Abstract
Rationale:
Several studies have demonstrated links between infectious diseases and cardiovascular conditions. Malaria and hypertension are widespread in many low- and middle-income countries, but the possible link between them has not been considered.
Objective:
In this article, we outline the basis for a possible link between malaria and hypertension and discuss how the hypothesis could be confirmed or refuted.
Methods and Results:
We reviewed published literature on factors associated with hypertension and checked whether any of these were also associated with malaria. We then considered various study designs that could be used to test the hypothesis. Malaria causes low birth weight, malnutrition, and inflammation, all of which are associated with hypertension in high-income countries. The hypothetical link between malaria and hypertension can be tested through the use of ecological, cohort, or Mendelian randomization studies, each of which poses specific challenges.
Conclusions:
Confirmation of the existence of a causative link with malaria would be a paradigm shift in efforts to prevent and control hypertension and would stimulate wider research on the links between infectious and noncommunicable disease.
Keywords: arterial stiffness, blood pressure, epidemiology, inflammation, malaria
Age standardized mean blood pressures (BPs) are higher in many parts of Asia and sub-Saharan Africa than in high-income countries.1 Despite the high burden of cardiovascular disease in low- and middle-income countries (LMIC), few studies have examined their pathophysiology or treatment.2,3 Although demographic and lifestyle changes including urbanization4 contribute substantially to the burden of hypertension in LMICs, examining factors unique to or more prevalent in LMIC settings might reveal new pathophysiological mechanisms that could aid efforts to control the condition.
Editorial, see p 7
In This Issue, see p 2
The rise of cardiovascular disease in LMIC is occurring against the background of continuing high burden of infectious diseases.5,6 Several studies in more developed settings have reported links between infectious or inflammatory conditions and cardiovascular disease. In this article, we outline the hypothesis that hypertension, the leading risk factor for death in LMIC,7 could be linked to one of the leading infectious conditions in the same region, malaria.
The Malaria–Hypertension Hypothesis
We postulate that malaria contributes to the burden of hypertension in LMIC in the following ways:
1. Malaria in pregnancy leads to low birth weight through pathophysiologically connected mechanisms.8 In areas with high malaria endemicity where women are likely to have acquired immunity to prevent most febrile episodes, low birth weight results from fetal growth restriction, which is a consequence of impaired uteroplacental blood flow9 and maternal anemia (which is itself because of malaria).10,11 Febrile malaria episodes that are more likely among women with low immunity are thought to induce uterine contractions, which are mediated by elevated levels of tumor necrosis factor-α leading to preterm birth.12,13 Malaria is also associated with hypertensive disorders of pregnancy such as gestational hypertension and preeclampsia in young primigravid women,14–16 and these are risk factors for low birth weight.17 Low birth weight children have an increased incidence of hypertension in later life.18–21 In a study conducted in Ibadan, Nigeria, infants of mothers who experienced malaria during pregnancy had a higher increase in BP levels during the first year of life compared with those who did not.22 Because BP levels track strongly through to adulthood, such differences could significantly influence the prevalence of adult hypertension.23–25 By virtue of its association with hypertensive disorders of pregnancy that are themselves risk factors for essential hypertension in women,26,27 malaria likely contributes to an intergenerational vicious cycle of disease susceptibility because hypertensive parents bear children who develop hypertension more frequently.28,29
2. Malaria is associated with stunting and malnutrition in childhood,30,31 which predisposes to the development of hypertension in later life.19,23,32
Although the biological pathways have not been fully characterized, postulated mechanisms involved in the development of hypertension after stunting and chronic malnutrition include reduced nephron numbers18 and premature senescence in the kidney, which is particularly prominent when there is rapid weight gain after growth restriction.33 In addition, Jamaican survivors of severe acute malnutrition in childhood were found at the age of 30 years to have markedly smaller left ventricular outflow tracts with reduced cardiac output in the presence of elevated peripheral resistance, a pattern of changes that is likely to lead to hypertension in later life.34
3. Malaria is a cause of chronic inflammation,35 and inflammation predisposes to cardiovascular diseases in high-income countries.36 In a prospective study of 20 525 female US health professionals, there was a linear relationship between baseline C-reactive protein levels and incident hypertension.37 Patients with inflammatory bowel disease and rheumatoid arthritis have increased arterial stiffness, which precedes hypertension.38–40 The link between inflammatory conditions and hypertension may be related to perturbations in the levels of endothelial-based growth factors. Angiopoietin-2 (Ang-2) is a multimeric ligand of the Tie 2 receptor, part of a vascular-specific tyrosine kinase signaling pathway that is essential for vessel development and stability.41 Ang-2 is predominantly secreted by endothelial cells and some smooth muscle cells in many inflammatory and angiogenic states (Figure). Ang-2 levels are elevated in children with severe malaria in several different settings35,42–45 and in returning travellers infected with malaria.46 Ang-2 levels predict cardiovascular disease in children with chronic kidney disease.47 Although no causal association has been established, several studies have demonstrated an association between Ang-2 levels and arterial stiffness and BP in adults.48,49
Figure.
