Abstract
The inner ear consists of two otocyst-derived, structurally and functionally distinct components: the dorsal vestibular and ventral auditory compartments. BMP signaling is required to form the vestibular compartment, but how it complements other required signaling molecules and acts intracellularly is unknown. Using spatially and temporally controlled delivery of signaling pathway regulators to developing chick otocysts, we show that BMP signaling regulates the expression of Dlx5 and Hmx3, both of which encode transcription factors essential for vestibular formation. However, although BMP regulates Dlx5 through the canonical SMAD pathway, surprisingly, it regulates Hmx3 through a non-canonical pathway involving both an increase in cAMP-dependent protein kinase A activity and the GLI3R to GLI3A ratio. Thus, both canonical and non-canonical BMP signaling establish the precise spatiotemporal expression of Dlx5 and Hmx3 during dorsal vestibular development. The identification of the non-canonical pathway suggests an intersection point between BMP and SHH signaling, which is required for ventral auditory development.
KEY WORDS: Dorsalization, Inner ear, Otocyst, Polarization, Signaling, Chicken
Summary: BMP signaling regulates the expression of Dlx5 and Hmx3, transcription factors essential for vestibular development, through the canonical pSMAD pathway and a non-canonical pathway, respectively.
INTRODUCTION
The inner ear is a morphologically complex three-dimensional organ with regionally specified sensory functions. It develops from the otocyst, an initially spherical epithelial primordium that forms adjacent to the caudal hindbrain. Growth factors secreted from neighboring structures, such as the neural tube and notochord, establish the dorsoventral (DV) patterning of the otocyst (Bok et al., 2007a; Groves and Fekete, 2012; Riccomagno et al., 2002, 2005; Wu and Kelley, 2012). Two signaling systems, the Wingless-type MMTV integration site family (WNT) and Sonic hedgehog (SHH), play particularly important roles in this process. Each is presumed to provide positional information across the DV axis of the otocyst by forming concentration gradients; namely, a dorsal-high to ventral-low gradient of WNT that patterns mainly the dorsal otocyst, and an opposing gradient of SHH that patterns mainly the ventral otocyst (Bok et al., 2005; Bok et al., 2007a; Ohyama et al., 2006; Riccomagno et al., 2002, 2005). Wnt1 and Wnt3a expressed in the dorsal neural tube, but not in the otocyst, are required for vestibular development (Riccomagno et al., 2005). SHH signaling, in contrast to WNT signaling, patterns the ventral otocyst and is required for formation of the cochlear duct. Absence of SHH signaling in Shh−/− mouse embryos, or ablation of the embryonic tissue secreting SHH (notochord and floor plate of the neural tube) in chick embryos, results in complete loss of the cochlear duct (Bok et al., 2005, 2007b; Brown and Epstein, 2011; Riccomagno et al., 2002).
SHH signaling is mediated by GLI transcription factors, of which there are two functionally distinct types: activators (GLIA) and repressors (GLIR) (Briscoe and Novitch, 2008; Ingham and McMahon, 2001). cAMP-dependent protein kinase A (PKA) phosphorylates full-length GLI, stimulating its proteolytic processing to GLIR (Chen et al., 1998; Wang et al., 2000). During development of the inner ear, GLI2 and GLI3 play crucial roles in mediating SHH signaling through their dual functions. In the ventral otocyst, which is closest to the source of SHH signaling, the unprocessed GLIA forms are expected to predominate, whereas in the dorsal otocyst, which is distant from the source of SHH signaling, partial proteolytic processing of the full-length form is expected to occur, generating the GLIR forms. Based on the analysis of mouse GLI mutant embryos, it was suggested that formation of the cochlear duct requires predominantly GLI2A and GLI3A, whereas formation of the vestibular system requires predominantly GLI3R (Bok et al., 2007a).
Although a simple model of otocyst DV patterning is appealing, in which opposing gradients of only two signaling pathways are required, other evidence suggests that patterning is more complex. For example, in the absence of SHH signaling, Dlx5, a gene normally expressed in the dorsal otocyst, is expanded ventrally; however, another dorsally expressed gene, Hmx3 (formerly Nkx5.1), is unaffected (Riccomagno et al., 2002). Moreover, Pax2 and Otx2, two genes expressed in the ventral otocyst, are expressed normally in the Wnt1/Wnt3a double mutant, rather than being expanded dorsally (Riccomagno et al., 2005). These results suggest that other signals in addition to WNTs and SHH provide regional information for otocyst DV patterning. Among the possible candidates are BMPs. BMPs are expressed in the roof plate of the hindbrain and in the adjacent dorsomedial margin of the otic cup (Oh et al., 1996; Wu and Oh, 1996), as well as within the wall of the dorsal otocyst itself (Chang et al., 2008; Oh et al., 1996; Wu and Oh, 1996). Chromatography beads coated with the BMP antagonist noggin and implanted into chick embryos adjacent to the dorsal otocyst inhibit the formation of the semicircular canals (Chang et al., 1999; Gerlach et al., 2000). Similarly, conditional knockout of Bmp4 in the developing mouse inner ear results in loss of the three sensory cristae and their associated semicircular canals, whereas the cochlear duct seems unaffected (Chang et al., 2008). Thus, in addition to WNT signaling, BMP signaling is required for development of dorsal otocyst structures.
In the dorsolateral otocyst, BMP signaling was suggested to maintain, either directly or indirectly, the expression of two homeobox genes, Dlx5 and Hmx3, which encode transcription factors required to form the primordial canal pouch (Hadrys et al., 1998; Merlo et al., 2002; Wang et al., 2004, 1998). However, it is not known how BMP signaling molecularly regulates the expression of these genes.
Here, we show that BMP signaling regulates both Dlx5 and Hmx3 expression in the dorsal otocyst and this occurs through distinct intracellular pathways. Specifically, the canonical phospho-SMAD (pSMAD) pathway regulates Dlx5 expression, whereas a non-canonical pathway that activates PKA and increases the ratio in the dorsal otocyst of GLI3R to GLI3A regulates Hmx3. Thus, both canonical and non-canonical BMP signaling are important for dorsal otocyst patterning, and the non-canonical pathway suggests a link that could be used to coordinate dorsal and ventral otocyst patterning signals.
RESULTS
Gain of BMP signaling inhibits cochlear duct development and induces Hmx3 and Dlx5 expression
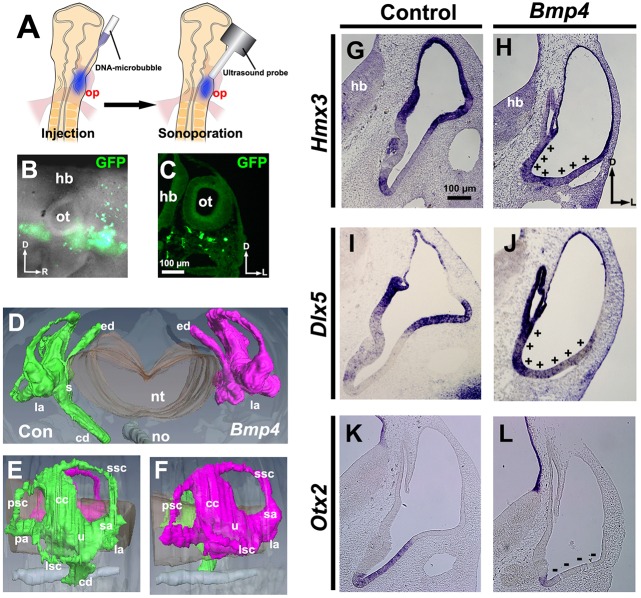
Previous loss-of-function studies showed that BMPs are required to form the vestibular system (Chang et al., 2008, 1999, 2002; Gerlach et al., 2000; Ohta et al., 2010). However, whether increasing BMP signaling dorsalizes the early otocyst is unknown. To determine this, we co-sonoporated Bmp4 and Gfp into the ventral head mesenchyme of chick embryos at HH stages 9-11 (Fig. 1A-C). Computer-aided three-dimensional reconstruction of the inner ears 5-6 days later showed that, in comparison with the control side, development of the cochlear duct on the sonoporated side was strongly inhibited, whereas both the vestibular system and endolymphatic duct were normal (Fig. 1D-F). Moreover, 60 h after sonoporation, the expression of two transcription factors normally restricted mainly to the dorsal otocyst, namely Hmx3 and Dlx5, was altered. Relative to embryos treated with Gfp only, the expression of both genes was expanded ventromedially within the otocyst of embryos treated with both Bmp4 and Gfp (Fig. 1G-J, plus signs). Moreover, expression of Otx2, a gene normally expressed ventrally in the nascent cochlear duct, was inhibited (Fig. 1K,L, minus signs). These results show that gain of BMP signaling ventrally is sufficient to dorsalize the ventral otocyst, suggesting that BMP signaling plays a role in establishing the dorsal polarity of the otocyst.
