Abstract
Objective
The relationship between adipose tissue fibrosis, adipocyte hypertrophy, and preadipocyte hyperplasia in the context of obesity, and the correlation of these tissue-based phenomena with systemic metabolic disease are poorly defined. The goal of this study was to define clarify the relationship between adipose tissue fibrosis, adipocyte hypertrophy, and preadipocyte hyperplasia in human obesity and determine the correlation of these adipose-tissue based phenomena with diabetes.
Methods
Visceral and subcutaneous adipose tissues from humans with obesity collected during bariatric surgery were studied with QRTPCR, immunohistochemistry, and flow cytometry for expression of collagens and fibrosis-related proteins, adipocyte size, and preadipocyte frequency. Results were correlated with clinical characteristics including diabetes status.
Results
Fibrosis was decreased, hypertrophy was increased, and preadipocyte frequency and fibrotic gene expression were decreased in adipose tissues from diabetic subjects compared to non-diabetic subjects. These differences were greater in visceral compared to subcutaneous adipose tissue.
Conclusions
These data are consistent with the hypothesis that adipose tissue fibrosis in the context of human obesity limits adipocyte hypertrophy and is associated with a reciprocal increase in adipocyte hyperplasia, with beneficial effects on systemic metabolism. These findings suggest adipose tissue fibrosis as a potential target for manipulation of adipocyte metabolism.
Keywords: adipose tissue, diabetes, fibrosis, hypertrophy, preadipocyte
Introduction
Adipocyte hypertrophy is an adaptive response to nutrient excess that maintains adipose tissue nutrient buffering capacity and protects other tissues from lipotoxicity. The correlation between adipocyte hypertrophy and metabolic disease is context-dependent: lean humans with smaller adipocytes manifest a worse metabolic response to overfeeding,1 suggesting that in the lean state, increased adipocyte size is beneficial and a metric for nutrient buffering capacity. In some patients with obesity, however, a hypertrophic threshold may be reached beyond which adipocyte buffering capacity is exceeded, leading to ectopic lipid deposition in peripheral tissues. Consistent with this concept, extreme adipocyte hypertrophy in obesity correlates directly with BMI and metabolic disease in humans and mice.2-7 Hypertrophy has pleiotropic effects on adipocyte function. Hypoxia may ensue with expansion beyond a diameter of 100 microns, the tissue diffusion distance of oxygen, inducing hypoxic-response genes, endoplasmic reticulum and oxidative stress, inflammation, and metabolic dysfunction.
The processes that regulate adipocyte hypertrophy are poorly understood. Excess extracellular matrix (ECM) deposition in the form of fibrosis is a feature of obese adipose tissue, and a few reports demonstrate an inverse correlation between adipocyte size and fibrosis8-10, suggesting a role for fibrosis in negatively regulating adipocyte hypertrophy. Most reports demonstrate that adipose tissue ECM content is increased in human and murine obesity10-13, but the relationship between fibrosis and metabolic disease is less well-defined. Correlations between adipose tissue fibrosis and metabolic disease are consistently seen in murine obesity,13-16 but data from humans are conflicting.8-10,17,18 Like hypertrophy, the relationship between fibrosis and metabolic disease is BMI-dependent: in lean subjects, increased fibrosis correlates with elevated diabetes risk,21 suggesting that in the lean state, fibrosis-induced limitation of adipocyte hypertrophy is maladaptive, while in obesity these same fibrotic changes may be adaptive once a specific hypertrophic threshold is reached.
Preadipocyte hyperplasia balances hypertrophy to regulate adipose tissue homeostasis. Decreased preadipocyte proliferative capacity is associated with obesity and metabolic disease in some2,4,6,20,21 but not all studies,5,22-24 suggesting that hyperplasia and hypertrophy may be reciprocally regulated. These conflicting data may derive from differences in human and murine models and anatomic adipose tissue depots studied. No reports have evaluated preadipocyte hyperplasia as a function of diabetes status in humans. The contribution of preadipocyte hyperplasia to metabolic disease and its relationship to adipose tissue fibrosis and adipocyte hypertrophy is not well-established.
The goal of this study was to clarify the relationship between adipocyte hypertrophy, adipose tissue fibrosis, and preadipocyte hyperplasia in humans with obesity, and determine the correlation of these adipose tissue-based characteristics with diabetes. We hypothesized that adipocyte hypertrophy and adipose tissue fibrosis would be positively associated with human diabetes, similar to preclinical models. We instead demonstrate decreased fibrosis in diabetic (DM) humans, along with increased adipocyte hypertrophy and decreased preadipocyte frequency. Our findings support a model in which decreased adipose tissue fibrosis in metabolic disease is associated with a tissue architecture that permits adipocyte hypertrophy and limits preadipocyte hyperplasia, leading to larger, metabolically-impaired adipocytes. These observations suggest that adipose tissue fibrosis may be beneficial in human obesity with respect to metabolic disease.
Methods
Human subjects
Human subjects undergoing bariatric surgery were enrolled with Institutional Review Board approval from the University of Michigan and Ann Arbor Veteran's Administration Hospital. Visceral adipose tissue (VAT) from the greater omentum and subcutaneous adipose tissue (SAT) from the abdominal skin incision were collected from 82 subjects at the beginning of operation and processed immediately. Diabetic (DM) subjects were defined by clinical diagnosis of type 2 diabetes requiring treatment with medication. Non-diabetic (NDM) subjects were defined by no clinical history of diabetes, normal HbA1c, and no diabetes-related medication use. Subjects with a diagnosis of pre-diabetes/insulin resistance and/or an elevated % hemoglobin A1c (HbA1c) but not treated with diabetes-related medication, or with type 1 diabetes, were excluded.
