Abstract
For over a century, embryologists who studied cellular motion in early amniotes generally assumed that morphogenetic movement reflected migration relative to a static extracellular matrix (ECM) scaffold. However, as we discuss in this Review, recent investigations reveal that the ECM is also moving during morphogenesis. Time-lapse studies show how convective tissue displacement patterns, as visualized by ECM markers, contribute to morphogenesis and organogenesis. Computational image analysis distinguishes between cell-autonomous (active) displacements and convection caused by large-scale (composite) tissue movements. Modern quantification of large-scale ‘total’ cellular motion and the accompanying ECM motion in the embryo demonstrates that a dynamic ECM is required for generation of the emergent motion patterns that drive amniote morphogenesis.
KEY WORDS: Amniote morphogenesis, Emergent patterns, Extracellular matrix dynamics, Tissue-scale motion
Summary: This Review considers the important, but often overlooked, role of the extracellular matrix in cell migration and tissue morphogenesis in amniotes.
Introduction
“That great Frenchman* first carried out the conception that living bodies … must be regarded as consisting of certain primary webs or tissues, out of which the various organs … are built up … each material having its peculiar composition and proportions. No man, one sees, can understand and estimate the entire structure or its parts … without knowing the nature of the materials”.
From Middlemarch (1872) by George Eliot. *Marie François Xavier Bichat (circa 1829).
The early amniote embryo is shaped by unabated tissue motion. In particular, all early developmental milestones in amniotes involve large (millimeter-scale) morphogenetic movements, including gastrulation, left-right symmetry breaking, neurulation, segmentation and caudal axis extension. These early landmark events create the foundation for organogenesis by sculpting the vertebrate body plan and transporting organ precursors to appropriate destinations within the embryo. What mechanisms, therefore, underlie primordial motion patterns? How do large-scale morphogenetic movements direct the assembly of tissues and organs? Understanding the forces that shape the embryo will require a multi-scale interdisciplinary approach. Decades of crucial molecular details notwithstanding, enormous gaps exist in our comprehension of amniote morphogenesis.
The conventional idea of cells actively crawling (‘migrating’) within a relatively static extracellular matrix (ECM) requires serious rethinking given recent evidence showing ECM motion during early amniote embryogenesis (discussed below). While the genetic code stipulates individual cellular capabilities, emergent system-wide complexities arise through the collective behavior of cells operating within a dynamic ECM – a physical framework that exhibits both mobility and fluctuating mechanical properties. The resulting tissue motion involves more than 10,000 cells, spans millimeters and occurs on minute-level time-scales (Czirok et al., 2006; Rozbicki et al., 2015). The morphogenetic ‘motion system’ is also characterized by complex evolutionarily acquired biomechanical and material properties. The properties emerge via interactions between two motile constituents (cells and ECM). Neither cells nor ECM alone exhibit the required emergent properties. An analogy is the formation of a new termite nest. No termite, and no person, can know the exact location and shape of the next nest – its shape will emerge from the actions of thousands of blind termites following a set of simple rules. Examining individual termites, cells, proteins or genes cannot lead to an understanding of the emergent nest-building process. Analogously, analyzing the behavior and property of individual cells alone cannot unravel the complexities of morphogenesis: fluctuating ECM material properties and collective biological motion govern the tissue-scale deformations that shape early amniote embryos and their organ primordia.
In this Review, we discuss our understanding of ECM motion during early morphogenesis and emphasize the importance of convective ECM/tissue displacement as an essential morphogenetic mechanism that is of equal importance to conventional cell migration. An appreciation of both cell-autonomous and tissue-level motion perspectives is needed to understand the biomechanical mechanisms that shape amniote embryos and, potentially, non-amniote embryos.
The role of ECM in tissue morphogenesis: a historical overview
Early studies of morphogenetic movements relied largely on tissue culture and optically accessible embryos such as the lancelet (amphioxus), chicken and Fundulus (killifish) for experimental manipulation (Abercrombie, 1977; Conklin, 1932; Harrison, 1910, 1912; Lewis, 1923; Spratt, 1948; Trinkaus, 1963). Subsequent planar cell culture studies, in which cellular motion was analyzed in the context of a static ECM scaffold, led to a common (and misleading) assumption that all migratory patterns observed in intact embryos arose via cells actively crawling through or upon the ECM (Bilozur and Hay, 1988; Hay, 1989). In the envisioned scenario, the cellular displacements within an embryo were ‘permitted’ by a passive ECM that acted primarily as a sort of molecular Styrofoam or rigid scaffold (Zagris, 2001). Data from other non-embryonic contexts, such as wound healing, cellular motility through hydrogels and cancer cell ‘invasive’ motility, reinforced the concept that the ECM is a passive scaffold allowing locomotion of cells (Chen et al., 1979; Tickle et al., 1978). Simultaneously, other ECM functions were described: an osmotically active adhesive scaffold for invasive cells (Camenisch et al., 2000; Toole, 2001); a warehouse for the sequestration, storage and presentation of growth factors during cell signaling (Chen et al., 2007; Ghosh and Brauer, 1996); and a medium for sensing and transducing mechanical signals while simultaneously maintaining tissue integrity and elasticity (Engler et al., 2006; Wang et al., 2009). Collectively, these studies reinforced the notion that the ECM is static and that cell motility, with respect to the ECM, is the source of all morphogenetic movements that shape embryos.
While a huge preponderance of work involved cell motility in vitro (i.e. where the ECM was static), significant progress was made on a hypothetical framework in which morphogenesis was viewed as a complex system of cells and their adhesive environment. One of the first such principles of spontaneous cellular organization was inspired by the work of Townes and Holtfreter, in which an in vitro assortment of amphibian embryonic cells demonstrated the ability to self-organize (Townes and Holtfreter, 1955). The self-organization of cell collectives, driven by differential adhesion, is a process by which a tissue-like aggregate forms via a gradual progression of equilibrium states. Steinberg proposed differential adhesion as a model for embryogenesis and histogenesis (Foty and Steinberg, 2005; Steinberg, 1963). The earliest experimental evidence suggesting a dynamic role for the ‘non-cellular environment’ (presumably ECM) during amniote morphogenesis came from the study by Bronner-Fraser (1982). This work showed that non-motile retinal pigment epithelial cells were translocated by convective tissue flow after introduction into neural crest migratory pathways. Meanwhile, Harrisson et al. (1985) observed transfer of ECM components between chick-quail chimeric blastoderms, thus showing a dynamic role for ECM in the early amniote embryo. In a related in vitro study, Newman et al. (1985) suggested that non-equilibrium chemical phenomena driven primarily by ECM fibrillogenesis, at the interface of two distinct pools of ECM constituents, might be sufficient to propel embedded cells by means of ‘matrix-driven translocation’. Taken together, these biophysical data suggest a complex and dynamic morphogenetic platform in which cells and ECM fiber assembly influence amniote embryogenesis. Although we largely limit our discussion to amniotes due to data availability, it is likely that similar emergent tissue-scale motion patterns, born from interactions between cells and a dynamic ECM, occur during non-amniote morphogenesis (e.g. see below regarding Hydra).
Tissue-scale motion includes ECM dynamics
Autonomous cellular motion is insufficient to explain the morphogenetic deformations of embryonic tissue revealed by live imaging (Li et al., 2015; Sato et al., 2010). Inevitably, cell fate-mapping studies in which only cell motion is tracked cannot reveal large-scale composite tissue motion, much less the actual degree of cell-autonomous motility. This is primarily because conventional cell motion tracking studies do not employ the wide mesoscale vantage point needed to unravel large-scale tissue deformations spanning multiple germ layers (Aleksandrova et al., 2015a), and because standard image processing algorithms do not quantify either cellular versus tissue motion or the overall motion of the tissue environment, i.e. cells plus their filamentous ECM scaffold (Zamir et al., 2005).
With the ability to distinguish cell-autonomous motility from convective displacements (Box 1), it is now possible to reformulate our understanding of amniote morphogenesis: large-scale or bulk tissue/ECM deformations are essential for the gradual morphogenetic transitions that characterize amniote tissue and organ formation. New quantitative data show that ECM constituents are in constant motion during embryogenesis. Our data show that intrinsic ECM motion spans from the molecular to the tissue level of organization. Studies using high optical resolution recording and one-second time intervals show local oscillations and deformations of ECM microfibrils. Superimposed on this ‘local’ micrometer per second scale motion is gradual fiber assembly and slower displacements that correlate with cellular motility and tissue drift, which occur at 5-15 min time-scales over 30-100 µm length scales (Czirok et al., 2006; Szabo et al., 2011).
Box 1. Extracting tissue motion from image sequences.
Tissue and cell movements can be computed using immunolabeled image sequences of ECM components. The similarity of two w×w image tiles, T1 and T2, can be characterized by their cross-correlation:
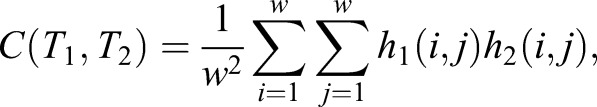 |
(1) |
where hn(i, j) denotes the brightness of pixel (i, j) within tile Tn.