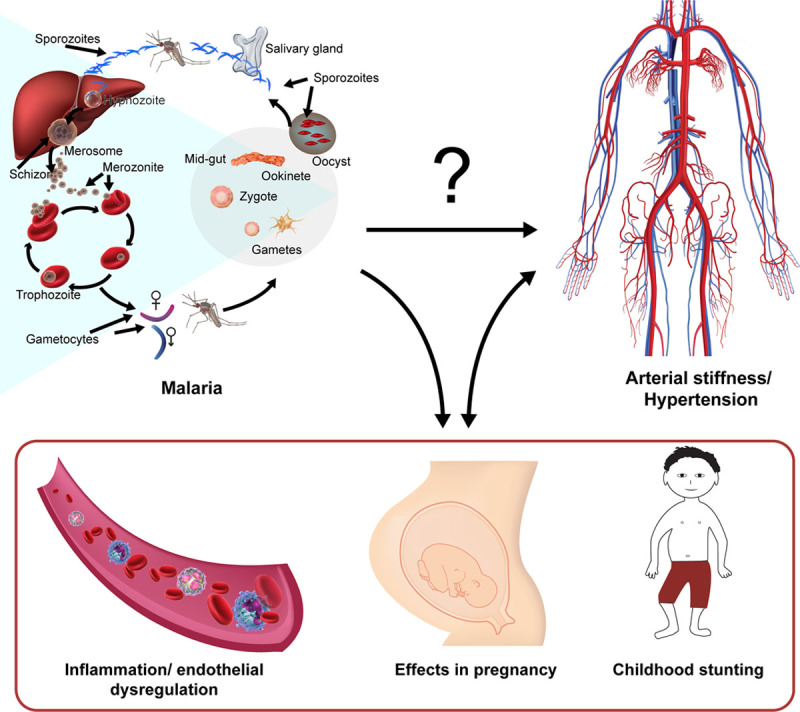
The malaria-high blood pressure hypothesis. Malaria is known to cause low birth weight, inflammation as well as stunting. All these factors have been separately associated with the development of high blood pressure in high-income countries. Studies are needed to confirm whether malaria contributes to the development of high blood pressure in low- and middle-income countries.
Testing the Hypothesis
Observational Studies
Ecological studies examining BP levels in relation to malaria incidence are hampered by the lack of finely scaled data on the relevant BP distributions. A worldwide study on BP levels had scarce raw data on BP from sub-Saharan Africa, where most malaria endemic countries are situated.1 In contrast, there are good epidemiological data on the spatial and temporal distribution of malaria.50
Although traditional case–control studies (with malaria as the exposure and hypertension as the outcome) could provide an efficient way to test the hypothesis, they are limited by the nonspecificity and nondurability of immunologic markers for malaria, a prerequisite for identifying individuals who have previously been exposed to malaria.51–53 This limitation also applies to the potential use of propensity scores54 to assemble groups that are comparable in their risk of malaria; in order to generate such scores reliable immunologic markers of malaria exposure would be needed.