QRTPCR
Equal amounts of RNA from adipose tissue, SVF, preadipocytes, or adipocytes was reverse-transcribed and studied with quantitative real-time polymerase chain reaction (QRTPCR) using Taqman primer-probes (Life Technologies Inc., Carlsbad, CA, USA), including actin as an endogenous control, on a StepOnePlus thermocycler (Applied Biosystems Inc., Foster City, CA, USA).
Adipose tissue fixation
Adipose tissue explants were fixed in 10% formalin, embedded in paraffin, and sectioned (5μm) onto charged glass slides and heat-treated for antigen retrieval. Subsequent analyses were performed by an observer blinded to patient clinical information.
Sirius Red staining
Slides were deparaffinized in xylene/ethanol, stained with Sirius Red/Fast Green dye (Chondrex Inc., Redmond, WA, USA) and visualized on an Olympus BX-51 inverted microscope with bright-field and plane-polarized light. Staining intensities of multiple 10× fields of identical size were analyzed from multiple slides from each sample, and staining normalized to tissue area i.e. total number of fields analyzed, quantified with ImageJ software, and averaged for each patient.
Adipocyte sizing
Fixed hematoxylin/eosin-stained slides were imaged on an Olympus IX-81 fluorescent microscope, captured as multiple TIFF-gray-scale images and analyzed with ImageJ software. Pixel areas of all individual cells were averaged for each patient. For sizing in fibrotic and non-fibrotic areas of tissue, fibrotic areas were chosen that contained visible bands of fibrosis and/or thickened intracellular spaces, while non-fibrotic areas chosen contained little or no fibrotic bands and closely-packed adipocytes. For each slide, six separate fibrotic and non-fibrotic areas were analyzed.
Immunohistochemistry
Slides were incubated overnight with primary antibodies to collagens 1a1, 3a1, 6a1, HIF-1α, or GLUT-1 (Thermo-Scientific Inc., Waltham, MA, USA), washed, incubated with secondary antibody (goat anti-rabbit Alexa-Fluor 488, Life Technologies Inc., Carlsbad, CA, USA), and coverslips mounted. At least six images captured per slide in grey-scale on an Olympus IX-81 fluorescent microscope using 10XB objective were analyzed using ImageJ Software. HIF-1α and GLUT-1 signals were normalized to adipocyte number; collagen signals were normalized to tissue area.
Stromal-vascular cell fraction (SVF), preadipocyte, and mature adipocyte isolation, flow cytometry
Adipose tissue was digested with Type II collagenase (175 units/ml PBS/2% BSA, Life Technologies Inc., Carlsbad, CA, USA) 60 minutes, 37°C, centrifuged, and the SVF cell pellet used for RNA or flow cytometry. Mature adipocytes were harvested from the adipocyte layer of collagenase-digested tissue followed by RNA isolation. Preadipocytes were isolated as described25: SVF were plated overnight, non-adherent cells discarded, and adherent cells passaged three times to enrich for preadipocytes followed by RNA isolation. Such cells demonstrate adipocyte differentiation capacity and retain depot- and patient-specific characteristics26.
For flow cytometry, SVF cells were stained with viable dye and antibodies and analyzed on a FACSCanto II flow cytometer (Becton-Dickinson Inc., Franklin Lakes, NJ, USA). Data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA) after exclusion of doublets and non-viable cells using fluorescence-minus-one controls with forward scatter/side scatter gates encompassing all cells with subsequent analysis of CD45- cells. Preadipocytes were defined as CD45-CD34+CD31-. Antibodies: CD45-FITC, CD31-APC-Cy7, CD34-PERCP-Cy5.5 (Biolegend Inc., San Diego, CA, USA), anti-rabbit secondary antibody-PE (Life Technologies, Inc., Carlsbad, CA, USA); CD140a-AlexaFluor 647 (BD Biosciences, Inc., San Jose, CA, USA).
Statistical analysis
Independent t-test was used to compare data between groups. Delta CT values were compared for QRTPCR data. Linear regression was used to determine correlations. Fisher's exact test was used to compare dichotomous patient demographic variables. Analysis of covariance using general linear models was used to compare adipocyte size, fibrosis, and preadipocyte frequency adjusting for age. Error bars on all figures represent standard error of the mean Due to limited tissue amounts, not all subjects were used for all experiments.
Results
Diabetes is associated with increased adipocyte hypertrophy in humans with obesity
To evaluate the relationship between adipocyte hypertrophy and diabetes, we examined VAT and SAT from DM and NDM subjects with obesity (Table 1). BMI was similar between subject groups; DM subjects were older than NDM subjects.
Table 1. Subject demographics.
