&E; I, H&E; reprinted with permission from Nature Publishing Group [7]).
3.4. CSF and ventricular abnormalities
Seven patients (22%) developed aseptic chemical meningitis following aneurysm treatment with polymer-coated coils [6,9,10,15]. CSF analysis in 6 of these patients confirmed pleocytosis from presumed polymer reaction [9,10,15]. Focal perianeurysmal lymphocytic meningitis was identified on histologic examination in the remaining patient who had undergone coil embolization of a cerebral aneurysm and had multifocal cerebral polymer emboli proven at autopsy [6]. Delayed secondary communicating hydrocephalus occurred as a result of meningitis in 3 (43%) of these patients [9,10].
3.5. Histopathologic features of degrading polymer
On light microscopic examination of hematoxylin and eosin (H&E)–stained biopsy and autopsy specimens, polymer deposits were predominantly basophilic, granular, and lamellar or amorphous in morphology [7]. However, they exhibited marked variation in coloration and shape with progressive volume loss, eosinophilia and vesicular change (Fig. 5) [18]. Early stages of polymer degradation included macrophagic > giant cell > granulomatous > lymphocytic responses. Later stages of degradation, identified in patients with prolonged reactions, included microabscess formation and/or neutrophilic response [5,7,14,17]. Heterogeneous reactions were often noted [14]. The variable histologic appearances and distinct inflammatory reactions were consistent with active biodegradation of polymeric materials [7,14,18].
Fig. 5.
Intravascular biodegradation of polymer emboli. Polymer emboli undergo biodegradative changes with marked variation in histologic appearances. Note progressive alteration in coloration, size, and shape; concomitant inflammation (predominantly intravascular histiocytic, giant cell, and neutrophilic response); microthrombus formation and/or fibrous response (images from cases 11 and 19) (H&E).
4. Discussion
Polymeric substances are widely used as coatings on endovascular catheters and vascular devices. Their unique properties enhance lubrication and biocompatibility of device surfaces and have enabled several innovative endovascular technologies. Nonetheless, complications associated with their use have increasingly been recognized. Early reports documented shedding of polymer coats from device surfaces upon percutaneous vascular insertion and illustrated potential for significant inflammatory changes, such as ulceration at sites of dermal access [19,20]. Intravascular dissociation of polymer microparticles, with unanticipated hematogenous spread to vital organs distant from access sites, has subsequently been recognized [4,6,7,11,13,14,16,17]. Although the overall significance of these phenomena has been unclear, accumulating evidence suggest that these complications are underrecognized iatrogenic causes of morbidity and mortality [6,7,11,13,14].
Polymer emboli involving the brain were first documented among patients who underwent therapeutic cerebral angiography [4]. In this initial report, the polymer emboli were thought to be incidental; however, the contributing device (Fastracker-18 infusion microcatheter, Target Therapeutics) was promptly discontinued by the manufacturer. The first fatality due to polymer embolism was reported by our group in 2009 [6]. This and subsequent reports documented polymer effects in patients following diverse neurointerventional procedures such as cerebral angiography, aneurysm coil embolization, aneurysm flow diversion, and intraarterial thrombolysis [5,7,13,14, 16,17,21]. Also at risk for cerebral polymer injuries are patients who undergo pulmonary venous and left heart catheterization as well as those with congenital or acquired right-to-left cardiac shunts who are subjected to a variety of vascular interventions. Thus, a myriad of devices may elicit neurologic injuries by introducing intravascular particles of foreign coating materials or by causing chemical responses locally or downstream from sites of endovascular device manipulation or implantation.
In a recent hospital autopsy-based investigation, we showed that the histologic prevalence of polymer embolism to the vital organs was 13%, with central nervous system involvement in 1% [14]; however, due to polymer biodegradation and sampling limitations, this study likely underestimated the true frequency of cerebral polymer phenomena. Notably, this postmortem analysis did not encompass any patients who had undergone endovascular aneurysm therapy or other therapeutic cerebral angiographic procedure, and included only rare patients who were status post diagnostic cerebral angiogram [14]. The prevalence of intracerebral polymer complications would likely be significantly greater among patients who undergo neurointerventional procedures.