For a pair of images, I1, I2, the displacement of tile T1 in image I1 is determined by maximizing the cross- correlation C(T1, T2) for all possible tiles T2 in image I2. Thus, for each tile T2 within I2
| (2) |
holds, where 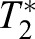 is the optimal image tile. The procedure then identifies
is the optimal image tile. The procedure then identifies 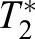 as the tile into which the original image tile T1 moved to. Repeating the procedure for distinct tiles T1, we obtain an array of estimated displacement vectors
as the tile into which the original image tile T1 moved to. Repeating the procedure for distinct tiles T1, we obtain an array of estimated displacement vectors 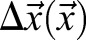 characterizing various image locations
characterizing various image locations 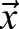 .
.
It is often useful to relate displacements to anatomical reference points as:
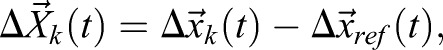 |
(3) |
where 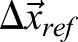 denotes the mean displacement of the reference points. To reconstruct a trajectory within the anatomical frame of reference, displacement values are accumulated as:
denotes the mean displacement of the reference points. To reconstruct a trajectory within the anatomical frame of reference, displacement values are accumulated as:
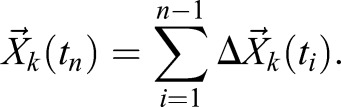 |
(4) |
Similarly, we can define active cell displacements as displacements relative to the local ECM scaffold:
| (5) |
This definition of active cell movement corresponds closely to the ‘conventional’ in vitro scenarios where cellular motility occurs on a fixed two-dimensional surface or within static gels. The application of Eqn (5) requires that we have two displacement values from the same location: one for the cells of interest and one for the ECM scaffold. Thus, it requires simultaneous imaging in multiple (at least two) optical modes, using two distinct fluorophores with no spectral overlap.
Cells can actively remodel, pull and drag the ECM. Therefore, the identification of ECM and tissue movements is an approximation. High temporal resolution recordings of ECM movements indicated that local cell activity yields quickly changing fluctuations, with a correlation time of less than a minute. By contrast, tissue movements can be identified as ECM movements that are persistent in time (autocorrelation time being in hours). This observation allows a more precise extraction of tissue movements by low-pass temporal filtering of the ECM displacement data. A practical possibility is a linear fit by minimizing the expression
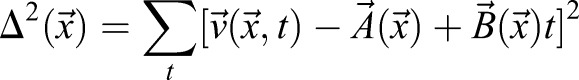 |
(6) |
for each location 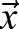 yielding
yielding 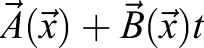 as a better estimate for tissue motion.
as a better estimate for tissue motion.
This latter view of morphogenesis involves tissue-scale deformations that are typically not observed in cell culture systems but can be readily analyzed in a native embryological context using widefield multi-spectral time-lapse imaging (Aleksandrova et al., 2015a,b; Rozbicki et al., 2015; Zamir et al., 2006). Active cell movements are defined as displacements relative to the local ECM scaffold, and are calculated as the difference between local cellular and global ECM movements (for an explanation of the underlying algorithms, see Box 1). Convective tissue movements, identified as temporally persistent ECM movements, are extracted by low-pass temporal filtering of the ECM displacement data over time-scales of hours. Computational tools allow the determination of cell-autonomous motility independent of large tissue-scale displacements. Accordingly, ECM dynamics can be quantified, not only during early embryogenesis (Czirok et al., 2006; Zamir et al., 2006, 2008) but also during organogenesis (Aleksandrova et al., 2015a, 2012), as discussed further below.
Tissue-scale morphogenetic motions during early amniote embryogenesis
In the following paragraphs, we discuss recent evidence obtained from avian embryos describing tissue-scale motion that demonstrates convective flow. The morphogenetic properties of a dynamic ECM are integral to tissue self-organization during embryogenesis. Most of our discussion will focus on bulk tissue flow; however, we also hypothesize that time-dependent material property change is an emergent morphogenetic mechanism.
Primitive streak formation and gastrulation
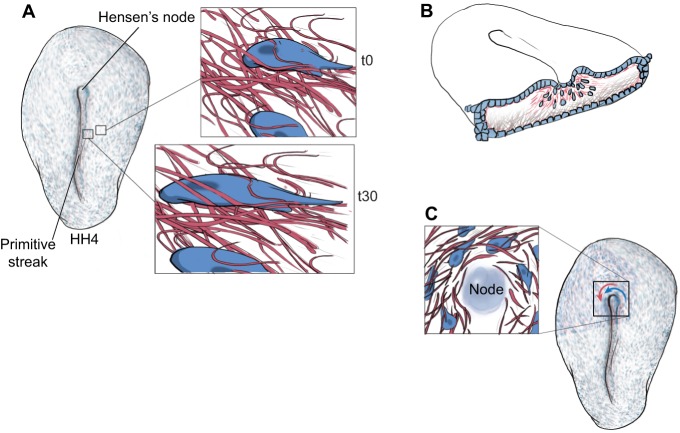
Formed just prior to gastrulation, the primitive streak is a dynamic self-reinforcing furrow of cells waiting to move ventrally (ingress) to form the mesendoderm. The formation of the primitive streak is initiated by a mediolateral epiblastic cell intercalation process, which marks the first global morphogenetic phenomenon within the flat disc-shaped, single cell-layered chick embryo (Voiculescu et al., 2007). Tissue-scale motion patterns of the ECM occur as early as primitive streak formation: epiblastic cells move in concert with the subepiblastic ECM during stages HH2 and HH3 (Fleury, 2012; Rozbicki et al., 2015; Zamir et al., 2008) (Fig. 1A). Indeed, there is little cell-autonomous displacement relative to the ECM because both components move together as a tissue composite. Particle image velocimetry and autocorrelation analyses (Box 1) revealed that, relative to the underlying ECM, the cells undergo a random walk with no apparent directional bias or persistence. In other words, most of the epiblastic displacement observed during primitive streak formation is in fact due to composite tissue-scale deformations (i.e. cells plus ECM).
Fig. 1.
Composite tissue (cells plus ECM) motion during early embryogenesis. (A) ECM motion is present in avian embryos as early as primitive streak formation. Time-lapse recordings of cells (blue) and ECM fibers (red) show both embryonic tissue components moving as a composite [compare insets at time (t) 0 and 30 min later], along with concomitant cell shape changes. (B) During gastrulation, ECM fibers (pink) move through the primitive streak (ingress) along with prospective mesendodermal cells (blue). See also Movie 1. (C) A third example of ECM fiber motion occurs near Hensen's node (the ‘organizer’ in birds). Nodal cells, adjacent epiblastic cells and ECM fibers move in unison (blue and red arrows). The organizer tissue undergoes rotational motion that determines left-right asymmetry with respect to the head-to-tail axis. Only counterclockwise motion is illustrated for simplicity; however, clockwise rotation of more remote epiblastic cells occurs in the peri-nodal region (see Cui et al., 2009b).
During gastrulation, germ layers (ectoderm, mesoderm and endoderm) are not only specified but are also molded into a body plan (Solnica-Krezel and Sepich, 2012). Locally occurring displacements of epiblastic cells collectively coordinate their epithelial-to-mesenchymal transition by a Nodal- and Wnt-planar cell polarity (PCP)-dependent (local signals) positive-feedback mechanism to mobilize their ingression via the primitive streak (Bertocchini and Stern, 2002; Voiculescu et al., 2007, 2014). The tissue-level deformations initiated during primitive streak formation continue unabated during gastrulation, thus promoting gradual ingression of mesendodermal precursors into the streak; simultaneously, fibronectin fibrils continue to ingress. Therefore, in the avian embryo, the ingression occurs at the tissue scale of organization, during which time the ECM is anything but static (Fig. 1B, Movie 1). Fluorescently labeled ECM constituents in gastrulae provide a passive in situ marker for quantifying convective displacements, and allow calculation of local cell-autonomous displacements versus bulk morphogenetic (tissue) motion. Indeed, to a first approximation, mesenchymal cells that are not moving relative to the ECM are not moving at all. It is now possible to resolve composite tissue morphogenetic movements into two components: (1) cell-autonomous motion; and (2) ECM flow. Such empirical data allowed the identification of a cell-autonomous motility gradient along the anteroposterior axis, with caudal cells moving faster than their cranial counterparts (Zamir et al., 2006). The increased density of ECM fluorescence and the reduced motility of more cranial regions suggest that the more mature (older) mesoderm might be hardening, altering the tissue material properties and thus reducing active cellular motility.