| DM (n=34) | NDM (n=48) | p-value | |
|---|---|---|---|
| Demographics, lab values | |||
| Gender (F/M, n) | 21/13 | 38/10 | 0.133 |
| Age (mean, years) | 49 | 41 | 0.002 |
| BMI (mean, kg/m2) | 47 | 47 | 0.619 |
| HbA1c (mean, %) | 7.1 | 5.8 | <0.001 |
| Fasting plasma glucose (mean, mg/dl) | 131 | 97 | <0.001 |
| Comorbid disease | |||
| Sleep apnea (n) | 27 | 22 | 0.003 |
| Hypertension (n) | 27 | 22 | 0.003 |
| Dyslipidemia (n) | 20 | 14 | 0.007 |
| Medication use | |||
| Beta-blocker (n) | 6 | 9 | 1.000 |
| Statin (n) | 17 | 5 | <0.001 |
| ACE inhibitor (n) | 8 | 6 | 0.239 |
| Thiazolidinedione (n) | 2 | 0 | 0.169 |
| Metformin (n) | 32 | 0 | <0.001 |
| Insulin (n) | 14 | 0 | <0.001 |
| Sulfonylurea (n) | 10 | 0 | <0.001 |
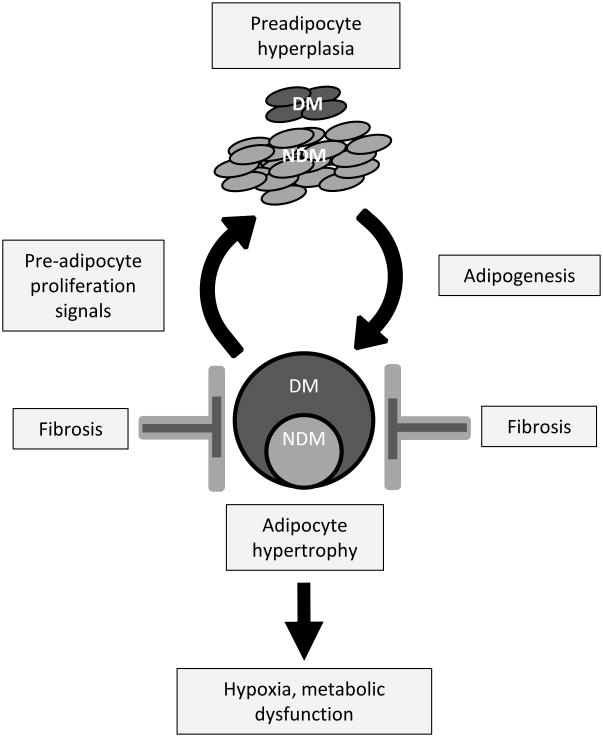
Age-adjusted mean adipocyte cross-sectional area was greater in DM compared to NDM subjects in VAT and SAT (Figure 1A). Adipocytes were larger in SAT than VAT, consistent with prior data.27,28 Linear regression analysis of all subjects (DM+NDM) demonstrated positive correlations between adipocyte size and HbA1c in VAT and SAT (Figure 1B). Linear regression analysis demonstrated no correlations between adipocyte size in VAT or SAT and age (data not shown). These data confirm a positive correlation between adipocyte hypertrophy and DM, consistent with prior studies.2,3,5-7
Figure 1. Adipocyte hypertrophy is increased in DM humans with obesity.
A. Above left: Age-adjusted adipocyte area in VAT and SAT in DM and NDM subjects; ordinate: mean adipocyte area, μm2; *: p<0.050 comparing DM and NDM groups. Below left: Representative VAT adipocyte sizing images from DM, NDM subjects; Right: Distribution of adipocyte size in VAT and SAT for DM and NDM subgroups, abscissas: adipocyte area, μm2; n=22 DM, 29 NDM subjects for VAT, and n=10 DM, 10 NDM subjects for SAT.
B. Linear regression analysis correlating adipocyte area with preoperative HbA1c (%).
C. Quantified HIF-1α, GLUT-1 immunohistochemistry staining intensity in VAT, SAT, ordinate: immunohistochemistry staining intensity normalized to adipocyte number, arbitrary units (A.U.); *: p<0.050 comparing DM and NDM groups; n=10 DM, 10 NDM subjects for VAT and SAT.
D. Representative HIF-1α and GLUT-1 Immunohistochemistry images (HIF-1α, GLUT-1: green) of SAT from DM and NDM subjects.
Since adipocyte hypertrophy may be associated with hypoxia, we studied hypoxia-inducible gene expression with immunohistochemistry. HIF-1α and GLUT-1 expression was increased in DM compared with NDM subjects in SAT but not VAT (Figure 1C, D). These findings suggest that SAT, given its larger adipocyte size, may be more susceptible to hypoxia-inducible gene expression than VAT, and that DM patients may have an exaggerated hypoxia-inducible gene expression response to hypertrophy, possibly due to larger adipocyte size.
Diabetes is associated with decreased adipose tissue fibrosis in humans with obesity
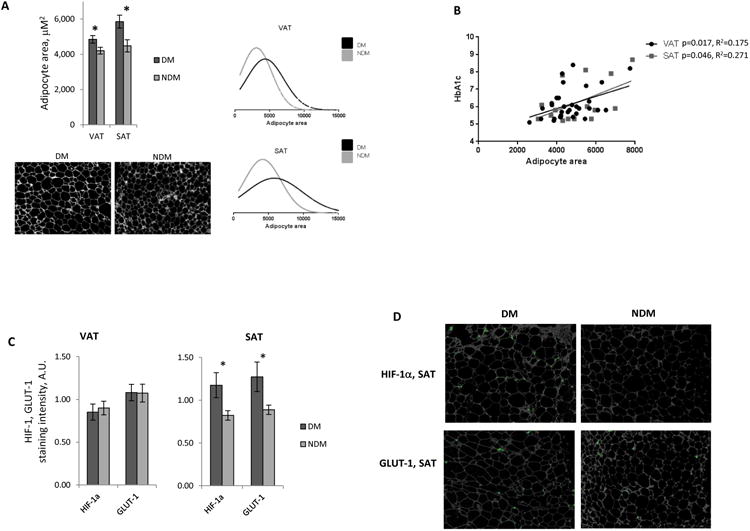
To determine the relationship between fibrosis and DM status, we evaluated ECM deposition in adipose tissues. Age-adjusted ECM deposition measured histologically by Sirius Red staining was lower in VAT and SAT from DM subjects compared to NDM subjects (Figure 2A). Immunohistochemistry revealed a trend towards lower COL1A1 protein levels in VAT from DM subjects, and lower COL3A1 protein levels in SAT from DM subjects (Figure 2B). Transcript levels of fibrotic genes COL1A1, COL6A1, LOX, MMP2, and TIMP-1 were lower, and MMP9 higher, in VAT from DM compared to NDM subjects; no differences in fibrotic transcript levels were observed between DM and NDM subjects in SAT (Figure 2C). Linear regression analysis of all subjects (DM+NDM) revealed that in VAT, COL1A1, COL3A1, COL6A1, and LOX expression correlated inversely with HbA1c (Figure 2D). No correlations were observed between SAT fibrotic transcripts and HbA1c, between fibrosis-related transcripts in VAT or SAT and age or BMI, or between Sirius Red staining in VAT or SAT and age, BMI, or HbA1c (data not shown). Overall, these data suggest that a decreased fibrotic profile in VAT and SAT associated with DM status in humans with obesity.