Individual reports and case series document pathologic effects associated with cerebral polymer phenomena however, a systematic review of these lesions and their outcomes has not been previously reported. In this study, we reviewed the literature and our archives and identified 3 primary patterns of cerebral polymer injuries: (1) inflammatory reactions, (2) embolic phenomena, and (3) arterial abnormalities [5,21]. Inflammatory sequelae occurred in 75% of patients and included large vessel arteritis, perianeurysmal inflammation, chemical meningitis, cerebral abscess, and granuloma formation [9–12,14–17]. Polymer embolic phenomena occurred in 66% of patients and were associated with multifocal ischemic or hemorrhagic infarcts that involved cerebral, cerebellar, brainstem, spinal, pituitary, or tumoral tissue downstream from sites of endovascular device utilization or implantation [4,6,7,10,12,13]. Infarcts either involved large territories or were small and scattered, depending upon the overall burden and size of polymer emboli and degree of secondary injuries. Inflammatory and embolic phenomena were most common at gray-white junctions, although scattered white matter lesions also occurred. Arterial pathologies were overall present in 75% of patients and further included large and/or small artery narrowing or obliteration [4,6,7,13,14,16,17], intraarterial thrombus formation [4,6,7], frank vasculitis (predominantly giant cell or granulomatous) [16], fibrinoid vascular necrosis [6,7,14], perivascular hemosiderin deposition [6], neovascularization [6], intravascular fibrous response [6,7], scattered fibrin thrombi [4], internal elastic lamina disruption, and perivascular rarefaction and gliosis [7,14]. Overall, the reactions were associated with an aberrant temporal pattern of inflammation, including early mononuclear and delayed neutrophilic reactions that are characteristic of polymer degradative change [14].
Ninety-seven percent of patients developed symptoms attributable to cerebral polymer phenomena. Presenting signs and symptoms included focal neurologic deficits (41%) [6,7,9–12,15–17], headache (22%) [9,10,16], constitutional symptoms (19%) [9,10,15], meningitis (16%) [9,10], seizure and involuntary movements (9%) [11,16], coma (6%) [4] and syncope (3%) [13]. Secondary reactions occurred within hours to days of contributing vascular procedures in 14 patients (44%). A notable finding was the delayed onset of symptoms, weeks to months following procedures, which occurred in 12 patients (38%). The majority of these patients exhibited delayed enhancing parenchymal lesions due to cerebral granulomas or abscesses downstream from sites of intervention. Delayed and persistent enhancing lesions, in our experience, may represent the most specific premortem radiologic pattern of polymer embolization. In 2 patients (6%), onset of symptoms occurred up to 9 months postprocedure [11,12]. Three patients (9%) developed persistent cerebral abscesses or granulomata with associated vasogenic edema and a waxing and waning radiologic course that persisted for up to 38 months [11,15,17]. One patient (3%) who exhibited multifocal intracranial and extracranial polymer microemboli at autopsy developed syndromic effects including clinical vasculitis, coagulopathy, and systemic inflammatory response syndrome that led to her demise [14]; 3 patients (9%) required ventriculoperitoneal shunt catheter placement for treatment of meningitis-induced communicating hydrocephalus [9,10]; 3 patients (9%) developed steroid or immunosuppressive dependency for management of symptomatic inflammation [15–17]; 2 patients (6%) suffered from steroid-induced adverse effects [16,17]; 5 patients (16%) died because of polymer-induced cerebral infarcts [6,13]; 3 additional patients who developed fatal cerebral infarcts (9%) exhibited polymer emboli at autopsy, within areas of infarcted brain [4]. In addition to increased morbidity and mortality, multiple patients required repeated imaging studies, additional surgical procedures, repeated hospitalizations, and long-term medical therapies and suffered long-term disability, which contributed to increased cost of medical care.