Symmetry breaking
Establishing left-right asymmetry is an early embryonic event, which occurs at or near the time of gastrulation, and sets the stage for anatomical asymmetries of the internal organs. In mice and rabbits – but not all vertebrate systems – the earliest steps of this process are initiated by the monocilia found on the cells of the posterior notochordal plate (Levin, 2005; Raya and Izpisua Belmonte, 2006). The primary cilia at the node, termed the nodal cilia, rotate to generate a leftward fluid flow, called the nodal flow, within a pit-like teardrop-shaped space that is devoid of subjacent endoderm (Lee and Anderson, 2008; Nonaka et al., 1998). The nodal flow stimulates signaling cascades that ultimately result in the induction of asymmetric gene expression (Komatsu and Mishina, 2013). Nodal flow-based mechanisms for left-right symmetry breaking were also demonstrated in fish and frog embryos (Essner et al., 2005; Schweickert et al., 2007). Nevertheless, a role for tissue displacement during determination of left-right asymmetry (see below) has not been ruled out in the case of embryos that manifestly exhibit nodal flow (Lee and Anderson, 2008).
Data from bird and pig embryos show that symmetry breaking proceeds readily in the absence of nodal cilia (Gros et al., 2009). The notochordal plate cells in avian and porcine embryos contain neither the cilia nor the morphology to generate nodal flow (Gros et al., 2009). Instead, cells expressing left-right sidedness genes in the avian embryo undergo rotational motion in the region of Hensen's node, which itself is physically swept along by other tissue-level displacements (Fig. 1C) (Cui et al., 2009b). Additional confirmation of a role for the ECM in symmetry breaking comes from the observation of left-right asymmetry in ECM fiber movements around Hensen's node (Szabo et al., 2011). Furthermore, asymmetric gene expression patterns are established by cell movements at the node due to the rearrangement in the relative orientations of cells expressing crucial genes (Cui et al., 2009b; Gros et al., 2009). Collectively, these empirical time-lapse data suggest that the asymmetric expression domains of Fgf8 and Shh in the vicinity of the avian node are primarily dependent on composite tissue motion around the node. In the absence of tissue deformations, cells mediating these molecular regulatory mechanisms do not break symmetry. Only after being brought into juxtaposition by tissue deformations does symmetry breaking proceed (Cui et al., 2009b; Gros et al., 2009).
Axis elongation
Convergent extension is one of the main drivers of body axis elongation. In this process, cells intercalate mediolaterally causing the thinning and lengthening of embryonic tissues (Keller et al., 2000; Shih and Keller, 1992). Although convergent extension seems to be at play in the elongation of the anterior part of the body in multiple species, studies in amniotes suggest that a different morphogenetic scenario accounts for the formation of the posterior part of the body (Benazeraf et al., 2010; Benazeraf and Pourquie, 2013). In the chicken embryo it has been shown that the paraxial mesoderm is crucial for posterior axis extension. To reach the presomitic mesoderm (PSM), cells leave the posterior axial progenitor region (future tailbud) with a proximodistal movement driven by chemotaxis (Yang et al., 2002). Meanwhile, the PSM tissue undergoes a continuous large-scale movement of elongation. Thus, from the point of view of a newly formed somite, PSM cells and their ECM fibrils (fibronectin and fibrillin 2) both move toward the posterior pole of the embryo (Movie 2). Furthermore, computational time-lapse imaging, whereby ECM and cell motions were analytically uncoupled, revealed a local decreasing gradient of non-directional cellular motility within the elongating PSM tissue (Benazeraf et al., 2010). In other words, cellular motility is more diffusive (less persistent) in the posterior part of the PSM compared with the anterior. Similarly, in the zebrafish embryo, increased variability of individual cell velocity has been observed at the posterior tip of the elongating tailbud, suggesting that this motion pattern is conserved among vertebrate species (Dray et al., 2013; Lawton et al., 2013). These data suggest that the material properties of the ECM in avian PSM might also differ along an anterior-to-posterior gradient, with the anteriormost ECM moving more slowly than the posteriormost ECM. In this scenario, the speed of random cell motion is potentially regulated by a gradient of ECM physical properties. Direct measurement of tissue material properties will be required to confirm that non-directional cell migration is slowed down as a consequence of increased tissue viscosity or stiffening.
The diffusive motion gradient is regulated by FGF signaling in the chicken PSM, and gain- and loss-of-function experiments showed that this non-equilibrium dynamic state is required for tissue elongation (Benazeraf et al., 2010; Delfini et al., 2005). These computational time-lapse data suggest that graded random motility is a collective cellular mechanism that can drive large-scale tissue deformations. Interestingly, FGF signaling also controls graded cellular velocity and rearrangements during avian limb bud elongation, suggesting that similar mechanisms might operate in other embryonic primordia (Gros et al., 2010). The coordinated growth, displacements and physical interactions of neighboring tissues (ectoderm, neural tube, lateral mesoderm, endoderm) are likely to play a significant role in the biophysical motion patterns that characterize axis extension. The systematic measurement of variables determining tissue biomechanics and ECM material properties will be required to gain a better understanding of distal axis elongation and other millimeter-scale morphogenetic processes.
The foregoing evidence shows that large-scale tissue deformations involving ECM mobility are key mechanisms that govern early morphogenetic patterning. Below, we discuss results that confirm ECM mobility during organogenesis.
Tissue-scale motion during amniote organogenesis
Heart formation
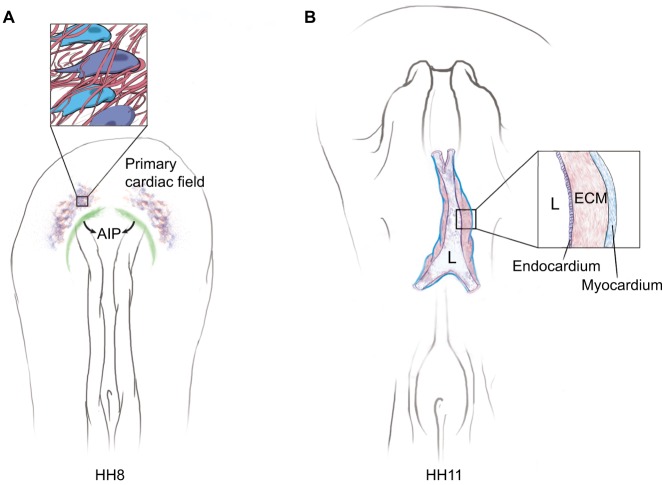
The cardiovascular system is the first functional organ system to develop. Avians, like humans, have high-performance, definitive, four-chambered hearts. During development, the right and left primary heart fields, which are initially planar structures within the anterior lateral plate mesoderm, are transformed into a bilayered midline tubular heart (tube-within-a-tube) separated by ECM (Drake et al., 1990; Wittig and Munsterberg, 2016). Amniote tubular heart formation involves the precise movement of the primordial cardiac cells via tissue motion (Cui et al., 2009a). Indeed, the autonomous motility of the endocardial cell precursors relative to their local ECM microenvironment is limited, and amounts to a slightly biased random walk. This finding was determined by analyzing the movements of ECM components (fibronectin, fibrillin 2) and fluorescently tagged TIE1-positive endocardial progenitors during avian embryogenesis (Aleksandrova et al., 2012). Likewise, myocardial cells transfected with a cardiac myosin light chain 2-driven fluorescent reporter (mito-RFP) were also shown to move towards the midline predominantly via tissue motion (i.e. with their surrounding ECM). Thus, the movements of both the endocardial and myocardial precursors are due mainly to large-scale tissue deformation, and not classically defined cell migration (Aleksandrova et al., 2015a).
This medial movement of bilateral myocardial progenitor fields is a primary consequence of foregut formation – a process that drives tangential shortening of the associated endoderm (i.e. regression of the anterior intestinal portal) (Fig. 2A) (Aleksandrova et al., 2015a; Varner and Taber, 2012). Time-lapse recordings show that as the myocardial progenitor fields approach the midline, the cells autonomously exert mechanical stresses within the tissue (Aleksandrova et al., 2015a). These forces give rise to at least two distinct active autonomous deformations, which together propel the anterior displacement of the myocardium (relative to the endoderm) and thereby reposition the nascent myocardial tube (Fig. 2B).
Fig. 2.
Tissue-scale motion dominates amniote heart formation. (A) Medial displacement of the primary cardiac field is driven by the centripetal forces (arrows) of anterior intestinal portal (AIP; green) regression. As the tissue moves the ECM fibers (red), endocardial progenitors (purple) and myocardial progenitors (blue) are propelled toward the midline (arrows). There is negligible independent cellular motility during AIP regression. See Movie 3 and Aleksandrova et al. (2012). (B) Driven by tissue deformation of cardiac progenitors and ECM, the right and left heart primordia fuse at the midline – the cardinal step in forming the tube-within-a-tube morphology of the amniote heart. Note that once fusion occurs, an expansive ECM (cardiac jelly) separates the myocardium and endocardium. Many of the ECM fibrils in the cardiac jelly (inset) were ‘born’ hours earlier and were subsequently transported into the tubular heart by the AIP-driven tissue deformation(s) (see Aleksandrova et al., 2015a). L, lumen of the primitive heart chamber.