Fig. 1.
Bmp4 overexpression expands Hmx3 and Dlx5 expression ventrally, attenuates Otx2 expression and blocks formation of the cochlea. (A-C) Transfection scheme. (A) Diagram showing sonoporation targeted to transfect head mesenchymal cells ventral to the otic placode. (B) Whole-mount view 12-18 h after sonoporation of Gfp. (C) Transverse section through another transfected chicken embryo. (D-F) Reconstruction (D, frontal view; E,F, lateral views) of serial transverse sections of inner ears (HH stage 30) collected after sonoporation of Bmp4. Green, control (Con); magenta, Bmp4 overexpression. Paint fills, n=3; paraffin serial sections, n=5; reconstruction, n=1. (G-L) In situ hybridization of transverse sections of otocysts collected after transfection when embryos reached HH stages 24-25. (G,H) Hmx3 expression. +, region of expanded Hmx3 expression. (I,J) Dlx5 expression. +, region of expanded Dlx5 expression. (K,L) Otx2 expression. −, region of reduced Otx2 expression. n=6 (G,H,K,L) or n=9 (I,J) control and experimental otocysts. The right-left axis of histological images and reconstructions are oriented here and subsequently to align with the experimental schema, simplifying data interpretation. D, dorsal; L, lateral; R, rostral; cc, common crus; cd, cochlear duct; ed, endolymphatic duct; hb, hindbrain; la, lateral ampulla; lsc, lateral semicircular canal; no, notochord; nt, neural tube; op, otic placode; ot, otocyst; pa, posterior ampulla; psc, posterior semicircular canal; s, saccule; sa, superior ampulla; ssc, superior semicircular canal; u, utricle.
Loss of BMP signaling inhibits dorsal otocyst development and attenuates Hmx3 and Dlx5 expression while expanding Otx2 expression dorsally
Previously, we reported that overexpression of noggin (Nog), a BMP antagonist, resulted in the complete loss of the three semicircular canals 5-6 days later, whereas the endolymphatic duct and cochlear duct formed essentially normally (Ohta et al., 2010). To further test the requirement of BMP signaling in patterning the dorsal otocyst, we blocked BMP signaling again by co-sonoporating Nog and Gfp into the dorsal head mesenchyme of chick embryos at HH stages 15-16 (Fig. 2A-C). First, we confirmed our previous result, once more showing the loss of the semicircular canals and the persistence of the endolymphatic duct and cochlear duct (Fig. 2D). Then, we asked whether BMP signaling is required for Hmx3 and Dlx5 expression in the dorsal otocyst. In otocysts treated with Nog and Gfp, formation of the primordial canal pouch was inhibited 48 h later, and both Hmx3 and Dlx5 expression was greatly diminished (Fig. 2E-H, black arrowheads), with the exception that Dlx5 was still expressed in the forming endolymphatic duct (Fig. 2H, red arrowhead). Moreover, Otx2 expression was expanded dorsally (Fig. 2I,J, red arrowheads) and was even expressed ectopically in the most dorsal otocyst cells (Fig. 2J, asterisk, inset). These results show that BMP signaling is required for both Hmx3 and Dlx5 expression, and that it restricts Otx2 expression to the ventral otocyst.
Fig. 2.
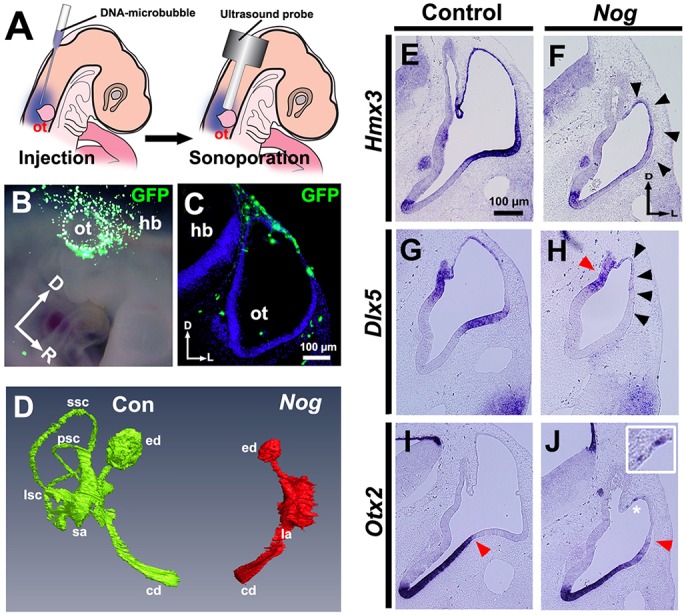
Nog overexpression attenuates Hmx3 and Dlx5 expression, expands Otx2 expression dorsally and blocks formation of the semicircular canals. (A-C) Transfection scheme. (A) Diagram showing sonoporation targeted to transfect head mesenchymal cells overlying the dorsal otocyst. (B) Whole-mount view 12-18 h after sonoporation of Gfp. The borders of the otocyst are indicated by white dots. (C) Transverse section through another transfected embryo. (D) Frontal view of a reconstruction of serial transverse sections of inner ears at HH stage 35 collected after sonoporation of Nog. Green, control (Con); red, Nog overexpression. Paint fills, n=3; paraffin serial sections, n=3; reconstruction, n=1. (E-J) In situ hybridization of transverse sections of otocysts collected after transfection when embryos reached HH stages 24-25. (E,F) Hmx3 expression. Arrowheads indicate region of reduced Hmx3 expression. (G,H) Dlx5 expression. Black arrowheads indicate region of abolished Dlx5 expression; red arrowhead indicates region (the developing endolymphatic duct) of persisting Dlx5 expression. (I,J) Otx2 expression. Asterisk indicates ectopic expression of Otx2 (enlarged in inset); red arrowhead indicates the dorsolateral limit of Otx2 expression, which is expanded further dorsally after Nog overexpression. (E-J) n=6 control and experimental otocysts. Abbreviations as in Fig. 1.
BMP signaling controls Dlx5 expression through the pSMAD pathway
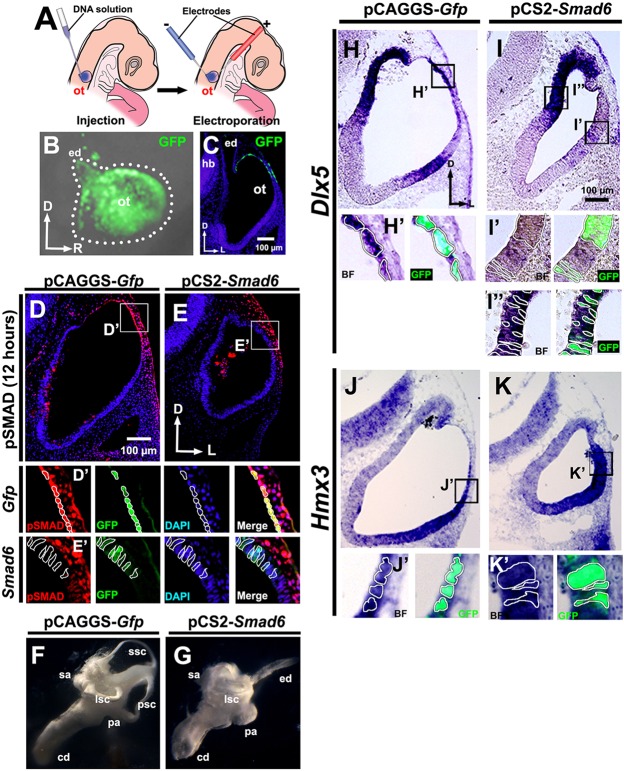
To determine whether BMP signaling controls Hmx3 and/or Dlx5 expression in the dorsal otocyst through its canonical pathway, we blocked pSMAD signaling in the dorsal otocyst by co-electroporating the inhibitory SMAD Smad6 and Gfp into the dorsolateral wall of the otocyst at HH stages 15-16 (Fig. 3A-C). Twelve hours later, pSMAD labeling was still present in the dorsal region of control otocysts transfected with Gfp alone (Fig. 3D,D′), but in otocysts co-transfected with Smad6 and Gfp the pSMAD labeling in the dorsal otocyst was absent (Fig. 3E,E′), confirming that overexpression of Smad6 blocked pSMAD signaling. Additionally, co-transfection of Smad6 and Gfp into the dorsal otocyst completely inhibited development of the semicircular canals, but development of the endolymphatic duct and cochlear duct was essentially unaffected (Fig. 3F,G).