Figure 2. Adipose tissue fibrosis is decreased in DM humans with obesity.
A. Sirius Red staining of human adipose tissue: left: quantified age-adjusted Sirius Red staining, ordinate: Sirius Red staining intensity, arbitrary intensity units (A.U.); *: p<0.005 comparing DM and NDM groups; right: representative Sirius Red-stained VAT from DM, NDM subjects; n=17 DM, 19 NDM subjects for VAT, and n=9 DM, 9 NDM subjects for SAT.
B. Immunohistochemistry for collagens 1, 3, and 6: top: quantified collagen staining intensity in VAT, SAT, ordinate: arbitrary intensity units; *: p<0.050, **: p<0.100 comparing DM and NDM groups; bottom: representative immunohistochemistry images of collagen 1-stained VAT and collagen 3-stained SAT from DM, NDM subjects; n=10 DM, 10 NDM subjects for VAT and SAT.
C. QRTPCR data from VAT, SAT whole tissue RNA; ordinate: fold difference in transcript levels in DM subjects relative to NDM subject group referent=1; *: p<0.050 comparing DM and NDM groups; n=12 DM, 15 NDM subjects for VAT, n=8 DM, 9 NDM subjects for SAT.
D. Linear regression analysis correlating HbA1c (%) with VAT fibrotic transcripts; ordinates: mean fold difference in transcript levels in DM subjects relative to mean transcript level in NDM subject group referent; all correlations with p<0.150 shown.
Adipocyte hypertrophy correlates inversely with fibrosis
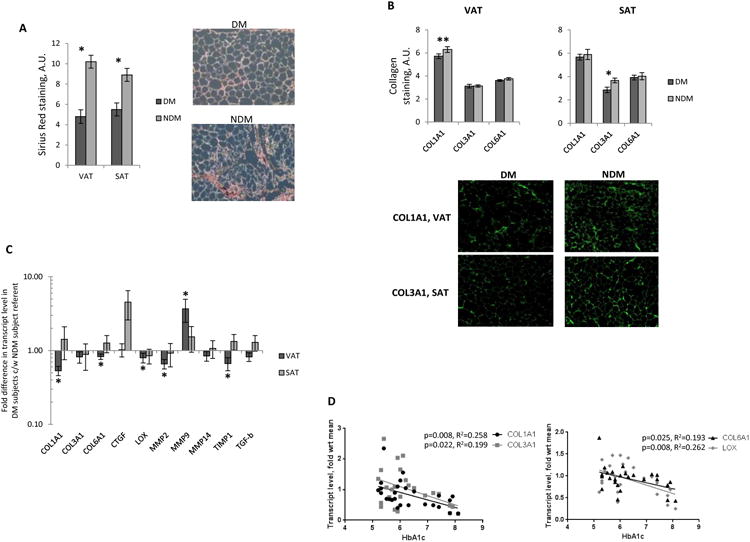
We next studied the relationship between adipocyte hypertrophy and adipose tissue fibrosis. Based on Sirius Red staining, adipocytes were smaller in fibrotic compared to non-fibrotic regions of tissue in DM and NDM subjects (Figure 3A). Linear regression analysis of all subjects (DM+NDM) revealed trends towards an inverse correlation between adipocyte size and fibrosis measured by Sirius Red staining in VAT and SAT (Figure 3B), as well as inverse correlations between adipocyte size and COL1A1, COL6A1, MMP2, MMP14, and TIMP-1 transcript levels, and a positive correlation between adipocyte size and MMP9 in VAT (Figure 3C). No correlations were observed between adipocyte size and COL3A1, CTGF, LOX, and TGF-β transcript levels in VAT; adipocyte size did not correlate with fibrotic transcript levels in SAT (data not shown). These associations support the concept that increased adipose tissue fibrosis restrains adipocyte hypertrophy in NDM patients, and that decreased fibrosis is permissive to adipocyte hypertrophy in DM patients.
Figure 3. Adipocyte hypertrophy correlates inversely with fibrosis in human adipose tissue.
A. Left: Representative images of non-fibrotic (top) and fibrotic (bottom) area of VAT; Right: Adipocyte area in fibrotic (F) and non-fibrotic (NF) areas of tissue; ordinate: mean adipocyte area, mM2; *: p<0.001 comparing adipocyte size in fibrotic and non-fibrotic areas; n=17 DM, 19 NDM subjects for VAT, and n=9 DM, 9 NDM subjects for SAT.
B. Linear regression analysis correlating adipocyte area with Sirius Red staining A.U. in VAT and SAT.
C. Linear regression analysis correlating adipocyte area with fibrotic transcript levels in VAT; ordinates: mean fold difference in transcript levels in DM subjects relative to mean transcript level in NDM subject group referent; all correlations with p<0.150 shown.
D. QRTPCR data from VAT, SAT whole tissue RNA; ordinate: fold difference in transcript levels in DM subjects relative to NDM subject group referent=1; *: p<0.050 comparing DM and NDM groups; n=12 DM, 10 NDM subjects for VAT, n=7 DM, 9 NDM subjects for SAT.
To explore the contribution of alterations in inflammation to fibrosis, we studied inflammation-related gene transcription in adipose tissues. Transcript levels of IL-4 were decreased in VAT but not SAT from DM subjects; no differences in macrophage-related or other inflammatory cytokine gene transcript levels were observed (Figure 3D).