Multiple dynamic datasets therefore establish the fact that large-scale tissue movements transport cardiac progenitor cells and presumptive cardiac jelly ECM to the midline, thus setting the stage for local cell-autonomous myocyte progenitor motility, ‘vasculogenesis’ of the endocardium, and expansion of the cardiac jelly space to accommodate the formation of primordial heart valves. The cells and the adjacent ECM are collectively transported to the midline via regression of the anterior intestinal portal (Movie 3), and not by autonomous cell migration.
Primary vasculogenesis
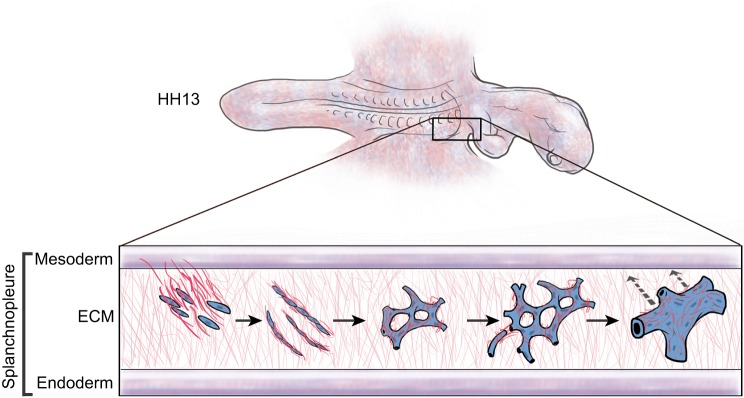
Vasculogenesis is the formation of endothelial tubes from naïve mesoderm. In amniotes, the most fundamental vascular pattern is a planar network of interconnected polygons (Poole and Coffin, 1989). Vessel formation precedes heart formation and there is no evidence – molecular or otherwise – that a prepattern specifies the future vascular pattern (Drake et al., 1997). Morphogenesis of a primary vascular network requires coordinated motion across numerous length scales (from µm to mm). Multiple behaviors are involved, including cell protrusive activity, cell locomotion, ECM assembly and macroscopic tissue deformations, all of which act in concert to create a unique emergent vascular pattern. Extensive time-lapse recordings from avian embryos showed that the primary vascular plexus is constructed from a combination of several biological motion behaviors (Fig. 3, Movie 4). Although a full description is beyond the scope of this article, major motion patterns include: (1) tissue deformations that convect primordial endothelial cells; (2) ‘vascular drift’, which occurs as a composite medial displacement of the entire vascular bed; (3) structural deformation/condensation of the polygonal network architecture; (4) cell-autonomous motion of primordial endothelial cells along existing vascular cords; and (5) subcellular extensions/retractions across avascular zones that form/remove connections within the polygonal network (Rupp et al., 2004; Sato et al., 2010). Multiple, large tissue-scale motions are strikingly evident, particularly during vascular fusion when smaller vessels coalesce into larger vessels. The afferent vascular network is constructed by the fusion of extraembryonic vasculature with the omphalomesenteric veins, a process that relies primarily on tissue deformations. Further composite tissue motion repositions the extraembryonic vasculature in order to ‘dock’ with the intraembryonic vasculature (Rupp et al., 2003). Moreover, the assembly of the dorsal aortae, which are part of the intraembryonic efferent network, requires both cell-autonomous and tissue-scale motions to position the great outflow vessels (Sato et al., 2010).
Fig. 3.
Tissue-scale motion during primary vasculogenesis. ECM motion is an integral component of the multi-stage primary vasculogenic process. Collective motion (ECM plus endothelium) influences all stages of primary vascular network formation. The process takes place within a splanchnopleural ECM that is expanding in three dimensions. Primordial endothelial progenitor cells move ventrally from the splanchnic mesoderm into the ECM and begin extension and protrusive behavior. Local ‘active’ motion of primordial endothelial cells leads to multicellular vascular cords (no lumen) that are subject to large-scale tissue drift/expansion (cells and ECM). As the lateral embryonic plate expands, the vascular cords coalesce into a hexagonal pattern, driven by both cellular motility and tissue motion, resulting in a vascular network composed of relatively small-caliber tubules. Further pattern formation involves tissue-scale remodeling of polygonal vascular networks. Specifically, a complex tissue-driven process begins whereby the lumens of some small-caliber vessels are forced together and fuse their respective lumens. Simultaneously, pruning results in the loss of other endothelial tubes. The result is morphogenesis of the great vessels, such as the aortae and the omphalomesenteric veins [see Movie 4 and Sato et al. (2010)]. During vasculogenesis, wide-scale tissue drift draws the intraembryonic and extraembryonic primary vasculature toward the midline (dashed arrows). Convergence of the extraembryonic vasculature toward the intraembryonic vasculature enables docking with the aortae and omphalomesenteric veins, resulting in a functional circulatory network. In amniotes the majority of morphogenetic motion experienced by a given endothelial cell is propelled by tissue-level events; meanwhile, local motility, cell extension and protrusive activity contribute to local shaping of individual vessels. Primary vascular pattern formation is emergent; there is no evidence that the process is predetermined or genetically regulated (see Perryn et al., 2008).
Time-lapse recordings from whole bird embryos and explanted mouse embryonic tissue both yield robust dynamic evidence that the de novo formation of a primary vascular pattern is emergent and not hard-wired (Perryn et al., 2008; Rupp et al., 2003). Cultured embryonic mouse allantoides (the sac-like mesoderm that will generate the umbilical cord) assemble an expanding primary vascular network driven by mesodermal tissue spreading across a tissue culture surface (Perryn et al., 2008). In sharp contrast, when left unperturbed in utero, the allantois forms an umbilical cord containing one artery and one vein. It is therefore impossible that the information required to form the allantois-derived polygonal vascular pattern is directly encoded in the murine genome. Instead, global mesodermal tissue expansion patterns in the presence of competent primordial endothelial cells stimulate the de novo formation of an emergent primary vascular pattern. It is worth noting that the morphological features of the mouse vascular pattern (in vitro) and primary quail vascular patterns (in vivo) are essentially the same. Thus, although it is necessary to identify and understand the requisite molecular and genetic regulatory mechanisms, it is equally important to understand the dynamic biophysical mechanisms required for patterning a new vascular bed (Czirok and Little, 2012; Gamba et al., 2003; Merks et al., 2006, 2008; Serini et al., 2003; van Oers et al., 2014). It is noteworthy that the tissue motion-based ‘vasculogenic’ patterns form within hours; in sharp contrast, static ‘angiogenesis-based’ in vitro systems require from days to weeks to construct a polygonal vascular network (e.g. within ECM hydrogels or retina-based preparations).
Organogenesis of neural crest derivatives
The neural crest is a transient population of motile multipotent cells that originates from the dorsal aspect of the neural tube and contributes to the cellular constituents of a multitude of organs (Mayor and Theveneau, 2013; Takahashi et al., 2013). The route along which neural crest cells move contains all major categories of ECM constituents (Perris and Perissinotto, 2000). The earliest empirical evidence suggesting in vivo ECM motion in the neural crest pathway came from the observation that non-motile retinal pigment epithelial cells were translocated ventrally upon their introduction into the avian embryo (Bronner-Fraser, 1982). Remarkably, the non-motile retinal pigment epithelial cells maintained their characteristic ‘static’ cuboidal morphology during the course of their translocation. These data suggest that the exogenous retinal pigment cells were being passively propelled, possibly by ECM tissue flow. The microenvironment associated with migrating neural crest is a dynamic system in the sense that major spatial and temporal changes in the distribution of ECM occur along the pathway (Perris et al., 1991). New optical approaches are being used to examine neural crest motility (Kulesa and McLennan, 2015). Nevertheless, the degree of active cell motility versus tissue flow-based motility remains an open question and one that awaits quantification using widefield recordings of neural crest locomotion in the context of a fluorescently labeled ECM.
Limb development and skeletogenesis
Recent evidence also suggests that, similar to axis elongation, morphogenesis of the avian limb may include collective tissue motion. Gros et al. (2010) demonstrated that an FGF/MAPK signaling-dependent gradient of cell velocity regulates continuous rearrangement of distal tip cells in limb buds. However, ECM motion was not monitored. Nevertheless, the subsequent elongation of growth cartilage during skeletogenesis is ipso facto dependent on dynamic ECM deposition and its subsequent expansion in both avian and, presumably, mammalian embryos (Kobayashi and Kronenberg, 2014; Li et al., 2015; Noonan et al., 1998). The tissue dynamics of cartilage and bone accretion, removal and displacement during skeletogenesis remain undocumented. However, with the development of new time-lapse imaging approaches that reveal a dynamic process of cartilage and bone accretion, skeletogenesis is expected to depend on a highly dynamic mesenchymal tissue (Dallas and Veno, 2012; Dallas et al., 2009; Kamel-ElSayed et al., 2015).