Fig. 3.
Smad6 overexpression inhibits Dlx5 expression in the dorsolateral otocyst, but not in the dorsomedial otocyst, fails to inhibit Hmx3 expression and blocks formation of the semicircular canals. (A-C) Transfection scheme. (A) Diagram showing electroporation targeted to the dorsolateral otocyst. (B) Whole-mount view 12-18 h after electroporation of Gfp. The borders of the otocyst are indicated by white dots. (C) Transverse section of the same embryo shown in B. (D-E′) pSMAD labeling of chick otocyst sections collected after electroporation. Boxed regions are shown in D′,E′, with borders of selected cells outlined. (F,G) Paint fills of chick inner ear at HH stage 30. Paint fills, n=5; paraffin serial sections, n=1. (H-K′) In situ hybridization for Dlx5 (H-I′) and Hmx3 (J-K′) expression on transverse sections of otocysts collected after transfection when embryos reached HH stages 20-21. Boxed regions are shown in H′-K′, with borders of selected cells outlined. n=6 (D,E,J,K) or n=11 (H,I) control and experimental otocysts. BF, brightfield; other abbreviations as in Fig. 1.
We next examined Hmx3 and Dlx5 expression in the otocyst at HH stages 20-21. Neither Dlx5 nor Hmx3 expression was affected after treatment with Gfp alone (Fig. 3H-K′). However, after treatment with Smad6 and Gfp, Dlx5 expression was abolished dorsolaterally (Fig. 3I,I′), with the exception that cells in the dorsomedial otocyst (i.e. those that form the endolymphatic duct) still expressed Dlx5 (Fig. 3I,I″). In striking contrast, all treated otocysts still expressed Hmx3 (Fig. 3J-K′). These results clearly show that BMP signaling regulates Dlx5 expression in the dorsolateral otocyst through the canonical pSMAD pathway, whereas Hmx3 expression is not regulated by this pathway, suggesting a role for a non-canonical BMP signaling pathway.
BMP signaling controls Hmx3 expression through a non-canonical pathway that involves GLI3 protein processing
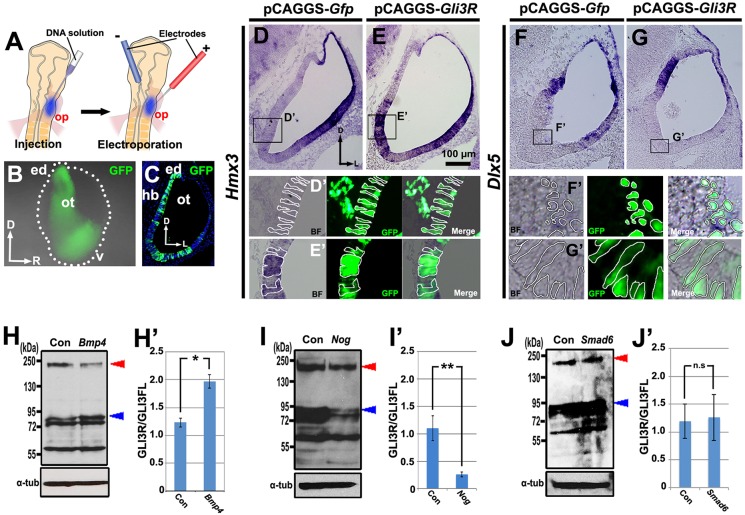
Analysis of inner ear phenotypes in GLI mutant mice suggested that GLIR and GLIA are present in the early otocyst as reciprocal DV protein gradients, with repressor being high dorsally and activator high ventrally (Bok et al., 2007a). This raises the possibility that such a gradient might control Hmx3 expression in the dorsolateral otocyst. To test this, we co-electroporated Gli3R and Gfp into the ventral otic epithelium of chick embryos at HH stages 9-11 (Fig. 4A-C) in order to alter the gradient by counteracting the higher level of Gli3A expected to be present in the ventral otocyst, and examined gene expression 24-30 h later at HH stages 20-21. In control otocysts transfected with Gfp alone, Hmx3 expression was unaltered (Fig. 4D,D′), but in otocysts co-transfected with Gli3R and Gfp, ectopic Hmx3 expression occurred ventromedially (Fig. 4E,E′). Dlx5 expression was unchanged in both control (Fig. 4F,F′) and Gli3R-transfected otocysts (Fig. 4G,G′). Thus, GLI3 signaling controls Hmx3 expression in the otocyst, but not Dlx5 expression.
Fig. 4.
Gli3R overexpression induces ectopic expression of Hmx3, but not of Dlx5, and increasing or decreasing BMP signaling alters the GLI3R/GLI3FL ratio in opposite directions. (A-C) Transfection scheme for D-G′,J,J′. (A) Diagram showing electroporation into the ventral otic placode to target the ventromedial otocyst. (B) Whole-mount view 18-24 h after electroporation of Gfp. The borders of the otocyst are indicated by white dots. (C) Transverse section of the same embryo shown in B. (D-G′) In situ hybridization for Hmx3 (D-E′) or Dlx5 (F-G′) expression on transverse sections of otocysts collected after transfection when embryos reached HH stages 20-21. Boxed regions are shown in D′-G′, with borders of selected cells outlined. (H-J′) Western blots and graphs of mean GLI3R/GLI3FL ratios in whole chick otocysts collected at the desired stage after overexpression of Gfp (Con) or Bmp4, Nog or Smad6. Blue arrowhead indicates GLI3R; red arrowhead indicates GLI3FL; α-tub, α-tubulin. Otocysts in H,H′ were from embryos transfected as in Fig. 1A, and those in I,I′ were from embryos transfected as in Fig. 2A. *P<0.05, **P<0.01; n.s., not statistically significant. n=5 (D,E,H-J) or n=6 (F,G) control and experimental otocysts. Error bars indicate s.d. d, dorsal (in B); v, ventral; other abbreviations as in Fig. 1.
To examine whether BMP signaling regulates the proteolytic processing of full-length GLI3 (GLI3FL) to increase the level of GLI3R, we altered the level of BMP signaling. Either Bmp4 or Nog was co-sonoporated with Gfp as described above (Fig. 1A, Fig. 2A). Otocysts were collected 48-60 h later, when they reached HH stages 24-25, and pooled for western blotting to detect GLI3 (Wang et al., 2000). After overexpression of Bmp4 and Gfp, the ratio of GLI3R to GLI3FL in otocysts was 1.8-fold greater than that of control otocysts due to an increase in GLI3 processing (i.e. an increase in GLI3R; Fig. 4H,H′). By contrast, after overexpression of Nog and Gfp, the ratio was dramatically decreased due to a reduction in GLI3R (Fig. 4I,I′). These results provide strong evidence that BMP signaling plays an important role in GLI3FL protein processing to increase the level of GLI3R. In addition, we asked whether BMP signaling through its pSMAD pathway affects GLI3FL processing. Smad6 and Gfp were co-electroporated into the dorsolateral otocyst as described above (Fig. 3A). Otocysts were again collected 24-30 h later when embryos reached HH stages 24-25 and pooled for western blotting to detect GLI3. The GLI3R/GLI3FL ratio was unchanged, showing that the canonical BMP/pSMAD pathway does not participate in GLI3FL processing (Fig. 4J,J′).