Mechanisms of polymer emboli formation are likely variable. The results of Fourier transform infrared spectroscopy indicate that polyvinylpyrrolidone, amaterial frequently used on sheaths and catheters, contributed to emboli formation in 2 patients [13]. Friction between devices, such as during coaxial, triaxial, or quadraxial catheterization techniques, is a likely precipitating factor. Excess friction between devices and vessel walls, such as during catheterization of tortuous or atherosclerotic vessels or during cannulation of distal small vessels, may also play a role. Repetitive scraping of coils against one another during pulsatile arterial flow has also been hypothesized to cause polymer dislodgement from treated aneurysms [11]. Because delayed reactions are noted exclusively in patients with implanted vascular devices such as stents, flow diverters, and aneurysm coils, it is possible that these harbor polymer materials originating from instruments used during their placement. Finally, manufacturing and patient-related factors are likely contributory. Avoidance of tight-fitting catheter and device combinations and dual groin punctures in favor of triaxial or quadraxial techniques are suggested for prevention [16]. A recent analysis suggests that microcatheter lumen aspiration following aneurysm coil deployment may reduce iatrogenic emboli [22]. Rapid withdrawal of catheters and guidewires should be avoided to prevent polymer dissociation. Proper use and storage of devices, and discontinuation of damaged devices are also advised. Steroids and immunomodulatory therapy (eg, mycophenolate and azathiprine) have been shown to effectively mitigate secondary inflammation, although they carry the risk of immunosuppressive side effects [9,10,15,16,17]. Ventriculoperitoneal shunt catheter placement has also been used to manage secondary hydrocephalus [9,10]. As no specific therapies are currently available, additional supportive measures should be implemented as needed.
This study highlights important features of intracranial polymer-induced complications (Table 3), although there are several limitations. Extent of tissue and CSF sampling was variable in each case, and inconsistencies in reporting necessitated omission of some reports from this analysis. Postprocedure imaging was not routinely performed, in some cases because patients had succumbed to death before imaging data could be obtained. As this was a retrospective investigation, detailed analysis of laboratory data was somewhat limited. In addition, long-term follow-up, including assessment of morbidity and efficacy of treatment modalities, was often unavailable. In spite of the limitations this review highlights clearly the need for numerous further investigations on the subject of iatrogenic polymer complications.
Table 3.
Summary of diagnosis, management and prevention of intracranial polymer-induced reactions
| Time of symptom onset: |
| Acute, subacute, or delayed postprocedure period(s) |
| Delayed ischemic or hemorrhagic infarcts in downstream treated vascular territories are suggestive |
| On postprocedure imaging, delayed enhancing inflammatory reactions in downstream treated vascular territories are highly suggestive, especially if persistent |
| Neuroradiologic or neuropathologic presentation(s): |
| Inflammatory reactions |
| Sterile perianeurysmal inflammation, sterile meningitis (ie, chemical meningitis), hydrocephalus |
| Sterile foreign body giant cell reaction, sterile granulomas (persistent/progressive), sterile abscesses |
| Embolic phenomena |
| Ischemic or hemorrhagic parenchymal infarcts |
| White matter changes |
| Arterial pathologies |
| Arterial obliteration, thrombosis, vasculitis, perivascular inflammation/rarefaction/hemosiderin, fibrinoid vascular necrosis, fibrin thrombi, luminal fibrosis, internal elastic lamina disruption |
| Supportive laboratory findings: |
| CSF: ↑ WBC, protein |
| Microbiology: cultures negative |
| Serum: ↑ WBC, ESR, CRP, p-ANCA, PT, PTT, D-dimer, FSP; ↓ fibrinogen |
| Identification of polymer on tissue sample: |
| H&E appearance: nonrefractile; nonpolarizable; predominantly basophilic, granular, and lamellated/ amorphous foreign body |
| Ancillary: Trichrome (blue), Congo red & mucicarmine (red) Spectroscopy: FTIR/Raman to identify polymer composition |
| Therapeutic options: |
| Steroids or immunosuppressives for inflammation, if indicated Surgical resection of persistent inflammatory lesion(s), if indicated Ventriculoperitoneal shunt for chemical meningitis, if indicated |
| Antiepileptics for seizure, if indicated |
| Antiplatelet therapy, if indicated |
| Supportive care |
| Preventative measures: |
| Avoidance of tight–fitting sheath/catheter/microcatheter /guidewire combinations |
| Use of dual groin punctures rather than triaxial/quadraxial or tight-fitting device combinations |
| Microcatheter lumen aspiration to reduce polymer microemboli |
| Avoidance of rapid withdrawal of guide wires and catheters |
| Discontinued use of damaged or deformed device(s) |
| Proper usage and storage of device(s) |
Abbreviation: FTIR, Fourier transform infrared.