Tissue-scale motion patterns in anamniotes and invertebrates – an open question
Mounting evidence for ECM/tissue motion provides impetus to the idea that ECM mobility is required for amniote embryogenesis. These data necessarily raise the question of whether morphogenetic ECM movements are exclusively limited to amniote developmental morphogenesis.
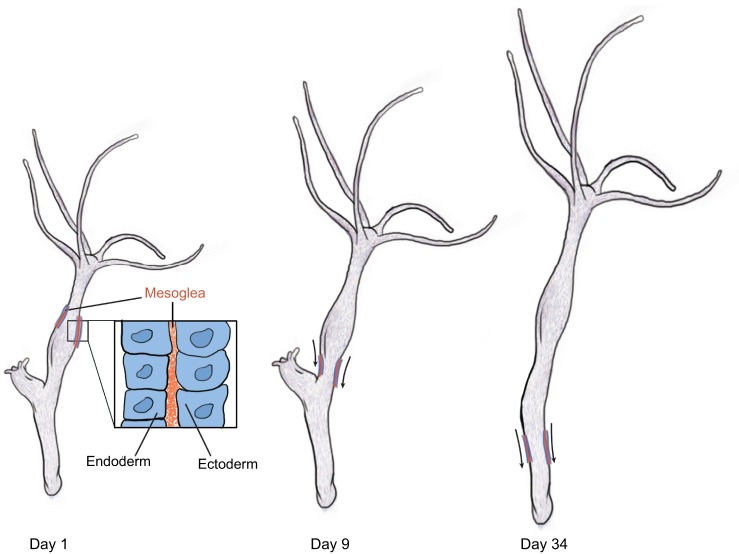
ECM components arose with metazoans (multicellular animals), with collagen, laminin and fibrillin 2 being conserved from sponges to humans, whereas fibronectin first appeared in the cartilaginous fishes (Ozbek et al., 2010). Despite the antiquity and ubiquity of ECM, data regarding ECM motion during metazoan morphogenesis is limited. Nonetheless, the evolutionary conservation of common ECM components and assembly processes across all extant eumetazoans (radiata and bilateria) (Fidler et al., 2014; Hynes, 2012) leaves tissue-scale motion in anamniotes (amphibia and fish) and invertebrates a distinct possibility. For example, in growing Hydra (an invertebrate with a relatively simple two cell-layered body plan), epithelial cells move towards the extremities and into the outgrowing buds, along with the associated primitive ECM (collagen 1 and laminin-rich mesoglea) via tissue-scale motion (Aufschnaiter et al., 2011). Thus, in the Hydra body column, the mesoglea is surprisingly dynamic, and is continuously displaced towards the ends of the animal (Fig. 4). Moreover, tissue-scale composite motion patterns may be involved during axis elongation of anamniotes as well (McMillen and Holley, 2015).
Fig. 4.
Hydra epithelia and mesoglea move together. Schematic of an experiment from Aufschnaiter et al. (2011) in which a graft containing fluorescently labeled mesoglea (primitive ECM, orange) and epithelial cells (blue) is displaced along the body column toward the aboral end of a Hydra (arrows) over the course of several days after grafting. The mesoglea and the epithelium (inset) move as a cohesive unit, demonstrating very early evolutionary evidence of morphogenetic tissue motion; the process involves extant ECM molecules such as collagen and laminin. A daughter hydra was seen budding off from the mother during the course of this experiment (day 1 and day 9).
Whether a highly dynamic ECM with tissue-scale motility is more prominent during early amniote morphogenesis compared with anamniote development is unclear. Phylogenomic evidence shows that vertebrates encode a wider repertoire of ECM components than protostomes and the basal metazoans (Adams, 2013). The richness of vertebrate ECM and its potential for structural/functional complexity are the result of gene duplication and domain shuffling leading to innovative fibril systems that are enabled by the presence of hyaluronan, fibronectin and several adaptor molecules (e.g. matrilins and tenascins). Hence, in the presence of a shared repertoire of ECM constituents between amniotes and anamniotes, we expect functional attributes such as tissue-scale bulk flow of ECM to have arisen early during vertebrate evolution, potentially before the amniote-anamniote split.
Terrestrial animals, in particular, might have faced the need for a multitude of ECM material properties, from tough to pliable and from rigid to elastic. Novel ECM material properties might thus have arisen over evolutionary time-scales. We therefore envision a scenario in which ancient molecular machinery (characteristic ECM peptide motifs) was available for embryos to experiment or tinker with at the tissue-scale of organization. Successful emergent ECM mechanical properties allowed new tissue motion and deformation patterns. Furthermore, existing ECM constituents could be mixed and matched in new ways (domain shuffling) to yield tissues with novel material properties. In our view, the evolution of novel motion patterns plus the ability to modify the properties of tissue composites (cells and ECM) over space and time typifies emergent morphogenetic phenomena (e.g. Holland, 1999; Kozel et al., 2006; Loganathan et al., 2012; Rozario and DeSimone, 2010; Szabo et al., 2011).
We speculate that reptile, bird and mammalian embryos might have evolved novel and productive ways to fold and deform because they benefited from the total life support provided in an amniotic cavity during their relatively long gestation. The progressive evolutionarily driven morphogenetic deformations reflect the emergence of novel tissue properties. If valid, this speculation raises the possibility that amniote embryogenesis might be partially regulated by an emergent set of biomechanical properties. The minimum requirements of a system would be collective cellular motion and/or shape change, and progressively changing (genetically selectable) tissue material properties. Acting in concert, these two variables could regulate millimeter-scale tissue deformation/folding during amniote embryogenesis. We speculate, for example, that when subjected to tissue-wide (cellular) convergent extension stresses, a highly crosslinked ECM, free of hyaluronan, will bend or fold differently than a hyaluronan-rich ECM that is free of intermolecular crosslinks (Spicer and Tien, 2004). Similarly, when embryonic cells collectively exert forces that result in a novel tissue folding pattern in response to an evolving biomechanical state, the result could be a new morphogenetic pattern(s) (e.g. a four-chambered heart).
A complex systems view of embryonic tissue motion – a speculation
The presence of ECM motion during morphogenetic movements raises intriguing questions about the origin of forces that drive ECM motility. In the context of composite tissue (cell plus ECM) motion, the motility of ECM is considered to be passive relative to active cell displacements. However, there are plausible physical scenarios in which the ECM might generate pushing or pulling forces. The answer might lie within the physical attributes of an ECM hydrogel and, in particular, the capacity of matrix molecules for spontaneous assembly (i.e. self-organization) (Newman and Tomasek, 1996). For in vitro matrix-driven translocation, the motion of collagen matrices could be partially accounted for by the properties of ‘percolation’. Percolation theory, which is a mathematical tool for the analysis of cluster formation in physical systems, has been used to demonstrate the sufficiency of relative concentrations of cells and matrix in the mesenchymal tissue to induce macroscopic ECM clusters (Forgacs et al., 1989). In the present context, the collagen microfibrils are engaged in percolation behavior, and thus the fibrils are able to assemble a cluster/network with a pathway of links that could be shown to span a hydrogel's entire spatial domain. Percolating networks exhibit dynamic properties such as surface tension and viscosity, which can change dramatically when individual contacts are made and broken frequently – as one could envision during morphogenetic movements in which both cells and matrix are in concerted motion. Unbalanced interfacial tension forces within this fluid-like tissue composite also contribute to ECM motility. As a result of these intrinsic physical forces, the ECM could be, at least theoretically, set in motion and possibly driven by interfacial tension forces.
Computational models have been widely utilized to test hypotheses of cellular self-organization or emergence during embryonic patterning (Heisenberg and Bellaiche, 2013; Morelli et al., 2012). We propose that, similar to tissue patterning (i.e. position-dependent differentiation of cells), morphogenesis (i.e. changes in position and shape of tissues) also needs to be subject to testing for emergent properties with computational models. Mechanical and statistical modeling approaches are already underway to dissect tissue morphogenesis with computational tools (Brodland et al., 2007; Czirok and Isai, 2014; Mehes and Vicsek, 2014; Mones et al., 2015; Newman, 2008; Steimel et al., 2016; Swat et al., 2012; Voiculescu et al., 2014). However, inclusive and falsifiable models for morphogenetic movements that incorporate ECM motility, in addition to cell motility, are scarce. Quantitation of ECM motion, separate from collective cell motion, during embryogenesis is only a first step in this direction.
Outlook
Increasing evidence for ECM motion compels us to consider how this motion contributes to the destiny of hundreds of thousands of embryonic cells, and how the resulting tissue deformations shape the embryo. At the very least, gradient-based mechanisms, which are commonly invoked for regulating patterning and morphogenesis, must be evaluated as dynamic processes due to persistent millimeter-scale ‘background’ ECM motion. In addition, ECM is not only dynamic in the sense of a moving entity, but also with respect to its macromolecular properties across the embryonic space and time continuum (Czirok et al., 2006; Loganathan et al., 2012; Szabo et al., 2011). It is inescapable that the material properties (ECM) of tissues fluctuate during the course of embryogenesis. For these reasons it is important to pose experimental hypotheses that focus on the contribution of dynamic material properties to tissue morphogenesis. A flexible tissue will deform differently than a stiff tissue. We end by stating that in order to understand the complexity of embryogenesis it will be necessary to test hypotheses using computational models spanning the tissue scale. Only then, in our opinion, will scientists begin to understand the emergent biological properties that regulate morphogenesis. Essential developmental mechanisms await discovery by a critical examination of the mesoscale – data beyond the scope of conventional molecular studies.