BMP signaling affects PKA activity, and increasing PKA activity inhibits cochlear duct development and induces Hmx3 but not Dlx5 expression
GLI3FL is phosphorylated by PKA, stimulating the proteolytic processing of GLI3FL to GLI3R (Chen et al., 1998; Wang et al., 2000). Thus, we next asked whether BMP signaling upregulates PKA activity. Either Bmp4 or Nog was co-sonoporated with Gfp as described above (Fig. 1A, Fig. 2A). Otocysts were collected 48-60 h later when embryos reached HH stages 24-25 and pooled for PKA activity assays. Bmp4 and Gfp overexpression increased PKA activity in the otocyst by more than 40% (Fig. 5A). In concert with this, Nog and Gfp overexpression decreased PKA activity by more than 50% (Fig. 5B). These results suggest that BMP signaling affects PKA activity in the developing otocyst.
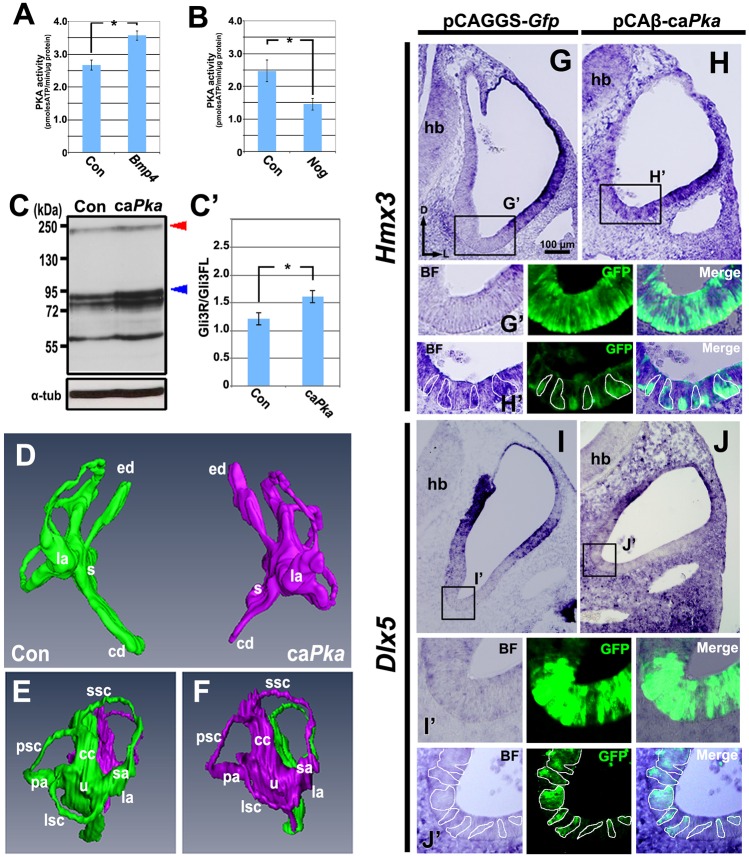
Fig. 5.
Increasing and decreasing BMP signaling alters PKA activity in opposite directions, and caPka overexpression induces ectopic expression of Hmx3, but not of Dlx5, and inhibits formation of the cochlea. (A,B) Mean levels of PKA activity in whole chick otocysts at HH stages 24-25 collected after overexpression of Bmp4 (transfected as in Fig. 1A; n=5) or Nog (transfected as in Fig. 1A; n=5), compared with controls (Con). (C,C′) Western blot and mean GLI3R/GLI3FL ratios in whole chick otocysts at HH stages 20-21 collected after overexpression of caPka (transfected as in Fig. 4A; n=5), compared with controls. Blue arrowhead, GLI3R; red arrowhead, GLI3FL; α-tub, α-tubulin. *P<0.05. Error bars indicate s.d. (D-F) Reconstruction (D, frontal view; E,F, lateral views) of serial transverse sections of inner ears at HH stage 30 (transfected as in Fig. 4A). Green, control ear (Con); magenta, caPka overexpression. Paraffin serial sections, n=7; reconstruction, n=1. (G-J′) In situ hybridization for Hmx3 (G-H′) or Dlx5 (I-J′) expression on transverse sections of otocysts collected after transfection when embryos reached HH stages 20-21 (transfected as in Fig. 4A). Boxed regions are shown in G′-J′, with borders of selected cells outlined. n=6 (G,H) or n=2 (I,J) control and experimental otocysts. Abbreviations as in Fig. 1.
We next asked whether increased PKA activity affects GLI3FL proteolytic processing in the otocyst. We co-electroporated the ventral otic epithelium, as described previously (Fig. 4A), with constitutively active PKA (caPka) and Gfp. Otocysts were collected 24-30 h later when they reached HH stages 20-21 and were pooled for western blotting to detect GLI3. Overexpression of caPka and Gfp resulted in an increased GLI3R/GLI3FL ratio due to excess production of GLI3R (Fig. 5C,C′). This suggests that PKA activity can regulate GLI3FL proteolytic processing, thereby changing the DV ratio of GLI3FL/GLI3R in the otocyst.
Computer-aided three-dimensional reconstruction of inner ears 5-6 days after caPka transfection showed that the cochlear duct was severely truncated, whereas the vestibular system and endolymphatic duct were normal (Fig. 5D-F), suggesting that increased PKA activity dorsalizes the ventral otocyst by altering regional gene expression. To address this, we transfected HH stage 9-11 otocysts ventrally (Fig. 4A) with caPka and Gfp, and 24-30 h later assessed Hmx3 and Dlx5 expression in otocysts at HH stages 20-21. In otocysts transfected with Gfp alone, Hmx3 expression was unaffected (Fig. 5G,G′), but in otocysts co-transfected with caPka and Gfp, Hmx3 was ectopically expressed in ventral cells (Fig. 5H,H′). By contrast, Dlx5 expression was unaffected in otocysts transfected with Gfp alone (Fig. 5I,I′) or after co-transfection with caPka and Gfp (Fig. 5J,J′). These results suggest that Hmx3, but not Dlx5, expression is regulated during dorsal patterning of the otocyst by a non-canonical BMP signaling pathway. Moreover, our results suggest that the non-canonical pathway involves the activation of PKA and increased GLI3 proteolytic processing.
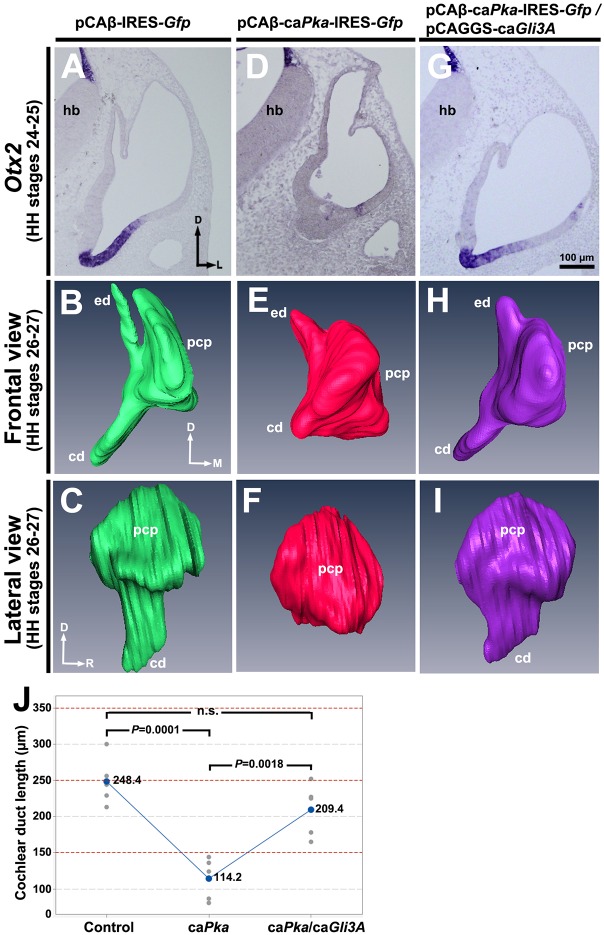
Inhibition of ventral otocyst development by caPka is rescued by caGli3A (Gli3AHIGH)
To determine whether caPka overexpression inhibited development of the cochlear duct specifically by increasing the level of GLI3R, we attempted to rescue ventral otocyst development after caPka overexpression by co-expressing caGli3A (Gli3AHIGH). caGli3A acts as a constitutively active transcriptional activator (Stamataki et al., 2005), so we expected that caGli3A would counteract the elevated GLI3R in the ventral otocyst that resulted from increased PKA activity. pCAβ-IRES-Gfp vector alone, pCAβ-caPka-IRES-Gfp, or pCAβ-caPka-IRES-Gfp and pCAGGS-caGli3A were electroporated into the ventromedial otocyst and embryos were collected 60 h later for in situ hybridization with Otx2 or 72 h later for computer-aided serial section reconstruction (Fig. 6A-I). As expected, Otx2 expression was unchanged after overexpression of vector alone (Fig. 6A) and the otocyst developed normally (Fig. 6B,C). Also as expected, Otx2 expression was essentially absent after overexpression of caPka (Fig. 6D) and development of the cochlear duct was inhibited (Fig. 6E,F). By contrast, co-overexpression of caPka and caGli3A (plus Gfp) restored virtually normal Otx2 expression (Fig. 6G) and cochlear duct development (Fig. 6H,I). Measurements of the cochlear duct showed that it did not statistically differ in length between control and rescued ducts, but that it differed statistically between control and caPka-treated ducts, as well as between caPka-treated and rescued ducts (Fig. 6J). Collectively, these results provide strong evidence that overexpression of caPka inhibited development of the cochlear duct specifically by increasing the level of GLI3R.