5. Conclusion
Polymer coatings have played an important role in the evolution of endovascular technologies and are used in millions of clinical procedures performed worldwide each year. Despite their utility, a variety of adverse effects related to their use have been recognized and reported. Herein, we review the spectrum of cerebral injuries that result from unanticipated polymer-induced complications in the acute, subacute, and delayed postprocedure clinical settings. Unexpected outcomes include death, which has now been documented in several cases. Cerebral polymer complications manifest through distinct patterns of injury, including (1) inflammatory reactions, (2) embolic phenomena, and (3) arterial pathologies. A heightened awareness of these patterns will facilitate earlier detection and further clarify clinical risks. Future systematic reporting and prospective quantitative analyses will elucidate etiological factors and help guide manufacturers, polymer chemists, and regulatory agencies in improving the safety and efficacy of new and emerging endovascular and polymer technologies.
| Documented neuroradiological finding(s) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acute multifocal embolic infarcts |
Hydrocephalus | Parenchymal hematoma |
White matter lesions |
Perianeurysmal enhancement |
Perianeurysm wall thickening |
Perianeurysm parenchymal edema |
Clot at aneurysm site |
Arterial narrowing or occlusion |
Abscess (ring enhancement) |
Granuloma (nodular enhancement) |
| + | ||||||||||
| + | ||||||||||
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| + | + | + | ||||||||
| + | + | + | + | |||||||
| + | ||||||||||
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| + | + | |||||||||
| + | ||||||||||
| + | + | |||||||||
| + | ||||||||||
| + | + | + | ||||||||
| + | + | |||||||||
| + | + | + | ||||||||
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| + | ||||||||||
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| + | ||||||||||
| + | + | |||||||||
| + | + | |||||||||
| + | + | |||||||||
| + | ||||||||||
| + | + | + | ||||||||
| + | ||||||||||
| + | + | |||||||||
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
Acknowledgments
Funding/Support: This work was supported by the New York State/United University Professions Joint Labor-Management Committees to R. I. M. R. I. M. is also supported by a grant from the National Institute of Neurological Disorders and Stroke (K08NS089830).
Footnotes
Competing interests: none.
This work was presented, in part, at the 89th annual meeting of the American Association of Neuropathologists (Charleston, SC) on June 22, 2013, and in part at the 52nd annual meeting of the American Society of Neuroadiology (Montreal, Canada) on May 22, 2014.
References
- 1.Mann T, Cubeddu G, Bowen J, et al. Stenting in acute coronary syndromes: a comparison of radial versus femoral access sites. J Am Coll Cardiol. 1998;32:572–576. doi: 10.1016/s0735-1097(98)00288-5. [DOI] [PubMed] [Google Scholar]
- 2.Murayama Y, Tateshima S, Gonzalez NR, et al. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: long-term experimental study. Stroke. 2003;34:2031–2037. doi: 10.1161/01.STR.0000083394.33633.C2. [DOI] [PubMed] [Google Scholar]
- 3.Gaba RC, Ansari SA, Roy SS, et al. Embolization of intracranial aneurysms with hydrogel-coated coils versus inert platinum coils: effects on packing density, coil length and quantity, procedure performance, cost, length of hospital stay and durability of therapy. Stroke. 2006;37:1443–1450. doi: 10.1161/01.STR.0000221314.55144.0b. [DOI] [PubMed] [Google Scholar]
- 4.Barnwell SL, D'Agostino AN, Shapiro SL, et al. Foreign bodies in small arteries after use of an infusión microcatheter. Am J Neuroradiol. 1997;18:1886–1889. [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta RI, Mehta RI, Mukherjee A, Castellani RJ. Hydrophilic polymer embolism and associated vasculopathy of the brain (abstract) J Neuropathol Exp Neurol. 2013;73:576. [Google Scholar]
- 6.Mehta RI, Mehta RI, Fishbein MC, et al. Intravascular polymer material following coil embolization of a giant cerebral aneurysm. HUM PATHOL. 2009;40:1803–1807. doi: 10.1016/j.humpath.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta RI, Mehta RI, Solis OE, et al. Hydrophilic polymer emboli: an under-recognized iatrogenic cause of ischemia and infarct. Mod Pathol. 2010;23:921–930. doi: 10.1038/modpathol.2010.74. [DOI] [PubMed] [Google Scholar]