Acknowledgements
This Review is dedicated to the memory of James H. Handelman, the past Executive Director of the Mathers Foundation. Throughout his 30-year career, Jim's enthusiasm and support for fundamental biological studies was a breath of fresh air to all who knew him. We thank the reviewers for providing essential feedback. The unpublished time-lapse sequence contained in Movie 1 was recorded in 2006 by Evan Zamir during a fellowship in the laboratory of C.D.L.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This article was supported by funds from the G. Harold and Leila Y. Mathers Foundation (C.D.L., B.J.R. and A.C.); the National Institutes of Health [NIH GM102801 to A.C.]; and the Hungarian Ministry of National Development [grant KTIA AIK 12-1-2013-0041 to A.C.]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.127886.supplemental
References
- Abercrombie M. (1977). Concepts in morphogenesis. Proc. R. Soc. B Biol. Sci. 199, 337-344. 10.1098/rspb.1977.0145 [DOI] [PubMed] [Google Scholar]
- Adams J. C. (2013). Extracellular matrix evolution: an overview. In Evolution of Extracellular Matrix (ed. Keeley F. W. and Mecham R. P.), pp. 1-25. Berlin: Springer. [Google Scholar]
- Aleksandrova A., Czirók A., Szabó A., Filla M. B., Hossain M. J., Whelan P. F., Lansford R. and Rongish B. J. (2012). Convective tissue movements play a major role in avian endocardial morphogenesis. Dev. Biol. 363, 348-361. 10.1016/j.ydbio.2011.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrova A., Czirok A., Kosa E., Galkin O., Cheuvront T. J. and Rongish B. J. (2015a). The endoderm and myocardium join forces to drive early heart tube assembly. Dev. Biol. 404, 40-54. 10.1016/j.ydbio.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrova A., Rongish B. J., Little C. D. and Czirók A. (2015b). Active cell and ECM movements during development. Methods Mol. Biol. 1189, 123-132. 10.1007/978-1-4939-1164-6_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufschnaiter R., Zamir E. A., Little C. D., Ozbek S., Munder S., David C. N., Li L., Sarras M. P. Jr and Zhang X. (2011). In vivo imaging of basement membrane movement: ECM patterning shapes Hydra polyps. J. Cell Sci. 124, 4027-4038. 10.1242/jcs.087239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénazéraf B. and Pourquié O. (2013). Formation and segmentation of the vertebrate body axis. Annu. Rev. Cell Dev. Biol. 29, 1-26. 10.1146/annurev-cellbio-101011-155703 [DOI] [PubMed] [Google Scholar]
- Bénazéraf B., Francois P., Baker R. E., Denans N., Little C. D. and Pourquié O. (2010). A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 466, 248-252. 10.1038/nature09151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocchini F. and Stern C. D. (2002). The hypoblast of the chick embryo positions the primitive streak by antagonizing nodal signaling. Dev. Cell 3, 735-744. 10.1016/S1534-5807(02)00318-0 [DOI] [PubMed] [Google Scholar]
- Bilozur M. E. and Hay E. D. (1988). Neural crest migration in 3D extracellular matrix utilizes laminin, fibronectin, or collagen. Dev. Biol. 125, 19-33. 10.1016/0012-1606(88)90055-3 [DOI] [PubMed] [Google Scholar]
- Brodland G. W., Viens D. and Veldhuis J. H. (2007). A new cell-based FE model for the mechanics of embryonic epithelia. Comput. Methods Biomech. Biomed. Eng. 10, 121-128. 10.1080/10255840601124704 [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. (1982). Distribution of latex beads and retinal pigment epithelial cells along the ventral neural crest pathway. Dev. Biol. 91, 50-63. 10.1016/0012-1606(82)90007-0 [DOI] [PubMed] [Google Scholar]
- Camenisch T. D., Spicer A. P., Brehm-Gibson T., Biesterfeldt J., Augustine M. L., Calabro A. Jr., Kubalak S., Klewer S. E. and McDonald J. A. (2000). Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Invest. 106, 349-360. 10.1172/JCI10272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Summerhayes I., Hsieh P. and Gallimore P. H. (1979). Possible role of fibronectin in malignancy. J. Supramol. Struct. 12, 139-150. 10.1002/jss.400120111 [DOI] [PubMed] [Google Scholar]
- Chen Q., Sivakumar P., Barley C., Peters D. M., Gomes R. R., Farach-Carson M. C. and Dallas S. L. (2007). Potential role for heparan sulfate proteoglycans in regulation of transforming growth factor-beta (TGF-beta) by modulating assembly of latent TGF-beta-binding protein-1. J. Biol. Chem. 282, 26418-26430. 10.1074/jbc.M703341200 [DOI] [PubMed] [Google Scholar]
- Conklin E. G. (1932). The Embryology of Amphioxus. J. Morphol. 54, 69-151. 10.1002/jmor.1050540103 [DOI] [Google Scholar]
- Cui C., Cheuvront T. J., Lansford R. D., Moreno-Rodriguez R. A., Schultheiss T. M. and Rongish B. J. (2009a). Dynamic positional fate map of the primary heart-forming region. Dev. Biol. 332, 212-222. 10.1016/j.ydbio.2009.05.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C., Little C. D. and Rongish B. J. (2009b). Rotation of organizer tissue contributes to left-right asymmetry. Anat. Rec. 292, 557-561. 10.1002/ar.20872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirok A. and Isai D. G. (2014). Cell resolved, multiparticle model of plastic tissue deformations and morphogenesis. Phys. Biol. 12, 016005 10.1088/1478-3975/12/1/016005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirok A. and Little C. D. (2012). Pattern formation during vasculogenesis. Birth Defects Res. C Embryol. Today 96, 153-162. 10.1002/bdrc.21010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirok A., Zamir E. A., Filla M. B., Little C. D. and Rongish B. J. (2006). Extracellular matrix macroassembly dynamics in early vertebrate embryos. Curr. Top. Dev. Biol. 73, 237-258. 10.1016/S0070-2153(05)73008-8 [DOI] [PubMed] [Google Scholar]
- Dallas S. L. and Veno P. A. (2012). Live imaging of bone cell and organ cultures. Methods Mol. Biol. 816, 425-457. 10.1007/978-1-61779-415-5_26 [DOI] [PubMed] [Google Scholar]
- Dallas S. L., Veno P. A., Rosser J. L., Barragan-Adjemian C., Rowe D. W., Kalajzic I. and Bonewald L. F. (2009). Time lapse imaging techniques for comparison of mineralization dynamics in primary murine osteoblasts and the late osteoblast/early osteocyte-like cell line MLO-A5. Cells Tissues Organs 189, 6-11. 10.1159/000151745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfini M.-C., Dubrulle J., Malapert P., Chal J. and Pourquie O. (2005). Control of the segmentation process by graded MAPK/ERK activation in the chick embryo. Proc. Natl. Acad. Sci. USA 102, 11343-11348. 10.1073/pnas.0502933102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C. J., Davis L. A., Walters L. and Little C. D. (1990). Avian vasculogenesis and the distribution of collagens I, IV, laminin, and fibronectin in the heart primordia. J. Exp. Zool. 255, 309-322. 10.1002/jez.1402550308 [DOI] [PubMed] [Google Scholar]
- Drake C. J., Brandt S. J., Trusk T. C. and Little C. D. (1997). TAL1/SCL is expressed in endothelial progenitor cells/angioblasts and defines a dorsal-to-ventral gradient of vasculogenesis. Dev. Biol. 192, 17-30. 10.1006/dbio.1997.8751 [DOI] [PubMed] [Google Scholar]
- Dray N., Lawton A., Nandi A., Jülich D., Emonet T. and Holley S. A. (2013). Cell-fibronectin interactions propel vertebrate trunk elongation via tissue mechanics. Curr. Biol. 23, 1335-1341. 10.1016/j.cub.2013.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L. and Discher D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677-689. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Essner J. J., Amack J. D., Nyholm M. K., Harris E. B. and Yost H. J. (2005). Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development 132, 1247-1260. 10.1242/dev.01663 [DOI] [PubMed] [Google Scholar]
- Fidler A. L., Vanacore R. M., Chetyrkin S. V., Pedchenko V. K., Bhave G., Yin V. P., Stothers C. L., Rose K. L., McDonald W. H., Clark T. A. et al. (2014). A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc. Natl. Acad. Sci. USA 111, 331-336. 10.1073/pnas.1318499111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury V. (2012). Clarifying tetrapod embryogenesis by a dorso-ventral analysis of the tissue flows during early stages of chicken development. Biosystems 109, 460-474. 10.1016/j.biosystems.2012.04.003 [DOI] [PubMed] [Google Scholar]