Fig. 6.
caGli3A rescues ventral otocyst development arrested by caPKA. (A,D,G) In situ hybridization of transverse sections for Otx2 after electroporation with vector alone, caPka, or caPka and caGli3A. n=5 control and experimental otocysts for each. (B,C,E,F,H,I) Reconstructions of serial transverse sections of developing chick inner ears collected after electroporation and shown in frontal (B,E,H) and lateral (C,F,I) views. For each of the three groups of otocysts: paraffin serial sections, n=4; reconstruction, n=1. (J) Cochlear duct length in control and transfected otocysts. n=5 in each of the three groups. pcp, primordial canal pouch; other abbreviations as in Fig. 1.
DISCUSSION
DV patterning of the otocyst involves the secretion of WNTs from the dorsal hindbrain and SHH from the notochord and floor plate of the hindbrain (Groves and Fekete, 2012; Wu and Kelley, 2012). In addition, considerable evidence supports an obligatory role for BMP signaling in dorsalization of the inner ear (Chang et al., 2008, 2002; Gerlach et al., 2000; Ohta et al., 2010). Here, we show that BMP signaling is essential for establishing otocyst dorsal polarity, and that at least two intracellular pathways are involved: a canonical pSMAD pathway, and a non-canonical pathway that activates PKA and increases the GLI3R/GLI3FL ratio by increasing the amount of GLI3R present in the dorsal otocyst. These two BMP-regulated pathways coordinate dorsalization of the otocyst, inducing expression of both Dlx5 and Hmx3, which encode transcription factors essential for normal formation of the vestibular system (Fig. 7).
Fig. 7.

Model showing the secreted factors and molecular pathways that regulate otocyst DV patterning. Lateral view (left) and oblique cross-sectional view (right) of the developing chick otocyst. Some of these factors (FGFs) are secreted by cells in tissues surrounding the otocyst (e.g. head mesenchyme), whereas others (WNTs and BMPs) are secreted by cells in the otocyst wall and surrounding tissues (e.g. dorsal spinal cord). Purple indicates the Dlx5 expression domain.
Interplay between dorsal signaling factors during inner ear development
In TOPGAL mouse embryos, β-GAL is detected in the dorsomedial portion of the otic epithelium, which is the region of the otocyst closest to the dorsal neural tube, the site of WNT secretion. Additionally, in tissue explants from such embryos, both β-GAL and Dlx5 respond in a dose-dependent manner to WNT/β-catenin signaling by expanding their expression ventrally in the otocyst (Riccomagno et al., 2005). These data clearly indicate that the dorsal otocyst responds to WNT signaling.
We showed previously that the BMP effector pSMAD is localized to the dorsolateral otocyst (Ohta et al., 2010), suggesting that BMPs also pattern the dorsal otocyst. When we inhibited BMP signaling using reagents that presumably would not inhibit WNT signaling (namely Nog or Smad6), the semicircular canals were partially or completely lost and expression of some dorsal otocyst genes was inhibited (e.g. both Hmx3 and Dlx5 with Nog, and only Dlx5 with Smad6). These results show that BMP signaling, like WNT signaling, is required to pattern the dorsal otocyst, and that neither growth factor alone is sufficient to effect full dorsal patterning. Moreover, in Wnt1−/−;Wnt3a−/− double-mutant mouse embryos, the size of the otocyst is severely reduced, but Bmp4 expression persists in the dorsolateral epithelium of the otocyst (Riccomagno et al., 2005), suggesting that the otocyst still receives BMP signals in these embryos. Nevertheless, these mutant embryos have severely disrupted inner ears lacking vestibular components. Thus, both WNT and BMP signaling are required for dorsal patterning of the otocyst and proper formation of the vestibular system.
Regulation of Dlx5 expression in the dorsolateral otocyst by canonical BMP signaling
In the early otocyst, Dlx5 is expressed throughout the forming semicircular canals and endolymphatic duct (Brown et al., 2005). At later stages, expression of Dlx5 in the canals becomes restricted to their nonsensory regions in a pattern complementary to that of their sensory patches, which express Bmp4; thus, Dlx5 and Bmp4 are expressed in discrete, non-overlapping regions (Brown et al., 2005). Dlx5 null mutant embryos have severe defects in the dorsal otocyst, lacking all three semicircular canals and forming a truncated endolymphatic duct (Merlo et al., 2002). Therefore, Dlx5 plays an essential role in formation of the dorsal otocyst.
In the present study, we showed that inhibition of canonical BMP signaling blocks Dlx5 expression. However, as mentioned above, Dlx5 expression is regulated in a dose-dependent manner by WNT/β-catenin signaling (Riccomagno et al., 2005). In addition, conditional inactivation of β-catenin in the otic placode of mouse embryos results in a dramatic reduction of Dlx5 expression, whereas conditional activation of the canonical WNT pathway causes an expansion of Dlx5 expression (Ohyama et al., 2006). Thus, it seems that the spatiotemporal pattern of Dlx5 expression in the developing inner ear is regulated by at least two pathways, i.e. both canonical BMP signaling and canonical WNT signaling. As mentioned above, Dlx5 is broadly expressed throughout the entire dorsal otocyst, including its dorsomedial region, which forms the endolymphatic duct, and its dorsolateral region, which forms the semicircular canals. Dlx5 expression in the dorsolateral region of the otocyst, a region that is relatively far from the dorsal neural tube as the source of WNT signaling, is likely to be regulated by BMP signaling, whereas Dlx5 expression in the dorsomedial region is likely to be regulated by WNT signaling, at least in part. Consistent with this, we showed that inhibition of BMP signaling in the otic epithelium reduced Dlx5 expression in the dorsolateral region but not in the dorsomedial region. Similar results have also been obtained in otic conditional Bmp4 knockout mice, in which Dlx5 expression is reduced in the canal pouch, but not more medially in the endolymphatic duct (see figure 3I in Chang et al., 2008). Thus, coordination of the two pathways is necessary for precise spatiotemporal expression of Dlx5 during vestibular development.
Regulation of Hmx3 expression in the dorsolateral otocyst by non-canonical BMP signaling
Hmx3 and Hmx2 (previously Nkx5.1 and Nkx5.2, respectively) are expressed in the dorsolateral otocyst, where they almost completely overlap with the Dlx5 expression domain (Herbrand et al., 1998). Disruption of either gene or both Hmx2 and Hmx3 gives rise to a phenotype similar to that of the Dlx5 mutant, that is, absence of the semicircular canals (Hadrys et al., 1998; Merlo et al., 2002; Wang et al., 2004, 1998). Both Dlx5 and Hmx3 play crucial roles in formation of the vestibular system (Merlo et al., 2002). However, Hmx3 expression seems to be regulated by a pathway that is distinct from that of Dlx5. As mentioned above, Dlx5 expression in the dorsal otocyst is likely to be regulated by both WNT and BMP signaling. However, Hmx3 expression is unlikely to require WNT signaling, as disruption of Wnt1 and Wnt3a, or ablation of the dorsal neural tube, does not affect Hmx3 expression (Riccomagno et al., 2005). Intriguingly, when we blocked BMP signaling with Nog, Hmx3 expression in the dorsolateral otocyst was attenuated but still present, whereas Dlx5 expression was almost extinguished. This suggests that regulation of Hmx3 expression is less sensitive to loss of BMP signaling than is Dlx5 expression. Similar results were obtained in Bmp4 conditional knockout mice, namely, strongly reduced Dlx5 expression in the dorsolateral otocyst, with persisting, but apparently weaker, Hmx3 expression (Chang et al., 2008). Collectively, these results indicate that BMP signaling alone is not sufficient to regulate full expression of Hmx3 in the dorsolateral otocyst.