- 8.Mehta RI, Mehta RI, Chun Y. Hydrophilic polymer embolism: an underrecognized iatrogenic cause of ischemia, inflammation, and coagulopathy. HUM PATHOL. 2015;46:488–489. doi: 10.1016/j.humpath.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyers PM, Lavine SD, Fitzsimmons BR, et al. Chemical meningitis after cerebral aneurysmtreatment using two second-generation aneurysm coils: report of two cases. Neurosurgery. 2004;55:1222. doi: 10.1227/01.neu.0000140987.71791.df. [DOI] [PubMed] [Google Scholar]
- 10.Im SH, Han MH, Kwon BJ, et al. Aseptic meningitis after embolization of cerebral aneurysms using hydrogel-coated coils: report of three cases. Am J Neuroradiol. 2007;28:511–512. [PMC free article] [PubMed] [Google Scholar]
- 11.Fealey ME, Edwards WD, Giannini C, et al. Complications of endovascular polymers associated with vascular introducer sheaths and metallic coils in 3 patients, with literature review. Am J Surg Pathol. 2008;32:1310–1316. doi: 10.1097/PAS.0b013e318165582a. [DOI] [PubMed] [Google Scholar]
- 12.Skolarus LE, Gemmete JJ, Braley T, et al. Abnormal white matter changes after cerebral aneurysm treatment with polyglycolic-polylactic acid coils. World Neurosurg. 2010;74:640–644. doi: 10.1016/j.wneu.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Hu YC, Deshmukh VR, Albuquerque FC, et al. Histopathological assessment of fatal ipsilateral intraparenchymal hemorrhages after the treatment of supraclinoid aneurysms with the pipeline embolization device. J Neurosurg. 2014;120:365–374. doi: 10.3171/2013.11.JNS131599. [DOI] [PubMed] [Google Scholar]
- 14.Mehta RI, Mehta RI, Choi JM, Mukherjee A, Castellani RJ. Hydrophilic polymer embolism and associated vasculopathy of the lung: prevalence in a retrospective autopsy study. HUM PATHOL. 2015;46:191–201. doi: 10.1016/j.humpath.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grewal SS, López Del Valle EM, Gupta V, Ramon N, Freeman WD, Tawk RG. Neurological changes with abnormal brain reactivity following coiling of cerebral aneurysm. Possible reactivity to endovascular devices and material? J Vasc Interv Neurol. 2015;8:28–36. [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro M, Ollenschleger MD, Baccin C, et al. Foreign body emboli following cerebrovascular interventions: clinical, radiographic, and histopathologic features. Am J Neuroradiol. 2015;36:2121–2126. doi: 10.3174/ajnr.A4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorentzen AO, Nome T, Bakke SJ, et al. Cerebral foreign body reaction after carotid aneurysm stenting. Interv Neuroradiol. 2016;22:53–57. doi: 10.1177/1591019915609171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuki I, Uchiyama N, Murayama Y, et al. Intravascular tissue reactions induced by various types of bioabsorbable polymeric materials: correlation between the degradation profiles and corresponding tissue reactions. Neuroradiology. 2010;52:1017–1024. doi: 10.1007/s00234-010-0657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozak M, Adams DR, Ioffreda MD, et al. Sterile inflammation associated with transradial catheterization and hydrophilic sheaths. Catheter Cardiovasc Interv. 2003;59:207–213. doi: 10.1002/ccd.10522. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian R, White CJ, Sternbergh WC, III, et al. Nonhealing wound resulting from a foreign-body reaction to a radial arterial sheath. Catheter Cardiovasc Interv. 2003;59:205–206. doi: 10.1002/ccd.10468. [DOI] [PubMed] [Google Scholar]
- 21.Mehta RI, Mehta RI, et al. O-893-Iatrogenic reactions due to polymer coatings originating from vascular devices: neuroradiological findings with neuropathological correlation (abstract); ASNR 52nd Annual Meeting Proceedings; 2014. pp. 357–358. [Google Scholar]
- 22.Kim DY, Park JC, Kim JK, et al. Microembolism after endovascular treatment of unruptured cerebral aneurysms: reduction of its incidence by microcatheter lumen aspiration. Neurointervention. 2014;10:67–73. doi: 10.5469/neuroint.2015.10.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]