- Forgacs G., Jaikaria N. S., Frisch H. L. and Newman S. A. (1989). Wetting, percolation and morphogenesis in a model tissue system. J. Theor. Biol. 140, 417-430. 10.1016/S0022-5193(89)80096-7 [DOI] [PubMed] [Google Scholar]
- Foty R. A. and Steinberg M. S. (2005). The differential adhesion hypothesis: a direct evaluation. Dev. Biol. 278, 255-263. 10.1016/j.ydbio.2004.11.012 [DOI] [PubMed] [Google Scholar]
- Gamba A., Ambrosi D., Coniglio A., de Candia A., Di Talia S., Giraudo E., Serini G., Preziosi L. and Bussolino F. (2003). Percolation, morphogenesis, and burgers dynamics in blood vessels formation. Phys. Rev. Lett. 90, 118101 10.1103/PhysRevLett.90.118101 [DOI] [PubMed] [Google Scholar]
- Ghosh S. and Brauer P. R. (1996). Latent transforming growth factor-beta is present in the extracellular matrix of embryonic hearts in situ. Dev. Dyn. 205, 126-134. [DOI] [PubMed] [Google Scholar]
- Gros J., Feistel K., Viebahn C., Blum M. and Tabin C. J. (2009). Cell movements at Hensen's node establish left/right asymmetric gene expression in the chick. Science 324, 941-944. 10.1126/science.1172478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J., Hu J. K.-H., Vinegoni C., Feruglio P. F., Weissleder R. and Tabin C. J. (2010). WNT5A/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Curr. Biol. 20, 1993-2002. 10.1016/j.cub.2010.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. G. (1910). The outgrowth of the nerve fiber as a mode of protoplasmic movement. J. Exp. Zool. 9, 787-846. 10.1002/jez.1400090405 [DOI] [PubMed] [Google Scholar]
- Harrison R. G. (1912). The cultivation of tissues in extraneous media as a method of morphogenetic study. Anat. Rec. 6, 181-193. 10.1002/ar.1090060404 [DOI] [Google Scholar]
- Harrisson F., Van Hoof J., Vanroelen C. and Vakaet L. (1985). Transfer of extracellular matrix components between germ layers in chimaeric chicken-quail blastoderms. Cell Tissue Res. 239, 643-649. 10.1007/BF00219243 [DOI] [PubMed] [Google Scholar]
- Hay E. D. (1989). Extracellular matrix, cell skeletons, and embryonic development. Am. J. Med. Genet. 34, 14-29. 10.1002/ajmg.1320340107 [DOI] [PubMed] [Google Scholar]
- Heisenberg C.-P. and Bellaïche Y. (2013). Forces in tissue morphogenesis and patterning. Cell 153, 948-962. 10.1016/j.cell.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Holland J. H. (1999). Emergence: From Chaos to Order. Cambridge: Helix Books. [Google Scholar]
- Hynes R. O. (2012). The evolution of metazoan extracellular matrix. J. Cell Biol. 196, 671-679. 10.1083/jcb.201109041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel-ElSayed S. A., Tiede-Lewis L. M., Lu Y., Veno P. A. and Dallas S. L. (2015). Novel approaches for two and three dimensional multiplexed imaging of osteocytes. Bone 76, 129-140. 10.1016/j.bone.2015.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Davidson L., Edlund A., Elul T., Ezin M., Shook D. and Skoglund P. (2000). Mechanisms of convergence and extension by cell intercalation. Philos. Trans. R. Soc. B Biol. Sci. 355, 897-922. 10.1098/rstb.2000.0626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T. and Kronenberg H. M. (2014). Overview of skeletal development. Methods Mol. Biol. 1130, 3-12. 10.1007/978-1-62703-989-5_1 [DOI] [PubMed] [Google Scholar]
- Komatsu Y. and Mishina Y. (2013). Establishment of left-right asymmetry in vertebrate development: the node in mouse embryos. Cell. Mol. Life Sci. 70, 4659-4666. 10.1007/s00018-013-1399-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel B. A., Rongish B. J., Czirok A., Zach J., Little C. D., Davis E. C., Knutsen R. H., Wagenseil J. E., Levy M. A. and Mecham R. P. (2006). Elastic fiber formation: a dynamic view of extracellular matrix assembly using timer reporters. J. Cell. Physiol. 207, 87-96. 10.1002/jcp.20546 [DOI] [PubMed] [Google Scholar]
- Kulesa P. M. and McLennan R. (2015). Neural crest migration: trailblazing ahead. F1000prime Rep. 7, 2 10.12703/P7-02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton A. K., Nandi A., Stulberg M. J., Dray N., Sneddon M. W., Pontius W., Emonet T. and Holley S. A. (2013). Regulated tissue fluidity steers zebrafish body elongation. Development 140, 573-582. 10.1242/dev.090381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. D. and Anderson K. V. (2008). Morphogenesis of the node and notochord: the cellular basis for the establishment and maintenance of left-right asymmetry in the mouse. Dev. Dyn. 237, 3464-3476. 10.1002/dvdy.21598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. (2005). Left-right asymmetry in embryonic development: a comprehensive review. Mech. Dev. 122, 3-25. 10.1016/j.mod.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Lewis W. H. (1923). Amniotic ectoderm in tissue-cultures. Anat. Rec. 26, 97-117. 10.1002/ar.1090260204 [DOI] [Google Scholar]
- Li Y., Trivedi V., Truong T. V., Koos D. S., Lansford R., Chuong C.-M., Warburton D., Moats R. A. and Fraser S. E. (2015). Dynamic imaging of the growth plate cartilage reveals multiple contributors to skeletal morphogenesis. Nat. Commun. 6, 6798 10.1038/ncomms7798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan R., Potetz B. R., Rongish B. J. and Little C. D. (2012). Spatial anisotropies and temporal fluctuations in extracellular matrix network texture during early embryogenesis. PLoS ONE 7, e38266 10.1371/journal.pone.0038266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor R. and Theveneau E. (2013). The neural crest. Development 140, 2247-2251. 10.1242/dev.091751 [DOI] [PubMed] [Google Scholar]
- McMillen P. and Holley S. A. (2015). The tissue mechanics of vertebrate body elongation and segmentation. Curr. Opin. Genet. Dev. 32, 106-111. 10.1016/j.gde.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méhes E. and Vicsek T. (2014). Collective motion of cells: from experiments to models. Integr. Biol. 6, 831-854. 10.1039/C4IB00115J [DOI] [PubMed] [Google Scholar]
- Merks R. M. H., Brodsky S. V., Goligorksy M. S., Newman S. A. and Glazier J. A. (2006). Cell elongation is key to in silico replication of in vitro vasculogenesis and subsequent remodeling. Dev. Biol. 289, 44-54. 10.1016/j.ydbio.2005.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merks R. M. H., Perryn E. D., Shirinifard A. and Glazier J. A. (2008). Contact-inhibited chemotaxis in de novo and sprouting blood-vessel growth. PLoS Comput. Biol. 4, e1000163 10.1371/journal.pcbi.1000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mones E., Czirók A. and Vicsek T. (2015). Anomalous segregation dynamics of self-propelled particles. New J. Phys. 17, 063013 10.1088/1367-2630/17/6/063013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli L. G., Uriu K., Ares S. and Oates A. C. (2012). Computational approaches to developmental patterning. Science 336, 187-191. 10.1126/science.1215478 [DOI] [PubMed] [Google Scholar]
- Newman T. J. (2008). Grid-free models of multicellular systems, with an application to large-scale vortices accompanying primitive streak formation. Curr. Top. Dev. Biol. 81, 157-182. 10.1016/S0070-2153(07)81005-2 [DOI] [PubMed] [Google Scholar]
- Newman S. A. and Tomasek J. J. (1996). Morphogenesis of connective tissues. In Molecular Components and Interactions (ed. Comper W. D.), pp. 335-369. Amsterdam: Harwood Academic Publishers. [Google Scholar]
- Newman S. A., Frenz D. A., Tomasek J. J. and Rabuzzi D. D. (1985). Matrix-driven translocation of cells and nonliving particles. Science 228, 885-889. 10.1126/science.4001925 [DOI] [PubMed] [Google Scholar]
- Nonaka S., Tanaka Y., Okada Y., Takeda S., Harada A., Kanai Y., Kido M. and Hirokawa N. (1998). Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829-837. 10.1016/S0092-8674(00)81705-5 [DOI] [PubMed] [Google Scholar]
- Noonan K. J., Hunziker E. B., Nessler J. and Buckwalter J. A. (1998). Changes in cell, matrix compartment, and fibrillar collagen volumes between growth-plate zones. J. Orthop. Res. 16, 500-508. 10.1002/jor.1100160416 [DOI] [PubMed] [Google Scholar]