In the current study, we showed that gain of GLI3R function in the otocyst induced ectopic expression of Hmx3, suggesting that GLI3R is a key factor positively regulating Hmx3 expression. This raises the question of which signaling pathways lead to the production of GLI3R. At least two pathways might be involved. One is the SHH pathway. According to an existing model of DV inner ear patterning (Bok et al., 2007a), the dorsal region of the otocyst should have a high concentration of GLI3R, as compared with the ventral region, because it should receive considerably less (or no) SHH signaling, which is generated from axial tissues ventral to the otocyst (Hui and Angers, 2011). Consistent with this, ShhP1 transgenic mouse embryos, which have ectopic SHH expression in the dorsal otic epithelium, have decreased Hmx3 expression (Riccomagno et al., 2002). Although not assessed by the authors in their study, we would expect that increased dorsal signaling of SHH would lead to a decrease in GLI3R dorsally. A second possible signaling pathway to regulate GLI3R is the non-canonical BMP pathway revealed in the current study. This pathway mediates PKA activation, which phosphorylates GLI3FL and results in production of GLI3R. Presumably, the higher amount of GLI3R maintained in the dorsal otocyst by both the lack of SHH signaling and active non-canonical BMP signaling would be necessary to induce the appropriate level of Hmx3 expression required for normal vestibular development.
Model of the molecular pathways regulating Dlx5 and Hmx3 expression in the developing otocyst
Our results show that canonical BMP signaling regulates Dlx5 expression in the dorsolateral otocyst (Fig. 7). Inhibition of SMAD signaling with Smad6 almost completely abolishes Dlx5 expression, whereas Dlx5 expression in the dorsomedial otocyst, including the endolymphatic duct, was not affected. Previous reports suggest that the homeobox gene Gbx2 is also involved in regulating Dlx5 expression. Gbx2 mutant mice lack Dlx5 expression in the dorsomedial domain of the otocyst, but expression in the dorsolateral domain persists (Lin et al., 2005). Moreover, WNT and FGF signaling have been suggested to play a role in inducing and maintaining Gbx2 expression, which in turn induces Dlx5 expression (Hatch et al., 2007; Riccomagno et al., 2005).
Our results also show that Hmx3 expression is ectopically induced in the ventral otocyst by gain of GLI3R function, suggesting that the function of GLI protein (i.e. as an activator or repressor) along the DV axis of the otocyst regulates Hmx3 expression (Fig. 7). According to a model proposed by Wu and colleagues, the dorsal region of the otocyst would be expected to have a high dose of GLI repressor due to the absence (or low level) of SHH signaling there (Bok et al., 2007a). Moreover, our results suggest that non-canonical BMP signaling increases PKA activity, which in turn would be expected to phosphorylate GLI3FL, resulting in its partial proteolysis and the production of GLI3R. Our rescue experiments strongly support this expectation. Collectively, our results suggest that the regulation of Hmx3 expression in the dorsolateral otocyst involves GLI3R-maintained crosstalk between SHH and BMP signaling.
A possible molecular pathway mediating PKA activation by BMP non-canonical signaling
Several studies have reported that BMP signaling can upregulate PKA activity (Gangopahyay et al., 2011; Lee and Chuong, 1997; Liu et al., 2005; Tee et al., 2010). However, the molecular pathway by which this occurs remains unclear. Because binding of cAMP(s) to the regulatory subunit(s) of PKA is thought to be indispensable for activation of PKA (Granot et al., 1980; Taylor et al., 1990), BMP signaling presumably interacts directly or indirectly with a G-protein-coupled receptor (GPCR) to increase the production of intracellular cAMP. The parathyroid hormone (PTH)/PTH-related protein (PTHrP) pathway is a likely candidate with potential to interact with BMP to control cAMP production. The PTH/PTHrP receptor can activate the Gαs subunit that activates the adenylate cyclase (AC)/PKA pathway in a ligand-dependent manner (Fujimori et al., 1992). Numerous reports have suggested that BMP signaling directly or indirectly regulates bone development by interacting with the PTH/PTHrP pathway (Minina et al., 2001; Susperregui et al., 2008; Zou et al., 1997). In the developing inner ear, PTHrP (also known as Pthlh) seems to be expressed in the dorsal epithelial wall, including the semicircular canals, but not in the endolymphatic duct, and it is also expressed in mesenchyme surrounding the cochlear duct (Karperien et al., 1996; Lee et al., 1995). By contrast, PTH receptor (also known as Pth1r) is diffusely expressed in both the inner ear epithelium and surrounding mesenchyme (Lee et al., 1995). Albright's hereditary osteodystrophy, a human congenital disease characterized by the inability to respond to PTH, involves hearing loss in some patients (Wilson and Trembath, 1994), but whether vestibular development is also affected is unknown. Although these results raise the possibility that the PTHrP pathway might function in BMP signaling in the inner ear, further studies are needed to directly test this.
MATERIALS AND METHODS
Embryos
Fertilized White Leghorn chick eggs were incubated at 38.5°C until embryos reached desired stages (Hamburger and Hamilton, 1951). All experiments were conducted using early chick embryos, at stages well before hatching, and were performed in compliance with the University of Utah IACUC requirements.
Western blotting
Otocysts were manually dissected from chick embryos at HH stages 20-24, pooled (5-20 for each lane), digested with dispase (GIBCO; 0.5 U/ml for 30-45 min at 37°C), and lysed with ice-cold RIPA buffer. Protein concentration was measured using the Pierce Coomassie Plus Bradford Assay Kit (ThermoFisher). Equal amounts of protein were loaded onto 7.5% SDS-PAGE gels, and western blotting was performed with GLI3 antibody as described previously (Pan and Wang, 2007; Wang et al., 2000). A monoclonal antibody to α-tubulin (1:2000; Sigma, T6199) was used as a loading control. Secondary antibodies were mouse IgGs conjugated with HRP (GE Healthcare, NA9310V).
Gene transduction
Electroporation was used to deliver genes encoding intracellular proteins to discrete, selected regions of the wall of the otocyst, and sonoporation was used to deliver genes encoding extracellular proteins to mesenchymal cells either directly ventral to the otic placode or dorsal to the otocyst. Chick eggs were windowed using standard techniques, the vitelline membranes were removed, the amnion (at stages when present) was opened over the otocyst, and embryos were either sonoporated (Ohta et al., 2003) or electroporated (Ohta et al., 2010) in ovo to transfect expression vectors.
For sonoporation, a DNA-microbubble mixture was prepared by adding 10 μl plasmid DNA solution (2.0-4.0 μg/μl) to 10 μl SonoVue (BRACCO), and the mixture was injected into the head mesenchyme adjacent to the otocyst using a glass micropipette (GD-1.2, Narishige). Stages were selected to target sonoporation ventrally (HH stages 9-11; Fig. 1A) or dorsally (HH stages 15-16; Fig. 2A). Injected embryos were immediately exposed to ultrasound using a 3-mm diameter probe (Sonitron 2000N, Nepagene) with an input frequency of 1 MHz, an output intensity of 2.0 W/cm2, and a pulse duty ratio of 20% for 60 s. Embryos were usually collected after an additional 48-60 h upon reaching HH stages 24-25.
For electroporation, the plasmid solution (2.0 μg/μl) contained 0.1% Fast Green for visualization, and was injected into the lumen of the developing otocyst (or cavity of the otic cup) using a glass micropipette. Stages were selected to target electroporation to the dorsolateral (HH stages 15-16; Fig. 3A) or ventromedial (HH stages 9-11; Fig. 4A) otocyst wall, and electrodes were positioned accordingly. In HH stage 9-11 embryos, the positive electrode was placed in the subcephalic pocket beneath the otic placode and the negative electrode was placed just above (dorsal to) the placode. In HH stage 15-16 embryos, the negative electrode was inserted into the otocyst lumen through the nascent endolymphatic duct and the positive electrode was placed just above (lateral to) the otocyst. Needle electrodes were used exclusively (tungsten, diameter 0.010 inch, length 3 inches, tapered tip of 12°, AC resistance of 5 MΩ) and were purchased from A-M Systems (Sequim, catalog no. 577300). Two 50-msec pulses at 10 volts were applied using a CUY21 electroporator (Nepa Gene). Embryos were usually collected 18-36 h later upon reaching HH stages 20-21.