A life-course epidemiological approach with longitudinal cohorts from the antenatal period or birth would allow the study of many of the postulated pathways through which malaria could be leading to hypertension in LMIC. Studies of pregnant women and children exposed to malaria in demographic surveillance systems with good quality ascertainment of exposure status are necessary. However, most demographic surveillance systems in LMIC were only set up in the last 15 years, and therefore may not have accumulated enough follow-up time to examine these outcomes, assuming that the biases of such retrospective studies can be overcome. Prospective surveillance, however, would also require long follow-up and be expensive to conduct.
Randomized Intervention Studies
Because malaria is of such great public interest, there have been and will continue to be a succession of randomized controlled trials of interventions tested at population level, such as vaccines, bed nets, and drug treatments. For those interventions that turn out to be effective it might be possible to examine their effect on arterial stiffness and BP. Prospective studies of interventions known to be effective as well as studies of controlled human malaria infection55 cannot be used here because although they satisfy the criterion of having 2 randomized groups with and without malaria, the fact that the vascular outcomes being tested are potentially irreversible pose an ethical challenge.56 As with observational studies, extended follow-up might be needed as vascular differences in trial groups because of the effects of malaria may take longer to be apparent compared with the antimalarial effects of the interventions.
Animal studies are hampered by the fact that murine models of malaria and hypertension are imperfect approximations of their human analogs that have complex pathophysiology.57,58
Genetic Studies
Mendelian randomization studies, where genetic polymorphisms are used as instrumental variables representing malaria exposure, would be particularly attractive for answering the question as they overcome many of the limitations of observational and intervention studies described above.59 Several hemoglobin polymorphisms provide some level of protection against malaria, including Hemoglobin C and S, and thalassemia.60–62 A comparison of arterial stiffness indices and BP in subjects with and without the polymorphisms in regions where they have been exposed to malaria in childhood would provide a robust test of the effect of malaria exposure on the development of hypertension.
An important prerequisite for using Mendelian randomization to make causal inferences about the effects of environmental exposures, is that the polymorphisms should not display pleiotropic effects, that is, they should not influence the outcome being studied through a pathway that is independent of the environmental exposure that they are being used as a proxy for.59 Some studies suggest that individuals with the sickle cell trait (SCT) are more likely to have cardiovascular events especially under extreme conditions, such as military training or athletics.63,64 A recent study among blacks found similar baseline BP in those with and without SCT, although on follow-up there was an increased incidence of chronic kidney disease in individuals with SCT.65 To exclude the possibility of confounding by pleiotropy, it may be necessary to include a control group that has not been exposed to malaria or use additional independent genetic polymorphisms, such as α thalassemia. If malaria causes hypertension and there are no pleiotropic effects of SCT, then one would expect to find higher BP in individuals without SCT compared with those with SCT in groups that have been exposed to malaria. Conversely, there would be no difference in BP based on trait status among those who have not been exposed to malaria.
Implications of the Hypothesis
Current efforts at understanding hypertension in LMIC have had a narrow focus anchored on traditional risk factors identified among populations in high-income countries. Confirmation of the causative role of malaria in elevating BP would be of immense scientific interest and could lead to a paradigm shift on how to control hypertension in LMIC.
Malaria is only one of many infectious diseases that have a high incidence across LMICs. The inflammatory pathways activated in malaria infection are similar to those of other illnesses.66 It is therefore likely that if malaria contributes to the burden of hypertension through inflammation, the same could be true of other chronic infections, such as HIV and tuberculosis, providing a novel impetus for the study and control of these infections. Currently, most treatment for infectious illnesses is focused on eliminating the pathogen with little regard for modulating the inflammatory responses that might result in adverse vascular consequences later. Elucidating these inflammatory pathways and their consequences would pave the way for trials of adjunctive therapy such as statins or specific cytokine antagonists to prevent adverse vascular remodeling as a result of infection.