- Ozbek S., Balasubramanian P. G., Chiquet-Ehrismann R., Tucker R. P. and Adams J. C. (2010). The evolution of extracellular matrix. Mol. Biol. Cell 21, 4300-4305. 10.1091/mbc.E10-03-0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perris R. and Perissinotto D. (2000). Role of the extracellular matrix during neural crest cell migration. Mech. Dev. 95, 3-21. 10.1016/S0925-4773(00)00365-8 [DOI] [PubMed] [Google Scholar]
- Perris R., Krotoski D., Lallier T., Domingo C., Sorrell J. M. and Bronner-Fraser M. (1991). Spatial and temporal changes in the distribution of proteoglycans during avian neural crest development. Development 111, 583-599. [DOI] [PubMed] [Google Scholar]
- Perryn E. D., Czirók A. and Little C. D. (2008). Vascular sprout formation entails tissue deformations and VE-cadherin-dependent cell-autonomous motility. Dev. Biol. 313, 545-555. 10.1016/j.ydbio.2007.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole T. J. and Coffin J. D. (1989). Vasculogenesis and angiogenesis: two distinct morphogenetic mechanisms establish embryonic vascular pattern. J. Exp. Zool. 251, 224-231. 10.1002/jez.1402510210 [DOI] [PubMed] [Google Scholar]
- Raya A. and Izpis ú a Belmonte J. C. (2006). Left-right asymmetry in the vertebrate embryo: from early information to higher-level integration. Nat. Rev. Genet. 7, 283-293. 10.1038/nrg1830 [DOI] [PubMed] [Google Scholar]
- Rozario T. and DeSimone D. W. (2010). The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 341, 126-140. 10.1016/j.ydbio.2009.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozbicki E., Chuai M., Karjalainen A. I., Song F., Sang H. M., Martin R., Knölker H.-J., MacDonald M. P. and Weijer C. J. (2015). Myosin-II-mediated cell shape changes and cell intercalation contribute to primitive streak formation. Nat. Cell Biol. 17, 397-408. 10.1038/ncb3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp P. A., Czirók A. and Little C. D. (2003). Novel approaches for the study of vascular assembly and morphogenesis in avian embryos. Trends Cardiovasc. Med. 13, 283-288. 10.1016/S1050-1738(03)00118-X [DOI] [PubMed] [Google Scholar]
- Rupp P. A., Czirók A. and Little C. D. (2004). alphavbeta3 integrin-dependent endothelial cell dynamics in vivo. Development 131, 2887-2897. 10.1242/dev.01160 [DOI] [PubMed] [Google Scholar]
- Sato Y., Poynter G., Huss D., Filla M. B., Czirók A., Rongish B. J., Little C. D., Fraser S. E. and Lansford R. (2010). Dynamic analysis of vascular morphogenesis using transgenic quail embryos. PLoS ONE 5, e12674 10.1371/journal.pone.0012674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweickert A., Weber T., Beyer T., Vick P., Bogusch S., Feistel K. and Blum M. (2007). Cilia-driven leftward flow determines laterality in Xenopus. Curr. Biol. 17, 60-66. 10.1016/j.cub.2006.10.067 [DOI] [PubMed] [Google Scholar]
- Serini G., Ambrosi D., Giraudo E., Gamba A., Preziosi L. and Bussolino F. (2003). Modeling the early stages of vascular network assembly. EMBO J. 22, 1771-1779. 10.1093/emboj/cdg176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J. and Keller R. (1992). Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development 116, 901-914. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L. and Sepich D. S. (2012). Gastrulation: making and shaping germ layers. Annu. Rev. Cell Dev. Biol. 28, 687-717. 10.1146/annurev-cellbio-092910-154043 [DOI] [PubMed] [Google Scholar]
- Spicer A. P. and Tien J. Y. L. (2004). Hyaluronan and morphogenesis. Birth Defects Res. C Embryo Today 72, 89-108. 10.1002/bdrc.20006 [DOI] [PubMed] [Google Scholar]
- Spratt N. T., Jr (1948). Development of the early chick blastoderm on synthetic media. J. Exp. Zool. 107, 39-64. 10.1002/jez.1401070103 [DOI] [PubMed] [Google Scholar]
- Steimel J. P., Aragones J. L., Hu H., Qureshi N. and Alexander-Katz A. (2016). Emergent ultra-long-range interactions between active particles in hybrid active-inactive systems. Proc. Natl. Acad. Sci. USA 113, 4652-4657. 10.1073/pnas.1520481113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. S. (1963). Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science 141, 401-408. 10.1126/science.141.3579.401 [DOI] [PubMed] [Google Scholar]
- Swat M. H., Thomas G. L., Belmonte J. M., Shirinifard A., Hmeljak D. and Glazier J. A. (2012). Multi-scale modeling of tissues using CompuCell3D. Methods Cell Biol. 110, 325-366. 10.1016/B978-0-12-388403-9.00013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó A., Rupp P. A., Rongish B. J., Little C. D. and Czirók A. (2011). Extracellular matrix fluctuations during early embryogenesis. Phys. Biol. 8, 045006 10.1088/1478-3975/8/4/045006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Sipp D. and Enomoto H. (2013). Tissue interactions in neural crest cell development and disease. Science 341, 860-863. 10.1126/science.1230717 [DOI] [PubMed] [Google Scholar]
- Tickle C., Crawley A. and Goodman M. (1978). Cell movement and the mechanism of invasiveness: a survey of the behaviour of some normal and malignant cells implanted into the developing chick wing bud. J. Cell Sci. 31, 293-322. [DOI] [PubMed] [Google Scholar]
- Toole B. P. (2001). Hyaluronan in morphogenesis. Semin. Cell Dev. Biol. 12, 79-87. 10.1006/scdb.2000.0244 [DOI] [PubMed] [Google Scholar]
- Townes P. and Holtfreter J. (1955). Directed movements and selective adhesion of embryonic amphibian cells. J. Exp. Zool. A Comp. Exp. Biol. 128, 53-120. 10.1002/jez.1401280105 [DOI] [PubMed] [Google Scholar]
- Trinkaus J. P. (1963). The cellular basis of Fundulus epiboly. Adhesivity of blastula and gastrula cells in culture. Dev. Biol. 7, 513-532. 10.1016/0012-1606(63)90139-8 [DOI] [PubMed] [Google Scholar]
- van Oers R. F. M., Rens E. G., LaValley D. J., Reinhart-King C. A. and Merks R. M. (2014). Mechanical cell-matrix feedback explains pairwise and collective endothelial cell behavior in vitro. PLoS Comput. Biol. 10, e1003774 10.1371/journal.pcbi.1003774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner V. D. and Taber L. A. (2012). Not just inductive: a crucial mechanical role for the endoderm during heart tube assembly. Development 139, 1680-1690. 10.1242/dev.073486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiculescu O., Bertocchini F., Wolpert L., Keller R. E. and Stern C. D. (2007). The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature 449, 1049-1052. 10.1038/nature06211 [DOI] [PubMed] [Google Scholar]
- Voiculescu O., Bodenstein L., Lau I.-J. and Stern C. D. (2014). Local cell interactions and self-amplifying individual cell ingression drive amniote gastrulation. eLife 3, e01817 10.7554/eLife.01817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Tytell J. D. and Ingber D. E. (2009). Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10, 75-82. 10.1038/nrm2594 [DOI] [PubMed] [Google Scholar]
- Wittig J. G. and Münsterberg A. (2016). The early stages of heart development: insights from chicken embryos. J. Cardiovasc. Dev. Dis. 3, 12 10.3390/jcdd3020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Dormann D., Münsterberg A. E. and Weijer C. J. (2002). Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev. Cell 3, 425-437. 10.1016/S1534-5807(02)00256-3 [DOI] [PubMed] [Google Scholar]
- Zagris N. (2001). Extracellular matrix in development of the early embryo. Micron 32, 427-438. 10.1016/S0968-4328(00)00011-1 [DOI] [PubMed] [Google Scholar]
- Zamir E. A., Czirók A., Rongish B. J. and Little C. D. (2005). A digital image-based method for computational tissue fate mapping during early avian morphogenesis. Ann. Biomed. Eng. 33, 854-865. 10.1007/s10439-005-3037-7 [DOI] [PubMed] [Google Scholar]
- Zamir E. A., Czirok A., Cui C., Little C. D. and Rongish B. J. (2006). Mesodermal cell displacements during avian astrulation are due to both individual cell-autonomous and convective tissue movements. Proc. Natl. Acad. Sci. USA 103, 19806-19811. 10.1073/pnas.0606100103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E. A., Rongish B. J. and Little C. D. (2008). The ECM moves during primitive streak formation--computation of ECM versus cellular motion. PLoS Biol. 6, e247 10.1371/journal.pbio.0060247 [DOI] [PMC free article] [PubMed] [Google Scholar]