Diabetes is associated with decreased preadipocyte frequency
To evaluate the hypothesis that deficits in adipogenic capacity contribute to diabetes, we studied preadipocyte frequencies in adipose tissues with flow cytometry (Figure 4A). Preadipocytes are defined as CD45-CD31-CD34+, within which CD140a+ defines a particularly adipogenic lineage.29, 30 Age-adjusted total preadipocyte and CD140a+ preadipocyte subpopulation frequencies in DM subjects compared to NDM subjects were decreased in VAT but not SAT. No differences were observed in the frequencies of CD140a+/CD140a- preadipocyte subpopulations as a percentage of total preadipocytes between DM and NDM subjects in VAT or SAT (Figure 4B). Linear regression analysis of all subjects (DM+NDM) revealed that preadipocyte frequency in VAT but not SAT correlated inversely with HBA1c (Figure 4C). Preadipocyte frequency in VAT and SAT did not correlate with age or BMI (data not shown). These data support a deficit in preadipocyte quantity in DM subjects.
Figure 4. Preadipocyte frequency and fibrotic gene expression is decreased in DM humans with obesity.
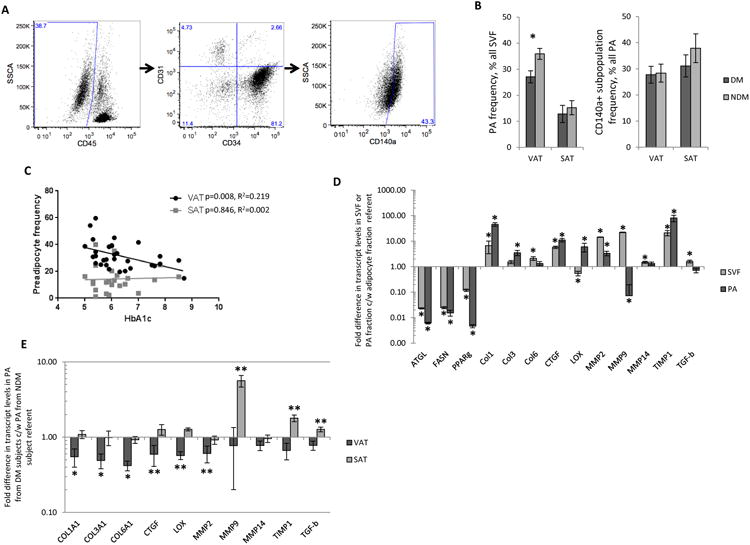
A. Representative scatterplots of VAT SVF demonstrating the flow cytometry gating strategy for preadipocytes (PA, CD45-CD31-CD34+); a large forward scatter-side scatter gate was used to encompass all viable cells (not shown), followed by gating on CD45- cells, followed by gating on CD31-CD34+ cells; the CD140a+ subpopulation within the CD31-CD34+ population was also analyzed.
B. Age-adjusted preadipocyte frequencies in human adipose tissues; ordinates: left: % of all viable SVF cells that are preadipocytes (PA, CD45-CD31-CD34+), right: % all PA that are CD140+; *: p<0.001 comparing DM and NDM groups; n=15 DM, 19 NDM subjects for VAT, n=10 DM, 14 NDM subjects for SAT.
C. Linear regression analysis correlating CD45-CD31-CD34+ preadipocyte frequencies (% of all SVF cells) in VAT and SAT with HbA1c (%).
D. QRTPCR data from RNA from mature adipocyte, SVF, and preadipocyte cell fractions from VAT; ordinate: fold difference in VAT transcript levels in SVF and PA fractions relative to mature adipocyte cell fraction referent=1; *: p<0.050 comparing SVF or PA fraction to adipocyte fraction referent; n=6 NDM subjects.
E. QRTPCR data from VAT and SAT preadipocyte RNA; ordinate: fold difference in transcript levels in PA from DM subjects relative to PA from NDM subject group referent=1; *: p<0.050, **: p<0.100 comparing transcript levels in PA from DM subjects relative to PA from NDM subject group referent; n=8 DM, 8 NDM subjects for VAT, n=6 DM, 10 NDM subjects for SAT.
To determine the primary source of fibrotic molecules in adipose tissue, transcript levels were compared between mature adipocyte, SVF, and preadipocyte cell fractions from VAT from NDM subjects. Transcript levels of adipocyte-specific genes ATGL, FASN, and PPAR-γ were highest in mature adipocytes, as expected. Transcript levels of COL1A1, COL3A1, CTGF, LOX, and TIMP-1 were highest in the preadipocyte fraction. Transcript levels of COL6A1, MMP2, MMP9, MMP14, and TGF-b were highest in SVF, suggesting that cells other than or in addition to preadipocytes within SVF contribute to expression of these molecules (Figure 4D).
To determine if decreased fibrosis in DM is due to decreased preadipocyte expression of fibrotic molecules or rather simply due to decreased preadipocyte numbers, transcript levels of fibrotic molecules were compared in identical amounts of input RNA from identical numbers of preadipocytes from VAT and SAT from DM and NDM subjects. Expression of COL1A1, COL3A1, and COL6A1 were decreased in VAT preadipocytes from DM subjects compared to NDM subjects, along with trends towards decreased CTGF, LOX, and MMP2 expression. In SAT preadipocytes in contrast, transcript levels of MMP9, TIMP-1, and TGF-β were increased in DM subjects compared to NDM subjects, although these differences only approached significance (Figure 4E).
Discussion
Adipocyte hypertrophy is a recognized feature of dysfunctional adipose tissue that is associated with increased cellular stress, decreased metabolic flexibility, and systemic diabetes. The mechanisms that regulate hypertrophy are unclear. In this study, we identify two potential features of DM patients that may contribute to increased adipocyte hypertrophy: (1) decreased adipose tissue fibrosis and (2) a smaller preadipocyte pool that limits the generation of new adipocytes.