Sources of Funding
A.O. Etyang, L. Smeeth, and J.A.G. Scott are funded by the Wellcome Trust (Fellowship numbers: 103951/Z/14/Z, 098532 and 098504). The Funders played no role in the preparation of this article.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- Ang-2
- angiopoietin-2
- BP
- blood pressure
- LMIC
- low and middle-income countries
- SCT
- sickle cell trait
In March 2016, the average time from submission to first decision for all original research papers submitted to Circulation Research was 14.97 days.
Novelty and Significance
What Is Known?
Malaria and high blood pressure are widespread in low- and middle-income countries.
Malaria is known to cause low birth weight, stunting, and inflammation.
Low birth weight, stunting, and inflammation are associated with the development of arterial stiffness and high blood pressure in developed countries.
What New Information Does This Article Contribute?
We outline the likely pathophysiological mechanisms of the hypothesized association between malaria and high blood pressure in low- and middle-income countries.
This hypothesis can be tested through well-designed ecological and cohort studies or using Mendelian randomization techniques.
Despite the knowledge that malaria causes low birth weight, inflammation, and stunting, all of which have been associated with the development of hypertension, no attempt has been made to link the 2 conditions. In this article, we review the literature in support of the hypothesis that malaria could be contributing to the widespread problem of hypertension in low- and middle-income countries. We also outline several ways in which this hypothesis could be proven or refuted. If proven, this link would be a paradigm shift in efforts to prevent and control high blood pressure in low- and middle-income countries.
References
- 1.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Pressure) National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 2.Holmes MD, Dalal S, Volmink J, Adebamowo CA, Njelekela M, Fawzi WW, Willett WC, Adami HO. Non-communicable diseases in sub-Saharan Africa: the case for cohort studies. PLoS Med. 2010;7:e1000244. doi: 10.1371/journal.pmed.1000244. doi: 10.1371/journal.pmed.1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim MM, Damasceno A. Hypertension in developing countries. Lancet. 2012;380:611–619. doi: 10.1016/S0140-6736(12)60861-7. doi: 10.1016/S0140-6736(12)60861-7. [DOI] [PubMed] [Google Scholar]
- 5.Mayosi BM, Flisher AJ, Lalloo UG, Sitas F, Tollman SM, Bradshaw D. The burden of non-communicable diseases in South Africa. Lancet. 2009;374:934–947. doi: 10.1016/S0140-6736(09)61087-4. doi: 10.1016/S0140-6736(09)61087-4. [DOI] [PubMed] [Google Scholar]
- 6.Etyang AO, Munge K, Bunyasi EW, et al. Burden of disease in adults admitted to hospital in a rural region of coastal Kenya: an analysis of data from linked clinical and demographic surveillance systems. Lancet Glob Health. 2014;2:e216–e224. doi: 10.1016/S2214-109X(14)70023-3. doi: 10.1016/S2214-109X(14)70023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 9.Dorman EK, Shulman CE, Kingdom J, Bulmer JN, Mwendwa J, Peshu N, Marsh K. Impaired uteroplacental blood flow in pregnancies complicated by falciparum malaria. Ultrasound Obstet Gynecol. 2002;19:165–170. doi: 10.1046/j.0960-7692.2001.00545.x. doi: 10.1046/j.0960-7692.2001.00545.x. [DOI] [PubMed] [Google Scholar]