For rescue experiments, the plasmid mixture was prepared by adding 2 μl pCAβ-caPka-IRES-Gfp (0.5 μg/μl) to 2 μl pCAGGS-caGli3A (Gli3AHIGH) (0.5 μg/μl) and electroporated into the ventromedial wall of the otocyst in chick embryos at HH stages 9-11. Non-rescued but experimentally treated otocysts were electroporated with pCAβ-caPka-IRES-Gfp (0.5 μg/μl) alone.
Expression vectors
pCAGGS-Gfp was obtained from H. Ogawa (Ogawa et al., 1995) and pCAGGS-Gli3AHIGH and pCAGGS-Gli3R were obtained from J. Briscoe (Stamataki et al., 2005). pCAGGS-Bmp4 (mouse) was described previously (Ohta et al., 2003). Full-length mouse Nog cDNA was obtained from A. McMahon (University of Southern California, CA, USA) and inserted into a pCAGGS vector. pCS2-SMAD6 (human) was purchased from Addgene (plasmid 14960). The constitutively active catalytic subunit of protein kinase A (PKA) was isolated from an RCAS(A) vector (from A. Munsterberg, University of East Anglia, Norwich, UK) using a ClaI site and subcloned into a ClaI site of pCAβ-IRES-Gfp (from A. Tucker, King's College London, London, UK).
Three-dimensional visualization of the inner ear
Inner ear morphology was visualized in two ways. First, paraffin serial transverse sections of chick embryos at HH stages 30-35 were stained with Hematoxylin and Eosin, photographed, and reconstructed three-dimensionally using Amira 5.1 software (FEI). Second, the otocyst lumen was filled with white paint, as described previously (Morsli et al., 1998), to create a cast of its three-dimensional shape. Phenotypes were readily detectable in serial sections, but computer-aided reconstructions and paint fills are shown in the figures to aid visualization of the complex three-dimensional morphology of the inner ear.
PKA activity assay
Dissected otocysts from embryos at HH stages 24-25 were pooled (20 per run), enzymatically digested as described above, and lysed in buffer comprising 25 mM Tris-HCl (pH 7.4), 0.5 mM EDTA, 0.5 mM EGTA and 10 mM β-mercaptoethanol, with one inhibitor cocktail tablet (Complete, Roche) added per 50 ml. PKA activity was measured using a PepTag Kit (Promega).
In situ hybridization on paraffin sections
Chick embryos were fixed with 4% paraformaldehyde and embedded in paraffin, and transverse sections were subjected to in situ hybridization using standard procedures with chick probes for Hmx3 (gift of Dr E. Bober, Max Planck Institute for Heart and Lung Research, Frankfurt, Germany), Dlx5 (gift of Dr W. Upholt, UCONN Health, Farmington CT, USA) and Otx2 (gift of Dr L. Bally-Cuif, Université Paris-Sud, Paris, France).
Immunolabeling
Transfected cells were detected in transverse paraffin sections with a monoclonal primary antibody against GFP (Roche, 11814460001; 1:250). pSMAD1/5/8 (Cell Signaling Technology, #9511) was detected with a rabbit polyclonal antibody, diluted 1:200 in blocking solution (0.1% sheep serum). Secondary antibodies conjugated to AlexaFluor 488 or AlexaFluor 594 (Invitrogen, A21202 or A11012, respectively) were diluted 1:1000 and imaged using an Olympus BX-51 microscope and DP-80 digital camera.
Statistical analysis
Sample size was based on past, similar experiments and feasibility. Statistical analyses were performed using Minitab Express. In each experiment, we used the Anderson–Darling test to evaluate whether the data fit a normal distribution, and the F-test to examine whether variances between the two groups (control and experiment) were similar. Based on this, means and standard deviations were calculated and are shown in the figures, with P-values obtained using a two-tailed, unpaired Student's t-test. When more than two groups were compared (Fig. 6J), means were compared statistically by one-way ANOVA.
Acknowledgements
Yukio Saijoh and Lisa Urness provided critical comments. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
S.O. conceived and executed all experiments, analyzed all data and drafted the manuscript. B.W. provided reagents and helped with experimental design, and provided input on the manuscript. S.L.M. provided input on experiments and data interpretation and edited the manuscript. G.C.S. assisted in experimental design, data analysis, and writing and editing the manuscript.
Funding
Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health [R01 DC011819 to S.L.M. and G.C.S.]; and the National Institute of General Medical Sciences of the National Institutes of Health [R01 GM114429 to B.W.]. Deposited in PMC for release after 12 months.
References
- Bok J., Bronner-Fraser M. and Wu D. K. (2005). Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development 132, 2115-2124. 10.1242/dev.01796 [DOI] [PubMed] [Google Scholar]
- Bok J., Dolson D. K., Hill P., Ruther U., Epstein D. J. and Wu D. K. (2007a). Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear. Development 134, 1713-1722. 10.1242/dev.000760 [DOI] [PubMed] [Google Scholar]
- Bok J., Brunet L. J., Howard O., Burton Q. and Wu D. K. (2007b). Role of hindbrain in inner ear morphogenesis: analysis of Noggin knockout mice. Dev. Biol. 311, 69-78. 10.1016/j.ydbio.2007.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J. and Novitch B. G. (2008). Regulatory pathways linking progenitor patterning, cell fates and neurogenesis in the ventral neural tube. Philos. Trans. R. Soc. B Biol. Sci. 363, 57-70. 10.1098/rstb.2006.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. S. and Epstein D. J. (2011). Otic ablation of smoothened reveals direct and indirect requirements for Hedgehog signaling in inner ear development. Development 138, 3967-3976. 10.1242/dev.066126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. T., Wang J. and Groves A. K. (2005). Dlx gene expression during chick inner ear development. J. Comp. Neurol. 483, 48-65. 10.1002/cne.20418 [DOI] [PubMed] [Google Scholar]
- Chang W., Nunes F. D., De Jesus-Escobar J. M., Harland R. and Wu D. K. (1999). Ectopic noggin blocks sensory and nonsensory organ morphogenesis in the chicken inner ear. Dev. Biol. 216, 369-381. 10.1006/dbio.1999.9457 [DOI] [PubMed] [Google Scholar]
- Chang W., ten Dijke P. and Wu D. K. (2002). BMP pathways are involved in otic capsule formation and epithelial–mesenchymal signaling in the developing chicken inner ear. Dev. Biol. 251, 380-394. 10.1006/dbio.2002.0822 [DOI] [PubMed] [Google Scholar]
- Chang W., Lin Z., Kulessa H., Hebert J., Hogan B. L. M. and Wu D. K. (2008). Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 4, e1000050 10.1371/journal.pgen.1000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Gallaher N., Goodman R. H. and Smolik S. M. (1998). Protein kinase A directly regulates the activity and proteolysis of cubitus interruptus. Proc. Natl. Acad. Sci. USA 95, 2349-2354. 10.1073/pnas.95.5.2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori A., Cheng S. L., Avioli L. V. and Civitelli R. (1992). Structure-function relationship of parathyroid hormone: activation of phospholipase-C, protein kinase-A and -C in osteosarcoma cells. Endocrinology 130, 29-36. [DOI] [PubMed] [Google Scholar]
- Gangopahyay A., Oran M., Bauer E. M., Wertz J. W., Comhair S. A., Erzurum S. C. and Bauer P. M. (2011). Bone morphogenetic protein receptor II is a novel mediator of endothelial nitric-oxide synthase activation. J. Biol. Chem. 286, 33134-33140. 10.1074/jbc.M111.274100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach L. M., Hutson M. R., Germiller J. A., Nguyen-Luu D., Victor J. C. and Barald K. F. (2000). Addition of the BMP4 antagonist, noggin, disrupts avian inner ear development. Development 127, 45-54. [DOI] [PubMed] [Google Scholar]
- Granot J., Mildvan A. S. and Kaiser E. T. (1980). Studies of the mechanism of action and regulation of cAMP-dependent protein kinase. Arch. Biochem. Biophys. 205, 1-17. 10.1016/0003-9861(80)90078-8 [DOI] [PubMed] [Google Scholar]