Data from humans addressing the correlation between adipose tissue fibrosis and metabolic disease are conflicting.8-10,13,17,18 Our data add to this literature and support the concept of fibrosis as an adaptive feature that preserves normal adipocyte function by restricting hypertrophy. Lackey et al. demonstrated decreased tensile strength and collagen expression in VAT from metabolically unhealthy compared to metabolically healthy patients with obesity.18 Divoux et al. observed an inverse correlation between VAT fibrosis and adipocyte size and serum hypertriglyceridemia in patients with obesity, but no correlation of fibrosis with glycemic parameters.9 These reports suggest a negative correlation between adipose tissue fibrosis and at least some aspects of metabolic disease, consistent with our findings. In contrast, Spencer et al. demonstrated that insulin resistance correlates directly with SAT fibrosis in NDM humans,10 while Vila et al. demonstrated increased fibrosis-related transcripts in SAT from humans with obesity, with highest levels in patients with metabolic syndrome.13 Finally Abdennour et al. observed increased SAT stiffness using tissue elastography in DM compared to NDM subjects, but no difference in immunohistochemical measures of fibrosis.17 Interpretation of this conflicting literature is complicated by subject heterogeneity and differences in tissue depots studied, definitions of metabolic disease, and methodologies and molecular targets used to measure fibrosis. In contrast to some studies, we used clinical diagnosis to define diabetes rather than provocative testing (e.g. glucose tolerance testing). Many reports, ours included, study bariatric surgery patients in whom it is common to optimize diabetes prior to surgery, a practice that normalizes serum measures of insulin resistance such as HbA1c, insulin, and glucose levels, confounding stratification of DM and NDM groups. An advantage of stratification by clinical diagnosis is capture of patients with clinically significant diabetes that might be masked by preoperative optimization of glucose homeostasis. In support of this, we did not observe correlations between preoperative HbA1c and fibrosis measured by Sirius Red staining despite a strong correlation of fibrosis with DM status, likely because HbA1c is at least partially corrected prior to surgery in many patients. We did however observe a negative correlation between transcript levels of specific collagens within VAT and HbA1c, suggesting that specific molecular targets may provide more specific and sensitive measures of tissue fibrosis in the context of metabolic dysfunction.
We observed depot-specific differences in fibrosis. Sirius Red staining was similar between VAT and SAT in DM and NDM subjects. However, fibrotic transcripts were decreased in DM subjects and correlated with HbA1c levels in VAT but not SAT. Furthermore, reduced Sirius Red staining in DM subjects was associated with decreased collagen 1 immunohistochemical staining in VAT but decreased collagen 3 staining in SAT; these differences were modest compared to DM/NDM differences in Sirius Red staining, suggesting that other collagens or fibrotic molecules contribute to overall differences in ECM deposition. Taken together, these data suggest qualitatively different mechanisms of fibrosis in VAT and SAT, and suggest that reduced fibrosis in VAT plays a more important role in diabetes, consistent with the well-established stronger association of diabetes with visceral rather than subcutaneous adiposity.
Adipocyte hypertrophy was increased in DM subjects with obesity, consistent with prior data.3,5,6 At the upper limits of adipocyte size, a threshold may be reached beyond which further hypertrophy impairs adipocyte metabolism. It has been suggested that adipose tissue fibrosis may be an adaptive response that limits extreme hypertrophy beyond this threshold, reducing adipocyte metabolic dysfunction.9 Differences in the adipose tissue fibrotic response among humans may therefore contribute to differing susceptibilities to metabolic disease. In our study, the observed positive correlation of hypertrophy with diabetes and inverse correlation with fibrosis support this hypothesis, and are consistent with multiple prior data that separately demonstrate similar relationships between adipocyte size and diabetes/insulin resistance,2-6,31 and between adipocyte size and fibrosis.8-10
Cellular hypoxia secondary to an oxygen diffusion defect is a putative mechanism by which hypertrophy induces adipocyte dysfunction.15, 32 In support of this concept, we demonstrate increased HIF-1α and GLUT-1 expression in DM subjects in SAT but not VAT, possibly because adipocytes are smaller in VAT and below the threshold for hypoxic gene expression. Adipose tissue hypoxia is controversial33, HIF-1α and GLUT-1 are regulated by hypoxia-independent mechanisms, and expression of hypoxia-related genes does not fully capture the pleiotropic effects of hypoxia on adipocyte function. Nonetheless, our data suggest a link between hypoxic gene expression and hypertrophy in the context of DM at the upper limits of adipocyte size in SAT but not in VAT.
We demonstrate decreased preadipocyte frequency in VAT from DM subjects in whom adipocytes are larger, consistent with previous data suggesting a reciprocal relationship between adipocyte hypertrophy and preadipocyte hyperplasia.2,4,6,20,21 No differences in relative proportions of CD140a+ cells within the preadipocyte population were observed between DM and NDM subjects, suggesting that this adipogenic subpopulation is down-regulated in DM in a non-disproportionate manner. No difference in preadipocyte frequency between DM and NDM subjects was observed in SAT, and the inverse correlation between preadipocyte frequency in VAT and HbA1c was not observed in SAT, reinforcing the dominance of VAT in dictating systemic metabolic phenotype.
Collagen transcript levels were decreased in purified VAT preadipocytes from DM relative to NDM subjects, paralleling whole tissue transcript data and suggesting that in addition to decreased numbers, a qualitative defect in preadipocyte fibrotic gene expression contributes to decreased VAT fibrosis in DM. While similar differences in expression of collagens were not observed in purified SAT preadipocytes, transcript levels of MMP9, TIMP-1, and TGF-β were increased in these cells from DM subjects. Further research will be required to corroborate these changes at the protein level and elucidate other changes in preadipocytes, but these data confirm that functional changes in preadipocytes contribute to the adipose tissue fibrotic milieu in diabetes, along with qualitative depot-specific differences.