- 10.Kalilani L, Mofolo I, Chaponda M, Rogerson SJ, Meshnick SR. The effect of timing and frequency of Plasmodium falciparum infection during pregnancy on the risk of low birth weight and maternal anemia. Trans R Soc Trop Med Hyg. 2010;104:416–422. doi: 10.1016/j.trstmh.2010.01.013. doi: 10.1016/j.trstmh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 12.Looareesuwan S, Phillips RE, White NJ, Kietinun S, Karbwang J, Rackow C, Turner RC, Warrell DA. Quinine and severe falciparum malaria in late pregnancy. Lancet. 1985;2:4–8. doi: 10.1016/s0140-6736(85)90056-x. doi: 10.1016/S0140-6736(85)90056-X. [DOI] [PubMed] [Google Scholar]
- 13.Aidoo M, McElroy PD, Kolczak MS, Terlouw DJ, ter Kuile FO, Nahlen B, Lal AA, Udhayakumar V. Tumor necrosis factor-alpha promoter variant 2 (TNF2) is associated with pre-term delivery, infant mortality, and malaria morbidity in western Kenya: Asembo Bay Cohort Project IX. Genet Epidemiol. 2001;21:201–211. doi: 10.1002/gepi.1029. doi: 10.1002/gepi.1029. [DOI] [PubMed] [Google Scholar]
- 14.Ndao CT, Dumont A, Fievet N, Doucoure S, Gaye A, Lehesran JY. Placental malarial infection as a risk factor for hypertensive disorders during pregnancy in Africa: a case-control study in an urban area of Senegal, West Africa. Am J Epidemiol. 2009;170:847–853. doi: 10.1093/aje/kwp207. doi: 10.1093/aje/kwp207. [DOI] [PubMed] [Google Scholar]
- 15.Duffy PE. Plasmodium in the placenta: parasites, parity, protection, prevention and possibly preeclampsia. Parasitology. 2007;134:1877–1881. doi: 10.1017/S0031182007000170. doi: 10.1017/S0031182007000170. [DOI] [PubMed] [Google Scholar]
- 16.Muehlenbachs A, Mutabingwa TK, Edmonds S, Fried M, Duffy PE. Hypertension and maternal-fetal conflict during placental malaria. PLoS Med. 2006;3:e446. doi: 10.1371/journal.pmed.0030446. doi: 10.1371/journal.pmed.0030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mol BW, Roberts CT, Thangaratinam S, Magee LA, de Groot CJ, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 18.Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes–a global concern. Nat Rev Nephrol. 2015;11:135–149. doi: 10.1038/nrneph.2014.251. doi: 10.1038/nrneph.2014.251. [DOI] [PubMed] [Google Scholar]
- 19.Wadsworth ME, Cripps HA, Midwinter RE, Colley JR. Blood pressure in a national birth cohort at the age of 36 related to social and familial factors, smoking, and body mass. BMJ. (Clin Res Ed) 1985;291:1534–1538. doi: 10.1136/bmj.291.6508.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewandowski AJ, Davis EF, Yu G, Digby JE, Boardman H, Whitworth P, Singhal A, Lucas A, McCormick K, Shore AC, Leeson P. Elevated blood pressure in preterm-born offspring associates with a distinct antiangiogenic state and microvascular abnormalities in adult life. Hypertension. 2015;65:607–614. doi: 10.1161/HYPERTENSIONAHA.114.04662. doi: 10.1161/HYPERTENSIONAHA.114.04662. [DOI] [PubMed] [Google Scholar]
- 21.Bertagnolli M, Luu TM, Lewandowski AJ, Leeson P, Nuyt AM. Preterm birth and hypertension: is there a link? Curr Hypertens Rep. 2016;18:28. doi: 10.1007/s11906-016-0637-6. doi: 10.1007/s11906-016-0637-6. [DOI] [PubMed] [Google Scholar]
- 22.Ayoola OO, Omotade OO, Gemmell I, Clayton PE, Cruickshank JK. The impact of malaria in pregnancy on changes in blood pressure in children during their first year of life. Hypertension. 2014;63:167–172. doi: 10.1161/HYPERTENSIONAHA.113.02238. doi: 10.1161/HYPERTENSIONAHA.113.02238. [DOI] [PubMed] [Google Scholar]
- 23.Cruickshank JK, Mzayek F, Liu L, Kieltyka L, Sherwin R, Webber LS, Srinavasan SR, Berenson GS. Origins of the “black/white” difference in blood pressure: roles of birth weight, postnatal growth, early blood pressure, and adolescent body size: the Bogalusa heart study. Circulation. 2005;111:1932–1937. doi: 10.1161/01.CIR.0000161960.78745.33. doi: 10.1161/01.CIR.0000161960.78745.33. [DOI] [PubMed] [Google Scholar]