- Groves A. K. and Fekete D. M. (2012). Shaping sound in space: the regulation of inner ear patterning. Development 139, 245-257. 10.1242/dev.067074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrys T., Braun T., Rinkwitz-Brandt S., Arnold H. H. and Bober E. (1998). Nkx5-1 controls semicircular canal formation in the mouse inner ear. Development 125, 33-39. [DOI] [PubMed] [Google Scholar]
- Hamburger V. and Hamilton H. L. (1951). A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49-92. 10.1002/jmor.1050880104 [DOI] [PubMed] [Google Scholar]
- Hatch E. P., Noyes C. A., Wang X., Wright T. J. and Mansour S. L. (2007). Fgf3 is required for dorsal patterning and morphogenesis of the inner ear epithelium. Development 134, 3615-3625. 10.1242/dev.006627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbrand H., Guthrie S., Hadrys T., Hoffmann S., Arnold H. H., Rinkwitz-Brandt S. and Bober E. (1998). Two regulatory genes, cNkx5-1 and cPax2, show different responses to local signals during otic placode and vesicle formation in the chick embryo. Development 125, 645-654. [DOI] [PubMed] [Google Scholar]
- Hui C.-C. and Angers S. (2011). Gli proteins in development and disease. Annu. Rev. Cell Dev. Biol. 27, 513-537. 10.1146/annurev-cellbio-092910-154048 [DOI] [PubMed] [Google Scholar]
- Ingham P. W. and McMahon A. P. (2001). Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059-3087. 10.1101/gad.938601 [DOI] [PubMed] [Google Scholar]
- Karperien M., Lanser P., de Laat S. W., Boonstra J. and Defize L. H. (1996). Parathyroid hormone related peptide mRNA expression during murine postimplantation development: evidence for involvement in multiple differentiation processes. Int. J. Dev. Biol. 40, 599-608. [PubMed] [Google Scholar]
- Lee Y.-S. and Chuong C.-M. (1997). Activation of protein kinase A is a pivotal step involved in both BMP-2- and cyclic AMP-induced chondrogenesis. J. Cell. Physiol. 170, 153-165. [DOI] [PubMed] [Google Scholar]
- Lee K., Deeds J. D. and Segre G. V. (1995). Expression of parathyroid hormone-related peptide and its receptor messenger ribonucleic acids during fetal development of rats. Endocrinology 136, 453-463. [DOI] [PubMed] [Google Scholar]
- Lin Z., Cantos R., Patente M. and Wu D. K. (2005). Gbx2 is required for the morphogenesis of the mouse inner ear: a downstream candidate of hindbrain signaling. Development 132, 2309-2318. 10.1242/dev.01804 [DOI] [PubMed] [Google Scholar]
- Liu H., Margiotta J. F. and Howard M. J. (2005). BMP4 supports noradrenergic differentiation by a PKA-dependent mechanism. Dev. Biol. 286, 521-536. 10.1016/j.ydbio.2005.08.022 [DOI] [PubMed] [Google Scholar]
- Merlo G. R., Paleari L., Mantero S., Zerega B., Adamska M., Rinkwitz S., Bober E. and Levi G. (2002). The Dlx5 homeobox gene is essential for vestibular morphogenesis in the mouse embryo through a BMP4-mediated pathway. Dev. Biol. 248, 157-169. 10.1006/dbio.2002.0713 [DOI] [PubMed] [Google Scholar]
- Minina E., Wenzel H. M., Kreschel C., Karp S., Gaffield W., McMahon A. P. and Vortkamp A. (2001). BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development 128, 4523-4534. [DOI] [PubMed] [Google Scholar]
- Morsli H., Choo D., Ryan A., Johnson R. and Wu D. K. (1998). Development of the mouse inner ear and origin of its sensory organs. J. Neurosci. 18, 3327-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Inouye S., Tsuji F. I., Yasuda K. and Umesono K. (1995). Localization, trafficking, and temperature-dependence of the Aequorea green fluorescent protein in cultured vertebrate cells. Proc. Natl. Acad. Sci. USA 92, 11899-11903. 10.1073/pnas.92.25.11899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.-H., Johnson R. and Wu D. K. (1996). Differential expression of bone morphogenetic proteins in the developing vestibular and auditory sensory organs. J. Neurosci. 16, 6463-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S., Suzuki K., Tachibana K. and Yamada G. (2003). Microbubble-enhanced sonoporation: efficient gene transduction technique for chick embryos. Genesis 37, 91-101. 10.1002/gene.10232 [DOI] [PubMed] [Google Scholar]
- Ohta S., Mansour S. L. and Schoenwolf G. C. (2010). BMP/SMAD signaling regulates the cell behaviors that drive the initial dorsal-specific regional morphogenesis of the otocyst. Dev. Biol. 347, 369-381. 10.1016/j.ydbio.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T., Mohamed O. A., Taketo M. M., Dufort D. and Groves A. K. (2006). Wnt signals mediate a fate decision between otic placode and epidermis. Development 133, 865-875. 10.1242/dev.02271 [DOI] [PubMed] [Google Scholar]
- Pan Y. and Wang B. (2007). A novel protein-processing domain in Gli2 and Gli3 differentially blocks complete protein degradation by the proteasome. J. Biol. Chem. 282, 10846-10852. 10.1074/jbc.M608599200 [DOI] [PubMed] [Google Scholar]
- Riccomagno M. M., Martinu L., Mulheisen M., Wu D. K. and Epstein D. J. (2002). Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 16, 2365-2378. 10.1101/gad.1013302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno M. M., Takada S. and Epstein D. J. (2005). Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 19, 1612-1623. 10.1101/gad.1303905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamataki D., Ulloa F., Tsoni S. V., Mynett A. and Briscoe J. (2005). A gradient of Gli activity mediates graded Sonic Hedgehog signaling in the neural tube. Genes Dev. 19, 626-641. 10.1101/gad.325905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susperregui A. R. G., Viñals F., Ho P. W. M., Gillespie M. T., Martin T. J. and Ventura F. (2008). BMP-2 regulation of PTHrP and osteoclastogenic factors during osteoblast differentiation of C2C12 cells. J. Cell. Physiol. 216, 144-152. 10.1002/jcp.21389 [DOI] [PubMed] [Google Scholar]
- Taylor S. S., Buechler J. A. and Yonemoto W. (1990). cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu. Rev. Biochem. 59, 971-1005. 10.1146/annurev.bi.59.070190.004543 [DOI] [PubMed] [Google Scholar]
- Tee J. B., Choi Y., Shah M. M., Dnyanmote A., Sweeney D. E., Gallegos T. F., Johkura K., Ito C., Bush K. T. and Nigam S. K. (2010). Protein kinase A regulates GDNF/RET-dependent but not GDNF/Ret-independent ureteric bud outgrowth from the Wolffian duct. Dev. Biol. 347, 337-347. 10.1016/j.ydbio.2010.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Van De Water T. and Lufkin T. (1998). Inner ear and maternal reproductive defects in mice lacking the Hmx3 homeobox gene. Development 125, 621-634. [DOI] [PubMed] [Google Scholar]
- Wang B., Fallon J. F. and Beachy P. A. (2000). Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100, 423-434. 10.1016/S0092-8674(00)80678-9 [DOI] [PubMed] [Google Scholar]
- Wang W., Grimmer J. F., Van De Water T. R. and Lufkin T. (2004). Hmx2 and Hmx3 homeobox genes direct development of the murine inner ear and hypothalamus and can be functionally replaced by Drosophila Hmx. Dev. Cell 7, 439-453. 10.1016/j.devcel.2004.06.016 [DOI] [PubMed] [Google Scholar]
- Wilson L. C. and Trembath R. C. (1994). Albright's hereditary osteodystrophy. J. Med. Genet. 31, 779-784. 10.1136/jmg.31.10.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D. K. and Kelley M. W. (2012). Molecular mechanisms of inner ear development. Cold Spring Harb. Perspect. Biol. 4, a008409 10.1101/cshperspect.a008409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D. K. and Oh S.-H. (1996). Sensory organ generation in the chick inner ear. J. Neurosci. 16, 6454-6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H., Wieser R., Massague J. and Niswander L. (1997). Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 11, 2191-2203. 10.1101/gad.11.17.2191 [DOI] [PMC free article] [PubMed] [Google Scholar]