Functionalities of fibrotic mediators are complex. Nonetheless, the observed decreased expression of LOX and TIMP-1 in DM VAT, generally considered pro-fibrotic mediators, is consistent with decreased fibrosis. We also observed decreased IL-4 transcript levels in DM VAT, but no differences in transcript levels of other inflammatory genes. Detailed study with more sensitive assays (e.g. flow cytometry, ELISA) will be necessary to elucidate the role of cytokines, macrophages, and other leukocytes in regulating adipose tissue fibrosis, but these initial data suggest a possible pro-fibrotic role for IL-4 within adipose tissue, consistent with similar functionality demonstrated in other tissues34,35. Caution must be exercised in extrapolating function from transcriptional data. Nonetheless, differentially expressed mediators represent targets for future studies.
Limitations in study population size precluded controlling for all potential confounders. DM subjects were older than NDM subjects but differences in adipocyte size, fibrosis, and preadipocyte frequency remained significant after adjusting for age, and regression analysis revealed no correlations of these measures with age. The prevalence of sleep apnea, hypertension, dyslipidemia, and medication use was higher in DM subjects, the latter of particular importance given that metformin and thiazolidinediones may attenuate fibrosis36,37. Larger studies will be necessary to rigorously address these and other potential confounders.
We demonstrate increased adipocyte hypertrophy and decreased adipose tissue fibrosis and preadipocyte frequency in DM humans with obesity. These data support a model in which adipose tissue fibrosis regulates the reciprocal balance between adipocyte hypertrophy and preadipocyte hyperplasia, governing adipose tissue expansion and systemic metabolic responses to obesity (Figure 5). Taken together our findings suggest that adipose tissue fibrosis plays a protective role with respect to diabetes in obesity and represents a target for manipulation of adipose tissue physiology and systemic metabolism.
Figure 5. Fibrosis regulates the balance between adipose tissue hyperplasia and hypertrophy.
Increased fibrosis in NDM adipose tissue constrains adipocyte hypertrophy, limiting adipocyte hypoxia and metabolic dysfunction, and providing signals that promote preadipocyte proliferation to maintain adipose tissue homeostasis. In DM tissue in contrast, decreased fibrosis permits increased adipocyte hypertrophy, resulting in adipocyte hypoxia and metabolic dysfunction, along with signals that inhibit preadipocyte proliferation.
What is known.
Adipocyte hypertrophy and adipose tissue fibrosis are established features of obese adipose tissue.
The relationship between adipose tissue fibrosis and diabetes is unclear, with discordance between murine and human data.
Adipose tissue homeostasis is dictated in part by the balance between adipocyte hypertrophy and preadipocyte hyperplasia.
What this study adds.
Measures of adipose tissue fibrosis correlate inversely with diabetes in humans with obesity.
Preadipocyte frequency and fibrotic gene expression is decreased and adipocyte hypertrophy is increased in visceral adipose tissue in diabetic humans with obesity.
Taken together, these findings suggest a protective role for adipose tissue fibrosis with respect to diabetes, and reinforce a reciprocal relationship between adipocyte hypertrophy and preadipocyte hyperplasia.
Acknowledgments
Funding: RWO: NIH grant R01DK097449, Michigan Metabolomics-Obesity Center/Michigan Nutrition-Obesity Research Center Pilot Grant; CNL: NIH grant DK090262; LAM: NIH grant T32DK101357; RWO and CNL: Michigan Institute for Clinical & Health Research T1 Bench to Bedside Translation Pilot Grant 2UL1TR000433.
Abbreviations
- BMI
body mass index
- DM
diabetic
- ECM
extracellular matrix
- HbA1c
hemoglobin A1c
- NDM
non-diabetic
- PA
preadipocyte
- QRTPCR
quantitative real-time polymerase chain reaction
- SAT
subcutaneous adipose tissue
- SVF
stromal-vascular cell fraction
- VAT
visceral adipose tisse
Footnotes
Disclosure: The authors have no relevant conflicts of interest.
References
- 1.Johannsen DL, Tchoukalova Y, Tam C, Covington JD, Xie W, Schwarz JM, et al. Effect of 8 weeks of overfeeding on ectopic fat deposition and insulin sensitivity: testing the adipose tissue expandability hypothesis. Diabetes Care. 2014;37(10):2789–97. doi: 10.2337/dc14-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotillard A, Poitou C, Torcivia A, Bouillot JL, Dietrich A, Klöting N, et al. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab. 2014;99(8):E1466–70. doi: 10.1210/jc.2014-1074. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson-Hogling D, Andersson DP, Bäckdahl J, Hoffstedt J, Rössner S, Thorell A, et al. Adipose tissue morphology predicts improved insulin sensitivity following moderate or pronounced weight loss. Int J Obes (Lond) 2015;39(6):893–8. doi: 10.1038/ijo.2015.18. [DOI] [PubMed] [Google Scholar]
- 5.Landgraf K, Rockstroh D, Wagner IV, Weise S, Tauscher R, Schwartze JT, et al. Evidence of early alterations in adipose tissue biology and function and its association with obesity-related inflammation and insulin resistance in children. Diabetes. 2014;64(4):1249–61. doi: 10.2337/db14-0744. [DOI] [PubMed] [Google Scholar]
- 6.Rydén M, Andersson DP, Bergström IB, Arner P. Adipose tissue and metabolic alterations: regional differences in fat cell size and number matter, but differently: a cross-sectional study. J Clin Endocrinol Metab. 2014;99(10):E1870–6. doi: 10.1210/jc.2014-1526. [DOI] [PubMed] [Google Scholar]
- 7.Veilleux A, Caron-Jobin M, Noël S, Laberge PY, Tchernof A. Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes. 2011;60(5):1504–11. doi: 10.2337/db10-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dankel SN, Svärd J, Matthä S, Claussnitzer M, Klöting N, Glunk V, et al. COL6A3 expression in adipocytes associates with insulin resistance and depends on PPARγ and adipocyte size. Obesity (Silver Spring) 2014;22(8):1807–13. doi: 10.1002/oby.20758. [DOI] [PubMed] [Google Scholar]