- 24.Juhola J, Magnussen CG, Viikari JS, Kähönen M, Hutri-Kähönen N, Jula A, Lehtimäki T, Åkerblom HK, Pietikäinen M, Laitinen T, Jokinen E, Taittonen L, Raitakari OT, Juonala M. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159:584–590. doi: 10.1016/j.jpeds.2011.03.021. doi: 10.1016/j.jpeds.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WC. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326:845. doi: 10.1136/bmj.326.7394.845. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Männistö T, Mendola P, Vääräsmäki M, Järvelin MR, Hartikainen AL, Pouta A, Suvanto E. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681–690. doi: 10.1161/CIRCULATIONAHA.112.128751. doi: 10.1161/CIRCULATIONAHA.112.128751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang NY, Young JH, Meoni LA, Ford DE, Erlinger TP, Klag MJ. Blood pressure change and risk of hypertension associated with parental hypertension: the Johns Hopkins Precursors Study. Arch Intern Med. 2008;168:643–648. doi: 10.1001/archinte.168.6.643. doi: 10.1001/archinte.168.6.643. [DOI] [PubMed] [Google Scholar]
- 29.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, Leeson P. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–e1561. doi: 10.1542/peds.2011-3093. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 30.Kang H, Kreuels B, Adjei O, Krumkamp R, May J, Small DS. The causal effect of malaria on stunting: a Mendelian randomization and matching approach. Int J Epidemiol. 2013;42:1390–1398. doi: 10.1093/ije/dyt116. doi: 10.1093/ije/dyt116. [DOI] [PubMed] [Google Scholar]
- 31.Snow RW, Molyneux CS, Njeru EK, Omumbo J, Nevill CG, Muniu E, Marsh K. The effects of malaria control on nutritional status in infancy. Acta Trop. 1997;65:1–10. doi: 10.1016/s0001-706x(96)00601-8. doi: 10.1016/S0001-706X(96)00601-8. [DOI] [PubMed] [Google Scholar]
- 32.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, Martyn CN, de Swiet M. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105:1088–1092. doi: 10.1161/hc0902.104677. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- 33.Luyckx VA, Bertram JF, Brenner BM, Fall C, Hoy WE, Ozanne SE, Vikse BE. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. 2013;382:273–283. doi: 10.1016/S0140-6736(13)60311-6. doi: 10.1016/S0140-6736(13)60311-6. [DOI] [PubMed] [Google Scholar]
- 34.Tennant IA, Barnett AT, Thompson DS, Kips J, Boyne MS, Chung EE, Chung AP, Osmond C, Hanson MA, Gluckman PD, Segers P, Cruickshank JK, Forrester TE. Impaired cardiovascular structure and function in adult survivors of severe acute malnutrition. Hypertension. 2014;64:664–671. doi: 10.1161/HYPERTENSIONAHA.114.03230. doi: 10.1161/HYPERTENSIONAHA.114.03230. [DOI] [PubMed] [Google Scholar]
- 35.Moxon CA, Chisala NV, Wassmer SC, Taylor TE, Seydel KB, Molyneux ME, Faragher B, Kennedy N, Toh CH, Craig AG, Heyderman RS. Persistent endothelial activation and inflammation after Plasmodium falciparum Infection in Malawian children. J Infect Dis. 2014;209:610–615. doi: 10.1093/infdis/jit419. doi: 10.1093/infdis/jit419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 37.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–2951. doi: 10.1001/jama.290.22.2945. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 38.Zanoli L, Cannavò M, Rastelli S, Di Pino L, Monte I, Di Gangi M, Boutouyrie P, Inserra G, Laurent S, Castellino P. Arterial stiffness is increased in patients with inflammatory bowel disease. J Hypertens. 2012;30:1775–1781. doi: 10.1097/HJH.0b013e3283568abd. doi: 10.1097/HJH.0b013e3283568abd. [DOI] [PubMed] [Google Scholar]