- 9.Divoux A, Tordjman J, le Lacasa D, Veyrie N, Hugol D, Aissat A, et al. Fibrosis in Human Adipose Tissue: Composition, Distribution, and Link With Lipid Metabolism and Fat Mass Loss. Diabetes. 2010;59L:2817–25. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, et al. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299(6):E1016–27. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourlier V, Sengenès C, Zakaroff-Girard A, Decaunes P, Wdziekonski B, Galitzky J, et al. TGFbeta family members are key mediators in the induction of myofibroblast phenotype of human adipose tissue progenitor cells by macrophages. PLoS One. 2012;7(2):e31274. doi: 10.1371/journal.pone.0031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M, et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008;9(1):R14. doi: 10.1186/gb-2008-9-1-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vila IK, Badin PM, Marques MA, Monbrun L, Lefort C, Mir L, et al. Immune cell Toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Rep. 2014;7(4):1116–29. doi: 10.1016/j.celrep.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 14.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29(16):4467–83. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, et al. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes. 2011;60(10):2484–95. doi: 10.2337/db11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, Goncalves-Marangoni R, et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun. 2014;5:3485. doi: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdennour M, Reggio S, Le Naour G, Liu Y, Poitou C, Aron-Wisnewsky J, et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. J Clin Endocrinol Metab. 2014;99(3):898–907. doi: 10.1210/jc.2013-3253. [DOI] [PubMed] [Google Scholar]
- 18.Lackey DE, Burk DH, Ali MR, Mostaedi R, Smith WH, Park J, et al. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am J Physiol Endocrinol Metab. 2014;306(3):E233–46. doi: 10.1152/ajpendo.00476.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henninger AM, Eliasson B, Jenndahl LE, Hammarstedt A. Adipocyte hypertrophy, inflammation and fibrosis characterize subcutaneous adipose tissue of healthy, non-obese subjects predisposed to type 2 diabetes. PLoS One. 2014;9(8):e105262. doi: 10.1371/journal.pone.0105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58(7):1550–7. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SM, Lun M, Wang M, Senyo SE, Guillermier C, Patwari P, et al. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 2014;20(6):1049–58. doi: 10.1016/j.cmet.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hauner H, Wabitsch M, Pfeiffer EF. Differentiation of adipocyte precursor cells from obese and nonobese adult women and from different adipose tissue sites. Horm Metab Res Suppl. 1988;19:35–39. [PubMed] [Google Scholar]
- 23.Maumus M, Sengenès C, Decaunes P, Zakaroff-Girard A, Bourlier V, Lafontan M, et al. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: influence of fat mass microenvironment and growth. J Clin Endocrinol Metab. 2008;93(10):4098–106. doi: 10.1210/jc.2008-0044. [DOI] [PubMed] [Google Scholar]
- 24.Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci USA. 2010;107(42):18226–31. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology. 2007;148(2):868–77. doi: 10.1210/en.2006-0687. [DOI] [PubMed] [Google Scholar]
- 26.Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, Forse RA, Chinnappan D, Martin-Ruiz C, von Zglinicki T, Kirkland JL. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55(9):2571–8. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 27.Drolet R, Richard C, Sniderman AD, Mailloux J, Fortier M, Huot C, et al. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int J Obes (Lond) 2008;32(2):283–91. doi: 10.1038/sj.ijo.0803708. [DOI] [PubMed] [Google Scholar]
- 28.O'Rourke RW, White AE, Metcalf MD, Olivas AS, Mitra P, Larison WG, et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54(6):1480–90. doi: 10.1007/s00125-011-2103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 2013;18(3):355–67. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sengenès C, Lolmède K, Zakaroff-Girard A, Busse R, Bouloumié A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol. 2005;205(1):114–22. doi: 10.1002/jcp.20381. [DOI] [PubMed] [Google Scholar]
- 31.O'Connell J, Lynch L, Cawood TJ, Kwasnik A, Nolan N, Geoghegan J, et al. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS One. 2010;5(4):e9997. doi: 10.1371/journal.pone.0009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood IS, de Heredia FP, Wang B, Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009;68(4):370–7. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- 33.Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124(1):67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 34.Peng H, Sarwar Z, Yang XP, Peterson EL, Xu J, Janic B, Rhaleb N, Carretero OA, Rhaleb NE. Profibrotic role for interleukin-4 in cardiac remodeling and dysfunction. Hypertension. 2015;66(3):582–9. 20. doi: 10.1161/HYPERTENSIONAHA.115.05627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan J, Zhang Z, Yang J, Mitch WE, Wang Y. JAK3/STAT6 Stimulates Bone Marrow-Derived Fibroblast Activation in Renal Fibrosis. J Am Soc Nephrol. 2015 Jun 1; doi: 10.1681/ASN.2014070717. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J, Shi J, Li M, Gui B, Fu R, Yao G, Duan Z, Lv Z, Yang Y, Chen Z, Jia L, Tian L. Activation of AMPK by metformin inhibits TGF-β-induced collagen production in mouse renal fibroblasts. Life Sci. 2015;127:59–65. doi: 10.1016/j.lfs.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Wu R, Zhang F, Xu Y, Liu B, Yang Y, Zhou H, Wang L, Wan K, Xiao X, Zhang X. Thiazolidinediones improve hepatic fibrosis in rats with non-alcoholic steatohepatitis by activating the adenosine monophosphate-activated protein kinase signalling pathway. Clin Exp Pharmacol Physiol. 2012;39(12):1026–33. doi: 10.1111/1440-1681.12020. [DOI] [PubMed] [Google Scholar]