- 39.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gkaliagkousi E, Gavriilaki E, Doumas M, Petidis K, Aslanidis S, Stella D. Cardiovascular risk in rheumatoid arthritis: pathogenesis, diagnosis, and management. J Clin Rheumatol. 2012;18:422–430. doi: 10.1097/RHU.0b013e31827846b1. doi: 10.1097/RHU.0b013e31827846b1. [DOI] [PubMed] [Google Scholar]
- 41.Thurston G, Daly C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb Perspect Med. 2012;2:a006550. doi: 10.1101/cshperspect.a006650. doi: 10.1101/cshperspect.a006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conroy AL, Glover SJ, Hawkes M, Erdman LK, Seydel KB, Taylor TE, Molyneux ME, Kain KC. Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: a retrospective case-control study*. Crit Care Med. 2012;40:952–959. doi: 10.1097/CCM.0b013e3182373157. doi: 10.1097/CCM.0b013e3182373157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain V, Lucchi NW, Wilson NO, Blackstock AJ, Nagpal AC, Joel PK, Singh MP, Udhayakumar V, Stiles JK, Singh N. Plasma levels of angiopoietin-1 and -2 predict cerebral malaria outcome in Central India. Malar J. 2011;10:383. doi: 10.1186/1475-2875-10-383. doi: 10.1186/1475-2875-10-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, Piera K, Price RN, Duffull SB, Celermajer DS, Anstey NM. Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci U S A. 2008;105:17097–17102. doi: 10.1073/pnas.0805782105. doi: 10.1073/pnas.0805782105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lovegrove FE, Tangpukdee N, Opoka RO, Lafferty EI, Rajwans N, Hawkes M, Krudsood S, Looareesuwan S, John CC, Liles WC, Kain KC. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One. 2009;4:e4912. doi: 10.1371/journal.pone.0004912. doi: 10.1371/journal.pone.0004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacMullin G, Mackenzie R, Lau R, Khang J, Zhang H, Rajwans N, Liles WC, Pillai DR. Host immune response in returning travellers infected with malaria. Malar J. 2012;11:148. doi: 10.1186/1475-2875-11-148. doi: 10.1186/1475-2875-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shroff RC, Price KL, Kolatsi-Joannou M, Todd AF, Wells D, Deanfield J, Johnson RJ, Rees L, Woolf AS, Long DA. Circulating angiopoietin-2 is a marker for early cardiovascular disease in children on chronic dialysis. PLoS One. 2013;8:e56273. doi: 10.1371/journal.pone.0056273. doi: 10.1371/journal.pone.0056273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marketou ME, Kontaraki JE, Tsakountakis NA, Zacharis EA, Kochiadakis GE, Arfanakis DA, Chlouverakis G, Vardas PE. Arterial stiffness in hypertensives in relation to expression of angiopoietin-1 and 2 genes in peripheral monocytes. J Hum Hypertens. 2010;24:306–311. doi: 10.1038/jhh.2009.95. doi: 10.1038/jhh.2009.95. [DOI] [PubMed] [Google Scholar]
- 49.David S, Kümpers P, Lukasz A, Kielstein JT, Haller H, Fliser D. Circulating angiopoietin-2 in essential hypertension: relation to atherosclerosis, vascular inflammation, and treatment with olmesartan/pravastatin. J Hypertens. 2009;27:1641–1647. doi: 10.1097/HJH.0b013e32832be575. doi: 10.1097/HJH.0b013e32832be575. [DOI] [PubMed] [Google Scholar]
- 50.Noor AM, Kinyoki DK, Mundia CW, Kabaria CW, Mutua JW, Alegana VA, Fall IS, Snow RW. The changing risk of Plasmodium falciparum malaria infection in Africa: 2000-10: a spatial and temporal analysis of transmission intensity. Lancet. 2014;383:1739–1747. doi: 10.1016/S0140-6736(13)62566-0. doi: 10.1016/S0140-6736(13)62566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akpogheneta OJ, Duah NO, Tetteh KK, Dunyo S, Lanar DE, Pinder M, Conway DJ. Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun. 2008;76:1748–1755. doi: 10.1128/IAI.01333-07. doi: 10.1128/IAI.01333-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 53.Ndungu FM, Olotu A, Mwacharo J, Nyonda M, Apfeld J, Mramba LK, Fegan GW, Bejon P, Marsh K. Memory B cells are a more reliable archive for historical antimalarial responses than plasma antibodies in no-longer exposed children. Proc Natl Acad Sci U S A. 2012;109:8247–8252. doi: 10.1073/pnas.1200472109. doi: 10.1073/pnas.1200472109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6:604–611. doi: 10.1161/CIRCOUTCOMES.113.000359. doi: 10.1161/CIRCOUTCOMES.113.000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spring M, Polhemus M, Ockenhouse C. Controlled human malaria infection. J Infect Dis. 2014;209(suppl 2):S40–S45. doi: 10.1093/infdis/jiu063. doi: 10.1093/infdis/jiu063. [DOI] [PubMed] [Google Scholar]
- 56.Jamrozik E, de la Fuente-Núñez V, Reis A, Ringwald P, Selgelid MJ. Ethical aspects of malaria control and research. Malar J. 2015;14:518. doi: 10.1186/s12936-015-1042-3. doi: 10.1186/s12936-015-1042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Craig AG, Grau GE, Janse C, Kazura JW, Milner D, Barnwell JW, Turner G, Langhorne J Participants of the Hinxton Retreat meeting on Animal Models for Research on Severe Malaria. The role of animal models for research on severe malaria. PLoS Pathog. 2012;8:e1002401. doi: 10.1371/journal.ppat.1002401. doi: 10.1371/journal.ppat.1002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen D, Coffman TM. The kidney and hypertension: lessons from mouse models. Can J Cardiol. 2012;28:305–310. doi: 10.1016/j.cjca.2012.01.002. doi: 10.1016/j.cjca.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 60.Hill AV, Allsopp CE, Kwiatkowski D, Anstey NM, Twumasi P, Rowe PA, Bennett S, Brewster D, McMichael AJ, Greenwood BM. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 61.Williams TN, Wambua S, Uyoga S, Macharia A, Mwacharo JK, Newton CR, Maitland K. Both heterozygous and homozygous alpha+ thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood. 2005;106:368–371. doi: 10.1182/blood-2005-01-0313. doi: 10.1182/blood-2005-01-0313. [DOI] [PubMed] [Google Scholar]
- 62.Malaria Genomic Epidemiology N. Reappraisal of known malaria resistance loci in a large multicenter study. Nat. Genet. 2014;46:1197–1204. doi: 10.1038/ng.3107. doi: 10.1038/ng.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris KM, Haas TS, Eichner ER, Maron BJ. Sickle cell trait associated with sudden death in competitive athletes. Am J Cardiol. 2012;110:1185–1188. doi: 10.1016/j.amjcard.2012.06.004. doi: 10.1016/j.amjcard.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Kark JA, Posey DM, Schumacher HR, Ruehle CJ. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med. 1987;317:781–787. doi: 10.1056/NEJM198709243171301. doi: 10.1056/NEJM198709243171301. [DOI] [PubMed] [Google Scholar]
- 65.Naik RP, Derebail MD, Franceschini MD, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA. 2015;21287:2115–2125.. doi: 10.1001/jama.2014.15063. doi: 10.1001/jama.2014.15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Page AV, Liles WC. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence. 2013;4:507–516. doi: 10.4161/viru.24530. doi: 10.4161/viru.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]


